Abstract
Crimean-Congo hemorrhagic fever virus (CCHFV) causes viral hemorrhagic fever with high case-fatality rates and is geographically widely distributed. Due to the requirement for a biosafety level 4 (BSL-4) laboratory and the lack of an animal model, knowledge of the viral pathogenesis is limited. Crimean-Congo hemorrhagic fever (CCHF) is characterized by hemorrhage and vascular permeability, indicating the involvement of endothelial cells (ECs). The interplay between ECs and CCHFV is therefore important for understanding the pathogenesis of CCHF. In a previous study, we found that CCHFV-infected monocyte-derived dendritic cells (moDCs) activated ECs; however, the direct effect of CCHFV on ECs was not investigated. Here, we report that ECs are activated upon infection, as demonstrated by upregulation of mRNA levels for E-selectin, vascular cell adhesion molecule 1 (VCAM1), and intercellular adhesion molecule 1 (ICAM1). Protein levels and cell surface expression of ICAM1 responded in a dose-dependent manner to increasing CCHFV titers with concomitant increase in leukocyte adhesion. Furthermore, we examined vascular endothelial (VE) cadherin in CCHFV-infected ECs by different approaches. Infected ECs released higher levels of interleukin 6 (IL-6) and IL-8; however, stimulation of resting ECs with supernatants derived from infected ECs did not result in increased ICAM1 expression. Interestingly, the moDC-mediated activation of ECs was abrogated by addition of neutralizing tumor necrosis factor alpha (TNF-α) antibody to moDC supernatants, thereby identifying this soluble mediator as the key cytokine causing EC activation. We conclude that CCHFV can exert both direct and indirect effects on ECs.
INTRODUCTION
Crimean-Congo hemorrhagic fever virus (CCHFV) belongs to the genus Nairovirus in the family Bunyaviridae. It is a viral hemorrhagic fever (VHF) and causes the severe human disease Crimean-Congo hemorrhagic fever (CCHF). The case-fatality rate of CCHF varies depending on the geographic region and transmission route but can be as high as 50% (20, 39, 45). Cardinal signs of CCHF include hemorrhage and increased vascular permeability accompanied by severe thrombocytopenia (45). Infection occurs by tick bites or contact with contaminated blood or tissues from patients or livestock (45). Among the medically important tick-borne viruses, CCHFV has one of the most widespread distributions (15, 25).
The endothelium is supposedly a major target in CCHF, as indicated by clinical features, such as hemorrhage and increased vascular permeability (39). In autopsies of deceased CCHF patients, viral antigen was present in endothelial cells (ECs) (6), and molecular endothelial activation markers correlated with disease severity (4, 27). The involvement of the endothelium for CCHF and other VHFs has classically been explained by one of two theories: virus infection activates endothelial cells (i) directly or (ii) indirectly via infected leukocytes releasing soluble mediators with concomitant activation of the endothelium (20, 34). Previously, studies have focused on the indirect role of the endothelium, since CCHFV infects dendritic cells and macrophages (11, 30) and soluble mediators from CCHFV-infected dendritic cells activated ECs (30). Further support for an indirect effect of ECs for other VHFs comes from the identification of tumor necrosis factor alpha (TNF-α) as a key mediator of endothelial cell activation (1, 8, 17). A direct effect on ECs was recently shown by hantaviruses, with loss of barrier integrity followed by increased permeability due to internalization of vascular endothelial (VE) cadherin (35), an important component of adherens junctions (40, 44). Endothelial barrier integrity is maintained by both tight junctions and adherens junctions, and deregulation of either can lead to increased vascular permeability (3). However, to our knowledge, nothing is known with regard to the effect of CCHFV infection on endothelial cells.
Elevated serum levels of the proinflammatory cytokines interleukin 6 (IL-6) and TNF-α were correlated with CCHF disease severity in several patient studies, and IL-8 levels were increased in a fatal case of CCHF in Greece (16, 28, 29, 33). These cytokines were also released from CCHFV-infected dendritic cells (DCs) and macrophages (11, 30). To our knowledge, no studies have been performed focusing on the ability of CCHFV to induce release of proinflammatory cytokines from ECs.
The activation of endothelial cells increases vascular permeability, initiates inflammatory responses, and recruits leukocytes by the upregulation of the leukocyte adhesion molecules ICAM1, vascular cell adhesion molecule 1 (VCAM1), and E-selectin (41). ICAM1 is minimally expressed on resting endothelial cells and is upregulated in response to inflammatory mediators, such as virus infection and the proinflammatory cytokines IL-1β, IL-6, and TNF-α (31, 38). The activation marker soluble ICAM1 (sICAM1) (42) correlated with CCHF disease severity in two patient studies (4, 27), although it is not yet known whether this marker is derived from endothelial cells or from leukocytes.
In this study, we present the first evidence, to our knowledge, that infected ECs can play a more specific role in mediating CCHF pathogenesis than previously presumed. We show that CCHFV efficiently infects and consequently activates ECs, which is characterized by upregulation of ICAM1 and release of soluble mediators, such as IL-6 and IL-8. Endothelial cell activation correlated with the degree of leukocyte adhesion in vitro. Both the virus-mediated EC activation and concomitant leukocyte adhesion occurred in a virus dose-dependent manner. Endothelial cell activation by virus-infected moDCs was dependent on TNF-α, whereas a similar paracrine mechanism for ECs could not be observed. We conclude that CCHFV can target endothelial cells both directly and indirectly by the effect of cytokines released from dendritic cells.
MATERIALS AND METHODS
Generation of virus stock and determination of virus titer.
Virus stock was produced by infecting Vero cells with CCHFV strain Ibar 10200, isolated from Hyalomma excavatum ticks in 1966 (5), and collecting supernatants at 48 h postinfection (p.i.). The virus titer was determined by fluorescence focus unit (FFU) assay. Harvested supernatants were serially diluted and added to Vero cells grown in 96-well plates (Sarstedt, Nürnbrecht, Germany). The supernatants were removed after 1 h absorption at 37°C and the cell layer was washed with phosphate-buffered saline (PBS). The cells were fixed in ice-cold 80% acetone at 24 h p.i., and fluorescent foci were generated by indirect immunofluorescence assay (IFA). All calculations of multiplicity of infection (MOI) are based on viral titration on Vero cells.
Endothelial cell infection and stimulation.
Human umbilical vein endothelial cells (HUVECs) (Lonza, Walkersville, MD) were cultured in gelatin-coated flasks (Invitrogen, Carlsbad, CA) containing either endothelial growth medium (EGM) (Lonza, Walkersville, MD) or EGM-2 (Promocell, Heidelberg, Germany) and used for experiments between passages 3 and 6. Confluent HUVECs were infected with CCHFV at an MOI of 10 or, to study the dose-response effect, at MOIs of 1, 10, and 20. As negative controls, cells were either left untreated (mock) or treated with UV-inactivated virus stock, which had been inactivated with UV irradiation for 1 min (UV mineral light lamp, model UVG-54; 254 nm; UVP, Upland, CA). Cells treated with either TNF-α at 2 ng/ml or 10 ng/ml (RnD Systems, United Kingdom) or 100 μg/ml lipopolysaccharide (LPS) (11) served as positive controls. Incubations were performed for up to 6, 24, 48, and 72 h p.i. at 37°C in a 5% CO2 humidified atmosphere. All handling of the virus and infected material occurred in a biosafety level 4 (BSL-4) laboratory at the Swedish Institute for Infectious Disease Control, Solna, Sweden.
IFA.
Determination of the virus titer and verification of viral infection of endothelial cells were performed by addition of primary rabbit anti-CCHFV nucleocapsid protein (NP) (2), followed by washing in PBS and further incubation with swine anti-rabbit fluorescein isothiocyanate (FITC)-conjugated antibody (Dako-Cytomation, Copenhangen, Denmark) for 1 h. Foci were quantified on a fluorescence microscope, enabling calculation of progeny virus titers.
HUVEC monolayers fixed with 4% formaldehyde and permeabilized with 0.1% Triton-X were incubated with a primary rabbit anti-CCHFV NP (2) and/or mouse anti VE-cadherin (Santa Cruz Biotechnology Inc.) for 1 h. Slides stained with VE-cadherin were then incubated with anti-rabbit Texas red-conjugated and anti-mouse FITC-conjugated antibodies. DAPI (4′,6-diamidino-2-phenylindole) (Sigma, St. Louis, MO) was added to stain cell nuclei. Slides were analyzed by immunofluorescence microscopy. Images were obtained with a Hamamatsu digital camera (Wasabi 1.4 Hamamatsu; Photonics, GmbH, Germany).
RNA isolation and cDNA generation.
Total RNA was extracted with chloroform from Trizol-treated cells (Invitrogen, Groeningen, Holland). A Qiagen Mini Viral RNA kit (Qiagen, Hilden, Germany) was used to extract RNA from the aqueous phase according to the manufacturer's instructions. cDNA was generated by reverse transcription of purified RNA with Superscript III and random primers (Invitrogen, Paisley, United Kingdom) or with an RT2 first-strand kit (SABiosciences Qiagen, MD) according to the manufacturer's instructions.
Relative quantitative PCR.
Time line studies for transcript analysis for ICAM1, VCAM1, E-selectin, CCHFV NP, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) levels were performed by using manually designed primers for ICAM1, CCHFV NP, and GAPDH (11), and VCAM1 (PPH00623E), E-selectin (PPH00683E), and GAPDH (PPH00150E) were relatively quantified by the use of RT2 quantitative PCR (qPCR) primer assays (SABiosciences Qiagen, MD). Analysis of VE-cadherin levels was performed using an RT2 Profiler PCR array Human Endothelial Cell Biology kit ((PAHS-015C) SABiosciences Qiagen, MD). The samples were amplified in an ABI 7900 HT sequence detection system (Applied Biosystems, Foster City, CA) with 40 cycles (95°C for 15 s and 60°C for 1 min), and a dissociation stage ended the program for quality control of samples. Analysis of the results was performed in SDS 2.3 (Applied Biosystems, Foster City, CA). All results from the individual qPCR assays were normalized against the GAPDH housekeeping gene. All results, apart from those for VE-cadherin, were calibrated against a noninfected control and analyzed by the 2−ΔCT method and then shown as the fold change from levels in noninfected cells to those in infected cells. The VE-cadherin results are normalized to housekeeping genes and analyzed by the 2−ΔCt method.
Western blotting.
Western blotting (WB) was performed as previously described (11). Briefly, lysis buffer was added to infected cells at designated time points, and polypeptides were separated on an SDS-PAGE gel. Electrophoresis was carried out at a constant voltage of 200 V. Proteins were transferred to a nitrocellulose membrane and blocked in 5% milk in PBS containing 0.1% Tween (PBS-T). Upon completion of blocking, the membranes were incubated with rabbit anti-calnexin (36), anti-CCHFV NP (2), anti-vesicular stomatitis virus (VSV) G (Santa Cruz; sc-138076), anti-ICAM1 (Santa Cruz; sc-7891); or anti-VE-cadherin (Santa Cruz; sc-52751). The membranes were developed using Amersham ECL plus Western blotting detection reagents (GE Healthcare, Buckinghamshire, United Kingdom) according to the manufacturer's protocol.
Enzyme immunoassay (EIA).
Confluent HUVECs were cultured in 96-well plates and treated as described above. The experiments were stopped at the indicated time points, and cell layers were fixed in 1.5% formaldehyde, followed by blocking in 5% milk in PBS. The cells were then incubated with antibodies against CD105 (RnD Systems, United Kingdom), ICAM1 (BD Pharmingen, NJ), VCAM1 (Beckman-Coulter, Marseille, France), E-selectin (RnD Systems, United Kingdom), and isotype control (BD Pharmingen, NJ) at 1 μg/ml in PBS. At the end of incubation, the plates were washed repeatedly with PBS to remove residual antibody, and a secondary antibody conjugated with horseradish peroxidase (HRP) (Bio-Rad, Hercules, CA) in 5% milk was added. The cell layer was washed in PBS, the above-mentioned cell membrane proteins were then detected by addition of 3,3′,5,5′-tetramethylbenzidine (TMB) for a maximum of 10 min, and the reaction was stopped by addition of 0.5 M H2SO4. Absorption was measured at 450 nm and plastic background at 620 nm with a Synergy HT microplate reader (Bio-Tek, VT) using KC4 software (Bio-Tek, VT). The nonspecific signal (isotype control) was subtracted from all results, followed by normalization against CD105, which is constitutively expressed in resting HUVECs (26), and ICAM1 levels are shown as percentages of CD105 expression. In some instances, the background expression of ICAM1 in nontreated cells was subtracted from the ICAM1 expression of treated ECs.
Leukocyte adhesion assay.
The method for detecting adhesion of leukocytes to endothelial cells under static conditions was developed and optimized for usage in the BSL-4 laboratory using the protocol described by Kiely et al. (22). Endothelial cell layers were treated with TNF-α (10 ng/ml), mock treated with cell medium, or infected with CCHFV or UV-inactivated CCHFV at an MOI of 10 as described above and incubated until 48 h postinfection. Leukocytes were isolated from buffy coats by Ficoll-Paque (GE Healthcare) density gradient centrifugation as described previously (11) and stained with 2 μM 2′,7″-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) in Hanks balanced salt solution containing 25 mM HEPES (Invitrogen Life Technologies) and 0.5% bovine serum albumin (Sigma). The leukocytes were finally suspended in RPMI 1640 medium containing 10% fetal calf serum (FCS) and 100 U/ml penicillin, 100 M streptomycin (RPMI 10%) (Invitrogen Life Technologies).
The endothelial cell layers were washed extensively with fresh medium and cocultured with BCECF-AM-stained leukocytes for 1 h at 37°C in a 5% CO2 humidified atmosphere. Nonadherent leukocytes were washed away with PBS. Lysis buffer (50 mM Tris-HCl, 0.1% SDS in water, pH 8.2 to 8.4) was added to the remaining cells, the fluorescence of BCECF-AM was detected by exciting the lysates at 485 nm, and emission was read at 528 nm in the Synergy HT microplate reader (Bio-Tek, VT) using KC4 software (Bio-Tek, VT).
Cytokine quantification.
Supernatants from cells treated as described above were collected at different time points and assayed for concentrations of IL-1β, TNF-α, IL-6 (Mabtech, Sweden), and IL-8 (RnD Systems, United Kingdom) by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions.
In vitro differentiation of monocyte-derived dendritic cells.
The generation of moDCs was performed as previously described (11). Peripheral blood mononuclear cells (PBMCs) were enriched for monocytes by plastic adherence, followed by culturing with RPMI 1640 containing 200 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech), 75 ng/ml IL-4 (RnD Systems, United Kingdom), and 10% FCS, 2 mM l-glutamine, 100 U/ml penicillin, 100 M streptomycin, and 2% HEPES (Invitrogen Life Technologies).
Paracrine endothelial cell activation and TNF-α neutralization assay.
Supernatants were conditioned by incubation with noninfected or infected HUVECs or moDCs for 48 h, followed by harvesting and storage at −80°C until further use. Confluent HUVECs were incubated with cell medium or virus stock (negative controls), TNF-α (2 or 10 ng/ml) (positive control), or the conditioned supernatants for 6 h. All stimulations were performed in triplicate. After exposure, the cells were fixed in formaldehyde, and detection of cell surface-bound ICAM1 was done according to the method described for EIA. Furthermore, conditioned supernatants from moDCs, cell medium, or TNF-α suspension (2 ng/ml) were preincubated with or without neutralizing antibody against TNF-α (5 μg/ml; catalog no. 554508; BD Pharmingen, NJ)) for 1 h at 37°C. These suspensions were then added to confluent EC layers and incubated for 6 h. Neutralization of activation was measured by upregulation of ICAM1 to the cell surface as described in the cell-based EIA protocol.
Statistical analysis.
The statistical analyses were performed using Graphpad Prism 5.0 software. All group comparisons were based on one-way analysis of variance (ANOVA) or Student's t test, and a P value of <0.05 was considered statistically significant.
RESULTS
Infected endothelial cells upregulate leukocyte adhesion molecules.
To study the kinetics of CCHFV infection and activation of ECs, we exposed ECs to a constant virus dose (MOI, 10) and followed the resulting upregulation of the leukocyte adhesion molecules E-selectin, VCAM1, and ICAM1. Transcript levels of CCHFV NP rapidly increased upon infection, with a concomitant increase in transcripts of these molecules, which peaked at 48 h p.i. (Fig. 1 A). However, when the cell surface levels of E-selectin, VCAM1, and ICAM1 were detected by EIA, only ICAM1 levels were increased significantly (P < 0,01) (Fig. 1B). Upregulation of ICAM1 occurred only in response to native virus, and not in response to UV-inactivated virus (MOI, 10). We then analyzed the sensitivity of the EIA method by comparing the responses of leukocyte adhesion molecules upon treatment with TNF-α (at different concentrations) for 6 h. As shown in Fig. 1C, the method detects E-selectin and VCAM1 at a very low level of TNF-α stimulation; however, the levels of VCAM1 and E-selectin were lower than those of ICAM1. Infectivity was stable throughout the EIA experiments performed and corresponded to approximately 80% of the ECs as determined by IFA (data not shown), whereas no infection could be detected in cells exposed to UV-inactivated virus.
Fig. 1.
Viral infection of ECs leads to upregulation of ICAM1. (A) Confluent HUVECs were treated with cell medium or infected with CCHFV at an MOI of 10 for 6, 24, 48, and 72 h p.i. Total RNA was then extracted from the Trizol-lysed cells, and cDNA was generated with random primers. Relative quantitative PCR was performed to analyze the transcript levels of the EC activation markers E-selectin, VCAM1, and ICAM1, and CCHFV NP RNA was detected to verify infection. The results are normalized against GAPDH and calibrated against a noninfected control sample. Transcripts for all leukocyte adhesion molecules increase at 48 h p.i. and then decrease at 72 h p.i. A minor difference can be observed at 24 h p.i. (approximately 2-fold increase), and no difference can be observed at 6 h p.i. The means and standard deviations (SD) of three independent experiments are shown. (B) Confluent HUVECs were treated with TNF-α (10 ng/ml), cell medium, or native or UV-inactivated virus (MOI, 10) for 48 h, followed by detection of cell surface E-selectin (i), VCAM1 (ii), or ICAM1 (iii). The results were normalized against the control cell surface protein CD105 and are illustrated as percentages of CD105 expression. The cell surface expression of ICAM1 on infected ECs was enhanced compared to noninfected ECs, whereas the increased expression of VCAM1 was not significant (ns), and no increase in E-selectin could be observed. The results are displayed as the means of triplicates plus SD. **, P < 0.01; ***, P < 0.001. The statistical tests applied were ANOVA and Dunnett's multiple-comparision test, and the stimulated cells are all relative to mock infection. (C) In order to determine and compare the sensitivity of the EIA method in detecting cell surface E-selectin, VCAM1, and ICAM1, TNF-α was 10-fold diluted and added to confluent HUVECs for 6 h, followed by fixation and staining for the above-mentioned adhesion molecules. The results were normalized against CD105 expression and are shown as percentages of CD105. The sensitivity of the EIA method is lowest for VCAM1, followed by E-selectin, with the strongest detection of ICAM1 cell surface expression.
EC activation with corresponding leukocyte adhesion occurs in a virus dose-dependent manner.
We studied the role of virus levels in the degree of EC activation and resulting leukocyte adhesion. We found a virus dose-dependent increase in total protein levels of ICAM1 at 48 and 72 h p.i. (Fig. 2 A). At 72 h p.i., the ICAM1 levels also increased at MOIs of 0.1 and 1, with the highest intensity observed at an MOI of 10. The CCHFV NP bands also increased with virus levels and days postinfection. To demonstrate the specificity of ICAM1 upregulation with CCHFV infection, we infected HUVECs with VSV at an MOI of 0.1. At 48 h p.i., cells were harvested for either WB or IFA. IFA demonstrated that almost all the cells were infected at 48 h p.i. (data not shown). As shown in Fig. 2B, we found that VSV did not induce ICAM1 protein expression. The expression of ICAM1 on the surfaces of ECs was analyzed by EIA at 48 h p.i. and was increased at different MOIs (MOI of 1, not significant; MOIs of 10 and 20, P < 0.001) (Fig. 2C). The infectivity of HUVECs was analyzed by Western blotting and staining for CCHFV NP. As shown in Fig. 2A, the level of infection increases with increasing MOIs.
Fig. 2.
Activation of endothelial cells is virus dose dependent. (A) Noninfected (mock) (M) and infected (MOIs, 0.1, 1, and 10) HUVECs were harvested at 6, 24, 48, and 72 h p.i., followed by protein separation by SDS-PAGE and staining for the proteins ICAM1 and CCHFV NP and the loading control calnexin. CCHFV NP is observed at increasing levels depending on the initial MOI, with the highest intensity at 72 h p.i. ICAM1 levels are increased upon exposure to LPS (L) (100 μg/ml) at 48 and 72 h p.i. and also upon infection with CCHFV at an MOI of 10 at 48 h p.i. At 72 h p.i., the ICAM1 levels increase in a virus dose-dependent manner. (B) Noninfected HUVECs (mock) and HUVECs infected with VSV at an MOI of 0.1 were harvested at 48 h p.i., followed by protein separation by SDS-PAGE and staining for the proteins ICAM1 and VSV-G and the loading control calnexin. VSV-G proteins are observed only in infected cells. ICAM1 protein expression is not induced by VSV in HUVECs at 48 h p.i. (C) HUVECs were incubated for 48 h with TNF-α (10 ng/ml), mock treated with cell medium, or infected with CCHFV at MOIs of 1, 10, and 20, followed by fixing and staining for ICAM1 on the cell surface. All results are normalized against CD105, and ICAM1 is shown as a percentage of CD105 expression. Each treatment was performed in triplicate for two independent experiments. Endothelial cells respond in a virus dose-dependent manner by upregulating ICAM1 on the cell surface. Statistical significance is shown (ns, not significant; ***, P < 0.001), and the error bars indicate standard deviations. The statistics were calculated by comparing stimulated cells to mock-infected cells, and the tests used were ANOVA followed by Dunnett's multiple-comparison test. (D) HUVECs were treated with TNF-α (10 ng/ml) or cell medium or infected with CCHFV at MOIs of 1, 10, and 20 or with UV-inactivated virus at an MOI of 20 and incubated for 48 h p.i., followed by addition of BCECF-AM-stained leukocytes for 1 h. Nonadherent leukocytes were washed away, followed by lysis of ECs and adherent leukocytes in lysis buffer. The intensity of fluorescence of BCECF-AM from lysed leukocytes in the lysates was then measured. The level of adhesion was calculated by normalizing against leukocytes adhered to nontreated ECs, and the results are shown as the fold change to leukocytes adhered to ECs pretreated with only medium. With increasing levels of virus, there is an increasing level of leukocyte adherence to infected endothelial cells. The results are means ± standard errors of the mean (SEM). ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. The statistics were calculated by comparing results from treated cells to those from the UV-inactivated virus control utilizing ANOVA followed by Dunnett's multiple-comparision test.
Resting PBMCs adhered to CCHFV-infected ECs at 48 h p.i. in a virus dose-dependent manner (MOI of 1, not significant; MOI of 10, P < 0.05; and MOI of 20, P < 0.01) (Fig. 2D). ECs challenged with UV-inactivated virus (MOI, 20) did not recruit more leukocytes than noninfected cells. Endothelial infection was verified by cells that were treated similarly and run in parallel to the EIA method and leukocyte adhesion assay, followed by fixing and IFA staining for CCHFV NP at the same time that PBMCs were added. Weak infection could be detected at an MOI of 1, whereas infection at an MOI of 10 approximated 80% and at an MOI of 20 approximated 90% infectivity (data not shown).
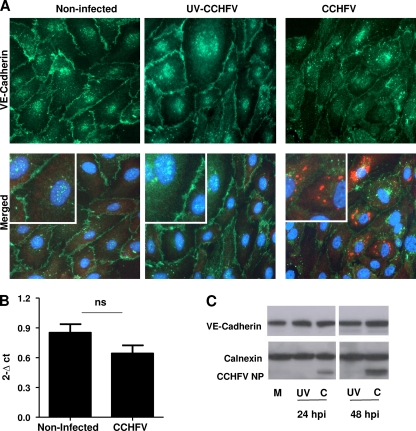
VE-cadherin localization in CCHFV-infected endothelial cells.
To study whether CCHFV infection of HUVECs could affect endothelial barrier integrity, we infected confluent cells with either UV-inactivated or native CCHFV at an MOI of 10. At 48 h p.i., the cells were fixed and analyzed for VE-cadherin by IFA as described in Materials and Methods. As shown in Fig. 3A, we observed some changes in the intensity and distribution of VE-cadherin between some of the infected cells. A PCR assay (Fig. 3B) and Western blotting (Fig. 3C) demonstrated that there was no significant downregulation or degradation of VE-cadherin between noninfected and CCHFV-infected ECs.
Fig. 3.
Analysis of VE-cadherin in CCHFV-infected ECs. (A) Confluent HUVECs were inoculated with native or UV-treated CCHFV at an MOI of 10 or left untreated. After 48 h p.i., the cells were fixed and stained for CCHFV NP and VE-cadherin and counterstained with DAPI to show the cell nucleus. A section of the IF picture was magnified to further illustrate the adherens junctions between neighboring cells (insets). VE-cadherin is localized to cell-cell junctions in all treatments, but the intensity could possibly appear to be lower for CCHFV-infected cells. (B) HUVECs were exposed to CCHFV at an MOI of 10 or to cell medium for 24 and 48 h p.i. Cell layers were harvested for analysis of VE-cadherin mRNA levels by relative quantitative PCR, and CCHFV NP RNA was detected to verify infection. The results are normalized against GAPDH and calculated according to the 2−ΔCT method. The results are displayed as the means and SD for three experiments. No significant difference between the mRNA levels of VE-cadherin of infected and noninfected cells could be observed. The statistical test applied was a paired t test, two-tailed, P = 0.08, although there were too few values for statistics. (C) Confluent HUVECs were inoculated with native or UV-treated CCHFV at an MOI of 10 or left untreated for 24 and 48 h p.i., followed by harvesting for determination of total protein levels of VE-cadherin. The proteins were separated by SDS-PAGE and stained for VE-cadherin, CCHFV NP, and the loading control calnexin. No difference in the levels of VE-cadherin can be observed between mock-treated (M), UV-inactivated (UV), and CCHFV-infected (C) ECs.
Proinflammatory cytokines are released in response to infection.
We decided to study the release of the proinflammatory cytokines IL-1β, IL-6, TNF-α, and IL-8 from CCHFV-infected ECs. Production of IL-1β and TNF-α could not be detected by ELISA or was below the limit of detection (data not shown). However, resting ECs showed weak constitutive production of IL-6, which was markedly increased upon infection (P < 0.01 to P < 0.001 at 24 to 72 h p.i., respectively) (Fig. 4A). We also found constitutive production of IL-8, which was only weakly increased upon infection (P < 0.05 at 24 and 72 h p.i., respectively) (Fig. 4B). Neither IL-6 nor IL-8 release was observed in cells treated with UV-inactivated virus (MOI, 10), but addition of LPS caused strong release of both cytokines (positive control).
Fig. 4.
CCHFV-infected ECs release proinflammatory cytokines. ECs were exposed to LPS (100 μg/ml), UV-inactivated or native CCHFV at an MOI of 10, or cell medium only (mock) for 6, 24, 48, and 72 h p.i. Supernatants were gathered at designated time points, and the release of IL-6 (A) and IL-8 (B) was quantified by ELISA analysis. Significantly increased levels of IL-6 were detected at 24 h p.i., with further increase over time. The background levels of IL-8 are high, but a significant difference in release is observed at 24 and 72 h p.i. The results are averages, and error bars indicate standard deviations (n = 4). Statistical significance is shown: *, P < 0.05; **, P < 0.01; ***, P < 0.001. The treated cells were compared statistically to the UV-inactivated CCHFV control, applying ANOVA, followed by Dunnett's multiple-comparision test.
The activation of endothelial cells by CCHFV is not mediated by an autocrine or paracrine mechanism.
It is not known whether the activation of infected ECs is caused by an autocrine or paracrine mechanism or induced directly by virus replication. In order to study this, we exposed resting HUVECs to conditioned medium harvested from CCHFV-infected (MOI, 10) or noninfected ECs at 48 h p.i., followed by ICAM1 detection with EIA. Conditioned medium harvested from CCHFV-infected (MOI, 10) or noninfected moDCs at 48 h p.i. was also included to verify the activation of ECs, as shown previously (11). The results are given in Fig. 5 and show that conditioned medium from CCHFV-infected HUVECs does not increase ICAM1 expression on resting ECs but conditioned medium from infected moDCs triggers upregulation of ICAM1 compared to noninfected moDCs (P < 0.05).
Fig. 5.
Activation of CCHFV-infected endothelial cells is not mediated by an autocrine or paracrine mechanism. Culture medium was conditioned by exposure to noninfected and CCHFV-infected (MOI, 10) HUVECs or moDCs for 48 h. The conditioned medium was then added to confluent layers of noninfected endothelial cells for 6 h of incubation. As controls, cells were either left untreated or treated with virus stock or recombinant TNF-α (10 ng/ml). After exposure, the cells were fixed and EIA stained for ICAM1 on the cell surface membrane. The values were normalized against CD105 expression, and the background results from untreated cells were subtracted. Conditioned medium from infected ECs did not activate endothelial cells as analyzed by ICAM1 expression. However, ECs were activated upon exposure to conditioned medium from infected moDCs. The conditioned medium from endothelial cells was derived from two independent experiments, each performed in quadruplicate, whereas the conditioned medium from dendritic cells was derived from moDCs generated from six independent donors. The error bars indicate standard deviations. The results are expressed as means and SD; ns, not significant; *, P < 0.05; ***, P < 0.001. Each statistical test was performed relative to the respective controls using the Wilcoxon signed-rank test.
The endothelial cell activation from infected dendritic cells is TNF-α dependent.
To identify the soluble mediator that caused the activation of endothelial cells by moDC-conditioned media, TNF-α was targeted, since it correlates with CCHF disease severity (16, 28). Conditioned medium from CCHFV-infected (MOI, 10) or noninfected moDCs harvested at 48 h p.i. was preincubated with or without a neutralizing antibody against TNF-α and then added to resting ECs. Cells treated with recombinant TNF-α served as a positive control. Preincubation with anti-TNF-α antibody effectively prevented upregulation of cell surface ICAM1 expression on ECs exposed to either moDC supernatant or recombinant TNF-α (P < 0.05 and P < 0.001, respectively) (Fig. 6).
Fig. 6.
CCHFV-infected dendritic cells activate ECs by a TNF-α-dependent mechanism. Conditioned medium from CCHFV-infected (MOI, 10) moDCs or noninfected MODCs was incubated with or without neutralizing antibody (5 μg/ml) targeting TNF-α for 1 h, followed by addition to confluent endothelial cells for 6 h. Control treatments included incubating TNF-α (2 ng/ml) or cell medium with or without neutralizing antibody. The moDCs were derived from four individual donors. The ECs were fixed and stained for cell surface-bound ICAM1. Background ICAM1 levels derived from ECs exposed to only cell culture medium were subtracted. The values displayed are the averages of triplicates, with the error bars indicating standard deviations. Activation of ECs by conditioned medium from infected moDCs is reduced upon preincubation with neutralizing antibody against TNF-α. The results are expressed as means and SD; ns, not significant; *, P < 0.05; ***, P < 0.001. The treated cells were compared to the respective control, and statistical significance was tested by the Wilcoxon signed-rank test.
DISCUSSION
Activation of ECs is one of the key processes that promote the initiation of inflammatory reactions, which involve leukocyte rolling, adhesion, and transmigration into inflamed sites (37). However, the direct interaction of CCHFV and ECs has been largely neglected, even though there are several indicators that ECs are involved in CCHF pathogenesis (4, 6, 27). To our knowledge, this is the first study focusing on the in vitro interaction between CCHFV and ECs. For the first time, we show a direct activation of ECs by CCHFV (Fig. 1), as indicated by ICAM1 upregulation of mRNA transcripts, total protein level, and consecutive cell surface expression. VCAM1 and E-selectin transcript levels were also upregulated at the same level as ICAM1, yet this could not be verified by cell surface studies by EIA. This could reflect a technical insensitivity of the EIA method, though the method has been used successfully in other studies to detect cell adhesion molecules on the cell surface (10, 18, 24).
The induction of ICAM1 cell surface expression occurred in a virus dose-dependent manner (Fig. 2A.). Furthermore, the ICAM1 induction was specific for CCHFV, as shown using VSV as a virus negative control, since VSV failed to induce ICAM1 expression (Fig. 2B). The relevance of the CCHFV-induced ICAM1 upregulation was further supported by results obtained from a biological assay in which leukocyte adhesion to infected endothelial cells was dependent on the initial virus dose (Fig. 2D). The adherence of leukocytes to infected ECs could be mediated not just by upregulated ICAM1, but possibly also by VCAM1 and E-selectin on the cell surface, which could not be detected by EIA. A direct correlation between the severity of disease for CCHF and a high viral load had already been observed in several patient studies (9, 14, 33), although the molecular mechanisms underlying this observation were not further elucidated. We tentatively speculate that the mechanism underlying the correlation between high viral load and disease severity in patients could be caused in part by the virus-enhanced endothelial cell activation, followed by excessive inflammatory reactions and leukocyte recruitment. The release of vasoactive mediators from neutrophils recruited by CCHFV-activated ECs could be a suggested mechanism for increased leakage (23). Additionally, dengue virus-infected ECs recruited PBMCs, followed by increased permeability of the endothelial cell layer (13), indicating one possible outcome for CCHFV-infected ECs interacting with PBMCs.
Previously, it was shown that CCHFV induces IL-6, IL-8, and TNF-α release from infected dendritic cells and macrophages (11, 30). These cytokines were also implicated as a marker of CCHF disease severity in patients (16, 28, 29, 33). We could not detect any TNF-α or IL-1β in supernatants collected from CCHFV-infected endothelial cells. However, the level of the inflammatory cytokine IL-6 was strongly increased in infected compared to noninfected cells, and a modest increase in IL-8 release was also found, although constitutive IL-8 secretion was already substantial (Fig. 4A). Our study adds ECs to the list of cells contributing to cytokine release upon CCHFV infection. It is possible that the increased IL-8 and IL-6 in CCHF patients derives in part from CCHFV-infected ECs.
In a previous article, we showed in an epithelial cell model of tight junctions that CCHFV does not disrupt barrier integrity, nor did infection result in internalization of critical tight-junction proteins (12). At the time, adherens junctions were not included in the study. In this study, we analyzed the localization of VE-cadherin, a component of adherens junctions, but did not observe any significant changes in the levels of VE-cadherin mRNA or protein between CCHFV-infected cells and noninfected cells. Although immunofluorescence (IF) staining of VE-cadherin demonstrates some internalization of the adherens junction protein, which may indicate that infection could increase vascular permeability, along with inducing and perpetuating inflammatory reactions. To prove this hypothesis, further studies utilizing permeability models are needed. Another member of the family Bunyaviridae, the hantavirus Andes virus, was recently shown to internalize VE-cadherin in the early stages of infection, with concomitant increase in permeability (35).
ECs constitutively express low levels of ICAM1, but expression can be upregulated upon exposure to proinflammatory cytokines or viral infection (7, 31). The molecular mechanism mediating induction of ICAM1 in CCHFV-infected endothelial cells is currently unknown. ICAM1 was not upregulated when resting ECs were incubated with medium from infected HUVECs (Fig. 5). Although endothelial cells lack the IL-6 receptor (IL-6R), which is found systemically as sIL-6R, it is possible in humans that IL-6 can induce ICAM1 expression in endothelial cells once its receptor is present (7, 21, 32). In our study, however, ICAM1 expression was most likely mediated by a direct mechanism caused by viral infection.
VHF disease severity, including CCHF, has been correlated in several patient studies with levels of TNF-α, indicating the important role of this cytokine in pathogenesis (16, 19, 28, 33, 43). We and others have identified moDCs as target cells for CCHFV infection (11, 30) and determined their ability to activate endothelial cells, as shown by ICAM1 expression (11). This observation was further confirmed by utilizing a different method, which allowed us to analyze ICAM cell surface expression (Fig. 5). We also determined the specific role of TNF-α in activating endothelial cells by incubating moDC-conditioned media with a neutralizing antibody directed against TNF-α, followed by addition of these supernatants to resting ECs. The cell surface expression of ICAM1 was abrogated in ECs incubated with infected moDC supernatants containing the neutralizing antibody (Fig. 6). A similar result has been observed for other viruses causing VHFs, where neutralization of TNF-α released from infected monocytes or present in patient sera inhibits endothelial activation and permeability (1, 8, 17). Although ECs are activated upon infection, as shown by our study, the indirect targeting by moDCs is also an important mediator of endothelial activation.
Previously, we have shown that CCHFV does not directly target tight junctions, nor does it cause leakage in an in vitro model utilizing the Madin-Darby Canine Kidney (MDCK) epithelial cell line (12). We also found that infected moDCs activate endothelial cells, pointing to indirect targeting of the endothelium as a cause of vascular leakage (11). Here, we highlight the ability of CCHFV to activate ECs, as shown by ICAM1 upregulation, leukocyte adhesion, and enhanced release of proinflammatory soluble mediators. We also confirmed the ability of moDCs to activate endothelial cells and identified TNF-α as the key cytokine mediating the increase in ICAM1 cell surface expression. CCHF pathogenesis is most likely a result of the complex interplay between direct and indirect effects on the endothelium induced by viral infection. However, further studies are needed to identify the sequence of molecular events and which cell types are the key contributors to pathogenesis.
ACKNOWLEDGMENT
This work was in part supported by Swedish Medical Research Council grant K2010-57X-0349-01-3 (to A.M.).
Footnotes
Published ahead of print on 1 June 2011.
REFERENCES
- 1. Anderson R., Wang S., Osiowy C., Issekutz A. C. 1997. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J. Virol. 71:4226–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson I., et al. 2004. Role of actin filaments in targeting of Crimean Congo hemorrhagic fever virus nucleocapsid protein to perinuclear regions of mammalian cells. J. Med. Virol. 72:83–93 [DOI] [PubMed] [Google Scholar]
- 3. Bazzoni G., Dejana E. 2004. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 84:869–901 [DOI] [PubMed] [Google Scholar]
- 4. Bodur H., et al. 2010. Evidence of vascular endothelial damage in Crimean-Congo hemorrhagic fever. Int. J. Infect. Dis. 14:e704–e707 [DOI] [PubMed] [Google Scholar]
- 5. Burt F. J., Swanepoel R. 2005. Molecular epidemiology of African and Asian Crimean-Congo haemorrhagic fever isolates. Epidemiol. Infect. 133:659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burt F. J., et al. 1997. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch. Pathol. Lab. Med. 121:839–846 [PubMed] [Google Scholar]
- 7. Caldenhoven E., et al. 1994. Stimulation of the human intercellular adhesion molecule-1 promoter by interleukin-6 and interferon-gamma involves binding of distinct factors to a palindromic response element. J. Biol. Chem. 269:21146–21154 [PubMed] [Google Scholar]
- 8. Cardier J. E., et al. 2005. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine 30:359–365 [DOI] [PubMed] [Google Scholar]
- 9. Cevik M. A., et al. 2007. Viral load as a predictor of outcome in Crimean-Congo hemorrhagic fever. Clin. Infect. Dis. 45:e96–e100 [DOI] [PubMed] [Google Scholar]
- 10. Chang C. H., Huang Y., Anderson R. 2003. Activation of vascular endothelial cells by IL-1alpha released by epithelial cells infected with respiratory syncytial virus. Cell Immunol. 221:37–41 [DOI] [PubMed] [Google Scholar]
- 11. Connolly-Andersen A. M., Douagi I., Kraus A. A., Mirazimi A. 2009. Crimean Congo hemorrhagic fever virus infects human monocyte-derived dendritic cells. Virology 390:157–162 [DOI] [PubMed] [Google Scholar]
- 12. Connolly-Andersen A. M., Magnusson K. E., Mirazimi A. 2007. Basolateral entry and release of Crimean-Congo hemorrhagic fever virus in polarized MDCK-1 cells. J. Virol. 81:2158–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dewi B. E., Takasaki T., Kurane I. 2008. Peripheral blood mononuclear cells increase the permeability of dengue virus-infected endothelial cells in association with downregulation of vascular endothelial cadherin. J. Gen. Virol. 89:642–652 [DOI] [PubMed] [Google Scholar]
- 14. Duh D., et al. 2007. Viral load as predictor of Crimean-Congo hemorrhagic fever outcome. Emerg. Infect. Dis. 13:1769–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ergonul O. 2006. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 6:203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ergonul O., Tuncbilek S., Baykam N., Celikbas A., Dokuzoguz B. 2006. Evaluation of serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-alpha in patients with Crimean-Congo hemorrhagic fever. J. Infect. Dis. 193:941–944 [DOI] [PubMed] [Google Scholar]
- 17. Feldmann H., et al. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harcourt B. H., Rota P. A., Hummel K. B., Bellini W. J., Offermann M. K. 1999. Induction of intercellular adhesion molecule 1 gene expression by measles virus in human umbilical vein endothelial cells. J. Med. Virol. 57:9–16 [DOI] [PubMed] [Google Scholar]
- 19. Heller M. V., Saavedra M. C., Falcoff R., Maiztegui J. I., Molinas F. C. 1992. Increased tumor necrosis factor-alpha levels in Argentine hemorrhagic fever. J. Infect. Dis. 166:1203–1204 [DOI] [PubMed] [Google Scholar]
- 20. Hoogstraal H. 1979. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 15:307–417 [DOI] [PubMed] [Google Scholar]
- 21. Kerr R., Stirling D., Ludlam C. A. 2001. Interleukin 6 and haemostasis. Br. J. Haematol. 115:3–12 [DOI] [PubMed] [Google Scholar]
- 22. Kiely J. M., Luscinskas F. W., Gimbrone M. A., Jr 1999. Leukocyte-endothelial monolayer adhesion assay (static conditions). Methods Mol. Biol. 96:131–136 [DOI] [PubMed] [Google Scholar]
- 23. Kumar P., et al. 2009. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev. Mol. Med. 11:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madonna R., Pandolfi A., Massaro M., Consoli A., De Caterina R. 2004. Insulin enhances vascular cell adhesion molecule-1 expression in human cultured endothelial cells through a pro-atherogenic pathway mediated by p38 mitogen-activated protein-kinase. Diabetologia 47:532–536 [DOI] [PubMed] [Google Scholar]
- 25. Maltezou H. C., Papa A. 2010. Crimean-Congo hemorrhagic fever: risk for emergence of new endemic foci in Europe? Travel Med. Infect. Dis. 8:139–143 [DOI] [PubMed] [Google Scholar]
- 26. Mutin M., Dignat-George F., Sampol J. 1997. Immunologic phenotype of cultured endothelial cells: quantitative analysis of cell surface molecules. Tissue Antigens 50:449–458 [DOI] [PubMed] [Google Scholar]
- 27. Ozturk B., et al. 2010. Evaluation of the association of serum levels of hyaluronic acid, sICAM-1, sVCAM-1, and VEGF-A with mortality and prognosis in patients with Crimean-Congo hemorrhagic fever. J. Clin. Virol. 47:115–119 [DOI] [PubMed] [Google Scholar]
- 28. Papa A., et al. 2006. Cytokine levels in Crimean-Congo hemorrhagic fever. J. Clin. Virol. 36:272–276 [DOI] [PubMed] [Google Scholar]
- 29. Papa A., Dalla V., Papadimitriou E., Kartalis G. N., Antoniadis A. 2009. Emergence of Crimean-Congo haemorrhagic fever in Greece. Clin. Microbiol. Infect. 16:843–847 [DOI] [PubMed] [Google Scholar]
- 30. Peyrefitte C. N., et al. 2010. Differential activation profiles of Crimean-Congo hemorrhagic fever virus- and Dugbe virus-infected antigen-presenting cells. J. Gen. Virol. 91:189–198 [DOI] [PubMed] [Google Scholar]
- 31. Roebuck K. A., Finnegan A. 1999. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 66:876–888 [DOI] [PubMed] [Google Scholar]
- 32. Romano M., et al. 1997. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6:315–325 [DOI] [PubMed] [Google Scholar]
- 33. Saksida A., et al. 2010. Interacting roles of immune mechanisms and viral load in the pathogenesis of Crimean-Congo hemorrhagic fever. Clin. Vaccine Immunol. 17:1086–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schnittler H. J., Feldmann H. 2003. Viral hemorrhagic fever—a vascular disease? Thromb. Haemost. 89:967–972 [PubMed] [Google Scholar]
- 35. Shrivastava-Ranjan P., Rollin P. E., Spiropoulou C. F. 2010. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J. Virol. 84:11227–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simon M., Falk K., Lundkvist Å., Mirazimi A. 2006. Exogenous nitric oxide inhibits Crimean Congo hemorrhagic fever virus. Virus Res. 120:184–190 [DOI] [PubMed] [Google Scholar]
- 37. Sprague A. H., Khalil R. A. 2009. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 78:539–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sumpio B. E., Riley J. T., Dardik A. 2002. Cells in focus: endothelial cell. Int. J. Biochem. Cell Biol. 34:1508–1512 [DOI] [PubMed] [Google Scholar]
- 39. Swanepoel R., et al. 1987. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am. J. Trop. Med. Hyg. 36:120–132 [DOI] [PubMed] [Google Scholar]
- 40. Venkiteswaran K., et al. 2002. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. Am. J. Physiol. Cell Physiol. 283:C811–C821 [DOI] [PubMed] [Google Scholar]
- 41. Vestweber D. 2007. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol. Rev. 218:178–196 [DOI] [PubMed] [Google Scholar]
- 42. Videm V., Albrigtsen M. 2008. Soluble ICAM-1 and VCAM-1 as markers of endothelial activation. Scand. J. Immunol. 67:523–531 [DOI] [PubMed] [Google Scholar]
- 43. Villinger F., et al. 1999. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J. Infect. Dis. 179(Suppl. 1):S188–S191 [DOI] [PubMed] [Google Scholar]
- 44. Vincent P. A., Xiao K., Buckley K. M., Kowalczyk A. P. 2004. VE-cadherin: adhesion at arm's length. Am. J. Physiol. Cell Physiol. 286:C987–C997 [DOI] [PubMed] [Google Scholar]
- 45. Whitehouse C. A. 2004. Crimean-Congo hemorrhagic fever. Antiviral Res. 64:145–160 [DOI] [PubMed] [Google Scholar]