Abstract
The 2003 monkeypox virus (MPXV) outbreak and subsequent laboratory studies demonstrated that the black-tailed prairie dog is susceptible to MPXV infection and that the ensuing rash illness is similar to human systemic orthopoxvirus (OPXV) infection, including a 7- to 9-day incubation period and, likely, in some cases a respiratory route of infection; these features distinguish this model from others. The need for safe and efficacious vaccines for OPVX in areas where it is endemic or epidemic is important to protect an increasingly OPXV-naïve population. In this study, we tested current and investigational smallpox vaccines for safety, induction of anti-OPXV antibodies, and protection against mortality and morbidity in two MPXV challenges. None of the smallpox vaccines caused illness in this model, and all vaccinated animals showed anti-OPXV antibody responses and neutralizing antibody. We tested vaccine efficacy by challenging the animals with 105 or 106 PFU Congo Basin MPXV 30 days postvaccination and evaluating morbidity and mortality. Our results demonstrated that vaccination with either Dryvax or Acambis2000 protected the animals from death with no rash illness. Vaccination with IMVAMUNE also protected the animals from death, albeit with (modified) rash illness. Based on the results of this study, we believe prairie dogs offer a novel and potentially useful small animal model for the safety and efficacy testing of smallpox vaccines in pre- and postexposure vaccine testing, which is important for public health planning.
INTRODUCTION
Although smallpox has been eradicated (5), orthopoxviruses (OPXVs), such as variola virus (VARV) (6, 32), monkeypox virus (MPXV) (3, 10, 57), vaccinia virus (VACV) (4, 76), and others (53, 68, 77), are a continuing public health concern (61, 67). Due to cellular and humoral protective effects (7, 15, 16, 46, 73), the traditional live VACV-based smallpox vaccines provide cross-protection against multiple OPXV threats (48). Unfortunately, the cessation of routine vaccination (37) has left much of the global population fully susceptible to OPXV infections (34), and the adverse effects of first- and second-generation vaccines (78) coupled with increases in the global immunocompromised population (59) underline the need for continued development and testing of safer smallpox vaccines (30).
Testing of smallpox vaccines requires the development of relevant animal models. Current smallpox vaccine testing animal models include ectromelia virus (ECTV) infection of mice (75), VACV infection of mice (63), rabbitpox virus (RPXV) and VACV infection of rabbits (1, 18), VARV and MPXV infection of nonhuman primates (NHP) (13, 19, 21, 25, 26, 31), and others (65, 74). Limitations of current models include abbreviated disease incubation periods and/or differences from human systemic orthopoxvirus disease presentation and progression The validation of alternative animal models for smallpox vaccine testing is an ongoing effort (2, 19, 39).
The MPXV outbreak in the United States in 2003 (3) identified a potential alternative model as it established that MPXV can be transmitted to black-tailed prairie dogs (Cynomys ludovicianus) (42). Investigations of the MPXV outbreak and epidemiological, immunological, and pathological studies of prairie dogs infected with MPXV (8, 12, 27, 28, 35, 38, 43, 57, 58, 64, 69, 74, 80) determined that this rodent species can manifest MPXV infection similar to human systemic OPXV disease. This disease model shares a respiratory route of infection, similar disease incubation period, and similar rash illness (20, 29, 38, 42, 80) with human MPXV and VARV infections, thus making it a potentially useful small animal model for testing vaccines and therapeutics (29, 70). A particular strength of this model is its 7- to 9-day incubation period, which is more similar to that seen in human disease than many other animal models. Thus, this may represent a novel small animal model system to test both pre- and postexposure vaccination scenarios.
This two-part study is the first to evaluate vaccine safety and efficacy in the prairie dog animal model. In the first part, vaccine safety was tested by inoculation of a small group of animals with first- (Dryvax), second- (Acambis2000), and third-generation (IMVAMUNE) VACV-based smallpox vaccines. We compared these live vaccine virus immunizations with similar administration of a high dose of the Western Reserve strain of VACV (VACV-WR) to determine vaccine safety. To demonstrate the induction of immune responses, we monitored the vaccination “take” or primary lesion formation at the site of inoculation (except in the case of IMVAMUNE, which is administered subcutaneously [s.c.] and is not known to elicit a “take” in other mammalian model systems) and the subsequent kinetics of anti-OPXV antibodies (Abs) in vaccinated animals.
The efficacies of these three smallpox vaccination regimens in protection from monkeypox disease in two scenarios were evaluated. The first MPXV challenge study (low dose) was performed on animals previously vaccinated with Dryvax, Acambis2000, or a single dose of IMVAMUNE. These animals were challenged with 1 ×105 PFU (17× the 50% lethal dose [LD50]) MPXV Republic of Congo (ROC). The second MPXV challenge (high dose) used a challenge dose of 1 ×106 PFU (170× the LD50) MPXV ROC and two prior doses of IMVAMUNE in that vaccination arm. In both experiments, the animals were challenged 30 to 40 days postvaccination, anesthetized, and sampled every 3 to 4 days and observed daily for mortality and morbidity. In addition, Ab levels were measured both pre- and postchallenge to evaluate the kinetics of vaccine-induced anti-OPXV Abs present in animals protected from MPXV challenge. In the control arm of both scenarios, animals were challenged with MPXV without prior vaccination. The results of this study establish the prairie dog as a novel animal model for the testing of smallpox vaccines and contribute to the body of knowledge regarding vaccine protection from OPXVs. These data are relevant to public health responses and bioterrorism planning in the case of a natural occurrence of OPXV disease or an accidental or intentional release of VARV.
MATERIALS AND METHODS
Animals.
For the vaccine safety evaluation, 18 animals (11 females, 7 males) were randomly selected from over 200 wild-caught, live-trapped, black-tailed prairie dogs captured in one trapping event in Boulder County, CO, for use in this study. Animal husbandry was performed as previously described (36). For the MPXV challenge portion of the initial study, the remaining 12 animals were transferred to a biosafety level 3 (BSL3) facility where trained personnel performed all work under enhanced animal BSL3 conditions. An additional 32 animals were then transferred to the same lab for the second challenge study. At the conclusion of each study, the animals were euthanized. Animals were cared for and managed in accordance with CDC Institutional Animal Care and Use Committee (IACUC) policies and procedures under an approved animal protocol.
Pathogen screening.
Screening for Yersinia pestis, Francisella tularensis, Bartonella spp., and Rickettsia spp. was outsourced to CDC's Division of Vector-Borne Infectious Diseases, Bacterial Diseases Branch Diagnostic and Reference Laboratory, and the Division of Viral and Rickettsial Diseases, Rickettsial Zoonoses Branch. Whole-blood samples were assayed by PCR as previously described (40, 62, 72). In addition, serum from these animals taken prior to study start was tested in an enzyme-linked immunosorbent assay (ELISA) to detect IgG Abs to OPXV species. All samples were negative for the pathogens tested, so no results are described.
Mini Mitter biotelemetry.
Surgery was performed on each animal in the vaccine safety study to implant a biotelemetry transponder as described previously (36). This procedure has been shown to cause no lasting discomfort beyond a short recovery period in multiple species (11, 23, 24, 49, 66). The ER-4000 E-Mitter transponder (Mini Mitter, Respironics, Bend, OR) was programmed to record temperature and gross motor activity levels at 5-min intervals, and data were analyzed using VitalView (Mini Mitter, Respironics) software. There were no differences in temperature or activity from established baseline levels (36) observed between any of the animal groups during the initial study. Therefore, to minimize potential discomfort to the animals, the transponders were not implanted in the second challenge study.
VACV-WR virus inoculations and smallpox vaccination.
The animals in the vaccine safety study were divided into six groups of three animals each. The Dryvax- and Acambis2000-vaccinated groups were inoculated with 2 × 105 PFU of vaccine in 10 μl of phosphate-buffered saline (PBS) via multiple puncture (m.p.; 15 strikes) between the shoulder blades using a tuberculin needle. The IMVAMUNE-vaccinated group was given 1 × 108 tissue culture infectious doses (TCID) of vaccine in 500 μl s.c. between the shoulder blades. The VACV-WR group was inoculated with 1 × 106 PFU of virus in 10 μl PBS by m.p. (15 strikes) between the shoulder blades. Two negative-control PBS groups of three animals each were inoculated with either 500 μl PBS s.c. or 10 μl via m.p. (15 strikes) between the shoulder blades. All vaccine doses were human doses, except for the single dose of IMVAMUNE, which is normally administered twice. The higher VACV-WR dose was equivalent to lethal doses used in mouse studies and was administered to cause VACV pathology for use as a positive control. The second challenge study groups were as follows: Dryvax (n = 10), Acambis2000 (n = 10), IMVAMUNE (n = 10), and PBS (n = 2). Vaccinations for the second challenge study (high dose) were performed identically to those described above, except that two doses of IMVAMUNE were administered at day −60 and day −30 prior to challenge (day 0). The animals were observed daily for inappetence and general health. Blood samples and vaccination site and oral swabs were taken every 3 to 4 days for 30 days to monitor disease progression.
MPXV virus challenges.
In the first experiment (low-dose challenge), all animals (Dryvax [n = 3], Acambis2000 [n = 3], IMVAMUNE [n = 3], and PBS [n = 3]) were challenged intranasally (i.n.) with 105 PFU of MPXV in 10 μl PBS administered by inserting 5 μl into each nare of sedated animals. In the second experiment (high-dose challenge), the dose was increased to 106 PFU of MPXV. In this second experiment, eight vaccinated animals from each group (Dryvax [n = 10], Acambis2000 [n = 10], and IMVAMUNE [n = 10]) were challenged, and the two remaining vaccinated animals were kept as vaccinated but uninfected negative controls (n = 6). Both animals in the PBS group (n = 2) were unvaccinated and used as virus challenge controls. The virus used in both challenges (Congo Basin MPXV strain, MPXVV-2003-38) was collected from a 2003 outbreak of MPXV in the Republic of Congo and previously sequenced (45). The virus was passaged twice in BSC-40 cells prior to seed pool production. Verification of inoculation titers for both experiments was accomplished by titration of the diluted virus aliquoted for use in challenge studies.
Morbidity measurements.
The principal investigator observed nasal involvement and full body lesion counts for each animal during sampling. The nasal involvement was measured using the following subjective scale: 1, mild inflammation localized to the nares with one or more lesions; 2, moderate inflammation with slight spread to the rest of the nasal area with one or more lesions; 3, severe inflammation with spread to the rest of the nasal and oral areas with multiple lesions; 4, draining lesions with or without secondary bacterial inflammation and involvement of both the nasal and oral areas. The lesion count was a full body manual count. Pictures of each animal taken at the time of observation were randomly examined during subsequent data analysis to ensure accuracy of reported results and to reduce bias.
Blood sampling and analysis.
Animals were anesthetized using isoflurane gas to effect in a chamber with maintenance of anesthesia via nose cone with the animal on a heating block to maintain core temperature in accordance with IACUC-approved protocols. The hind limb/groin area was sprayed with 70% isopropanol, and a 28-gauge needle was used to collect 1 ml of blood from the saphenous vein; the sample was distributed to a tube containing EDTA (Fisher Scientific) for hematological analysis on a CBC-Diff veterinary hematology system (Heska, Fort Collins, CO) or to a serum separation tube (Fisher Scientific) for chemistry analysis on a Piccolo serum chemistry analyzer (Abaxis, Union City, CA). There were no significant differences from established baseline values (36) observed in any of the animal groups. Therefore, no results are reported.
Real-time PCR and tissue infectivity culture of virus.
Blood and swab samples were assayed by real-time PCR (RT-PCR) that targeted the conserved OPXV E9L (DNA polymerase) gene as previously described (44). VACV DNA was used as the positive control. RT-PCR results (in fg/ml) were converted to genome equivalents (GE)/ml by using the conversion factor of 1 fg = 50 GE (41). It was previously demonstrated that RT-PCR detection of viral DNA is significantly more sensitive than detection of PFU (28). Therefore, specimens were first tested for the presence of OPXV DNA by RT-PCR and, if positive, were evaluated for viable virus by tissue culture. RT-PCR-positive samples were titrated using serial 10-fold dilutions onto BSC-40 cell monolayers, incubated at 37°C and 5% CO2 for 72 h, and stained with crystal violet and formalin to reveal plaques to determine viral titer. Tissue culture results were measured in PFU/ml.
ELISAs.
A modified version of the ELISA was used for analysis of anti-OPXV immunoglobulin types A and G (35). Microtiterplates (Immulon II; Dynatech) were coated with crude VACV (Wyeth strain) on one-half of the plate and an equal volume of BSC-40 cell lysate diluted on the other half and incubated overnight. After inactivation, plates were blocked, followed by three PBST (PBS–0.05% Tween 20) washes. Prairie dog serum (diluted 1:50 to 1:1,518,750 in assay diluent) was added to both halves of the plates and incubated. Plates were washed, and ImmunoPure A/G conjugate (Pierce) was added and incubated. Plates were washed, and peroxidase substrate (Kirkegaard & Perry Laboratories) was added and allowed to develop. After development, stop solution (Kirkegaard & Perry Laboratories) was added, and absorbance was read on a spectrophotometer at 450 nm. Values reported represent the averages of duplicate wells for each sample. Positive human vaccinee sera were used as assay controls. The BSC-40 cell lysate half of each plate was used to generate a cutoff value (COV) for each plate by averaging all the values of the BSC-40 lysate half and adding two standard deviations (SD). Specimens were considered positive if the test sample's value was above the COV. Additionally, any plate that was outside 2 SD of the mean of all the plates for either the lysate background optical density (OD), lysate background SD, or the positive control OD at 1:12,150 was discarded. The end point titer for each animal at each time point was determined based on the highest dilution that was positive and was used to calculate the geometric mean titer (GMT) and SD for each group at each time point (all SDs were <0.5 log from the mean), and data are reported on a log scale.
HCS-GFP neutralization assay.
Neutralizing antibody (NAb) titers against VACV were measured by using a previously validated and described green fluorescent protein (GFP)-based assay (33). Briefly, a VACV-WR strain expressing GFP (WR-GFP) was incubated with serum, and then the treated virus was used to infect cells. After 24 h, formalin-fixed plates were sealed and run on the ArrayScan high-content screening (HCS) reader with target acquisition software (Thermo Scientific, PA). The HCS-GFP assay detects the percentage of GFP-producing responder cells (R), and this value is then normalized to control wells to produce the relative percent responders (RPR) titer. The reported values below are the 50% RPR GMT previously correlated to the inhibitory concentration that neutralizes 50% of viral infection (ID50) in a traditional plaque reduction neutralization titer (PRNT) assay (33). The 50% RPR titer was calculated using a modified variable slope sigmoidal equation (Hill equation, Levenberg Marquardt algorithm) and Prism 5.0 software (GraphPad) with goodness of fit to this sigmoidal curve (represented by R2) calculated by the least-squares method. Serum samples were used to obtain a time point for each animal at day 0 or 3, 7 or 10, 14 or 17, 21 or 24, and 28 days post-MPXV challenge; EDTA blood samples were used if serum was not available for a 3-day window (day 0 or 3, 7 or 10, etc.). All data used in this analysis had assay-specific R2 values greater than 0.9000.
Statistical analysis.
Prism 5.0 (GraphPad) was used for statistical analyses. Data to be analyzed passed the D'Agostino-Pearson omnibus test, which measures the deviation from a predicted Gaussian distribution by skewness (asymmetry) and kurtosis (shape), and thus further statistical analyses were performed assuming a Gaussian “normal” distribution (41). The ordinary two-way analysis of variance (ANOVA) parametric assay with a Bonferroni post test was used to compare data for weight loss, nasal involvement, lesion counts, viral DNA, and infectious virus among the unvaccinated, uninfected, and vaccinated groups at each time point. Comparisons of morbidity and mortality among groups were accomplished using survival curves with P values determined by the Kaplan-Meier method.
RESULTS
Smallpox vaccines are safe for use in the prairie dog model.
Several disease markers, including weight loss, were observed to evaluate the safety of smallpox vaccines in this model. As shown in Fig. 1A, the group of animals inoculated with VACV-WR demonstrated a statistically significant weight loss on day 15 of infection compared to the PBS group (P = 0.0296; two-way ANOVA). For the VACV-WR-infected animals, the mean weight loss was 25.06 ± 12.03 g (∼5%). The PBS group did not exhibit a significant weight loss after mock vaccination, nor did those animals vaccinated with Dryvax, Acambis2000, or IMVAMUNE.
Fig. 1.
VACV-WR pathogenicity in prairie dogs. (A) Percent weight loss, determined by measuring animal weights every 3 days postvaccination and comparing those to the weight of the animal on the day of inoculation. Significant weight loss in the VACV-WR-infected group of ∼5% was determined by two-way ANOVA (P = 0.0296). (B) Photographs of VACV-WR-inoculated prairie dogs are arranged to show representative data demonstrating the appearance and resolution of ocular infections (top row), secondary oral lesions (middle row), and secondary groin lesions (bottom row). (C) Summary chart illustrating many of the salient events during VACV-WR infection in the prairie dog.
Other disease markers of OPXV infections include lesion formation and ocular involvement. To ensure that human smallpox vaccine doses did not cause disease in these animals, we “modeled” vaccinia virus infection in prairie dogs by infecting them with a large dose of VACV-WR (1 × 106 PFU). This dose, which is 5 times larger than the vaccine doses, was given to ensure that some disease was seen, for comparison with human doses of replication-competent Dryvax or Acambis2000 vaccines (2 × 105 PFU). In the VACV-WR-infected group, 2 of 3 animals showed signs of ocular involvement, and one of these formed disseminated lesions. The groups vaccinated with Dryvax, Acambis2000, IMVAMUNE, or PBS showed no disseminated lesion formation or ocular involvement. Figure 1B shows these findings in the context of the disease course of VACV-WR infection in the prairie dog. Ocular involvement appeared at day 11 of infection, peaked at day 14, and cleared by day 21. Disseminated lesions were found in the oral area (observed on day 14, cleared by day 21) and the groin area (observed on day 17, cleared by day 21). Tissue samples taken at necropsy (day 28) for the three VACV-WR-infected animals and three PBS animals were negative for pathology by hematoxylin and eosin staining and negative for OPXV by immunohistochemistry staining (data not shown).
Figure 1C summarizes the time course of VACV-WR disease pathology in the prairie dog. Additional data used to formulate the disease time course (described here, but data are not shown) included OPXV DNA in blood and infectious virus cultured from oral swab eluates. The only group of animals that tested positive for viral DNA in blood was the VACV-WR-infected group (∼60,000 GE/ml). Infectious virus was detected on day 11 of infection in oral swabs from the VACV-WR-infected group (1 of 3 animals; 102.5 PFU) and Dryvax-vaccinated group (1 of 3; 102.5 PFU). On day 14, infectious virus was found in the VACV-WR-infected animals (2 of 3; 101 to 102 PFU) and Dryvax-vaccinated animals (1 of 3; 103.5 PFU). By day 23, the only infectious virus cultured from oral swabs was found in the WR-VACV group (1 of 3 animals; 101 PFU). On day 28, all blood samples were negative for viral DNA, and all oral swabs were negative for viral DNA and infectious virus (data not shown).
Smallpox vaccination induces humoral immune responses in prairie dogs.
Traditional methods of measuring the induction of an immune response after smallpox vaccination are an observable “take” (formation of lesion, or jennerian pustule, at site of inoculation) and the induction of anti-OPXV Abs. No take was seen in animals inoculated with IMVAMUNE, as it was administered s.c. and usually undergoes no more than one round of replication in mammalian tissue. For the VACV-WR-infected and Dryvax-vaccinated animals, the lesion formation initiated on days 2 to 4 and scab detachment occurred at ∼day 19 to 20. In the Acambis2000-vaccinated animals, the lesion formation initiated around day 5 to 7 and scab detachment was observed around days 22 to 25. The take lesions in VACV-WR-infected and Dryvax- and Acambis2000-vaccinated animals were similar in size, at approximately 0.5 cm in diameter (data not shown). RT-PCR performed on DNA extracted from eluates of swabs of the take site detected high levels of OPXV DNA (∼600,000 GE/ml) in VACV-WR-infected animals (through day 21) and Dryvax-vaccinated animals (through day 8), with Acambis2000-vaccinated animals having lower levels of OPXV DNA (∼60,000 GE/ml) through day 8. Infectious virus was only cultured from the swabs of the VACV-WR primary lesion (102.5 PFU/ml) on day 8 (data not shown).
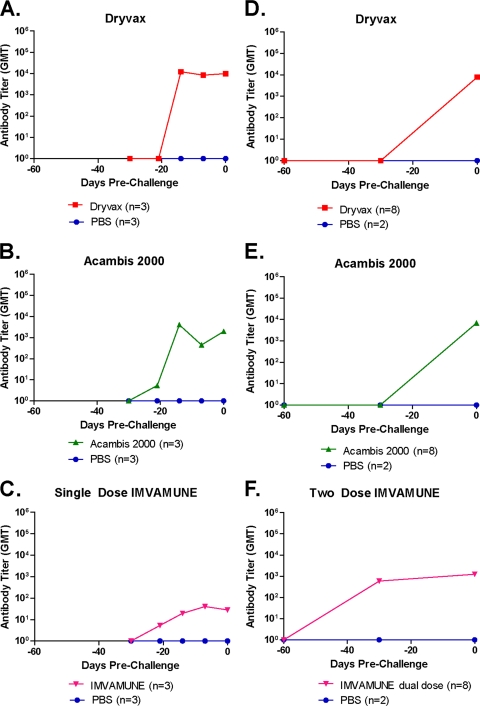
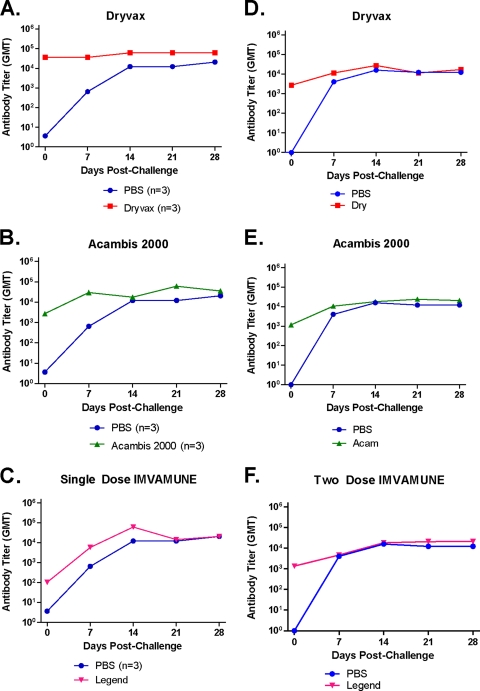
Administration of smallpox vaccines to prairie dogs induces the formation of anti-OPXV IgG Abs (Fig. 2). To measure Ab kinetics, we sampled sera from each animal at multiple time points by ELISA both before and after MPXV challenge. For low-dose (105 PFU) MPXV-challenged animals (Fig. 2A to C) previously vaccinated at day −28, anti-OPXV Abs were detectable in all animals from day −14. For the Dryvax-vaccinated animals, the GMT at day −14 was 1.22 × 104 (Fig. 2A). The GMT for Acambis2000-vaccinated animals (Fig. 2B) was slightly lower, at 4.05 × 103. The Ab level for the group of animals receiving a single dose of IMVAMUNE (Fig. 2C) was still lower, with a GMT of 1.96 × 101 on day −14. On the day of challenge (day 0), the GMTs of anti-OPXV Abs were 3.55 × 104 for Dryvax-vaccinated, 2.74 × 103 for Acambis20000-vaccinated, and 1.04 × 102 for one-dose IMVAMUNE-vaccinated animals.
Fig. 2.
Vaccination-induced anti-OPXV antibody kinetics prechallenge. ELISAs were performed to measure anti-OPXV antibody titers present in the sera of vaccinated animals prior to challenge. In the low-dose group (single IMVAMUNE), all vaccinations took place on day −30 (A to C [n = 3]). For the high-dose study (two doses of IMVAMUNE), the initial IMVAMUNE dose was given at day −60, and the second dose of IMVAMUNE and the Dryvax or Acambis2000 doses were given at day −30 (D to F [n = 8]). Day 0 was the day of MPXV challenge. The GMT per group is graphed on a log scale. Results for sera collected from animals vaccinated with Dryvax (A and D), Acambis2000 (B and E), and IMVAMUNE (C and F) are shown.
In animals challenged with the high dose (106 PFU) of MPXV (Fig. 2D to F), the IMVAMUNE-vaccinated animals received the first of two doses of IMVAMUNE 60 days prior to challenge (day −60). By day −30, the first dose of IMVAMUNE had induced Abs in this group to a GMT of 1.39 × 102 (Fig. 2F). On day −30, these animals were boosted with a second dose of IMVAMUNE, and the other groups of animals were vaccinated with Dryvax or Acambis2000. On the day of challenge (day 0), the GMT for Dryvax-vaccinated animals was 2.68 × 103 (Fig. 2D). Acambis2000-vaccinated animals had a slightly lower GMT of 1.18 × 103 (Fig. 2E); values for both of these groups were similar to what was seen in the low-dose challenge group. Importantly, the GMT for the 2-dose IMVAMUNE-vaccinated animals was 1.35 × 103, 12.9-fold higher than the level of Abs induced in the low-dose challenge group, for which animals received only a single dose of IMVAMUNE.
Vaccinated animals produce Anti-OPXV Abs faster than unvaccinated animals post-MPXV challenge.
Figure 3 shows the absolute count and kinetics of postchallenge Abs for vaccinated and unvaccinated animals for each vaccination regimen in both low-dose (Fig. 3A to C) and high-dose challenges (Fig. 3D to F) as measured by ELISA. In the low-dose (105 PFU) MPXV-challenged animals, the inoculation of animals with MPXV led to a rapid anamnestic increase in anti-OPXV Abs in animals in the Dryvax and Acambis2000 groups (Fig. 3A and B), reaching GMTs of 3.55 × 104 and 3.00 × 104, respectively, by day 7 postchallenge. Interestingly, the GMT of the IMVAMUNE-vaccinated group was 5- to 6-fold lower at 5.84 × 103. The unvaccinated group had a GMT of 6.49 × 102 on day 7. In the high-dose study (106 PFU), all vaccinated animals (Fig. 3D, E, and F) again demonstrated an anamnestic response, with group GMTs for Dryvax animals of 1.13 × 104, for Acambis2000 animals of 1.06 × 104, and for IMVAMUNE animals of 4.65 × 103 by day 7. The unvaccinated animals challenged with the high dose of MPXV generated a 6-fold-higher level of Abs than the unvaccinated animals challenged with the lower MPXV dose (4.05 × 103 versus 6.49 × 102). In summary, Dryvax and Acambis2000 vaccination induced total Abs to levels that were higher than in unvaccinated animals on day 7 in both the low- and high-dose studies. IMVAMUNE-induced total Abs were higher than in unvaccinated animals in the low-dose study, but not the high-dose study.
Fig. 3.
MPXV challenge-induced anti-OPXV antibody kinetics. ELISAs were performed to measure antibody titers of anti-OPXV antibodies present in the sera of animals. The GMT per group is graphed on a log scale. Serum was collected on day 0, day 7, day 14, day 21, and day 28 from each animal in the low-dose (single IMVAMUNE; n = 3; A to C) and high-dose (two-dose IMVAMUNE; [n = 8]; D to F) challenge groups. Data for animals vaccinated with Dryvax (A and D), Acambis2000 (B and E), and IMVAMUNE (C and F) are shown.
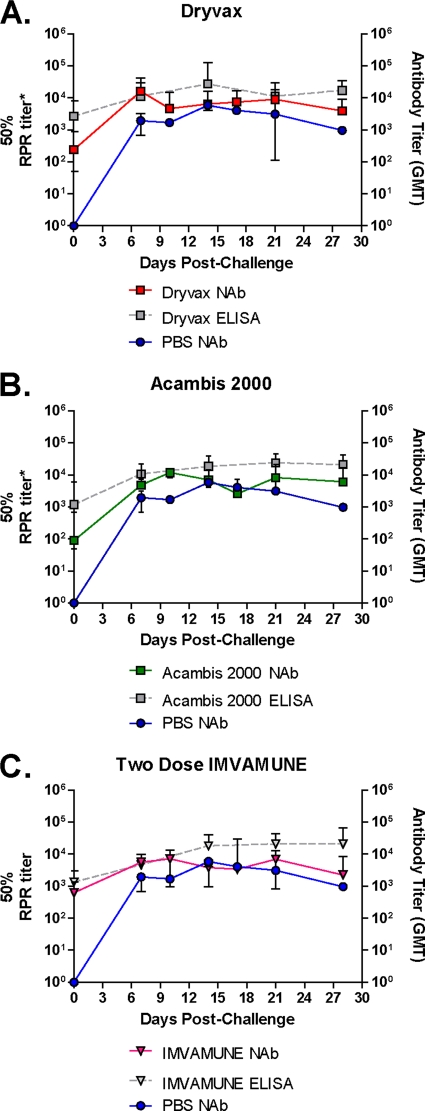
The HCS-GFP neutralization assay (33) was used to measure NAb function for all animals in the high-dose challenge group in order to establish that the Ab kinetics we observed in the ELISA (described above) were also true for NAbs. Figure 4 shows the geometric mean NAb titers for each vaccine group (Dryvax [n = 8], Acambis2000 [n = 8], and IMVAMUNE [n = 8]) compared to the geometric mean NAb titer for the unvaccinated PBS group (n = 2). The ELISA data for the high-dose study reported above are recapitulated in Fig. 4 to allow comparison between ELISA and NAb kinetics.
Fig. 4.
MPXV challenge-induced anti-OPXV neutralizing anti- body response. Animals from the Dryvax (A), Acambis2000 (B), and IMVAMUNE (C) groups were evaluated by HCS-GFP neutralization assay to measure NAb function. GMTs (which correlate with 50% PRNT titers [32]) for each group are shown, along with antibody GMTs obtained from ELISAs (gray) and unvaccinated control animals (blue).
These results show that Dryvax, Acambis2000, and IMVAMUNE animals had NAb titers of 2.38 × 102, 8.94 × 101, and 6.26 × 102 on day 0, respectively. On day 7 these titers had increased to 1.61 × 104, 4.79 × 103, and 5.58 × 103, respectively. The NAb response to MPXV challenge in the unvaccinated animals rose to 1.94 × 103 by day 7. In summary, Dryvax-vaccinated animals had a Nab titers 8.9-fold higher than unvaccinated animals on day 7, and the NAb levels of Acambis2000- and IMVAMUNE-vaccinated animals were, respectively, 2.5- and 3-fold higher than in unvaccinated animals on day 7.
All vaccines protected prairie dogs from weight loss in both low- and high-dose MPXV challenges.
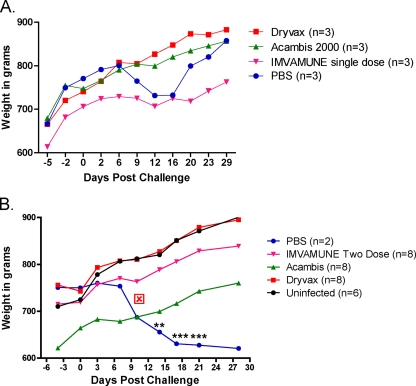
Both low-dose (105 PFU) and high-dose (106 PFU) MPXV challenges resulted in a demonstrable weight loss in the unvaccinated animals, beginning after day 6 (Fig. 5). The low-dose MPXV-challenged unvaccinated animals recovered and began to regain weight by day 21, with an average weight loss of 39.0 g ± 21.1 g (∼5%) (Fig. 5A), while the high-dose MPXV-challenged unvaccinated animals fared poorly, with one death recorded on day 11. The second unvaccinated animal became very ill with multiple lesions and severe nasal involvement and never recovered from a weight loss of 94.5 ± 15 g (∼13%) (Fig. 5B) before being euthanized. The weight loss for high-dose unvaccinated animals was greater than, and highly statistically different from, that observed in the unchallenged animals at days 14, 17, and 21 (P < 0.01 and P < 0.001; two-way ANOVA). Importantly, none of the animals previously vaccinated with Dryvax, Acambis2000, or IMVAMUNE showed a significant weight loss compared to the unchallenged animals at either challenge dose.
Fig. 5.
Weight loss after MPXV challenge. Group mean animal weights (in grams) were determined for Dryvax-, Acambis2000-, or IMVAMUNE-vaccinated animals or mock-vaccinated (PBS) or uninfected animals in the low-dose (A) and high-dose (B) MPXV challenge groups. All groups were compared by using a two-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Differences among smallpox vaccines in protection of animals from nasal involvement and rash illness in MPXV challenge.
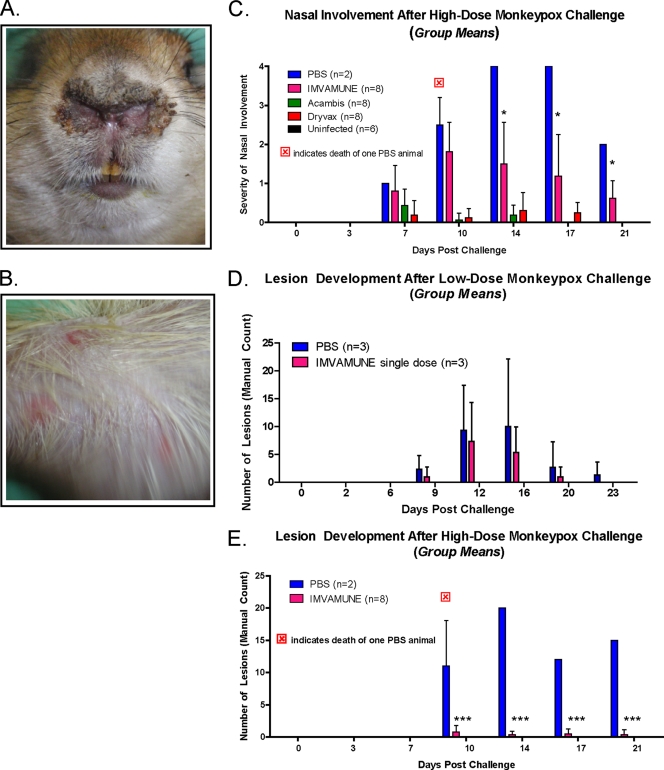
Figure 6 shows representative samples of the nasal area lesions/inflammation, which appeared by day 7 at the site of inoculation (Fig. 6A). Systemic infection then led to disseminated lesions (Fig. 6B), initially in the groin and axillary regions (by day 9) and then on the dorsal and ventral surfaces (by day 11) of the animal. Except for those in the nasal area (site of inoculation), the lesions progressed through the normal stages of OPXV lesions: macular, vesicular, pustular, and then drying and desquamation. Nasal lesions remained at the vesicular stage and were the first to form and last to heal, often developing into open sores with a moderate incidence of secondary bacterial infections. Ocular infections developed in some animals, but these may have been secondary infections caused by self-inoculation and did not differ significantly among experimental groups (data not shown).
Fig. 6.
Morbidity measurements in MPXV-challenged animals. Representative photos of nasal lesions (A) (with a score of 3 [see the text for a description of the subjective scale used]) and secondary lesions (B) are shown. Nasal lesions from high-dose challenge (C) and secondary lesion counts from low-dose (D) and high-dose (E) challenged animals are also shown for comparison of these pathogenicity markers among vaccinated, unvaccinated, and uninfected groups. All groups were compared with a two-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In the low-dose MPXV-challenged animals, nasal involvement was minimal in the unvaccinated and IMVAMUNE single-dose-vaccinated animals and nonexistent in the Dryvax- and Acambis2000-vaccinated animals (data not shown). However, in the high-dose, MPXV-challenged animals (Fig. 6C), most animals had nasal involvement: (unvaccinated [2 of 2], IMVAMUNE two-dose group [8 of 8], Dryvax [5 of 8], and Acambis2000 [6 of 8]). All vaccinated animals had less nasal symptomatology than unvaccinated high-dose MPXV-challenged animals, but of these, only the IMVAMUNE two-dose-vaccinated animals and unvaccinated animals showed significantly higher levels of nasal involvement than the unchallenged animals at days 14 (mean scores of 2 and 4, respectively), 17 (scores of 1.5 and 4), and 21 (scores of 1 and 2) (P < 0.05; two-way ANOVA).
Disseminated lesion formation was measured by a manual count of body lesions. Dryvax- and Acambis2000-vaccinated animals were completely clear of body lesions in both challenge studies. A comparison of lesion formation in the low-dose MPXV-challenged animals demonstrated that only the unvaccinated animals (3 of 3 animals, 2 to 24 lesions) and IMVAMUNE-vaccinated animals (3 of 3 animals, 3 to 14 lesions) showed any rash burden, and there was no statistical difference between these two groups when one dose of IMVAMUNE was administered (Fig. 6D). In the high-dose MPXV-challenged animals, the unvaccinated (2 of 2 animals, 2 to 20 lesions) and IMVAMUNE two-dose-vaccinated animals (6 of 8 animals, 1 to 2 lesions) were again the only groups to form lesions, but the IMVAMUNE-vaccinated animals had significantly less rash illness than unvaccinated animals when two doses of IMVAMUNE were administered (P < 0.001; two-way ANOVA) (Fig. 6E).
Differences in viral DNA in blood and infectious virus in oral swabs among animals vaccinated with different smallpox vaccines.
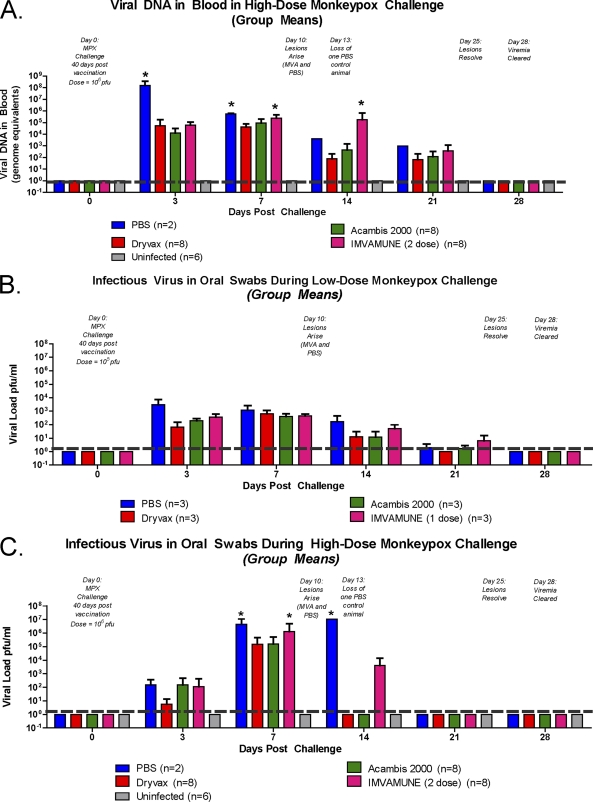
We measured the OPXV DNA load in blood from vaccinated and unvaccinated animals in both the low- and high-dose MPXV challenge groups. Although the assay is a generic OPXV DNA assay, the DNAemia detected is referred to here as MPXV, since any OPXV DNA attributable to the vaccine had cleared by the time of the MPXV challenge (data not shown). In the low-dose MPXV challenge group that received the one-dose IMVAMUNE vaccination (data described here but not shown), the viral DNA load in blood on day 3 in all animals was 103 GE/ml. Viral DNA load peaked at days 7 to 9 with all groups, having similar levels (104.5 GE/ml). By day 21, the viral DNA in all animals was detectable at very low levels (<102 GE/ml), and the levels among the groups were again indistinguishable. The viral DNA levels decreased below detectable levels by day 28 in all challenged animals. The differences in MPXV DNA load, measured in blood, between vaccinated and unvaccinated animals did not reach statistical significance at any time point (data not shown). Similar to the low-dose challenge group, the MPXV DNA load in blood in the high-dose MPXV-challenged animals (Fig. 7 A) showed similar kinetics and demonstrated that there was no statistical difference between Dryvax-vaccinated, Acambis2000-vaccinated, and uninfected animals at any time point. In contrast to the low-dose MPXV challenge, we observed statistically significant differences in DNAemia among the animal groups. On day 3 all vaccinated animal groups (104 GE/ml) had lower levels of viral DNA in blood than the unvaccinated animals (108 GE/ml) (P < 0.05; two-way ANOVA). By day 7, both the IMVAMUNE two-dose-vaccinated animals and the unvaccinated animals had significantly higher levels of MPXV DNA (106 GE/ml) in blood than Dryvax- or Acambis2000-vaccinated animals (105 GE/ml) (P < 0.05; two-way ANOVA). Surprisingly, on day 14, the IMVAMUNE two-dose-vaccinated animals continued to have significantly more MPXV DNA (106 GE/ml) than did the Dryvax-vaccinated (102 GE/ml) and Acambis2000-vaccinated animals (103 GE/ml) and even the surviving unvaccinated animal (104 GE/ml) (all with P values of <0.05; two-way ANOVA).
Fig. 7.
Viral loads in blood (DNA) and oral swabs (in PFU/ml) in MPXV-challenged vaccinated and unvaccinated animals. RT-PCR of DNA extracted from blood samples from high-dose challenge (A) and infectious virus cultured from the eluate of oral swabs during low-dose challenge (B) and high-dose challenge were analyzed for differences in viral loads among vaccinated, unvaccinated, and uninfected animal groups. Comparisons among groups were made using a two-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We performed quantitative plaque assays with extracts from oral swabs to compare levels of infectious virus among the experimental groups. In the low-dose MPXV-challenged animals, the amount of infectious virus in oropharyngeal swabs showed no statistically significant differences among the groups at any time point, although some trends were noted (Fig. 7B). On day 3, all previously vaccinated groups (102.5 to 103 PFU/ml) had slightly lower levels of infectious virus in oral swabs than did unvaccinated animals (104 PFU/ml). On day 7 (103 PFU/ml) and day 14 (102 PFU/ml), all animal groups had virtually indistinguishable levels of infectious virus in oral swabs, and by day 21 all animals had low levels of infectious virus (<101 PFU/ml). In contrast, differences between unvaccinated and vaccinated groups were seen in the high-dose MPXV-challenged animals. On day 3, the unvaccinated animals, Acambis2000- and IMVAMUNE two-dose-vaccinated animals had infectious viral titers of 103 PFU/ml, with Dryvax-vaccinated animals having an infectious viral load of 102 PFU/ml. On day 7, IMVAMUNE two-dose and unvaccinated challenged animals had significantly (P < 0.05; two-way ANOVA) higher amounts of infectious virus (106 PFU/ml) in oral samplings than either Dryvax- or Acambis2000-vaccinated animals (105 PFU/ml). On day 14, the only infectious virus seen in the oral swabs was in unvaccinated animals (107 PFU/ml) and IMVAMUNE two-dose-vaccinated animals (104 PFU/ml), although only the unvaccinated animals were significantly different than the uninfected controls (P > 0.05; two-way ANOVA). By day 21, all animals had cleared the virus.
RT-PCR of DNA extracted from oral swab eluates for MPXV was also performed and showed similar kinetics and group differences in both the low- and high-dose challenges, with viral DNA in oral swabs (in GE/ml) typically 0.5 to 1.0 log higher than the infectious virus (in PFU/ml) results (data described but not shown). There were no statistically significant differences in the MPXV DNA levels among any of the animal groups that received the low-dose MPXV challenge. On days 3 and 7, all groups had similar amounts of DNA (105 GE/ml), with viral DNA levels dropping on days 14 and 21 (103 GE/ml) and viral clearance by day 28. In the high-dose MPXV challenge groups, no statistical difference in oral swab-derived MPXV DNA was seen between Dryvax-vaccinated, Acambis2000-vaccinated, or uninfected animals at any time point. Similar to results for infectious virus in oral swabs, on day 7 the IMVAMUNE two-dose-vaccinated animals had significantly more (P < 0.01; two-way ANOVA) MPXV DNA (107 GE/ml) than Dryvax-vaccinated or Acambis2000-vaccinated animals (105 GE/ml) detected in oral swabs.
Differences in mortality and rash illness among animals vaccinated with different smallpox vaccines.
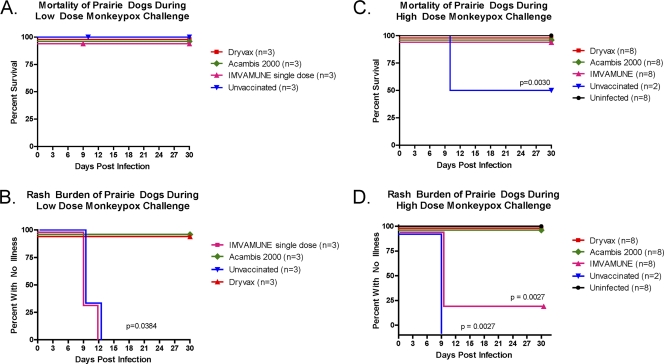
In both low- and high-dose MPXV challenge groups, the Dryvax, Acambis2000, and IMVAMUNE vaccinations protected animals that received MPXV challenge from death, but efficacy varied when measured by rash burden (Fig. 8). In the low-dose MPXV challenge groups, no animals, including the unvaccinated animals, died after the MPXV challenge (Fig. 8A). Using rash illness as a marker of morbidity in low-dose MPXV-challenged animals (Fig. 8B), neither Dryvax- nor Acambis2000-vaccinated animals presented with lesions. In contrast, all the unvaccinated animals (3 of 3 animals, 2 to 24 lesions) and IMVAMUNE one-dose-vaccinated animals (3 of 3 animals, 3 to 14 lesions) challenged with MPXV presented with lesions around days 9 to 12. These groups were statistically indistinguishable from each other and had significantly more rash illness than the Dryvax- and Acambis2000-vaccinated groups (P = 0.0384; Kaplan-Meier survival analysis).
Fig. 8.
Mortality and rash burden in vaccinated and unvaccinated MPXV-challenged prairie dogs. Mortality (A and C) and rash burden (B and D) of both low-dose (A and B) and high-dose (C and D) challenge groups are shown. The Dryvax, Acambis2000, and IMVAMUNE single and two-dose regimens are compared to unvaccinated and unchallenged animals. Comparison of mortality and morbidity curves was accomplished using the Kaplan-Meier method.
After a high-dose MPXV challenge, the Dryvax (8 of 8 animals), Acambis2000 (8 of 8 animals), IMVAMUNE two-dose (8 of 8 animals), and uninfected groups (8 of 8 animals) survived, whereas 1 of 2 unvaccinated animals died on day 10 (Fig. 8C). The 50% mortality was less than the 100% that was expected. Even with this observed mortality, the unvaccinated group manifested significantly higher mortality (P = 0.0030; Kaplan-Meier survival analysis) than any vaccinated group.
In the rash illness analysis for this study (Fig. 8D), the unvaccinated animals (2 of 2 animals, 16 to 20 lesions) showed lesion formation beginning around days 9 to 12, as did the IMVAMUNE two-dose group (6 of 8 animals, 1 to 2 lesions), but all animals (8 of 8) in the Dryvax- and Acambis2000-vaccinated groups were lesion free. Both the Dryvax- and Acambis2000-vaccinated groups were statistically indistinguishable from uninfected controls or each other, whereas both the unvaccinated and IMVAMUNE two-dose-vaccinated groups included significantly more animals presenting with rash illness than either the Dryvax- or Acambis2000-vaccinated groups (P = 0.0027; Kaplan-Meier survival analysis). While the unvaccinated and IMVAMUNE-vaccinated groups were not statistically significantly different from each other in the number of animals that exhibited rash illness (2/2 and 6/8), the number of lesions on individual IMVAMUNE vaccinated animals (1 to 2 lesions) was lower than the lesion counts on individual unvaccinated animals (16 to 20 lesions). All animals in all groups at either dose cleared lesions around day 21. In summary, all vaccines protected the animals from mortality in these studies. Animals that received Dryvax or Acambis2000 vaccination were protected completely from morbidity, whereas IMVAMUNE-vaccinated animals exhibited (modified) rash illness.
DISCUSSION
Protection of an increasingly vulnerable population (60) and the development of safer smallpox vaccines (22) remain ongoing research concerns. In this study, we attempted to (i) establish the black-tailed prairie dog as a novel smallpox vaccine testing model and (ii) contribute to the body of knowledge regarding protection of first-, second-, and third-generation smallpox vaccines when used prophylactically.
To establish the prairie dog as a valid vaccine model, we first evaluated the safety of current smallpox vaccines in this model by comparing any disease manifestations and virus shedding to those of animals infected with a high-dose of VACV-WR. We demonstrated that VACV-WR (106 PFU) m.p. infection in prairie dogs caused weight loss, local and disseminated lesions, ocular infections, presence of viral DNA in blood, and viral DNA and infectious virus in oral swabs. Conversely, m.p. inoculation of prairie dogs with human doses of Dryvax or Acambis2000 (105 PFU; 5 times less virus than the VACV-WR infection) or s.c. inoculation of IMVAMUNE (108 TCID50) did not induce any demonstrable illness (Fig. 1), indicating that any disease we see in MPXV challenge studies is due to challenge virus and not vaccine.
To compare vaccine-induced immune responses between one- and two-dose IMVAMUNE regimens, we measured total anti-OPXV IgG antibodies for all three vaccines at the peak of antibody induction (with day 0 at 30 days postvaccination). At this time point, vaccine-induced total antibodies were greater in Dryvax-vaccinated (3.55 × 104) and Acambis2000-vaccinated (2.74 × 103) animals than in animals vaccinated with one dose of IMVAMUNE (1.04 × 102). However, 30 days after a second dose of IMVAMUNE was administered, total anti-OPXV Abs in these animals (1.35 × 103) were at levels similar to Dryvax-vaccinated (2.68 × 103) or Acambis2000-vaccinated (1.18 × 103) animals (Fig. 2). Our results confirmed the boosting effect of the second dose of IMVAMUNE (17), as Abs induced with a single dose were 12.9-fold lower than the level induced by two doses.
Because vaccine-induced NAbs are considered a vital immune response for protection against monkeypox challenge in NHP (15) and to better compare our experiments to previously reported animal studies, we measured NAb titers for animals vaccinated with Dryvax, Acambis2000, or two doses of IMVAMUNE. Our results showed that two-dose IMVAMUNE (6.26 × 102) induced a higher peak level of NAbs (with day 0 at 30 days after the second vaccination) than Dryvax (2.38 × 102) or Acambis2000 (8.94 × 101). This is consistent with previous reports that “first-generation” vaccines induce similar higher levels of anti-VACV NAbs than “second-generation” vaccines (22), and it recapitulates findings (17) where, in human volunteers, two doses of MVA generated higher levels of NAbs than Dryvax alone. Similarly, in MPXV-infected NHP (13) and RPXV-infected rabbit challenge models (18), a two-dose MVA regimen elicited higher levels of NAbs than Dryvax. In summary, the observation that vaccinated animal serum showed induction of total Abs and NAbs consistent with previous studies, coupled with the evidence of a “take” seen in Dryvax-vaccinated and Acambis2000-vaccinated animals, led us to hypothesize that smallpox vaccination would be efficacious in protecting prairie dogs from MPXV challenge.
To determine if these vaccines protected the animals from death from MPXV challenge, we administered a high- or low-dose MPXV challenge to various vaccinated animal groups and compared survival to that observed in unvaccinated controls. All previously vaccinated animals survived and manifested less morbidity than was seen in unvaccinated animals, indicating that the vaccines were protective. Although these were designed as 17× and 170× LD50 studies, we did not observe 100% lethality for unvaccinated challenged animals. Our sample sizes for each group were chosen to minimize animal usage while maintaining statistical significance and used mortality estimates based on an LD50 of 5.9 × 103 PFU/ml, calculated using the Reed-Muench equation (27). Based on multiple publications (27, 29, 70) and additional unpublished work in our lab (P. Hudson, C. L. Hutson, and S. K. Smith), we expected a lethal challenge with unvaccinated control animals requiring euthanasia around day 12 of the study. Although that did not occur, the one control animal in the high-dose challenge study that survived to the end of the study (day 28) was severely ill and had not fully recovered from illness at the study end point. Although all vaccinated survivor animals were gaining weight and their lesions had resolved by study day 28, the survivor control animal barely maintained 87% of its initial weight and its lesions were incompletely resolved at day 28; however, the surviving control animal did not fully meet euthanasia criteria. Despite this, there was a statistically significant difference between survival of any of the vaccinated group arms and unvaccinated control arm in the high-dose MPXV-challenged animals (Fig. 8), leading us to conclude that all three vaccines were protective against mortality in this animal model.
In order to identify broad differences in the abilities of each vaccine to protect the animals from MPXV-induced illness, we evaluated the effects of vaccination on weight loss, inflammation at the site of inoculation of MPXV challenge virus, and disseminated lesions. Unvaccinated animals had demonstrable weight loss (Fig. 5) and nasal involvement and rash illness (Fig. 6) at both doses. The Dryvax- and Acambis2000-vaccinated animals demonstrated no weight loss (Fig. 5) and no disseminated rash illness (Fig. 6) regardless of MPXV challenge dose. After high-dose MPXV challenge, the Dryvax- and Acambis2000-vaccinated animals had very mild nasal involvement throughout the illness course compared to the unvaccinated animals (Fig. 6), indicating the overall effectiveness of Dryvax and Acambis2000 against systemic OPXV disease. This recapitulates findings from an NHP intravenous (i.v.) challenge study with ∼10 times the LD50 (3.8 × 107 PFU), which demonstrated that vaccination with Acambis2000 was protective against both death and rash illness in macaques. All protective markers in the Acambis2000-vaccinated animals were virtually indistinguishable from the Dryvax-vaccinated animals challenged with the same dose (47), which is similar to what we observed in both our 17× LD50 and 170× LD50 challenge studies.
The prairie dogs that were previously vaccinated with one or two doses of IMVAMUNE did not show a demonstrable weight loss (Fig. 5) after MPXV challenge. Interestingly, both groups of IMVAMUNE-vaccinated/MPXV-challenged animals did have significantly more signs of illness than the Dryvax- or Acambis2000-vaccinated/MPXV-challenged animals (Fig. 6), although this illness was attenuated in IMVAMUNE-vaccinated compared to unvaccinated animals. This recapitulates findings from previously reported animal studies that demonstrated that two-dose IMVAMUNE vaccination, although protective against mortality, does not prevent all morbidity upon MPXV challenge. In one NHP ∼10× LD50 (5 × 107 PFU) MPXV i.v. challenge study, 6 of 6 MVA-vaccinated animals developed lesions, compared to none of the Dryvax-vaccinated animals (13), which is comparable the results of our high-dose (106 PFU) prairie dog study when we used a two-dose IMVAMUNE regimen. Using this same NHP model, a single dose of MVA 30 days prior to challenge resulted in 4 of 4 MVA-vaccinated animals developing lesions, whereas the Dryvax-vaccinated animals did not (14). This was similar to what we observed in our low-dose (105 PFU) study when we administered a single dose of IMVAMUNE.
Because the i.v. route of infection used in the studies above is not typical of human systemic OPXV infection, and our model uses an i.n. route of infection, we also compared our study to an NHP study that used intratracheal (i.t.) administration of 107 PFU MPXV after vaccination with Elstree-RIMV or 2 doses of MVA-BN. In this study, no lesions were seen in animals vaccinated with Elstree-RIMV, but 1 (of 6) MVA-BN-vaccinated animals developed rash illness, similar to 3 (of 3) unvaccinated control animals (47). In the RPXV-infected rabbit animal model, vaccination with Dryvax or two doses of IMVAMUNE protected against death from an ∼500× LD50 aerosolized RPXV challenge, but a comparison of rash illness cannot be done, as the rabbits succumbed to disease prior to cutaneous rash formation (18). We feel that our results from this study of first-, second-, and third-generation vaccines in the prairie dog model augment results found in comparable animal model studies, which have shown that while MVA vaccines protect from mortality, some “breakthrough” of disease, albeit attenuated, does occur.
To attempt to better understand this attenuated disease and the effects of the vaccines on the level and duration of viral load/viral shedding, we measured DNAemia by RT-PCR in blood and infectious virus by tissue culture from oropharyngeal secretions and compared these results among vaccinated and unvaccinated animal groups (Fig. 7). A previous report for an NHP study using i.t. administration of 107 PFU MPXV after vaccination with Elstree-RIMV or two doses of MVA-BN indicated that the MVA-BN-vaccinated animals had viral loads (in plasma and throat swabs) that were higher than in the Elstree-RIMV-vaccinated animals but less than the unvaccinated controls (47). In a similar study, using two-doses of MVA and an i.v. MPXV (107 PFU) challenge, MVA-vaccinated animals had viral genome levels (GE/ml) in blood that were significantly higher than the Dryvax-vaccinated animals but significantly lower than unvaccinated control animals (13). We observed a similar result in our high-dose (106 PFU), two-dose IMVAMUNE study, which showed that IMVAMUNE- vaccinated animals had MPXV DNAemia levels that were statistically indistinguishable from those observed in unvaccinated animals and were slightly but significantly higher than those observed in Dryvax- and Acambis2000-vaccinated animals from day 7 through day 14. In addition, the infectious viral load in oral swabs was statistically indistinguishable between IMVAMUNE-vaccinated animals (106 PFU) and unvaccinated control animals (>106 PFU) and higher than Dryvax-vaccinated (105 PFU) or Acambis2000-vaccinated (105 PFU) animals on day 7. On day 14, only the unvaccinated (>106 PFU) and IMVAMUNE-vaccinated animals (>103 PFU) had any infectious virus in oral swabs, although notably, the IMVAMUNE-vaccinated animals excreted ∼3 logs less virus. Our conclusions are that after challenge with high-dose MPXV, IMVAMUNE-vaccinated animals have similar DNAemia in blood but presumably shed less virus than unvaccinated animals, but they have more DNAemia in blood and presumably shed more virus and for a longer duration than Dryvax- or Acambis2000-vaccinated animals. Further studies measuring infectious virus in blood and transmission studies using MPXV-challenged animals after various vaccination regimens would help to clarify the significance of these observations.
Our study and the NHP studies referenced above indicate that even though MVA induces NAbs to levels similar to Dryvax by 30 days after the last vaccination, animals vaccinated with MVA, but not Dryvax, still exhibit attenuated rash illness upon challenge. While this may be a function of the high levels of challenge dose used in the study, it is more likely an indication of differences in the MVA-induced immune response, which fail to constrain viral replication before the secondary viremia, and seeding of the skin, with virus. In OPXV infections, examination of the effects and interactions of the humoral and cellular responses induced by smallpox vaccines is an active area of research (51, 52, 73). NAbs appear to be more necessary for protection from challenge than cellular responses (9, 15, 17, 54, 56), but there is compelling evidence that intramuscular administration of MVA protects B cell-deficient mice (79), indicating that MVA can protect irrespective of Abs. The level of protection attributed to each immune response component appears to be highly variable across animal models (51), as MVA-vaccinated macaques have been shown to have lower cellular immune responses than Dryvax-vaccinated macaques (54), but human MVA vaccinees have higher VACV-specific CD8 T cell responses (56). In addition, no correlation between survival and cellular cytokine production was seen in VACV-WR-challenged mice (50), indicating a need for more studies of cellular immunity in animal models of smallpox vaccination (73). In our study, all vaccinated animals, regardless of challenge dose (17× or 170× the LD50) or vaccine regimen (one or two doses of IMVAMUNE), had higher levels of total Abs and NAbs than unvaccinated animals on day 0, and these responses increased by day 7 due to a robust anamnestic response (Fig. 3 and 4). This may indicate that the attenuated disease seen in IMVAMUNE-vaccinated animals is due to differences in the vaccine-induced cellular response. We are currently performing studies to generate the immunological reagents necessary to study the cellular immune response in this model in an attempt to understand what role the cellular immune response plays in vaccine-induced protection.
Differences seen in protection from illness by these vaccines could also be due to differences in the neutralizing targets of vaccine-induced Abs. Protein microarrays comparing MVA or Dryvax vaccination in humans and macaques have indicated that the Ab profiles induced by each vaccine are broadly similar (10), and macaques vaccinated with MVA or Dryvax mount similar Ab responses to the L1, A33, and B5 proteins (13). In another study, MVA vaccination induced similar levels of NAbs as Dryvax, with MVA inducing an increased NAb response to extracellular enveloped virion proteins (56). In our study, we observed that the IMVAMUNE-vaccinated animals had similar numbers of NAbs but lower total Abs on day 7 postchallenge compared to the Dryvax- or Acambis2000-vaccinated animals. IMVAMUNE-vaccinated animals also had the highest level of NAbs on day 0, but Dryvax- and Acambis2000-vaccinated animals had levels of NAbs that equaled or surpassed those of the IMVAMUNE-vaccinated animals by day 7. Interpretations of these observations are complicated by the use of different VACV strains in each assay (Wyeth for ELISA, WR for HCS-GFP), and we are currently investigating the proteins targeted by Abs induced by IMVAMUNE, Dryvax, and Acambis2000 in order to better understand this observation and to determine if differences in NAb targets correlate to the differences in protection from rash illness seen in this model.
Although MPXV-infected NHP are a frequently used animal model for the testing of smallpox vaccines and recent research continues to improve this model (19), the expense of these animals and their relatively complicated husbandry make an inexpensive, easily obtainable, easily husbandried small animal model—like the prairie dog—attractive as an alternate model for use for initial testing of second- and third-generation vaccines. Comparisons with other small animal models, (1, 2, 50) show that the pathogenesis of MPXV in prairie dogs is more similar to systemic OPXV infection in humans than other small animal models in both the route of transmission of challenge virus and length of viral incubation period. This 7- to 9-day incubation period is especially important, as it potentially makes the MPXV-infected prairie dog an ideal model for testing postexposure therapies, as have recently been tested in ECTV-infected mice (55). In addition, unlike the recently described MPXV-infected STAT1−/− mice (71) and inbred mouse models (2), the prairie dog is an immunocompetent and natural host for MPXV. Although these animals have not been successfully bred in captivity, they are easily available; although there are a limited number of commercially available reagents for this model, the fact that the prairie dog is an outbred animal model should offer a more realistic view of the variability in protection that will occur in any human vaccination program. These characteristics make the prairie dog a useful small animal model for evaluation of pre- and postexposure medical countermeasures, such as vaccination.
In summary, our results demonstrate that the MPXV-challenged prairie dog animal model offers a novel alternative small animal model to further explore humoral and cellular immunity and the mechanisms of protection of prophylactic smallpox vaccination, and possibly to assess postexposure vaccine efficacy. In this study, Dryvax or Acambis2000 completely, and single-dose IMVAMUNE partially, protected animals from rash illness from a low-dose systemic OPXV challenge administered 30 days prior to challenge. Similarly, a two-dose IMVAMUNE vaccination regimen, Dryvax, and Acambis2000 regimens all protected animals from death from a high-dose systemic OPXV challenge when vaccination regimens were completed 30 days prior to challenge. In the high-dose challenge study, the amount of rash illness in IMVAMUNE-vaccinated animals was subjectively 10-fold lower than that seen in the unvaccinated animals but was significantly higher than that seen in Dryvax-vaccinated, Acambis2000-vaccinated, or uninfected animals.
ACKNOWLEDGMENTS
This work was supported by program funds from the Centers for Disease Control and Prevention.
We acknowledge the assistance of the following individuals in accomplishing this project: Gregory Langham, Pauline Cates, and Christopher Meshida of the Lawrenceville Animal Resources Branch (ARB) staff and Allison Williams, Brock Martin, and Eddie Jackson of the Roybal ARB staff for animal husbandry.
Footnotes
Published ahead of print on 1 June 2011.
REFERENCES
- 1. Adams M. M., Rice A. D., Moyer R. W. 2007. Rabbitpox virus and vaccinia virus infection of rabbits as a model for human smallpox. J. Virol. 81:11084–11095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Americo J. L., Moss B., Earl P. L. 2010. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J. Virol. 84:8172–8180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous. 2003. Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin, 2003. MMWR Morb. Mortal. Wkly. Rep. 52:537–540 [PubMed] [Google Scholar]
- 4. Anonymous. 2009. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:532–536 [PubMed] [Google Scholar]
- 5. Anonymous. 1980. World Health Organization on smallpox. J. Trop. Med. Hyg. 83:47–48 [PubMed] [Google Scholar]
- 6. Behbehani A. M. 1983. The smallpox story: life and death of an old disease. Microbiol. Rev. 47:455–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bray M., Buller M. 2004. Looking back at smallpox. Clin. Infect. Dis. 38:882–889 [DOI] [PubMed] [Google Scholar]
- 8. Croft D. R., et al. 2007. Occupational risks during a monkeypox outbreak, Wisconsin, 2003. Emerg. Infect. Dis. 13:1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crotty S., et al. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171:4969–4973 [DOI] [PubMed] [Google Scholar]
- 10. Davies D. H., et al. 2008. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus Ankara is comparable to that of Dryvax. J. Virol. 82:652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dimarco N. M., Dart L., Sanborn C. B. 2007. Modified activity-stress paradigm in an animal model of the female athlete triad. J. Appl. Physiol. 103:1469–1478 [DOI] [PubMed] [Google Scholar]
- 12. Dubois M. E., Slifka M. K. 2008. Retrospective analysis of monkeypox infection. Emerg. Infect. Dis. 14:592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Earl P. L., et al. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182–185 [DOI] [PubMed] [Google Scholar]
- 14. Earl P. L., et al. 2008. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. U. S. A. 105:10889–10894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edghill-Smith Y., et al. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11:740–747 [DOI] [PubMed] [Google Scholar]
- 16. Ennis F. A., Cruz J., Demkowicz W. E., Jr., Rothman A. L., McClain D. J. 2002. Primary induction of human CD8+ cytotoxic T lymphocytes and interferon-gamma-producing T cells after smallpox vaccination. J. Infect. Dis. 185:1657–1659 [DOI] [PubMed] [Google Scholar]
- 17. Frey S. E., et al. 2007. Clinical and immunologic responses to multiple doses of IMVAMUNE (modified vaccinia Ankara) followed by Dryvax challenge. Vaccine 25:8562–8573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garza N. L., et al. 2009. Evaluation of the efficacy of modified vaccinia Ankara (MVA)/IMVAMUNE against aerosolized rabbitpox virus in a rabbit model. Vaccine 27:5496–5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goff A. J., et al. 2011. A novel respiratory model of infection with monkeypox virus in cynomolgus macaques. J. Virol. 85:4898–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guarner J., et al. 2004. Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 10:426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hahon N., Wilson B. J. 1960. Pathogenesis of variola in Macaca irus monkeys. Am. J. Hyg. (Lond.) 71:69–80 [DOI] [PubMed] [Google Scholar]
- 22. Handley L., Buller R. M., Frey S. E., Bellone C., Parker S. 2009. The new ACAM2000 vaccine and other therapies to control orthopoxvirus outbreaks and bioterror attacks. Expert Rev. Vaccines 8:841–850 [DOI] [PubMed] [Google Scholar]
- 23. Harkin A., O'Donnell J. M., Kelly J. P. 2002. A study of VitalView for behavioural and physiological monitoring in laboratory rats. Physiol. Behav. 77:65–77 [DOI] [PubMed] [Google Scholar]
- 24. Hebert J., et al. 2008. Thermoregulation in pronghorn antelope (Antilocapra americana, Ord) in winter. J. Exp. Biol. 211:749–756 [DOI] [PubMed] [Google Scholar]
- 25. Hooper J. W., et al. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huggins J., et al. 2009. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 53:2620–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hutson C. L., et al. 2010. Dosage comparison of Congo Basin and West African strains of monkeypox virus using a prairie dog animal model of systemic orthopoxvirus disease. Virology 402:72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hutson C. L., et al. 2007. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am. J. Trop. Med. Hyg. 76:757–768 [PubMed] [Google Scholar]
- 29. Hutson C. L., et al. 2009. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J. Gen. Virol. 90:323–333 [DOI] [PubMed] [Google Scholar]
- 30. Jacobs B. L., et al. 2009. Vaccinia virus vaccines: past, present and future. Antiviral Res. 84:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jahrling P. B., et al. 2004. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc. Natl. Acad. Sci. U. S. A. 101:15196–15200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jenner E. 1801. An inquiry into the causes and effects of the variola vaccine, a disease discovered in some of the western counties of England, particularly Gloucestershire, 3rd ed Printed for the author by D. N. Shury, London, England [Google Scholar]
- 33. Johnson M. C., Damon I. K., Karem K. L. 2008. A rapid, high-throughput vaccinia virus neutralization assay for testing smallpox vaccine efficacy based on detection of green fluorescent protein. J. Virol. Methods 150:14–20 [DOI] [PubMed] [Google Scholar]
- 34. Karem K. L., et al. 2007. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin. Vaccine Immunol. 14:1318–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karem K. L., Reynolds M., Olson V., Li Y., Damon I. K. 2006. Monkeypox outbreak diagnostics and implications for vaccine protective effect. Nat. Med. 12:495–496 [DOI] [PubMed] [Google Scholar]
- 36. Keckler M. S., et al. 2010. Physiologic reference ranges for captive black-tailed prairie dogs (Cynomys ludovicianus). J. Am. Assoc. Lab. Anim. Sci. 49:274–281 [PMC free article] [PubMed] [Google Scholar]
- 37. Kempe C. H. 1972. The end of routine smallpox vaccination in the United States. Pediatrics 49:489–492 [PubMed] [Google Scholar]
- 38. Kile J. C., et al. 2005. Transmission of monkeypox among persons exposed to infected prairie dogs in Indiana in 2003. Arch. Pediatr. Adolesc. Med. 159:1022–1025 [DOI] [PubMed] [Google Scholar]
- 39. Kramski M., et al. 2010. A novel highly reproducible and lethal nonhuman primate model for orthopox virus infection. PLoS One 5:e10412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kugeler K. J., Pappert R., Zhou Y., Petersen J. M. 2006. Real-time PCR for Francisella tularensis types A and B. Emerg. Infect. Dis. 12:1799–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kulesh D. A., et al. 2004. Smallpox and pan-orthopox virus detection by real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler and the Cepheid Smart Cycler platforms. J. Clin. Microbiol. 42:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Langohr I. M., Stevenson G. W., Thacker H. L., Regnery R. L. 2004. Extensive lesions of monkeypox in a prairie dog (Cynomys sp). Vet. Pathol. 41:702–707 [DOI] [PubMed] [Google Scholar]
- 43. Lewis M. W., Graham M. B., Hammarlund E., Hanifin J., Slifka M. K. 2007. Monkeypox without exanthem. N. Engl. J. Med. 356:2112–2114 [DOI] [PubMed] [Google Scholar]
- 44. Li Y., Olson V. A., Laue T., Laker M. T., Damon I. K. 2006. Detection of monkeypox virus with real-time PCR assays. J. Clin. Virol. 36:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Likos A. M., et al. 2005. A tale of two clades: monkeypox viruses. J. Gen. Virol. 86:2661–2672 [DOI] [PubMed] [Google Scholar]
- 46. Mack T. M., Noble J., Jr., Thomas D. B. 1972. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 21:214–218 [DOI] [PubMed] [Google Scholar]
- 47. Marriott K. A., et al. 2008. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine 26:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayr A. 2003. Smallpox vaccination and bioterrorism with pox viruses. Comp. Immunol. Microbiol. Infect. Dis. 26:423–430 [DOI] [PubMed] [Google Scholar]
- 49. Mertens A., Stiedl O., Steinlechner S., Meyer M. 2008. Cardiac dynamics during daily torpor in the Djungarian hamster (Phodopus sungorus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 294:R639–R650 [DOI] [PubMed] [Google Scholar]
- 50. Meseda C. A., et al. 2005. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 339:164–175 [DOI] [PubMed] [Google Scholar]
- 51. Moss B. 2011. Smallpox vaccines: targets of protective immunity. Immunol. Rev. 239:8–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moutaftsi M., et al. 2010. Uncovering the interplay between CD8, CD4 and antibody responses to complex pathogens. Future Microbiol. 5:221–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nedunchelliyan S., Reddy D. S., Venkataraman K. S. 1992. Buffalo pox infection in man. Indian J. Public Health 36:57. [PubMed] [Google Scholar]
- 54. Nigam P., et al. 2007. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology 366:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paran N., et al. 2009. Postexposure immunization with modified vaccinia virus Ankara or conventional Lister vaccine provides solid protection in a murine model of human smallpox. J. Infect. Dis. 199:39–48 [DOI] [PubMed] [Google Scholar]
- 56. Parrino J., et al. 2007. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine 25:1513–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reynolds M. G., et al. 2007. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg. Infect. Dis. 13:1332–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reynolds M. G., et al. 2006. Clinical manifestations of human monkeypox influenced by route of infection. J. Infect. Dis. 194:773–780 [DOI] [PubMed] [Google Scholar]
- 59. Richard J. L., Grimes D. E. 2008. Bioterrorism: class A agents and their potential presentations in immunocompromised patients. Clin. J. Oncol. Nurs. 12:295–302 [DOI] [PubMed] [Google Scholar]
- 60. Rimoin A. W., et al. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. U. S. A. 107:16262–16267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ryan C. P. 2008. Zoonoses likely to be used in bioterrorism. Public Health Rep. 123:276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sackal C., et al. 2008. Bartonella spp. and Rickettsia felis in fleas, Democratic Republic of Congo. Emerg. Infect. Dis. 14:1972–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sakhatskyy P., et al. 2008. Immunogenicity and protection efficacy of subunit-based smallpox vaccines using variola major antigens. Virology 371:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sale T. A., Melski J. W., Stratman E. J. 2006. Monkeypox: an epidemiologic and clinical comparison of African and US disease. J. Am. Acad. Dermatol. 55:478–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schultz D. A., Sagartz J. E., Huso D. L., Buller R. M. 2009. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology 383:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Serkova N. J., Rose J. C., Epperson L. E., Carey H. V., Martin S. L. 2007. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol. Genomics 31:15–24 [DOI] [PubMed] [Google Scholar]
- 67. Sinclair R., Boone S. A., Greenberg D., Keim P., Gerba C. P. 2008. Persistence of category A select agents in the environment. Appl. Environ. Microbiol. 74:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singh R. K., et al. 2007. Buffalopox: an emerging and re-emerging zoonosis. Anim. Health Res. Rev. 8:105–114 [DOI] [PubMed] [Google Scholar]
- 69. Sivapalasingam S., et al. 2007. Immunological memory after exposure to variola virus, monkeypox virus, and vaccinia virus. J. Infect. Dis. 195:1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Smith S. K., et al. 2009. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob. Agents Chemother. 53:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stabenow J., et al. 2010. A mouse model of lethal infection for evaluating prophylactics and therapeutics against monkeypox virus. J. Virol. 84:3909–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stevenson H. L., et al. 2003. Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: Ceratophyllidae and Pulicidae) using multiplex polymerase chain reaction. J. Med. Entomol. 40:329–337 [DOI] [PubMed] [Google Scholar]
- 73. Terajima M., et al. 2008. Vaccinia virus-specific CD8(+) T-cell responses target a group of epitopes without a strong immunodominance hierarchy in humans. Hum. Immunol. 69:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tesh R. B., et al. 2004. Experimental infection of ground squirrels (Spermophilus tridecemlineatus) with monkeypox virus. Emerg. Infect. Dis. 10:1563–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tian T., et al. 2009. Overexpression of IL-1α in skin differentially modulates the immune response to scarification with vaccinia virus. J. Investig. Dermatol. 129:70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Trindade G. S., Emerson G. L., Carroll D. S., Kroon E. G., Damon I. K. 2007. Brazilian vaccinia viruses and their origins. Emerg. Infect. Dis. 13:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vorou R. M., Papavassiliou V. G., Pierroutsakos I. N. 2008. Cowpox virus infection: an emerging health threat. Curr. Opin. Infect. Dis. 21:153–156 [DOI] [PubMed] [Google Scholar]
- 78. Wells T. S., et al. 2008. Self-reported adverse health events following smallpox vaccination in a large prospective study of US military service members. Hum. Vaccin. 4:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wyatt L. S., Earl P. L., Eller L. A., Moss B. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. U. S. A. 101:4590–4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xiao S. Y., et al. 2005. Experimental infection of prairie dogs with monkeypox virus. Emerg. Infect. Dis. 11:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]