Abstract
Daily preexposure prophylaxis (PrEP) with Truvada (emtricitabine [FTC] and tenofovir disoproxil fumarate [TDF]) is a novel HIV prevention strategy recently found to reduce HIV incidence among men who have sex with men. We used a macaque model of HIV transmission to investigate if Truvada maintains prophylactic efficacy against an FTC-resistant isolate containing the M184V mutation. Five macaques received a dose of Truvada 3 days before exposing them rectally to the simian/human immunodeficiency virus mutant SHIV162p3M184V, followed by a second dose 2 h after exposure. Five untreated animals were used as controls. Virus exposures were done weekly for up to 14 weeks. Despite the high (>100-fold) level of FTC resistance conferred by M184V, all five treated animals were protected from infection, while the five untreated macaques were infected (P = 0.0008). Our results show that Truvada maintains high prophylactic efficacy against an FTC-resistant isolate. Increased susceptibility to tenofovir due to M184V and other factors, including residual antiviral activity by FTC and/or reduced virus fitness due to M184V, may all have contributed to the observed protection.
TEXT
Oral administration of antiretroviral drugs before human immunodeficiency virus (HIV) exposure (preexposure prophylaxis [PrEP]) is a promising intervention to protect high-risk HIV-1-negative people from becoming infected (5, 12, 14). A recently completed trial with daily Truvada (a combination of emtricitabine [FTC] and tenofovir disoproxil fumarate [TDF]) among HIV-seronegative men who have sex with men (MSM) has provided the first indication that oral PrEP is protective (15). In this trial, the incidence of HIV-1 was reduced by 44% among participants that took Truvada; efficacy was substantially higher (73%) for study participants who reported >90% adherence (15). Ongoing clinical trials with different high-risk populations will soon inform if PrEP may also prevent HIV acquisition by other routes of transmission (12).
In areas with widespread access to antiretroviral therapy, drug-resistant viruses are prevalent and frequently transmitted (13). Exposure to an HIV-1 strain that is already resistant to FTC or tenofovir (TFV) is a potential threat for the success of PrEP with Truvada. TDF, FTC, and the closely related drug lamivudine (3TC) are important components of first-line therapy and have been extensively used for treatment. The overall prevalence of the TFV resistance reverse transcriptase (RT) mutation K65R in patients failing antiretroviral treatment has remained low (3%) and relatively stable during the past few years, although long duration of suboptimal therapy with TDF or stavudine (d4T) has been associated with higher frequencies of K65R (20, 21). In contrast, the M184V mutation, associated with FTC and 3TC resistance, is one of the most prevalent nucleoside RT inhibitor (NRTI) resistance mutations seen in patients who fail treatment (4, 23). Consequently, M184V-containing viruses are frequently transmitted and commonly seen among drug-naive, newly diagnosed HIV-infected persons (27).
Assessing the impact of circulating M184V viruses on PrEP efficacy in humans is difficult and often not feasible because it requires sampling early during infection and M184V tends to rapidly revert and become undetectable due to its high fitness costs (3, 6, 9, 28). Reversion of M184V to the wild type (WT) limits the accurate assessment of the impact of this mutation on PrEP effectiveness. Simian/human immunodeficiency virus (SHIV) infection of macaques is a well-established model of HIV transmission that can be used to explore the potential impact of M184V on the efficacy of Truvada. Using a repeat low-dose rectal SHIV transmission model, we have demonstrated the efficacy of Truvada in preventing transmission of a WT SHIV162P3 isolate in macaques (10, 11). This model was recently validated by the results of the iPrEX clinical trial with Truvada in humans, which showed similar efficacy among highly adherent participants (15). Here we used the same model to explore if in macaques Truvada maintains efficacy against an FTC-resistant SHIV isolate containing M184V.
The M184V mutation was introduced in the SHIV162p3 background by site-directed mutagenesis as recently described (7). Although one single-nucleotide change is sufficient to generate M184V, we introduced 2 nucleotide changes (ATG to GTT) to minimize reversion of M184V in vitro and in vivo after infection. Briefly, M184V was introduced (QuikChange II XL; Stratagene) in a pVP1 plasmid that contains the 5′ portion of SIVmac239 (kindly provided by Cecilia Cheng-Mayer from the Aaron Diamond AIDS Research Center) (7). The infectious viruses SHIV162P3 and SHIV162P3M184V were generated in human embryonic kidney (HEK-293T) cells after ligation of the plasmid pVP1 or pVP1M184V with the plasmid pSHIVp3gp160, which contains the gp160 region of SHIV162P3 (16–19). Virus stocks were expanded in CD8-depleted rhesus peripheral blood mononuclear cells (PBMCs) and stored in liquid nitrogen until use. A full phenotypic characterization of SHIV162P3M184V and the concurrently generated WT SHIV162P3 isolate has been recently reported (7). Similar to the case with HIV-1, M184V conferred high-level (>100-fold) resistance to FTC and increased susceptibility (3.3-fold) to TFV in TZM-bl cells. The phenotypic drug susceptibility profile determined by an enzymatic RT activity assay was similar (7). M184V reduced the susceptibility to FTC-triphosphate (FTC-TP) by >100-fold and increased the susceptibility to TFV-diphosphate (TFV-DP) by 1.3-fold (Table 1). Thus, the susceptibility of SHIV162P3M184V to TFV and FTC was similar to that seen in HIV-1 (22, 25).
Table 1.
Phenotypic susceptibility of SHIV162P3M184V to FTC and TFV using a TZM-bl assay and a heteropolymeric reverse transcriptase activity assay
| Isolate | IC50 (μM)a |
|||
|---|---|---|---|---|
| TZM-bl assay |
RT activity assay |
|||
| FTC | TFV | FTC-TP | TFV-DP | |
| SHIV162P3 | 0.1 | 1.67 | 0.22 | 0.30 |
| SHIV162P3M184V | 78.2 (>100)1 | 0.50 (0.3) | 25.4 (>100) | 0.24 (0.8) |
Fold change in IC50 (50% inhibitory concentration) relative to that for wild-type SHIV162p3 is shown in parentheses.
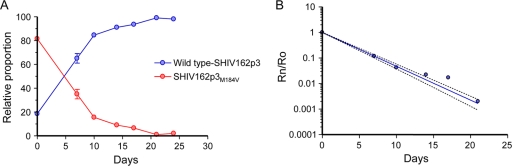
In HIV-1, the M184V mutation reduces virus fitness (6). We confirmed the deleterious effect of M184V on simian immunodeficiency virus (SIV) by using a competitive SHIV162p3 replication assay. In this experiment, an 80:20 mixture of SHIV162P3M184V and the concurrently generated WT SHIV162P3 was used to infect 5 × 105 Rhesus PBMCs at a multiplicity of infection of 0.002. Changes in the relative proportion of the two competing variants were then monitored over time by sequence analysis of viruses from culture supernatants (6). Figure 1 shows that SHIV162P3M184V was rapidly outcompeted by the WT isolate, demonstrating that, as in HIV-1, M184V in SIV is associated with a high fitness cost (6). The fitness difference among the two viruses was calculated by plotting the changes in relative proportions over time (6). SHIV162P3M184V was found to be 30-fold (95% confidence interval [CI] = 28.4 to 32.7) less fit than WT SHIV162p3 (Fig. 1).
Fig. 1.
Competition dynamics among WT SHIV162P3 and SHIV162P3M184V. The experiment was initiated at an 80/20 mutant-to-WT ratio. (A) Changes in the relative proportions of the two competing variants over time measured in a single competition experiment. (B) Fitness vector. To calculate the fitness vector, the proportion of SHIV162P3M184V with respect to WT SHIV (Rn) was divided by its ratio in the initial mixture (Ro), and this value (Rn/Ro) was plotted versus the time in days.
We next evaluated the transmissibility of SHIV162P3M184V in Indian rhesus macaques. Five macaques were exposed to SHIV162P3M184V rectally by nontraumatic inoculation of 1 ml of culture supernatant into the rectal vault via a sterile gastric feeding tube of adjusted length (10, 11, 24). Anesthetized macaques remained recumbent for at least 15 min after each inoculation. Macaques were monitored weekly for evidence of infection by molecular and serologic testing using a synthetic-peptide enzyme immunoassay (EIA) (Genetic Systems HIV-1/HIV-2; Bio-Rad, Redmond, WA) assay and real-time RT-PCR or PCR assays specific for SHIV RNA or DNA, respectively (24). Virus exposures were stopped when a macaque became SHIV RNA positive. We used a virus dose of 40 50% tissue culture infective doses (TCID50) since SHIV162P3M184V has a 4-fold-lower virus infectivity/virion particle ratio than WT SHIV162P3, which is usually dosed at 10 TCID50 (10). The Institutional Animal Care and Use Committee (IACUC) of the Centers for Disease Control and Prevention (CDC) approved this study.
All 5 rhesus macaques exposed to 40 TCID50 of SHIV162P3M184V were infected during repeated rectal exposures. The median number of challenges required to infect the animals (3 challenges; 95% CI, 2 to 11) was similar to that seen in 34 historical controls exposed to 10 TCID50 of the parental WT SHIV162P3 isolate (2 challenges; 95% CI, 1 to 13; P = 0.47, Wald chi-square test) (10) (not shown). Figure 2 shows the kinetics of acute viremia seen in the 5 infected macaques. The median peak virus load was 5.7 log10 viral RNA (vRNA) copies/ml and ranged from 5.2 to 7.3 log10 copies/ml. Seroconversion was observed in all animals within 2 to 5 weeks after the first detectable vRNA; proviral DNA was consistently detected in all 5 animals (not shown). Sequence analysis of viruses from plasma collected 3 to 36 weeks after infection confirmed the presence of the M184V mutation for all 5 macaques, demonstrating an absence of reversion of M184V at infection. Figure 2 also shows that peak virus loads in the SHIV162P3M184V infections were significantly lower than those seen in historical macaques infected with the parental WT SHIV162P3 isolate (7.1 log10 RNA copies/ml; range = 5.3 to 8.9; P = 0.04, Wilcoxon test).
Fig. 2.
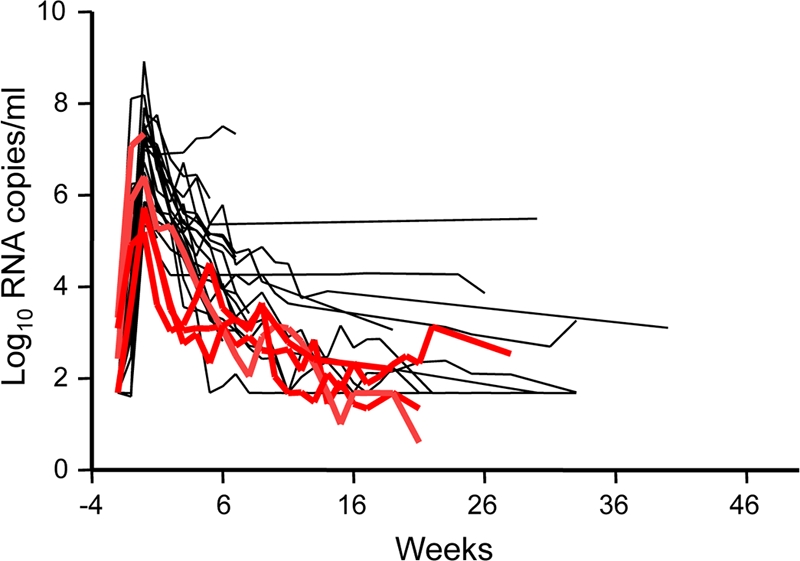
Blunted acute viremia in untreated control macaques infected with SHIV162P3M184V. Individual virus load kinetics (n = 5; red lines) are compared to those seen in historical macaques infected with the parental WT SHIV isolate (n = 22; dark lines). Time zero indicates the peak plasma virus load. Median peak viremia was significantly lower in M184V infections (5.7 log10 RNA copies/ml, compared to 7.1 log10 RNA copies/ml in macaques infected with WT SHIV; P = 0.04). One SHIV162P3M184V-infected animal has limited follow-up because it had to be euthanized for reasons unrelated to the study.
The prophylactic efficacy of Truvada against SHIV162P3M184V was evaluated with 5 rhesus macaques that received two weekly doses of Truvada. The two Truvada doses were given 3 days before and 2 h after each of the weekly virus exposures, which were done for 14 consecutive weeks. Truvada was administered orally by gavage to anesthetized macaques via a gastric feeding tube (10, 11, 24). We recently showed that the same Truvada regimen and dosing strategy was highly effective in preventing transmission of the WT SHIV162P3 isolate (5 out of 6 animals protected) (10). Figure 3 shows that all 5 macaques that received Truvada and were exposed to SHIV162P3M184V were also protected from infection after the 14 virus challenges (P = 0.0008 relative to results for untreated controls, Fisher's exact test), as indicated by an absence of seroconversion and detection of SHIV RNA or proviral DNA during the weekly virus exposures and a follow-up period of 20 weeks. These findings demonstrate the protective effect of Truvada against this FTC-resistant mutant.
Fig. 3.
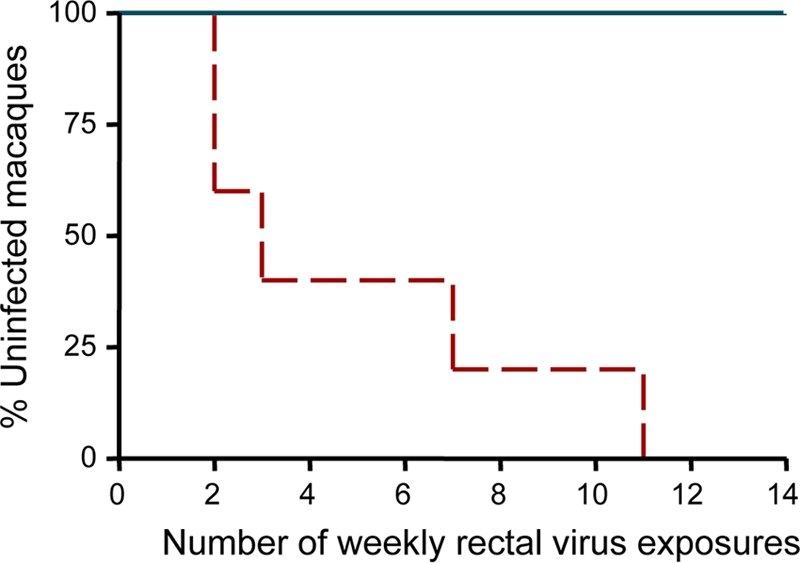
Prophylactic efficacy of Truvada against SHIV162P3M184V. Each survival curve represents the cumulative percentage of uninfected macaques as a function of weekly rectal exposures. Protected animals remained seronegative and viral RNA/DNA negative during the 14 exposures and a follow-up period of 20 weeks. Truvada prevented infection by SHIV162P3M184V (P = 0.0008).
Several PrEP human clinical trials with oral or topically administered antiretroviral drugs are now at various stages of completion (12). These studies will provide valuable information, including safety, efficacy for the major routes of HIV transmission, and acceptability of oral and topical drug dosing (12). The recent findings that a 1% TFV gel provided 38% protection against vaginal HIV transmission and that daily oral Truvada provided 44% protection from HIV among MSM are the first indications that PrEP may be a feasible strategy for preventing sexual HIV transmission if delivered as part of a comprehensive package of prevention services (1, 15).
The widespread use of 3TC and FTC and their low genetic barrier for resistance development have resulted in a high frequency of M184V for patients who fail highly active antiretroviral therapy (HAART) (4). A high frequency of M184V in HAART failures increases the risk of M184V resistance transmission and likely explains the frequent finding of M184V in newly diagnosed, drug-naive persons that are infected with drug-resistant viruses (26, 27). It is therefore essential to understand if exposure to M184V-containing viruses could potentially reduce the efficacy of PrEP with Truvada. The high protection seen in our animals that were exposed to this mutant and received only two doses of Truvada is reassuring and suggests that Truvada may still retain prophylactic efficacy in this setting. The high efficacy seen with only two weekly doses of Truvada strongly suggests that daily Truvada regimens might also be efficacious.
Several possible reasons could explain the high protection by Truvada seen in our macaques. As in the case of HIV-1, M184V was associated with increased susceptibility to TFV. Since TFV accumulates in rectal tissues, a 2- to 3-fold increase in virus susceptibility for TFV may increase the effective TFV dose at the mucosa and render TFV more efficient in blocking infection (10). Low virus fitness due to M184V might also facilitate blocking of these viruses with drug and limit their ability to establish and propagate a local infection, thus indirectly increasing PrEP efficacy. Although our infectivity adjustments based on the ratio between virus infectivity and virion particles were sufficient to recapitulate the transmission efficiency of WT SHIV162P3, peak M184V viremias in our untreated macaques were still significantly lower. Lower acute viremias were indeed consistent with the reduced virus fitness of SHIV162P3M184V seen in our competitive replication assay.
The high efficacy of Truvada against M184V viruses might also reflect residual antiviral activity of FTC or high FTC concentrations in rectal tissues. Extracellular concentrations of FTC in rectal secretions or tissues from rhesus macaques and humans have been found to be higher than blood plasma for up to 14 days postdose, likely reflect FTC trapping into mucus originating from drug elimination in feces (10; K. B. Patterson, H. A. Prince, E. Kraft, A. Jones, S. Paul, N. J. Shaheen, M. Spacek, P. E. Heidt, S. Reddy, J. Rooney, M. S. Cohen, and A. D. M. Kashuba, presented at the 18th International AIDS Conference, Vienna, Austria, 18 to 23 July 2010). High FTC concentrations in rectal tissues might offer some residual antiviral activity, as previously noted in patients who fail 3TC-containing regimens and have viruses containing M184V (23). Additional efficacy studies with a TDF-only regimen will inform on the role of FTC in the observed protection against SHIV162P3M184V.
Our results may not be generalized to all isolates that contain M184V or to other routes of transmission. While M184V by itself increases the susceptibility for TFV, this effect may be reduced in viruses that also contain other resistance-associated mutations (29). For instance, combinations of M184V with thymidine analog mutations, including M41L, T21Y, and L210W or D67N, K70R, and T215F, result in reduced susceptibility to TFV. FTC and TFV exposure also differs in vaginal and rectal tissues, which may potentially impact residual antiviral activity by FTC against M184V viruses and/or effective tissue TFV concentrations.
Our transmissibility data with SHIV162P3M184V expand our previous observations with a smaller number of animals showing that SHIV162P3M184V dose increases based on virus infectivity/total virion particle ratios were sufficient to recapitulate the transmission efficiency of the parental WT isolate (7). These findings further suggest that transmissibility of M184V-containing viruses may be less efficient than WT virus transmission. A lower transmission efficiency for M184V viruses has been suggested in a case-controlled study comparing resistance genotypes between potential transmitters and recent HIV seroconverters (8). However, these types of studies are challenging, since the population of potential transmitters is difficult to define and M184V viruses tend to revert shortly after transmission (2). Our study is the first to provide in vivo evidence in macaques suggesting that M184V mutants may indeed be less transmissible. Since M184V viremias were lower than WT viremias, our results also suggest that acute M184V virus infections might have reduced risks for secondary virus transmission.
In summary, we show in a validated macaque model of rectal SHIV transmission that PrEP with Truvada retains high efficacy against a highly FTC-resistant isolate. These findings are reassuring, since they suggest that exposure to FTC-resistant viruses does not necessary translate into a failure of Truvada to prevent infection in humans. Increased susceptibility to TFV due to M184V and possibly other factors, including residual antiviral activity by FTC and/or reduced virus fitness due to M184V, may all have contributed to the protection observed in our animals. These results illustrate how PrEP efficacy against drug-resistant viruses can be influenced by drug pharmacokinetics and pharmacodynamics, complex phenotypic drug susceptibility profiles, and virus replicative capacity.
Acknowledgments
We thank Katherine Paul for serving as the attending veterinarian for this animal study protocol and Cecilia Cheng-Mayer for providing us with plasmids pVP1 and pSHIVp3gp160.
This work was partially supported by Interagency Agreement Y1-AI-0681-02 between the CDC and NIH.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 1 June 2011.
REFERENCES
- 1. Abdool Karim Q., et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brenner B. G., et al. 2002. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J. Virol. 76:1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cane P. A. 2005. Stability of transmitted drug-resistant HIV-1 species. Curr. Opin. Infect. Dis. 18:537–542 [DOI] [PubMed] [Google Scholar]
- 4. Cheung P. K., Wynhoven B., Harrigan P. R. 2004. 2004: which HIV-1 drug resistance mutations are common in clinical practice? AIDS Rev. 6:107–116 [PubMed] [Google Scholar]
- 5. Cohen M. S., Gay C., Kashuba A. D., Blower S., Paxton L. 2007. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann. Intern. Med. 146:591–601 [DOI] [PubMed] [Google Scholar]
- 6. Cong M., Heneine W., García-Lerma J. G. 2007. The fitness cost of mutations associated with human immunodeficiency virus type 1 drug resistance is modulated by mutational interactions. J. Virol. 81:3037–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cong M., et al. 2011. Generation and mucosal transmissibility of emtricitabine- and tenofovir-resistant SHIV162p3 mutants in macaques. Virology 412:435–440 [DOI] [PubMed] [Google Scholar]
- 8. de Mendoza C., et al. 2004. Evidence for differences in the sexual transmission efficiency of HIV strains with distinct drug resistance genotypes. Clin. Infect. Dis. 39:1231–1238 [DOI] [PubMed] [Google Scholar]
- 9. Deval J., et al. 2004. Mechanistic basis for reduced viral and enzymatic fitness of HIV-1 reverse transcriptase containing both K65R and M184V mutations. J. Biol. Chem. 279:509–516 [DOI] [PubMed] [Google Scholar]
- 10. García-Lerma J. G., et al. 2010. Intermittent prophylaxis with oral Truvada protects macaques from rectal SHIV infection. Sci. Transl. Med. 2:14ra4. [DOI] [PubMed] [Google Scholar]
- 11. García-Lerma J. G., et al. 2008. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 5:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García-Lerma J. G., Paxton L., Kilmarx P., Heneine W. 2010. Oral pre-exposure prophylaxis for HIV prevention. Trends Pharmacol. Sci. 31:74–81 [DOI] [PubMed] [Google Scholar]
- 13. Geretti A. M. 2007. Epidemiology of antiretroviral drug resistance in drug-naïve persons. Curr. Opin. Infect. Dis. 20:22–32 [DOI] [PubMed] [Google Scholar]
- 14. Grant R. M., et al. 2005. Promote HIV chemoprophylaxis research, don't prevent it. Science 309:2170–2171 [DOI] [PubMed] [Google Scholar]
- 15. Grant R. M., et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harouse J. M., et al. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J. Virol. 75:1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harouse J. M., Gettie A., Tan R. C., Blanchard J., Cheng-Mayer C. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816–819 [DOI] [PubMed] [Google Scholar]
- 18. Hsu M., et al. 2005. A CCR5-tropic simian-HIV molecular clone capable of inducing AIDS in rhesus macaques. J. Acquir. Immune Defic. Syndr. 40:383–387 [DOI] [PubMed] [Google Scholar]
- 19. Luciw P. A., Pratt-Lowe E., Shaw K. E., Levy J. A., Cheng-Mayer C. 1995. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc. Natl. Acad. Sci. U. S. A. 92:7490–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez-Cajas J. L., Pai N. P., Klein M. B., Wainberg M. A. 2009. Differences in resistance mutations among HIV-1 non-subtype B infections: a systematic review of evidence (1996–2008). J. Int. AIDS Soc. 12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McColl D. J., Chappey C., Parkin N. T., Miller M. D. 2008. Prevalence, genotypic associations and phenotypic characterization of K65R, L74V and other HIV-1 RT resistance mutations in a commercial database. Antivir. Ther. 13:189–197 [PubMed] [Google Scholar]
- 22. Schinazi R. F., et al. 1993. Characterization of human immunodeficiency viruses resistant to oxatiolate-cytosine nucleosides. Antimicrob. Agents Chemother. 37:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shafer R. W., Schapiro J. M. 2008. HIV-1 drug resistance mutations: an updated framework for the second decade of HAART. AIDS Rev. 10:67–84 [PMC free article] [PubMed] [Google Scholar]
- 24. Subbarao S., et al. 2006. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J. Infect. Dis. 194:904–911 [DOI] [PubMed] [Google Scholar]
- 25. Tisdale N. S., Kemp D., Parry N. R., Larder B. A. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of the reverse transcriptase. Proc. Natl. Acad. Sci. U. S. A. 90:5653–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weinstock H., et al. 2004. The epidemiology of antiretroviral drug resistance among drug-naive HIV-1 infected persons in 10 U.S. cities. J. Infect. Dis. 189:2174–2180 [DOI] [PubMed] [Google Scholar]
- 27. Wheeler W. H., et al. 2010. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U. S.-2006. AIDS 24:1203–1212 [DOI] [PubMed] [Google Scholar]
- 28. White K. L., et al. 2002. Molecular mechanisms of resistance to human immunodeficiency virus type 1 with reverse transcriptase mutations K65R and K65R+M184V and their effects on enzyme function and viral replication capacity. Antimicrob. Agents Chemother. 46:3437–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolf K., et al. 2003. Tenofovir resistance and resensitization. Antimicrob. Agents Chemother. 47:3478–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]