Abstract
The events leading to death in severe cases of Lassa fever (LF) are unknown. Fatality seems to be linked to high viremia and immunosuppression, and cellular immunity, rather than neutralizing antibodies, appears to be essential for survival. We previously compared Lassa virus (LV) with its genetically close but nonpathogenic homolog Mopeia virus (MV), which was used to model nonfatal LF. We showed that strong and early activation of antigen-presenting cells (APC) may play a crucial role in controlling infection. Here we developed an in vitro model of dendritic-cell (DC)-T-cell coculture in order to characterize human T-cell responses induced by MV- or LV-infected DCs. Our results show very different responses to infection with LV and MV. MV strongly and durably stimulated CD8+ and CD4+ T cells, showing early and high activation, a strong proliferative response, and acquisition of effector and memory phenotypes. Furthermore, robust and functional CD4+ and CD8+ cytotoxic T lymphocytes (CTL) were generated. LV, however, induced only weak memory responses. Thus, this study allows an improved understanding of the pathogenesis and immune mechanisms involved in the control of human LV.
INTRODUCTION
Lassa fever (LF), a viral hemorrhagic fever, represents a major public health concern in West Africa, with about 300,000 cases and 5,000 to 6,000 deaths each year (49). LF is caused by an Old World arenavirus, Lassa virus (LV) (15). Humans become infected through contact with peridomestic rodents (Mastomys sp.), which serve as the reservoir host (49). Interhuman transmission then occurs via mucosal/cutaneous contact or nosocomial infection. There are no approved vaccines or effective drugs against this virus, except for ribavirin, which has been used in the field with only modest efficacy, due to limited availability and the difficulty of initiating therapy very early after infection (48).
The severity of the disease ranges from asymptomatic infection to fatal hemorrhagic fever (29). Nonspecific signs appear in patients after a 6- to 12-day incubation period. In the most severe cases, leading to death, more-specific symptoms of hypotensive, hypovolemic, and hypoxic shock are then observed, but the pathogenesis of LF remains unclear (25). The damage to the endothelium and other organs is not severe enough to account for terminal shock and death, which seem rather to depend on the host response (13). Elevated viremia and immunosuppression seem to characterize severe LV infections. Other features observed in patients and nonhuman primates (NHP) include structural changes, cellular depletion of secondary lymphoid tissues, necrosis of the splenic marginal zone, transitory lymphopenia, and abolition of mitogenic T-cell proliferation (7, 25, 27, 28).
In survivors, in contrast, symptoms disappear 10 to 15 days after onset, although about one-third of survivors may suffer from deafness, a common complication of LF (22). LV infection in humans seems to be controlled primarily by T-cell responses. Memory CD4+ T cells directed against the viral nucleoprotein and glycoproteins circulate in LV-seropositive subjects (66, 67), whereas neutralizing antibodies are detected at low titers only after recovery, and the production of specific immunoglobulin G (IgG) is not correlated with recovery (39). Furthermore, T-cell responses, but not antibody production, are correlated with protection of NHP against a lethal LV challenge after immunization and with the survival of naïve animals with LF (7, 26, 33). We and others have shown that dendritic cells (DC) and macrophages (MP) are the main targets of LV (6, 46). The infection of DC leads to a massive release of LV, without inducing cell activation, cell maturation, or the production of cytokines. Similarly, MP are productively infected with LV but are not activated, except for modest type I interferon (IFN) production (6, 8). Viral tropism for antigen-presenting cells (APC) probably plays a role in the defective cellular responses observed in severe cases. The lack of DC maturation after LV infection may lead to defective T-cell responses, since antigen (Ag) presentation by immature DC (iDC) induces tolerance (35).
Mopeia virus (MV) is closely related to LV, sharing 75% amino acid identity, and is also isolated from the same reservoir (12). However, MV is naturally attenuated and nonpathogenic for humans (75). Moreover, infection of NHP with MV protects against a lethal challenge with LV, confirming their close relationship (26). Therefore, the use of MV as a nonpathogenic counterpart of LV is justified and probably yields more consistent and more significant differences than comparison of the AV strain with another LV strain of lower pathogenicity. Indeed, the pathogenicities of the numerous LV strains are not well characterized and probably not markedly different. We have shown that the responses of APC to MV infection differ considerably from those observed with LV. MP were strongly activated shortly after MV infection and produced large amounts of type I IFN, whereas partial activation and moderate levels of IFN production were observed in MV-infected DC (58). Type I IFNs play an important role in antiviral defense and are also mediators of CD8+ T-cell responses (42). Thus, different responses of APC to LV and its attenuated counterparts may underlie the different adaptive immune responses and subsequent differences in pathogenicity between the two viruses.
Despite the crucial role played by T cells in LV infection, little is known about these responses. The locations of zones of endemicity and the serious health threat posed by these viruses have hampered the investigation of cellular responses in patients. Due to the lack of rodent models reproducing the immune responses of LV-infected humans, NHP are the only relevant model. As an alternative to clinical studies in humans and to NHP models, which are difficult and expensive to manipulate under biosafety level 4 (BSL-4) conditions, we compared here the human T-cell responses induced by LV-infected autologous DC with those induced by MV-infected DC, as a model of nonfatal LF. In vitro generation of human T-cell responses mediated by DC pulsed with a tumoral or viral Ag or infected with viruses has already been described (9, 17, 24, 30, 36, 62) and has recently been optimized (54). In fact, in vitro-induced T-cell responses are now recognized as a powerful alternative to in vivo models for evaluating the immunogenicity of vaccines or pathogens (9, 54). In our model, T cells were restimulated twice using LV or MV Ag-pulsed DC to amplify the cellular responses after a first round of culture with virus-infected iDC or mature DC (mDC). This approach has allowed an exhaustive analysis of CD4+ and CD8+ T-cell responses in humans. Such a study of closely related viruses differing in their pathogenicity helps to clarify the immune mechanisms involved in the control of LF.
MATERIALS AND METHODS
Viruses.
LV AV strain from the serum of a patient (34) and MV AN 23166 strain isolated from a Mastomys sp. (75) were subjected to four passages on Vero E6 cells at 37°C under a 5% CO2 atmosphere in Dulbecco's modified Eagle medium supplemented with 50 IU/ml penicillin-streptomycin, 1% nonessential amino acids (all from Invitrogen, Cergy-Pontoise, France), and 2% AB+ human serum (Etablissement Français du Sang, Lyon, France). Cell-free supernatants were harvested 3 or 4 days later for LV and MV, respectively, and were used as infectious virus stocks with a viral titer of 2.5 × 107 focus-forming units (FFU)/ml. BSL-4 facilities (Laboratoire P4, Inserm-Jean Mérieux, Lyon, France) were used for all experiments with LV, whereas MV was manipulated in BSL-2 facilities. For restimulation experiments, viruses were inactivated for 2 h at 60°C and were subjected to three freeze-thaw cycles to obtain noninfectious LV and MV stocks, which were manipulated in facilities similar to those used for their virulent counterparts. Three independent experiments with MV were nevertheless conducted in BSL-4 facilities in order to verify that similar results were obtained in the different facilities. Vero E6 cells, LV, and MV were not contaminated with mycoplasma. The AV strain of LV was preferred to the Josiah strain because the two strains are closely related, and the former was recently isolated from a fatally infected patient. Therefore, this strain is very close to the LV currently circulating in zones of endemicity, and it has been subjected to a limited number of passages since its isolation from the patient's serum (34). In addition, both strains are considered highly pathogenic LV strains (4).
Titration assays.
LV and MV titers were determined in culture supernatants using the Vero E6 cell line as described previously (6, 58). Infectious foci were counted, and results are expressed as focus-forming units per milliliter.
Purification of monocytes and lymphocytes.
Blood samples from healthy donors were obtained from the Etablissement Français du Sang. Peripheral blood mononuclear cells were isolated after density gradient centrifugation with Ficoll-Paque (GE Healthcare BioSciences AB, Uppsala, Sweden). Autologous plasma (AP) was heated for 30 min at 56°C and was centrifuged for 20 min at 1,200 × g before being used in the culture medium. Monocytes were then separated from peripheral blood lymphocytes (PBL) by centrifugation on 50% Percoll (GE Healthcare) in phosphate-buffered saline (PBS). Each fraction was washed separately three times in RPMI 1640-GlutaMAX I supplemented with 50 IU/ml penicillin-streptomycin, 1% nonessential amino acids, 10 mM HEPES (full RPMI), and 4% AB+ human serum. PBL were frozen in RPMI supplemented with 10% dimethyl sulfoxide (Sigma-Aldrich, Saint-Quentin-Fallavier, France) and 20% AP and were then stored in liquid nitrogen. Monocytes were purified by immunomagnetic depletion as described previously (6) and were cultured at 1 × 106/ml in full RPMI containing 10% AP and 2,000 IU/ml recombinant human granulocyte-macrophage colony-stimulating factor plus 1,000 IU/ml recombinant human interleukin 4 (rhIL-4) (all from PeproTech, Rocky Hill, NJ) to obtain iDC. Half of the amount of cytokines and 40% of the culture medium were renewed every 48 h, and DC were harvested 6 days later.
In vitro DC-T-cell coculture.
We established an in vitro model of DC-T-cell coculture with three rounds of stimulation. The first round involved DC infection with infectious LV or MV. DC were then cocultured with T cells from the same blood donor at a ratio of 1 DC to 10 T lymphocytes. T cells were cocultured with iDC or mDC. DC were harvested after 6 days of culture and were infected by incubating cell pellets with either virus-free Vero E6 cell supernatants (mock) or infectious LV or MV, at a multiplicity of infection (MOI) of 2, for 1 h at 37°C with regular shaking. Cells were then washed once and were cultured in full RPMI supplemented with 1 mM sodium pyruvate (Invitrogen) and 10% AP (T-cell medium). Cells were grown at a density of 2 × 105/ml. Two vials of iDC were frozen in AP containing 10% dimethyl sulfoxide for further restimulation. For experiments conducted with mDC, recombinant human tumor necrosis factor alpha (rhTNF-α) (2,500 IU/ml) and rhIL-1β (50,000 IU/ml) (both from PeproTech) were added to the culture medium of DC 2 h before coculture with T cells. PBL were thawed and washed three times in T-cell medium. PBL were depleted of B and NK cells using CD19 (Dynal, Oslo, Norway) and CD56 (Immunotech) antibodies, respectively, coupled to immunomagnetic beads (Dynal). Purified T cells (CD4+ and CD8+) were then added, at a density of 2 × 106/ml, to plates already containing DC. The second and third rounds of stimulation were carried out in the same way 9 and 19 days after the first round, using inactivated LV or MV or culture medium (mock stimulation) to stimulate thawed DC. These DC were then cocultured with the T cells harvested from the first or second stimulation with virus-infected, virus-stimulated, or mock-infected DC. In some cases, T cells cultured during the first stimulation with mock-infected iDC were subjected to second and third rounds of stimulation with inactivated-LV- or inactivated-MV-stimulated DC as a control. We replaced 30% of the culture medium with fresh medium every 2 or 3 days. On day 2 of each stimulation, 10 IU/ml (for the first stimulation) or 5 IU/ml (second and third stimulations) of rhIL-2 and rhIL-7 (both from PeproTech) was added to the culture medium. For some experiments (two independent experiments with different donors), direct contacts between mock-, LV-, or MV-infected iDC and naïve T cells were abrogated during the first stimulation using Transwell cell culture inserts with a 0.4-μm pore size (Costar, Corning, NY). A second round of stimulation was then performed as described above.
To evaluate the abilities of MV- or LV-specific T cells to control viral replication in DC, T cells were harvested 9 days after the first stimulation with mock-, MV-, or LV-infected DC. CD4+ and CD8+ T cells were purified by depleting CD8+ and CD4+ T cells using anti-CD8 and anti-CD4 antibodies, respectively, coupled to immunomagnetic beads (Dynal) from a part of harvested T cells. Four million total CD4+ or CD8+ T cells were cultured in 12-well plates with 2 × 105 thawed iDC infected 1 h earlier with MV or LV at a multiplicity of infection of 0.2. Cell-free supernatants were harvested each day for virus titration.
Flow cytometric analysis of T cells.
We evaluated the expression of cell surface and intracellular molecules involved in activation, costimulation, or the memory phenotype of T cells at days 2, 5, and 8 after each stimulation. T cells were harvested, centrifuged, and resuspended in PBS with 5% AB+ human serum. Cell surface markers were then stained with 0.1 μg/ml monoclonal antibodies (MAb) for 25 min at 4°C. MAb conjugated with fluorescein isothiocyanate (FITC) (anti-CD3, -CD8, and -CD45RA; Beckman Coulter, Villepinte, France), phycoerythrin (PE) (anti-CD3, anti-CD45RO [both from Beckman Coulter], and anti-CD154, -HLA-DR, and -CCR7 [BD Biosciences, Le-Pont-de-Claix, France]), PE-cyanine 5 (PC5) (anti-CD3 [Beckman] and anti-CD69 and -CD137 [BD Biosciences]), PE-cyanine 7 (PC7) (anti-CD3, -CD4, and -CD8; Beckman Coulter), or Alexa Fluor 647 (A647) (anti-CD3, -CD4, and -CD8; BD Biosciences) were used. Isotype controls were performed with the following irrelevant MAb: FITC-conjugated IgG1, PE-conjugated IgG2a, A647-conjugated IgG1κ (BD Biosciences), PC5-conjugated IgG1, and PC7-conjugated IgG1 (Beckman Coulter). T cells were subsequently washed with PBS containing 2.5% fetal calf serum (Eurobio, Courtaboeuf, France) and were resuspended in 1% paraformaldehyde in PBS. After surface marker staining, T cells destined for intracellular staining were permeabilized using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions. Permeabilized T cells were then incubated for 25 min at 4°C with PE-conjugated anti-Ki-67, FITC-conjugated anti-perforin, or PE-conjugated anti-granzyme B (anti-GrzB) (BD Biosciences) MAb before being washed and resuspended in 1% paraformaldehyde in PBS. Data were obtained using a four-color cytometer (EPICS-XL [Beckman Coulter] or FACSCalibur [BD Biosciences]) and were analyzed with Expo32 ADC Software (Applied Cytometry Systems, Dinnington, Sheffield, United Kingdom).
T-cell proliferation assay.
We measured the incorporation of bromodeoxyuridine (BrdU) in T cells at day 5 of each stimulation using the FITC BrdU Flow kit (BD Biosciences). BrdU labeling was performed by incubating T lymphocytes overnight with BrdU at a final concentration of 10 μM in culture medium. T cells were then harvested, washed, and stained as described previously with the following cell surface marker-specific MAb: PE-conjugated anti-CD3, PC5-conjugated-anti-CD4, or PC7- or A647-conjugated anti-CD8. Cells were subsequently washed, fixed, permeabilized, and treated with DNase to expose incorporated BrdU according to the manufacturer's instructions. Finally, incorporated BrdU was detected by flow cytometry using a specific FITC-conjugated anti-BrdU MAb.
Analysis of mRNA by quantitative reverse transcription-PCR (qRT-PCR).
Total RNA was extracted from a coculture of 1 × 106 T cells and 1 × 105 mock-, LV-, or MV-infected DC according to the instructions of the RNeasy kit manufacturer (Qiagen, Hilden, Germany). Potential genomic DNA contamination was minimized by treatment with DNase I (RNase-free DNase set; Qiagen). We synthesized cDNA by reverse transcription using extracted RNA, SuperScript II reverse transcriptase, oligo(dT), a deoxynucleoside triphosphate (dNTP) mixture, RNaseOUT, dithiothreitol (DTT), and 5× RT buffer (all from Invitrogen). cDNA was then quantified, using β-actin as a reference, by quantitative PCR (qPCR) on an ABI Prism thermocycler, model 7000 (Applied Biosystems, Courtaboeuf, France) using TaqMan Universal Master Mix and commercial TaqMan primers and probes for β-actin, IL-12p35, IL-12p40, CXCL-10, gamma interferon (IFN-γ), IL-2, TNF-α, IL-4, IL-10, FasL, and GrzB. Other TaqMan assays were carried out with the following primers and probes: for IFN-β, primers 5′-TCTCCACGACAGCTCTTTCCA-3′ and 5′-ACACTGACAATTGCTGCTTCTTTG-3′ and probe 5′-AACTTGCTTGGATTCCT-3′; for IFN-α1, primers 5′-GTGGTGCTCAGCTGCAAGTC-3′ and 5′-TGTGGGTCTCAGGGAGATCAC-3′ and probe 5′-AGCTGCTCTCTGGGC-3′; and for IFN-α2, primers 5′-CAGTCTAGCAGCATCTGCAACAT-3′ and 5′-GGAGGGCCACCAGTAAAGC-3′ and probe 5′-ACAATGGCCTTGACCTT-3′. Genomic DNA contamination was checked by testing for amplification in RNA samples without the reverse transcriptase step. Relative mRNA levels for each amplified cDNA were calculated as the mean of 2−ΔCT for five independent experiments, where CT is the cycle threshold and −ΔCT is the CT for the gene − the CT for β-actin.
Detection of cytokines in supernatants.
The following commercial enzyme-linked immunosorbent assay (ELISA) kits were used according to the manufacturer's instructions to quantify the amounts of protein in the supernatants harvested from mock-, LV-, or MV-infected DC-T-cell cocultures: the human CXCL-10 BD OptEIA set (BD Biosciences) and the human GrzB ELISA kit (Bender MedSystems). Cytokine concentrations are expressed in nanograms per milliliter.
ELISPOT assays.
T cells cocultured with mock-, LV-, or MV-infected APC were analyzed for IFN-γ or GrzB production 2 days after each round of stimulation (IFN-γ) or after the second stimulation (GrzB). This was done using the commercial human IFN-γ and GrzB enzyme-linked immunosorbent spot (ELISPOT) sets (BD Biosciences) in accordance with the manufacturer's instructions. T cells (2 × 104) were cocultured in T-cell medium in a precoated 96-well plate with 2 × 103 DC. For GrzB ELISPOT assays, CD4+ and CD8+ T cells were purified as described above and were used in addition to total T cells. Two days later, IFN-γ or GrzB released by T lymphocytes was detected with a specific MAb and was visualized by an immunoenzymatic process. Colored spots were automatically counted using Axioplan 2 imaging and KS ELISPOT software (Carl Zeiss MicroImaging GmbH, Göttingen, Germany).
Statistical analysis.
One-way analysis of variance (ANOVA) was used to compare mock-, LV-, and MV-infected conditions in the different experiments. The Student t test was used to compare MV titers (see Fig. 7F). Differences between two sets of data were considered to be significant when the P value was less than 0.05. Standard errors of the means (SEM) were also calculated for some experiments. Correlations between data were calculated using the Spearman rank order correlation test. All statistical tests were performed using SigmaStat software (SyStat Software Inc., San Jose, CA).
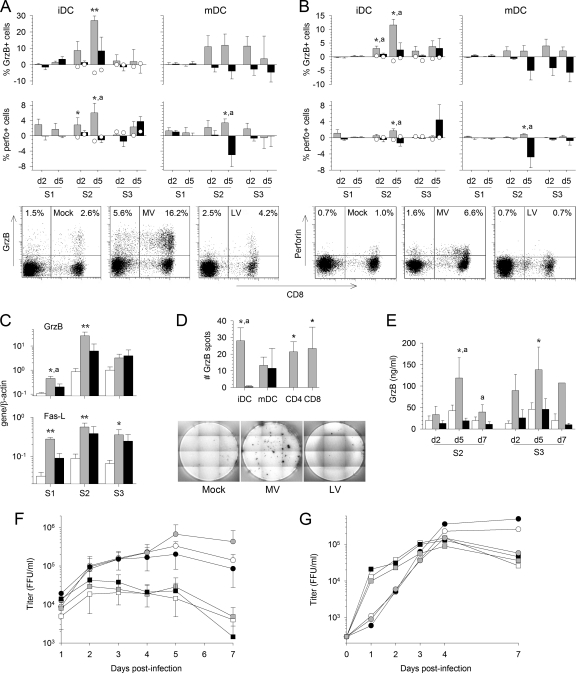
Fig. 7.
Induction of CTL by MV-infected DC. The expression of GrzB and perforin (perfo) at the surfaces of CD3+ CD8+ (A) or CD3+ CD4+ (B) T cells was analyzed by flow cytometry as for Fig. 3C. The percentage of T cells cultured with MV-infected DC (shaded bars) or LV-infected DC (filled bars) that express the molecule minus the percentage of T cells cultured with mock-infected DC that express it is represented. Results are means and SEM from five independent experiments using different donors. Significant differences are indicated as for Fig. 1. The expression of GrzB and perforin in control T cells that had first been stimulated with mock-infected iDC was quantified after restimulation with DC pulsed with inactivated MV (open circles on shaded bars) or with inactivated LV (open circles on filled bars) and is presented as the mean from two independent experiments using different donors. Dot plots showing the expression of CD8 and GrzB or perforin among CD3-gated cells 5 days after the second stimulation with mock-, MV-, or LV-infected iDC are presented. (C) GrzB and FasL mRNA synthesis was evaluated by qRT-PCR 2 days after each round of stimulation in T cells cultured with mock (open bars)-, MV (shaded bars)-, or LV (filled bars)-infected iDC. The means and SEM from five independent experiments using different donors are shown. Results are expressed as target gene/β-actin ratios. Asterisks indicate significant differences between mock-infected and infected cells as for Fig. 1. (D) (Top) ELISPOT assay results showing the number of GrzB spots for 104 T cells first stimulated with MV- or LV-infected iDC or mDC and then restimulated with MV (shaded bars)- or LV (filled bars)-pulsed DC, respectively, minus the number of spots for T cells stimulated with mock-infected DC. Results were obtained with total T cells (iDC and mDC) or with purified CD3+ CD4+ or CD3+ CD8+ T cells after the second stimulation and are means and SEM from five independent experiments using different donors. (Bottom) Results of a representative GrzB ELISPOT experiment after stimulation of T cells with mock-stimulated, MV-pulsed, or LV-pulsed iDC. (E) Detection of GrzB by ELISA in supernatants of T cells cultured with mock (open bars)-, MV (shaded bars)-, or LV (filled bars)-infected iDC obtained on day 2 (d2), d5, and d7 of the second and third stimulations. The means and SEM from five independent experiments using different donors are shown. Results are expressed in nanograms per milliliter, and significant differences are indicated as for Fig. 1. (F and G) Titration of MV particles in supernatants of MV-infected iDC (F) or of LV particles in supernatants of LV-infected iDC (G) cultured with total T cells (open symbols) or purified CD3+ CD4+ (filled symbols) or CD3+ CD8+ (shaded symbols) T cells that had first been stimulated by mock-infected (circles), MV-infected (squares) (F), or LV-infected (squares) (G) iDC. The means and SEM from four independent experiments using different donors are shown for MV. The means from two independent experiments using different donors are shown for LV. Results are expressed as the number of focus-forming units (FFU) per milliliter.
RESULTS
Establishment and optimization of the in vitro model.
Our model was set up according to previous studies and was optimized with our own experimental conditions (data not shown). Monocyte-derived DC were used as APC because DC are the most efficient cells at inducing primary T-cell responses (54, 76). In addition, DC are the main LV target and therefore are probably the primary APC during LF. We used an AP-supplemented medium to avoid background T-cell activation (36, 54, 62). Finally, IL-2 and IL-7 were added to allow optimal survival of T cells, including mock-stimulated T cells, without nonspecific proliferation (30, 36). We used total T cells rather than purified CD4+ or CD8+ T cells to generate cellular responses that were relevant to natural immunity (31, 54, 62, 76). T cells were restimulated with DC pulsed with inactivated virus rather than with a peptide pool in order to study the whole response and not only the T cells specific for selected immunodominant peptides or peptide pools derived from a single Ag. Comparison of different DC/T-cell ratios led us to choose 1:10, because this ratio gave the best responses against MV and has been used frequently in previous studies (30, 36). Indeed, although responses were slightly less robust using a ratio of 1:40, no significant response was observed with ratios of 1:100 and 1:1,000 (data not shown). We compared iDC and mDC to evaluate viral induction of iDC maturation, and therefore to evaluate the immunogenicity of virus-infected iDC, using cytokine-matured virus-infected DC as a positive control.
DC respond to MV, but not to LV, and cooperate with T cells.
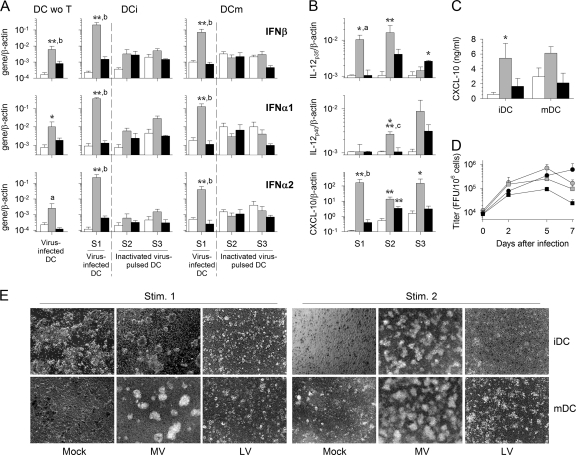
We detected a high level of type I IFN mRNA transcription 2 days after the first stimulation in response to MV but not to LV when T cells were cultured with iDC or mDC (Fig. 1A). MV-infected DC produced significantly more IFN-α1, IFN-α2, and IFN-β mRNA when T cells were present in the culture. In contrast, type I IFN mRNA synthesis was not upregulated after the second and third rounds of stimulation, when inactivated MV and inactivated LV were used to stimulate DC. Isolated iDC do not produce IL-12 mRNA in response to MV or LV infection (6, 58). In the presence of T cells, synthesis of IL-12p35 mRNA was observed in response to MV after the first and second rounds of stimulation, and the levels were significantly higher than those in mock- and LV-stimulated cultures (Fig. 1B). After the second and third stimulations, more-modest expression was detected in response to LV. Similarly, expression of IL-12p40 mRNA was significantly induced in MV-infected iDC after the second round of stimulation. In LV-infected iDC, this induction was observed only after the third stimulation and was nonsignificant (Fig. 1B). Finally, synthesis of CXCL-10 mRNA was strongly upregulated in response to MV after the first stimulation, and this upregulation continued during the second and third rounds of stimulation (Fig. 1B). In response to infection with LV, synthesis of CXCL-10 also occurred after the first and second rounds of stimulation, but at a lower level than that with MV infection. The transcription of these three genes did not increase when mDC were used (data not shown). Accordingly, CXCL-10 was released in the supernatants from iDC and mDC cocultures only in response to MV during the first round of stimulation (Fig. 1C). These results indicate that DC are activated after MV infection and that, to a lesser extent, they are activated after LV infection, but only after the second and third rounds of stimulation. In addition, the presence of T cells seems to substantially enhance the activation of iDC induced by MV infection. The detection of increased amounts of LV and MV particles in culture supernatants (Fig. 1D) confirmed that DC are productively infected by both viruses, as previously reported (6, 58).
Fig. 1.
Activation of infected DC and cooperation with T cells. (A) The expression of IFN-β (top), IFN-α1 (center), and IFN-α2 (bottom) mRNA was quantified 2 days after the first (S1), second (S2), and third (S3) stimulations in mock (open bars)-, MV (shaded bars)-, or LV (filled bars)-infected iDC and mDC. Whereas the first stimulation of T cells was performed using MV- or LV-infected DC, the second and third stimulations were performed using inactivated-virus-stimulated DC. Type I IFN mRNA production by mock-, MV-, or LV-infected iDC cultured without T cells (DC wo T) is also shown. Results are expressed as the gene/β-actin ratio. (B) Expression of IL-12p35, IL-12p40, and CXCL-10 mRNA by iDC 2 days after the first, second, and third stimulations. (C) The amounts of CXCL-10 released by iDC and mDC cultured with T cells were quantified by ELISA 2 days after the first stimulation. Results, expressed in nanograms per milliliter, are means and SEM from five independent experiments using different donors. Significant differences between mock-infected and infected cells are indicated by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001), and significant differences between Mopeia and Lassa virus-infected cells are indicated by lowercase letters (a, P < 0.05; b, P < 0.01; c, P < 0.001). (D) Titration of MV (shaded symbols) and LV (filled symbols) particles in culture supernatants after the first stimulation of T cells with MV- or LV-infected iDC (circles) or mDC (squares). Results, expressed in focus-forming units per 106 cells, are means and SEM from five independent experiments using different donors. (E) Microscopic photographs of mock-, MV-, or LV-infected cocultures of T cells with iDC or mDC 4 days after the first stimulation (Stim. 1) and of T-cell cocultures with iDC or mDC that were either mock stimulated or pulsed with inactivated MV or inactivated LV 6 days after the second stimulation. Magnification, ×60.
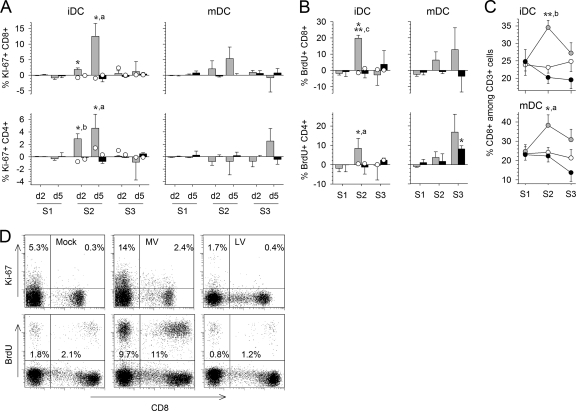
Examination of DC-T-cell cultures under microscopy confirmed the activation and cooperation of cells in response to MV. Indeed, T cells clustered massively around iDC and mDC in the presence of MV after each round of stimulation (Fig. 1E). In contrast, the appearance of DC-T-cell cultures in the presence of LV was similar to that with mock stimulation: no T cells clustered around iDC or mDC.
T cells are activated early and strongly in response to MV but not to LV.
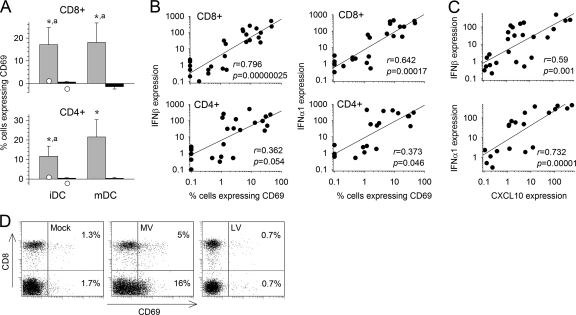
Upregulation of CD69 expression was detected in both CD4+ and CD8+ T cells 2 days after the first stimulation when T cells were cultured with MV-infected iDC or mDC, but not in response to LV (Fig. 2A and D). In control cultures consisting of T cells first stimulated by mock-infected iDC and then restimulated twice with inactivated MV- or LV-stimulated iDC, no CD69 expression was observed. Type I IFN is able to induce CD69 expression by T cells independently of Ag recognition (2). Accordingly, the numbers of CD4+ and CD8+ T cells expressing CD69 correlated strongly with the levels of type I IFN mRNA in the respective cocultures (Fig. 2B). Furthermore, type I IFN mRNA expression also correlated with the levels of CXCL-10 mRNA (Fig. 2C). These results suggest that massive, probably bystander activation of T cells occurs early after stimulation with MV-infected iDC and mDC, but not with LV-infected cells.
Fig. 2.
Expression of CD69 and correlation with type I IFN and CXCL10 expression. (A) The expression of CD69 on CD3+ CD8+ (top) and CD3+ CD4+ (bottom) T cells cultured with MV (shaded bars)- or LV (filled bars)-infected iDC or mDC was analyzed by flow cytometry after 2 days. The percentage of T cells cultured with virus-infected DC that expressed CD69 minus the percentage of T cells cultured with mock-infected DC that expressed CD69 was calculated. Results are means and SEM from five independent experiments using different donors. Significant differences are indicated as for Fig. 1. The expression of CD69 in control T cells that had first been stimulated with mock-infected iDC was quantified 2 days after restimulation with DC pulsed with inactivated MV (open circles on shaded bars) or inactivated LV (open circles on filled bars); these values are means from two independent experiments using different donors. (B) Correlation between IFN-β or IFN-α1 mRNA expression (as in Fig. 1A) and the percentage of CD69-expressing CD8+ and CD4+ T cells observed 2 days after coculture of MV- or LV-infected iDC and naïve T cells, represented by linear regression with the correlation coefficient (r) and the probability of correlation (P). (C) Correlation between IFN-β or IFN-α1 mRNA expression and CXCL-10 mRNA synthesis (as in Fig. 1B) by MV- or LV-infected iDC cultured with naïve T cells 2 days after infection. (D) Dot plots showing expression of CD8 and CD69 among CD3-gated cells 2 days after the first stimulation with mock-, MV-, or LV-infected iDC.
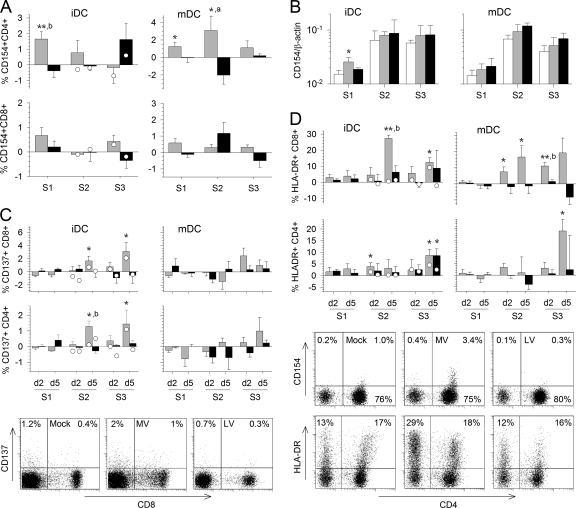
We then analyzed the expression of activation or costimulation markers at the T-cell surface. On the fifth day of the first round of stimulation, CD154 expression was significantly upregulated at the surfaces of CD4+ T cells cultured with MV-infected iDC or mDC but not with LV-infected cells (Fig. 3A). During the second round of stimulation, expression of CD154 in response to MV persisted only in the presence of mDC. After the last round of stimulation, an inconsistent and nonsignificant increase in CD154 expression was observed in response to LV-infected iDC and in control T cells stimulated twice with inactivated LV. In addition, CD154 was not significantly expressed on CD8+ T cells (Fig. 3A). Significant upregulation of CD154 mRNA was detected in T cells only after the first stimulation with MV-infected iDC, not after the next stimulation (Fig. 3B).
Fig. 3.
Expression of activation markers on the T-cell surface. The expression of several molecules involved in T-cell activation was analyzed by flow cytometry during the three rounds of stimulation (S1 to S3). T cells were cultured with MV (shaded bars)- or LV (filled bars)-infected iDC or mDC for the first stimulation and were restimulated twice with inactivated-MV- or inactivated-LV-pulsed DC, respectively. The percentage of T cells cultured with infected DC and restimulated with inactivated-virus-pulsed DC that express the markers minus the percentage of T cells cultured and restimulated with mock-infected DC and restimulated with culture medium that express the markers was calculated. Results are means and SEM from five independent experiments using different donors. Significant differences between mock-infected and infected cells are indicated as for Fig. 1. The expression of the respective marker in control T cells that had first been stimulated with mock-infected iDC was quantified 2 and 5 days after restimulation with DC pulsed with inactivated MV (open circles on shaded bars) or with inactivated LV (open circles on filled bars) and is expressed as the mean from two independent experiments using different donors. (A) The expression of CD154 on CD3+ CD4+ (top) and CD3+ CD8+ (bottom) T cells cultured with iDC or mDC is shown for 5 days after each stimulation. (B) CD154 mRNA synthesis was evaluated by qRT-PCR 2 days after each round of stimulation in T cells cultured with mock (open bars)-, MV (shaded bars)-, or LV (filled bars)-infected iDC. The means and SEM from five independent experiments using different donors are shown. Results are expressed as the gene/β-actin ratio. (C and D) The expression of CD137 (C) and HLA-DR (D) by CD3+ CD8+ and CD3+ CD4+ T cells cultured with iDC or mDC is shown for day 2 (d2) and d5 of the three rounds of stimulation. Dot plots showing the expression of CD8 and CD137 or of CD4 and CD154 or HLA-DR among CD3-gated cells 5 days after the second stimulation with mock-, MV-, or LV-infected iDC are presented.
CD137 expression was significantly upregulated on CD8+ T cells and, to a lesser extent, on CD4+ T cells in response to MV-infected iDC during the second and third rounds of stimulation (Fig. 3C). Similarly, in control CD4+ and CD8+ T cells, upregulation of CD137 expression was also observed 5 days after the second stimulation with inactivated MV. This suggests that priming with inactivated-MV-stimulated iDC is sufficient to induce CD137 expression. In contrast, no significant upregulation of CD137 expression was observed in the presence of MV-infected mDC or LV-infected cells (Fig. 3C).
We observed modest upregulation of HLA-DR expression on the surfaces of CD4+ and CD8+ T cells after the first round of stimulation with iDC infected by MV and, to a lesser extent, in cells cultured with LV-infected iDC; this was not observed, however, in the presence of mDC (Fig. 3D). During the second and third rounds of stimulation with MV-infected cells, there were significant increases in HLA-DR expression on CD8+ T cells, with levels peaking on the fifth day of the second stimulation. No major differences were observed between iDC and mDC. In the presence of LV-stimulated iDC, a limited increase in HLA-DR expression was sometimes observed on T cells on day 5 of the second or third round of stimulation. Maturation of DC decreased this response of CD8+ T cells to LV. HLA-DR expression was also upregulated at the surfaces of CD4+ T cells in response to MV, with levels reaching a peak after the third stimulation. This effect was again particularly marked when MV-infected mDC were used, but not in the presence of LV-infected mDC. Finally, HLA-DR was expressed only on a small number of control T cells after the second stimulation with inactivated-MV-stimulated iDC (Fig. 3D). Thus, these results suggest that CD8+ and CD4+ T cells are strongly activated after stimulation with MV-infected DC, but not with LV-infected DC.
T cells undergo a robust proliferative response to MV but not to LV.
During the first stimulation, we were unable to detect any proliferation of CD8+ or CD4+ T cells (Fig. 4A and B). Proliferation, measured by an increase in Ki-67 expression, began on the second day of the second stimulation only in response to MV (Fig. 4A). Proliferation reached a maximum on the fifth day, with a higher percentage of BrdU- and Ki-67-positive cells in CD8+ T cells than in CD4+ T cells (Fig. 4A, B, and D). Culture with mDC did not result in a higher proliferation rate during the same period. However, while T cells stimulated with MV-infected iDC failed to proliferate during the third stimulation, proliferation was maintained in T cells stimulated with mDC (Fig. 4B). Of note, we observed a higher proportion of CD8+ T cells in MV-stimulated cultures than in their mock- or LV-infected counterparts (Fig. 4C). Culture with LV-infected cells led to very limited incorporation of BrdU during the third stimulation, particularly for CD4+ T cells and mDC, and similar results were obtained for control T cells after stimulations with inactivated-MV- or inactivated-LV-pulsed DC (Fig. 4A, B, and D). Thus, MV induced a strong proliferative response in CD4+ and CD8+ T cells, which persisted throughout the experiment with mDC.
Fig. 4.
Proliferation of T cells. (A and B) The proliferation of T cells cultured with MV (shaded bars)- or LV (filled bars)-infected iDC or mDC was quantified by flow cytometry. Values were normalized by subtraction of T-cell proliferation after culture with mock-infected DC. Results are means and SEM from five independent experiments using different donors. Significant differences are indicated as for Fig. 1. (A) Ki-67 was detected at day 2 (d2) and d5 after each stimulation. (B) The incorporation of BrdU in proliferating CD3+ CD8+ and CD3+ CD4+ T cells was analyzed at day 5 of each stimulation. The expression of Ki-67 and the incorporation of BrdU in control T cells that had first been stimulated with mock-infected iDC was quantified after restimulation with DC pulsed with inactivated MV (open circles on shaded bars) or inactivated LV (open circles on filled bars). Data are means from two independent experiments using different donors. (C) The percentage of CD8+ T cells observed 5 days after each stimulation among CD3+ cells cocultured with mock (open circles)-, MV (shaded circles)-, or LV (filled circles)-infected iDC or mDC is indicated. (D) Dot plots showing expression of CD8 and Ki-67 or BrdU among CD3-gated cells 5 days after the second stimulation with mock-, MV-, or LV-infected iDC.
Memory T-cell differentiation in response to MV.
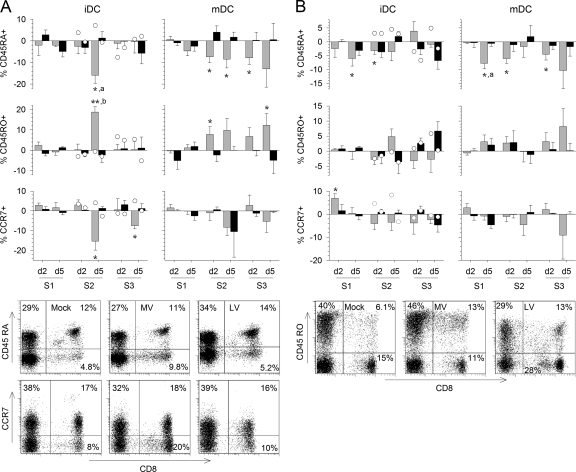
In the presence of MV-infected cells, we observed a decrease in CD45RA surface expression in CD8+ and CD4+ T cells, which was correlated with an increase in CD45RO expression (Fig. 5A and B). CD45RA levels on CD4+ and CD8+ T cells began to decrease during the first stimulation, and a more marked decrease was observed for CD8+ T cells during the second stimulation. Downregulation of CD45RA continued throughout the third stimulation only when T cells were cultured with mDC. We observed a similar pattern for CD45RO upregulation, except that the increase in CD45RO expression in CD4+ T cells was not significant. In addition, a decrease in CCR7 expression on the surfaces of CD8+ and CD4+ T cells was observed in the presence of MV-infected DC but was significant only for CD8+ T cells cultured with iDC (Fig. 5A and B). In contrast, in response to LV-infected iDC, only a small reduction in CD45RA levels, correlating with a small increase in CD45RO levels, was observed, essentially for CD4+ T cells during the third round of stimulation. No response was observed in cells stimulated with LV-infected mDC. The expression of CCR7 at the surfaces of T cells was not significantly reduced in the presence of LV-infected DC (Fig. 5A and B). Finally, a modest drop in CD45RA expression and a modest upregulation of CD45RO expression were observed in control T cells after the second stimulation with iDC pulsed with inactivated MV and, to a lesser extent, with inactivated LV (Fig. 5A and B). These results show that CD8+ and CD4+ T cells acquire a memory phenotype in response to MV from the time of the second stimulation, which can be maintained only when T cells are stimulated with mDC. T cells also acquired a memory phenotype in response to LV, but the effect was less pronounced and was detected after a longer delay.
Fig. 5.
Acquisition of a memory phenotype by T cells. The expression of CD45RA, CD45RO, and CCR7 at the surfaces of CD3+ CD8+ (A) or CD3+ CD4+ (B) T cells was analyzed by flow cytometry as for Fig. 3C. The percentage of T cells cultured with MV-infected DC (shaded bars) or LV-infected DC (filled bars) that express the molecule minus the percentage of T cells cultured with mock-infected DC that express it is represented. Results are means and SEM from five independent experiments using different donors. Significant differences are indicated as for Fig. 1. The expression of CD45RA, CD45RO, and CCR7 in control CD8+ and CD4+ T cells that had first been stimulated with mock-infected iDC was quantified after restimulation with DC pulsed with inactivated MV (open circles on shaded bars) or with inactivated LV (open circles on filled bars) and is expressed as the mean from two independent experiments using different donors. d, day. Dot plots showing the expression of CD8 and CD45RA, CD45RO, or CCR7 among CD3-gated cells 5 days after the second stimulation with mock-, MV-, or LV-infected iDC are presented.
Production of cytokines by T cells.
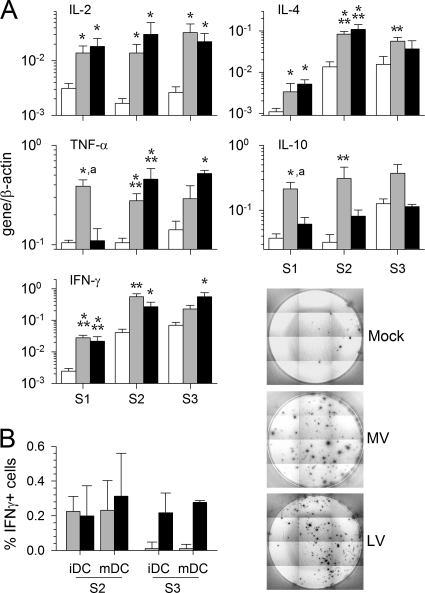
For both infectious viruses, during the first round of stimulation, T cells synthesized more mRNA encoding IFN-γ, IL-2, and IL-4 than mock-stimulated T cells (Fig. 6A). Similar patterns of upregulation persisted during the second and third rounds of stimulation. However, upregulation of TNF-α and IL-10 mRNA synthesis differed in response to MV versus LV. TNF-α and IL-10 mRNA upregulation was observed after the first stimulation with MV and continued at high levels over the second and third rounds of stimulation. In response to LV, TNF-α mRNA levels increased considerably after the second stimulation, and the increase persisted during the third round of stimulation; IL-10 mRNA synthesis, however, showed a pattern similar to that of control T cells, except for a moderate but significant upregulation during the second round of stimulation. Similar results were obtained with MV- and LV-infected mDC (data not shown). Thus, mRNA synthesis by T cells cultured with MV- versus LV-infected APC appeared to differ most after the first stimulation, for which infectious viruses rather than inactivated viruses were used.
Fig. 6.
mRNA synthesis and cytokine production after MV or LV infection. (A) Cytokine mRNA synthesis was evaluated by qRT-PCR 2 days after each round of stimulation (S1 to S3) in T cells cultured with mock (open bars)-, MV (shaded bars)-, or LV (filled bars)-infected iDC. The means and SEM from five independent experiments using different donors are shown. Results are expressed as gene/β-actin ratios. Significant differences are indicated as for Fig. 1. (B) Determination of the number of IFN-γ-producing T cells by ELISPOT assay. (Left) The graph shows the number of spots for 104 T cells that were first stimulated with MV- or LV-infected iDC or mDC and were then restimulated with MV (shaded bars)- or LV (filled bars)-pulsed DC, respectively, minus the number of spots for T cells stimulated with mock-infected DC. Results were obtained after the second and third stimulations and are represented as means and SEM from five independent experiments using different donors. (Right) Results of a representative experiment to detect IFN-γ production by T cells after the second stimulation with culture medium-, MV-, or LV-pulsed iDC.
The percentage of T cells secreting IFN-γ was also analyzed using an ELISPOT assay. About 0.2 to 0.4% of T cells produced IFN-γ in response to MV or LV after the second stimulation with either iDC or mDC (Fig. 6B). In contrast, after the third stimulation, IFN-γ was detected only in response to LV-infected DC.
MV-infected DC induce strong and efficient cytotoxic CD4+ and CD8+ T-cell responses.
To determine whether virus-infected DC were able to induce cytotoxic T lymphocytes (CTL), the intracellular expression of GrzB and perforin was evaluated by flow cytometry. High proportions of CD8+ and CD4+ T cells expressed GrzB and perforin in response to MV-infected DC after the second stimulation (Fig. 7A and B). A notable proportion of T cells continued to express GrzB when stimulated with MV-infected mDC. In contrast, no upregulation of GrzB or perforin expression occurred after LV stimulation, except for a modest, nonsignificant response observed at the end of the third round of stimulation. Similarly, control T cells stimulated twice with inactivated-virus-pulsed iDC failed to express GrzB and perforin (Fig. 7A and B), demonstrating that only virulent-MV-infected DC induce CTL differentiation.
In agreement with these findings, strong and highly significant synthesis of GrzB and FasL mRNA was detected as early as 2 days after the first stimulation of T cells with MV-infected iDC (Fig. 7C). These mRNA levels increased after the second stimulation and then returned to nonsignificant values for GrzB mRNA but persisted at high levels for FasL mRNA (Fig. 7C). Similar results were obtained with MV-infected mDC (data not shown). Stimulation with LV-infected iDC also resulted in notable, but nonsignificant, levels of GrzB and FasL synthesis by T cells (Fig. 7C).
The ability of T cells primed with MV- or LV-infected DC to lyse inactivated-virus-pulsed iDC was evaluated by a GrzB ELISPOT assay. MV-infected iDC induced efficient CTL, as shown by the significant number of GrzB spots detected (Fig. 7D). In contrast, LV-stimulated T cells failed to secrete GrzB. Similar results were obtained with mDC (data not shown). To confirm that both CD4+ and CD8+ MV-specific CTL were induced, CD4+ and CD8+ T cells were purified after the first round of stimulation. GrzB was secreted significantly by both cell subtypes after contact with inactivated-MV-pulsed iDC (Fig. 7D). These results were confirmed by the detection of GrzB in supernatants during the second and third rounds of stimulation with MV, but not with LV (Fig. 7E).
To determine whether MV- or LV-stimulated CTL could control viral replication in DC, T cells primed with virus- or mock-infected iDC were restimulated with virus-infected iDC. The release of MV or LV particles was evaluated by titration of the supernatant. The level of infectious MV particles dropped significantly when infected DC were cultured in the presence of MV-primed total T cells (Fig. 7F). This was also the case for infected DC cultured in the presence of CD4+ and CD8+ T cells purified after priming (Fig. 7F). Thus, MV-infected DC induce strong and efficient CD4+ and CD8+ CTL, which are able to lyse MV-infected DC and control viral replication. In contrast, LV-stimulated T cells failed to inhibit LV replication in DC (Fig. 7G).
In order to generate T-cell responses, direct contacts between MV-infected DC and naïve T cells are mandatory. Indeed, separation of the two cell populations during the first stimulation using transwell inserts inhibited the activation and proliferation of CD8+ and CD4+ T cells. This was indicated by the observations that there was no upregulation of HLA-DR, CD137, CD154, GrzB, perforin, or Ki-67 and no incorporation of BrdU 5 days after the second round of stimulation with inactivated-MV-pulsed DC (data not shown). Similarly, no T-cell response was observed after the second stimulation when LV-infected DC and T cells were separated during the first stimulation (data not shown).
Finally, we determined whether the different T-cell responses observed in the presence of MV and LV resulted from the different types of type I IFN responses induced by these two viruses. We neutralized type I IFN and the corresponding receptor (CD118) during the first stimulation of T cells with MV-infected iDC (data not shown). Following this intervention, the expression levels of CD137, CD154, HLA-DR, GrzB, and perforin were all similar. Furthermore, T cells showed equivalent proliferation 5 days after their restimulation with inactivated-virus-pulsed DC. These observations suggest that type I IFN is not essential for the induction of robust T-cell responses against MV. Moreover, the addition of rhIFN-α2b during the first stimulation of T cells with LV-infected DC did not induce a substantial and efficient T-cell response. These results suggest that although type I IFNs are probably involved in the differences of pathogenicity between LV and MV, these mediators alone are not responsible for the differences in T-cell responses observed between these two viruses.
DISCUSSION
Despite their likely importance for the control of LF, T-cell responses in patients are poorly understood. Activation and production of type I IFN by arenavirus-infected APC are inversely correlated with the pathogenicity of the virus (6, 8, 58). It is thus important to determine the effects of these innate immune responses on adaptive immunity against LV. As an alternative to studies with NHP and clinical studies with humans, we describe here an in vitro model in which naïve human T cells were stimulated with virus-infected DC. We compared the responses obtained with this highly pathogenic virus with those induced by MV, a closely related arenavirus that is nonpathogenic for humans. In vitro generation of human T-cell responses in order to obtain specific CTL for immunotherapy and to analyze specific T-cell responses has been widely reported (9, 24, 30, 36, 38, 47, 54, 62, 76). This approach could be an alternative to human clinical studies or animal models in the analysis of cellular antiviral immune responses or the immunogenicity of a candidate vaccine (9, 38, 54, 71).
Only infectious MV induced robust type I IFN mRNA expression in DC cultured with T cells. In addition to their antiviral effects, type I IFNs enhance T-cell responses by acting on both APC and T cells (43, 44). IL-12 mRNA was produced by DC in response to MV. This response was delayed and more modest with LV. IL-12 is crucial for the induction of T helper 1 (Th1) and CD8+ T-cell responses. Most of this mRNA expression occurred after restimulation, suggesting that interactions between DC and activated T cells are involved in this response and play a role in DC activation (68). Indeed, type I IFN synthesis by MV-infected iDC was much more robust when T cells were present than it was in isolated iDC, and no IL-12 mRNA was produced by isolated MV-infected iDC (58). Although type I IFN and IL-12 are not evidence of specific T-cell responses, their production by DC when T cells are present suggests cross talk between the two cell populations. Finally, large amounts of CXCL-10 were produced in MV-stimulated cultures. This may also favor the development of efficient T-cell responses, since CXCL-10 mediates the attraction of activated T lymphocytes and is associated with Th1 and CTL responses (53). Accordingly, T cells formed large clusters around DC in MV-stimulated cultures. In summary, DC are activated early and strongly, and interact with T cells, in response to MV, and DC are probably more suited to inducing T-cell responses against MV than against LV.
Many T cells expressed CD69, an early activation marker, 2 days after the first contact with MV-infected DC. In contrast, no expression was observed after stimulation with LV. We found a strong correlation between CD69 and expression of type I IFN. The levels of CXCL-10 and type I IFN mRNAs were also correlated, suggesting that type I IFN may play a role in the induction of T-cell responses against MV. However, the lack of suppression of the responses after neutralization of type I IFNs suggests that these mediators are nevertheless dispensable for the generation of cellular responses to MV. MV induces earlier and stronger expression of CD154 and CD137 by T cells than does LV. CD154 is expressed early and transiently by CD4+ T cells after antigenic stimulation. This may explain the low number of CD154-expressing cells and the modest levels of mRNA expression that we observed. CD154 is also involved in APC activation and B-cell help (59, 61). CD137 appears later after activation of T cells and is required for their effector functions (21). The preferential expression of CD154 by CD4+ T cells and of CD137 by CD8+ T cells is not surprising, since these molecules are potent costimulators for these different subtypes of T cells (16, 64). We did not detect Foxp3 mRNA or protein by use of qRT-PCR and flow cytometry (data not shown), suggesting a lack of regulatory T-cell induction (51). A large number of T cells expressed HLA-DR in response to MV; this response was modest with LV and was observed only in the presence of iDC. HLA-DR is expressed by activated T cells and is often associated with proliferation (41, 65). Accordingly, HLA-DR expression was significantly correlated with BrdU incorporation in MV-stimulated CD8+ T cells (r=0.687; P < 0.001). In response to MV, we observed only a small proportion of T cells expressing costimulatory molecules but found high proportions of HLA-DR+ and proliferating cells. This discrepancy may be related to different dynamics in the surface expression of the molecules rather than to specific versus nonspecific T-cell responses (37). Interestingly, during infection with the closely related lymphocytic choriomeningitis virus (LCMV), almost all activated T cells are LCMV specific and do not result from bystander activation (56).
One of the most marked differences in the responses to LV versus MV is the strong proliferation of T cells induced by MV-infected DC. The enrichment in CD8+ T cells confirms that MV is an efficient inducer of CD8+ T-cell responses, like LCMV (56). T cells stimulated with mDC continued to proliferate after the third stimulation, indicating a substantially longer response than with iDC. This exclusive ability of MV to induce strong proliferation of T cells may be a major factor underlying the difference in pathogenicity between the two viruses and is probably due to the efficient activation of DC and T cells after infection. Indeed, the clonal expansion of T cells occurs only after the recognition of three signals: the triggering of T-cell receptor (TCR), costimulation of T cells by APC, and stimulation by inflammatory cytokines (23). T-cell proliferation in vivo may allow efficient and early control of viral replication and lysis of infected cells.
The observation that T-cell activation and proliferation occurred mostly after the first restimulation—but neither during the days following stimulation with MV-infected DC nor in control T cells restimulated twice with inactivated-virus-pulsed DC—suggests that T-cell responses were probably due to MV-specific activated T cells and not to nonspecific reactivation of cross-reactive memory cells. However, further experiments, such as restimulation of T cells with virus-derived peptides, would be required in order to demonstrate definitely that MV-specific primary responses are observed.
Differences in the acquisition of memory cell markers were also found. After a second round of stimulation with MV-infected iDC, we observed a reduction in CD45RA levels at the surfaces of CD8+ T cells, which was strictly correlated with an increase in CD45RO levels and a decrease in CCR7 levels. These modifications were enhanced by a third round of stimulation with MV-pulsed mDC. Similar but smaller changes in CD45 expression were observed for CD4+ T cells. In contrast, upon stimulation with LV, only slight changes in the surface expression of these markers were observed. Interestingly, naïve cells are thought to be CD45RA+ CD45RO−, and memory cells are thought to be CD45RA− CD45RO+ (10). Finally, the downregulation of CCR7 observed in CD8+ T cells in response to MV may correspond to the induction of effector-memory cells (60).
Effector functions of T cells involve cytokine production and cytotoxic function development. In response to LV and MV, T cells synthesize mainly Th1/T cytotoxic 1 (Tc1) cytokines, such as IFN-γ, IL-2, and TNF-α (55). Such a preference for Th1/Tc1 cytokines may be consistent with the induction of cell-mediated immunity (55). IFN-γ and TNF-α display direct antiviral activity but are also able to activate MP; IFN-γ can also activate and increase T helper responses or CTL proliferation and induce memory T cells (57, 72-74). We also observed a strong increase in the level of IL-4 mRNA synthesis in response to LV and MV and a strong increase in the level of IL-10 mRNA synthesis in response to MV only. These cytokines are characteristic of a Th2/Tc2 profile, suggesting the presence of a so-called Th0 phenotype, composed of a mixture of Th1 and Th2 profiles and potentially representing a less differentiated stage of T cells (55). The strong increase in IL-10 mRNA transcription in response to MV may prevent excessive activation of APC or T cells by inhibiting the production of inflammatory or Th1 cytokines, respectively. In turn, these actions may therefore impede immunopathological events (11, 32). IL-10 also limits vascular permeability and edemas, which is important in viral hemorrhagic fevers (45). However, IL-10 is also crucially involved in the establishment of viral persistence (14). Persistent infection with LV or MV has not been described previously, but further investigations would be required to determine if this phenomenon occurs, at least in our model. PD-1/PD-L1 interactions are also involved in viral persistence (14), and upregulation of PD-1 expression in T cells has been correlated with chronic infection (69). Moreover, PD-1 mRNA synthesis was not upregulated in MV- and LV-stimulated cultures (data not shown).
Our results indicate robust induction of CTL by MV- but not LV-infected DC. Indeed, naïve T cells do not contain cytotoxic granules, and GrzB and perforin expression occurs only after TCR stimulation and proliferation of T cells (40, 50). To confirm that these CTL were functional and able to lyse MV-infected targets, we measured the release of GrzB after contact of primed T cells with MV-stimulated DC. Indeed, secretion of GrzB occurs specifically when CTL are activated by a specific target (63, 77). MV-infected DC can generate a high number of CD8+ and CD4+ CTL able to lyse MV-stimulated iDC. A higher number of GrzB-containing T cells was observed by flow cytometry than the number of GrzB spots detected in the ELISPOT assay. This discrepancy could be explained by the need for CTL to express both perforin and GrzB simultaneously in order to lyse a target but also by the fact that the number of spots is limited by the number of target cells (effector-to-target cell [E:T] ratio, 10:1) and by the level of probability that a T cell will encounter a target. CD8+ and CD4+ CTL were also highly efficient at controlling MV replication in DC, as seen by the 10-fold drop in the level of viral release when MV-primed T cells were cultured with MV-infected DC in comparison with mock-primed T cells. These results demonstrate that specific and efficient CTL are induced by MV- but not LV-infected DC, and they suggest that the T-cell responses observed with MV-infected DC may be virus specific. This correlation between CTL induction and nonpathogenic infection is consistent with the known role of CTL in the control of arenavirus infections (5, 26). The presence of CD4+ T cells in the CTL population is consistent with recent reports showing that generation of CD4+ CTL is a common event during viral infection and could be crucial for virus control (1, 70). Furthermore, CD4+ CTL seem to represent a mature phenotype of terminal differentiation (3, 18), highlighting once more the strong immunogenicity of MV. The CD4+ T-cell response probably facilitates the induction of effector and cytotoxic responses in CD8+ T cells (19) but may also have an effector role by itself (18). Conversely, upon stimulation with LV, CD4+ T cells are less strongly activated and do not proliferate, and only a small proportion of cells undergo late differentiation into memory cells, probably in numbers insufficient to induce CTL.
The induction of responses by MV-infected iDC suggests at least partial activation/maturation of iDC. However, this is not in line with the weak activation of DC that we observed after MV infection (58). Bidirectional interactions, leading to the reciprocal activation of MV-infected iDC and T cells, may explain these discrepancies. Indeed, this cross talk, mediated by cytokines and surface molecules, is essential for APC and T-cell activation (52) and seems to occur in our model. Mature DC induced longer-lasting memory responses than iDC, suggesting that MV does not induce full activation/maturation of iDC. The lack of persistence after the third stimulation of T cells primed with iDC may also be due to activation-induced cell death because of repeated stimulation with DC, as observed in a similar in vitro model (36). In contrast, neither immature nor mature LV-infected DC were able to induce substantial responses, suggesting that LV has immunosuppressive properties. This is consistent with the lack of activation of DC after LV infection (6, 46) and with the previous findings of defective T-cell proliferation in response to mitogens during LF in macaques (28). Although the mechanisms involved in the absence of immunogenicity of LV are still unclear, they are clearly not linked to a lack of replication of LV in DC, since similar amounts of viral particles were released after infection with LV and MV. In addition, infection of DC with LV or MV at the MOI used here leads to the infection of almost all cells after 48 h (6, 58). The stronger immunogenicity of MV than of LV cannot be linked to mediators possibly present in the supernatants of infected Vero cells that we used as virus stocks. Indeed, we analyzed the responses of T cells stimulated with iDC infected and pulsed with MV particles purified on a 20% sucrose cushion and found no difference from the responses induced by the crude MV stock (data not shown).
In conclusion, we demonstrated that MV and LV, although very closely related, induce different T-cell responses. MV induces the activation and proliferation of T cells and their differentiation into effector and memory cells. However, LV does not activate T cells and does not induce T-cell proliferation or differentiation into effector cells, although a few cells differentiate into memory cells. These differences may be linked to the differential responses of APC to the two viruses. However, further investigations are required, since our study does not fully explain why strikingly different adaptive immune responses occurred with the two viruses.
In comparing two closely related arenaviruses, our aim was to model immune responses that are induced in LF patients who either survive without severe symptoms (MV model) or develop fatal disease (LV model). Although we cannot conclude that surviving patients develop immune responses similar to those observed with MV in our model, these results are consistent with our findings in cynomolgus monkeys. Indeed, control of LV infection was associated with robust LV-specific CD4+ and CD8+ T-cell responses that were not observed during fatal infection (7). Although our model probably does not re-create all the aspects of virus-DC interactions in a patient, the generation of T-cell responses using iDC is a relevant approach to studying the immunogenicity of viral antigens (9, 30, 38, 62, 71, 76). The comparison of MV, as a surrogate of “nonpathogenic LV,” and the pathogenic AV strain of LV (4, 34) is justified by their close evolutionary relationship, their common natural host (12), and the cross-protection induced in NHP (26). However, this study does not entirely explain the different issues observed after human infection with LV. These range from the subclinical course of infection to fatal outcomes, and the reason why some patients are able to control the virus and others are not remains unclear. One hypothesis is that DC infected with an LV strain of low pathogenicity would induce stronger T-cell responses than DC infected with a pathogenic strain, such as AV or Josiah. It would therefore be interesting to use this in vitro model to compare LV strains of different pathogenicities. Other hypotheses could attribute differences in responses to LV to the inoculum dose (7), the route of infection, the type of cells that are first infected (8), previously acquired immunity against LV (66, 67), and heterologous immunity (20). However, it is unlikely that major histocompatibility complex (MHC) differences account for the responses we observed. Although most of our results were generated by testing cells from each donor with either MV or LV, three independent experiments with different donors gave results similar to those obtained when cells from the same donor were tested for LV and MV responses: strong responses with MV and a lack of response with LV (data not shown). The kinetics of the T-cell responses that we observed with MV is consistent with the in vivo induction of T-cell responses. Nevertheless, the responses described here may differ in some respects from those in a whole body, with secondary lymphoid organs and the diversity of tissues and cells. Overall, this model appears to be a suitable tool for the study of LF pathogenesis in humans and associated immune responses.
ACKNOWLEDGMENTS
We thank C. Clegg and G. Lloyd (both from the Centre for Applied Microbiology and Research, Porton Down, Salisbury, United Kingdom) for the gift of MV, S. Becker (Institut für Virologie, Philipps-Universität Marburg, Marburg, Germany) for providing the AV strain of LV, and T. G. Ksiazek (Department of Pathology, The University of Texas Medical Branch, Galveston, TX), P. E. Rollin (Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, GA), and P. Jahrling (Integrated Research Facility at Fort Detrick, Division of Clinical Research, NIAID, NIH, National Interagency Biodefense Campus, Fort Detrick, Frederick, MD) for giving us the LV-specific antibodies. We are grateful to the Etablissement Français du Sang for providing blood samples and to the Laboratoire P4, Inserm-Jean Mérieux, team for their assistance.
This work was supported by the Délégation Générale pour l'Armement (0034053). D.P. is a fellow recipient of the Délégation Générale pour l'Armement.
Footnotes
Published ahead of print on 1 June 2011.
REFERENCES
- 1. Adhikary D., et al. 2006. Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins. J. Exp. Med. 203:995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alsharifi M., et al. 2005. Type I interferons trigger systemic, partial lymphocyte activation in response to viral infection. J. Immunol. 175:4635–4640 [DOI] [PubMed] [Google Scholar]
- 3. Appay V., et al. 2002. Characterization of CD4+ CTLs ex vivo. J. Immunol. 168:5954–5958 [DOI] [PubMed] [Google Scholar]
- 4. Asper M., et al. 2004. Inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus. J. Virol. 78:3162–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Badovinac V. P., Hamilton S. E., Harty J. T. 2003. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 18:463–474 [DOI] [PubMed] [Google Scholar]
- 6. Baize S., et al. 2004. Lassa virus infection of dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172:2861–2869 [DOI] [PubMed] [Google Scholar]
- 7. Baize S., et al. 2009. Early and strong immune responses are associated with control of viral replication and recovery in Lassa virus-infected cynomolgus monkeys. J. Virol. 83:5890–5903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baize S., et al. 2006. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 8:1194–1202 [DOI] [PubMed] [Google Scholar]
- 9. Barba-Spaeth G., Longman R. S., Albert M. L., Rice C. M. 2005. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J. Exp. Med. 202:1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beverley P. C. 1991. CD45 isoform expression: implications for recirculation of naïve and memory cells. Immunol. Res. 10:196–198 [DOI] [PubMed] [Google Scholar]
- 11. Bogdan C., Vodovotz Y., Nathan C. 1991. Macrophage deactivation by interleukin 10. J. Exp. Med. 174:1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bowen M. D., Peters C. J., Nichol S. T. 1997. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol. Phylogenet. Evol. 8:301–316 [DOI] [PubMed] [Google Scholar]
- 13. Bray M. 2005. Pathogenesis of viral hemorrhagic fever. Curr. Opin. Immunol. 17:399–403 [DOI] [PubMed] [Google Scholar]
- 14. Brooks D. G., et al. 2008. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl. Acad. Sci. U. S. A. 105:20428–20433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buckley S. M., Casals J. 1970. Lassa fever, a new virus disease of man from West Africa. III. Isolation and characterization of the virus. Am. J. Trop. Med. Hyg. 19:680–691 [DOI] [PubMed] [Google Scholar]
- 16. Bukczynski J., Wen T., Ellefsen K., Gauldie J., Watts T. H. 2004. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc. Natl. Acad. Sci. U. S. A. 101:1291–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carbonneil C., et al. 2003. Dendritic cells generated in the presence of granulocyte-macrophage colony-stimulating factor and IFN-α are potent inducers of HIV-specific CD8 T cells. AIDS 17:1731–1740 [DOI] [PubMed] [Google Scholar]
- 18. Casazza J. P., et al. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castellino F., Germain R. N. 2006. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu. Rev. Immunol. 24:519–540 [DOI] [PubMed] [Google Scholar]
- 20. Chen H. D., et al. 2001. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 2:1067–1076 [DOI] [PubMed] [Google Scholar]
- 21. Croft M. 2003. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 14:265–273 [DOI] [PubMed] [Google Scholar]
- 22. Cummins D., et al. 1990. Acute sensorineural deafness in Lassa fever. JAMA 264:2093–2096 [PubMed] [Google Scholar]
- 23. Curtsinger J. M., Johnson C. M., Mescher M. F. 2003. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165–5171 [DOI] [PubMed] [Google Scholar]
- 24. Dauer M., et al. 2005. FastDC derived from human monocytes within 48 h effectively prime tumor antigen-specific cytotoxic T cells. J. Immunol. Methods 302:145–155 [DOI] [PubMed] [Google Scholar]
- 25. Edington G. M., White H. A. 1972. The pathology of Lassa fever. Trans. R. Soc. Trop. Med. Hyg. 66:381–389 [DOI] [PubMed] [Google Scholar]
- 26. Fisher-Hoch S. P., Hutwagner L., Brown B., McCormick J. B. 2000. Effective vaccine for Lassa fever. J. Virol. 74:6777–6783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fisher-Hoch S. P., McCormick J. B., Sasso D., Craven R. B. 1988. Hematologic dysfunction in Lassa fever. J. Med. Virol. 26:127–135 [DOI] [PubMed] [Google Scholar]
- 28. Fisher-Hoch S. P., et al. 1987. Physiological and immunologic disturbances associated with shock in a primate model of Lassa fever. J. Infect. Dis. 155:465–474 [DOI] [PubMed] [Google Scholar]
- 29. Fisher-Hoch S. P., et al. 1995. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ 311:857–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fonteneau J.-F., et al. 2001. Generation of high quantities of viral and tumor-specific human CD4+ and CD8+ T-cell clones using peptide pulsed mature dendritic cells. J. Immunol. Methods 258:111–126 [DOI] [PubMed] [Google Scholar]
- 31. Gaucher D., et al. 2008. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 205:3119–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gazzinelli R. T., et al. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 157:798–805 [PubMed] [Google Scholar]
- 33. Geisbert T. W., et al. 2005. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Günther S., et al. 2000. Imported Lassa fever in Germany: molecular characterization of a new Lassa virus strain. Emerg. Infect. Dis. 6:466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hawiger D., et al. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ho W. Y., Nguyen H. N., Wolfl M., Kuball J., Greenberg P. D. 2006. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naïve repertoire. J. Immunol. Methods 310:40–52 [DOI] [PubMed] [Google Scholar]
- 37. Hochweller K., Anderton S. M. 2005. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur. J. Immunol. 35:1086–1096 [DOI] [PubMed] [Google Scholar]
- 38. Huang X.-L., et al. 2003. Priming of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cell responses by dendritic cells loaded with HIV-1 proteins. J. Infect. Dis. 187:315–319 [DOI] [PubMed] [Google Scholar]
- 39. Johnson K. M., et al. 1987. Clinical virology of Lassa fever in hospitalized patients. J. Infect. Dis. 155:456–463 [DOI] [PubMed] [Google Scholar]
- 40. Kelso A., et al. 2002. The genes for perforin, granzymes A-C and IFN-γ are differentially expressed in single CD8+ T cells during primary activation. Int. Immunol. 14:605–613 [DOI] [PubMed] [Google Scholar]
- 41. Ko H.-S., Fu S. M., Winchester R. J., Yu D. T. Y., Kunkel H. G. 1979. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J. Exp. Med. 150:246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kolumam G. A., Thomas S. Y., Thompson L. J., Sprent J., Murali-Krishna K. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Le Bon A., et al. 2006. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J. Immunol. 176:4682–4689 [DOI] [PubMed] [Google Scholar]
- 44. Le Bon A., et al. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009–1015 [DOI] [PubMed] [Google Scholar]
- 45. Li L., Elliott J. F., Mosmann T. R. 1994. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J. Immunol. 153:3967–3978 [PubMed] [Google Scholar]
- 46. Mahanty S., et al. 2003. Impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J. Immunol. 170:2797–2801 [DOI] [PubMed] [Google Scholar]
- 47. Mannering S. I., McKenzie J. L., Hart D. N. J. 1998. Optimisation of the conditions for generating human DC initiated antigen specific T lymphocyte lines in vitro. J. Immunol. Methods 219:69–83 [DOI] [PubMed] [Google Scholar]
- 48. McCormick J. B., et al. 1986. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 314:20–26 [DOI] [PubMed] [Google Scholar]
- 49. McCormick J. B., Webb P. A., Krebs J. W., Johnson K. M., Smith E. S. 1987. A prospective study of the epidemiology and ecology of Lassa fever. J. Infect. Dis. 155:437–444 [DOI] [PubMed] [Google Scholar]
- 50. Meng Y., Harlin H., O'Keefe J. P., Gajewski T. F. 2006. Induction of cytotoxic granules in human memory CD8+ T cell subsets requires cell cycle progression. J. Immunol. 177:1981–1987 [DOI] [PubMed] [Google Scholar]
- 51. Mills K. H. G. 2004. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 4:841–855 [DOI] [PubMed] [Google Scholar]
- 52. Miro F., et al. 2006. T cell-dependent activation of dendritic cells requires IL-12 and IFN-γ signaling in T cells. J. Immunol. 177:3625–3634 [DOI] [PubMed] [Google Scholar]
- 53. Moser B., Loetscher P. 2001. Lymphocyte traffic control by chemokines. Nat. Immunol. 2:123–128 [DOI] [PubMed] [Google Scholar]
- 54. Moser J. M., et al. 2010. Optimization of a dendritic cell-based assay for the in vitro priming of naïve human CD4+ T cells. J. Immunol. Methods 353:8–19 [DOI] [PubMed] [Google Scholar]
- 55. Mosmann T. R., Sad S. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138–146 [DOI] [PubMed] [Google Scholar]
- 56. Murali-Krishna K., et al. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177–187 [DOI] [PubMed] [Google Scholar]
- 57. Nacy C. A., Meierovics A. I., Belosevic M., Green S. J. 1991. Tumor necrosis factor-alpha: central regulatory cytokine in the induction of macrophage antimicrobial activities. Pathobiology 59:182–184 [DOI] [PubMed] [Google Scholar]
- 58. Pannetier D., Faure C., Georges-Courbot M. C., Deubel V., Baize S. 2004. Human macrophages, but not dendritic cells, are activated and produce type I interferons in response to Mopeia virus infection. J. Virol. 78:10516–10524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Quezada S. A., Jarvinen L. Z., Lind E. F., Noelle R. J. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307–328 [DOI] [PubMed] [Google Scholar]
- 60. Romero P., et al. 2007. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 178:4112–4119 [DOI] [PubMed] [Google Scholar]
- 61. Roy M., Waldschmidt T., Aruffo A., Ledbetter J. A., Noelle R. J. 1993. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 151:2497–2510 [PubMed] [Google Scholar]
- 62. Schlienger K., Craighead N., Lee K. P., Levine B. L., June C. H. 2000. Efficient priming of protein antigen-specific human CD4+ T cells by monocyte-derived dendritic cells. Blood 96:3490–3498 [PubMed] [Google Scholar]
- 63. Shafer-Weaver K., et al. 2003. The Granzyme B ELISPOT assay: an alternative to the 51Cr-release assay for monitoring cell-mediated cytotoxicity. J. Transl. Med. 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Skov S., Bonyhadi M., Odum N., Ledbetter J. A. 2000. IL-2 and IL-15 regulate CD154 expression on activated CD4 T cells. J. Immunol. 164:3500–3505 [DOI] [PubMed] [Google Scholar]
- 65. Speiser D. E., et al. 2001. Human CD8+ T cells expressing HLA-DR and CD28 show telomerase activity and are distinct from cytolytic effector T cells. Eur. J. Immunol. 31:459–466 [DOI] [PubMed] [Google Scholar]
- 66. ter Meulen J., et al. 2000. Characterization of human CD4+ T cell clones recognizing conserved and variable epitopes of the Lassa virus nucleoprotein. J. Virol. 74:2186–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. ter Meulen J., et al. 2004. Old and New World arenaviruses share a highly conserved epitope in the fusion domain of the glycoprotein 2, which is recognized by Lassa virus-specific human CD4+ T-cell clones. Virology 321:134–143 [DOI] [PubMed] [Google Scholar]
- 68. Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146 [DOI] [PubMed] [Google Scholar]
- 69. Urbani S., et al. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398–11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van Leeuwen E. M. M., Remmerswaal E. B. M., Heemskerk M. H. M., ten Berge I. J. M., van Lier R. A. W. 2006. Strong selection of virus-specific cytotoxic CD4+ T-cell clones during primary human cytomegalovirus infection. Blood 108:3121–3127 [DOI] [PubMed] [Google Scholar]
- 71. Weissman D., et al. 2000. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J. Immunol. 165:4710–4717 [DOI] [PubMed] [Google Scholar]
- 72. Whitmire J. K., Benning N., Whitton J. L. 2005. Early IFN-γ signaling directly enhances primary antiviral CD4+ T cell responses. J. Immunol. 175:5624–5628 [DOI] [PubMed] [Google Scholar]
- 73. Whitmire J. K., Eam B., Benning N., Whitton J. L. 2007. Direct interferon-γ signaling dramatically enhances CD4+ and CD8+ T cell memory. J. Immunol. 179:1190–1197 [DOI] [PubMed] [Google Scholar]
- 74. Whitmire J. K., Tan J. T., Whitton J. L. 2005. Interferon-γ acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 201:1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wulff H., McIntosh B., Hamner D., Johnson K. 1977. Isolation of an arenavirus closely related to Lassa virus from Mastomys natalensis in south-east Africa. Bull. World Health Organ. 55:441–444 [PMC free article] [PubMed] [Google Scholar]
- 76. Zarling A. L., Johnson J. G., Hoffman R. W., Lee D. R. 1999. Induction of primary human CD8+ T lymphocyte responses in vitro using dendritic cells. J. Immunol. 162:5197–5204 [PubMed] [Google Scholar]
- 77. Zuber B., et al. 2005. Detection of human perforin by ELISpot and ELISA: ex vivo identification of virus-specific cells. J. Immunol. Methods 302:13–25 [DOI] [PubMed] [Google Scholar]