Abstract
Effective, safe, and affordable rabies vaccines are still being sought. Newcastle disease virus (NDV), an avian paramyxovirus, has shown promise as a vaccine vector for mammals. Here, we generated a recombinant avirulent NDV La Sota strain expressing the rabies virus glycoprotein (RVG) and evaluated its potential to serve as a vaccine against rabies. The recombinant virus, rL-RVG, retained its high-growth property in chicken eggs, with titers of up to 109.8 50% egg infective doses (EID50)/ml of allantoic fluid. RVG expression enabled rL-RVG to spread from cell to cell in a rabies virus-like manner, and RVG was incorporated on the surface of the rL-RVG viral particle. RVG incorporation did not alter the trypsin-dependent infectivity of the NDV vector in mammalian cells. rL-RVG and La Sota NDV showed similar levels of sensitivity to a neutralization antibody against NDV and similar levels of resistance to a neutralization antibody against rabies virus. Animal studies demonstrated that rL-RVG is safe in several species, including cats and dogs, when administered as multiple high doses of recombinant vaccine. Intramuscular vaccination with rL-RVG induced a substantial rabies virus neutralization antibody response and provided complete protection from challenge with circulating rabies virus strains. Most importantly, rL-RVG induced strong and long-lasting protective neutralization antibody responses to rabies virus in dogs and cats. A low vaccine dose of 108.3 EID50 completely protected dogs from challenge with a circulating strain of rabies virus for more than a year. This is the first study to demonstrate that immunization with an NDV-vectored vaccine can induce long-lasting, systemic protective immunity against rabies.
INTRODUCTION
Rabies virus (RV), which belongs to the genus Lyssavirus of the family Rhabdoviridae, causes a fatal neurologic disease in humans and animals (13). More than 55,000 people die of rabies each year, with about 95% of those deaths occurring in Asia and Africa (61). The number of human deaths attributed to rabies worldwide is greater than that from avian influenza, polio, meningococcal meningitis, Japanese encephalitis, yellow fever, and severe acute respiratory syndrome combined (60).
Rabid dogs remain the main source of human exposure (22, 61). The most cost-effective strategy for preventing rabies in humans is to eliminate rabies in dogs through animal vaccination (37). However, the vaccination rate of dogs in many developing countries is low, especially in rural areas, mainly due to low economic development and the high costs of vaccine (33). A live attenuated RV strain (SAG-2) and a recombinant vaccinia virus expressing the rabies virus glycoprotein (RVG) have been licensed and shown to be effective oral vaccines (27, 58). However, the recombinant vaccinia virus expressing RVG can cause intense skin inflammation and systemic vaccinia virus infection in humans (1, 43), and SAG-2 induces a low level of virus-neutralizing antibody (VNA) responses, which complicates evaluation of its efficiency (22, 61). Therefore, effective, safe, and low-cost rabies vaccines are still being sought (19, 40, 47, 63, 67).
Newcastle disease virus (NDV) is a member of the genus Avulavirus of the family Paramyxoviridae. NDV strains are classified as nonvirulent (lentogenic), moderately virulent (mesogenic), or highly virulent (velogenic) for poultry (2). This virulence is mainly determined by the amino acid sequence of the protease cleavage site of the fusion (F) protein precursor (51). Lentogenic strains contain fewer basic amino acids at this site and can only be cleaved by trypsinlike extracellular proteases that are largely confined to the respiratory tract, whereas highly virulent strains are cleaved by ubiquitous intracellular proteases, potentially resulting in systemic infection (48). Currently, lentogenic strains, such as the La Sota strain, are used as live attenuated vaccines against NDV in poultry (3).
The development of a reverse genetics system has provided a method to generate recombinant NDV-based live virus-vectored vaccines (10, 46). NDV has been actively developed (25, 26, 28, 50, 57) and evaluated for its potential as a vaccine vector for the control of human and animal diseases (7, 10). NDV does not usually infect mammals because of host range restriction, and it is antigenically distinct from mammalian paramyxoviruses, suggesting that mammals would not be susceptible to NDV. After intranasal or intratracheal inoculation with a mesogenic or lentogenic strain of NDV, African green and rhesus monkeys experienced only a low-level, asymptomatic infection of the respiratory tract with little or no virus shedding (17). Studies with mice have shown that NDV expressing the protective antigens of H1 influenza virus, H5 highly pathogenic avian influenza virus, and human respiratory syncytial virus (RSV) were immunogenic and provided protection from challenges (25, 41, 44, 45). Moreover, recombinant NDV expressing SARS coronavirus S or H5 avian influenza virus hemagglutinin were safe and immunogenic in nonhuman primates and also provided protection from challenges (8, 15, 16, 18). These results strongly indicate that NDV is a promising vaccine vector for humans and other mammals. Here, we generated a recombinant NDV expressing RVG, the major antigen for protective immune responses against RV (13). The feasibility of this recombinant NDV to serve as a live-virus-vectored rabies vaccine for mammalian animals was evaluated.
MATERIALS AND METHODS
Viruses and cells.
HEp-2 cells, BHK-21 cells, Vero cells, A549 cells, and neuroblastoma cells (NA) of A/J mouse origin were grown in Eagle's minimum essential medium containing 10% fetal bovine serum (FBS). The Vero-adapted RV Evelyn-Rokitnicki-Abelseth (ERA) strain and the RV CVS-11 strain came from the China Veterinary Culture Collection. The NDV vector virus rL was rescued from the genomic cDNA of the NDV La Sota vaccine strain (25). Recombinant NDV strains were grown and titrated in 9-day-old specific-pathogen-free (SPF) embryonated chicken eggs by inoculation of the allantoic cavity. Recombinant NDV was also grown and titrated in BHK-21 or NA cells in 6-well or 96-well plates in fresh Opti-MEM (Invitrogen Corp., Carlsbad, CA) with or without 1 μg/ml of TPCK (tosylsulfonyl phenylalanyl chloromethyl ketone) trypsin (Sigma, St. Louis, MO). The infection of NDV in BHK-21 and NA cells was detected by using an indirect immunofluorescence assay (IFA) and observing the cells under an immunofluorescence microscope. The RV ERA and CVS-11 strains were propagated and titrated in Vero cells and BHK-21 cells, respectively. The modified vaccinia virus strain Ankara expressing the T7 RNA polymerase (64) was grown and titrated in primary chicken embryo fibroblasts. The RV street virus GX/09, isolated from the brain of a dog that died of rabies in Guangxi Province of China in 2009, was propagated and titrated in the brains of adult mice as previously described (56). Recombinant vesicular stomatitis virus (VSV) expressing enhanced green fluorescent protein, rVSV-EGFP was generated and prepared as previously described (38). Viral titration results were calculated by using the method of Reed and Muench (52). All viruses were stored at −70°C before use.
Plasmid construction and virus rescue.
To construct the full-length recombinant NDV genomic cDNA, the cDNA that represents the open reading frame (ORF) of the G gene of the RV ERA virus was amplified from the genome RNA by using the primer pair 5′-GACTGTTTAAACTTAGAAAAAATACGGGTAGAAGTGCCACCATGGTTCCTCAGGCTCTCCTG-3′ and 5′-GACTGTTTAAACTCACAGTCTGGTCTCACCCCCACTC-3′, in which the gene end and gene start sequences of NDV (underlined), the optimal Kozak sequence (italic), and the Pme I restriction sites (bold) were included. The G gene of ERA was introduced into the NDV genome of pLa through the introduction of a Pme I site in the P-M intergenic region at nucleotide position 3165 of the NDV genome, as described previously (25). The resultant plasmid was designated pLa-RVG and used for virus rescue by using reverse genetics as described previously (25). The presence of rescued virus and RVG expression in recombinant NDV were confirmed by IFA. The recovered viruses were also confirmed by sequencing the entire viral genome. The resultant recombinant virus was designated rL-RVG.
Immunofluorescence.
NDV infection of cultured cells was detected by IFA by using mouse or chicken serum against NDV as previously described (25). For confocal assays, BHK-21 cells were grown in 24-well plates or plated on coverslips in 35-mm-diameter dishes and infected with NDV rL or rL-RVG. At 24 h postinfection, cells were fixed in ice-cold 3% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature and then washed with PBS three times. Cells were blocked in PBS containing 1% (wt/vol) bovine serum albumin (BSA) at 4°C for 1 h. Cells were then incubated with mouse serum against RV or chicken serum against NDV for 30 min at room temperature. Cells were then washed three times with PBS containing 0.05% Tween 20 and stained with a TRITC (tetramethyl rhodamine isocyanate)-conjugated goat anti-mouse antibody (Sigma) or an FITC (fluorescein isothiocyanate)-conjugated rabbit anti-chicken antibody (Sigma) for 30 min. Cells were washed three times with PBS, and their nuclei stained with DAPI (4′,6′-diamidino-2-phenylindole). Cells were analyzed with a fluorescence microscope or confocal laser microscope. Images were acquired with a Zeiss (Thornwood, NY) Axioskop microscope equipped for epifluorescence with a Sensys charge-coupled device camera (Photometrics, Tucson, AZ) by using IPLab software (Scanalytics, Vienna, VA).
Western blotting.
BHK-21 cells were infected with rL and rL-RVG at a multiplicity of infection (MOI) of 10. At 36 h postinfection, lysates were prepared for the analysis of cell-associated proteins. For the analysis of virion-associated proteins, virus particles were isolated as previously described (44) from the allantoic fluid of SPF chicken eggs infected with rL or rL-RVG. Briefly, the allantoic fluid was clarified by low-speed centrifugation, layered onto a 40%-to-60% (wt/vol) sucrose gradient, and centrifuged at 90,000 × g for 90 min (Beckman Coulter, Fullerton, CA). The resulting band of virus particles was isolated and resuspended in PBS. As a control, allantoic fluid from mock-infected eggs was processed in parallel. Proteins from the lysates of infected cells or from purified virus particle preparations were separated by using SDS–10% PAGE under denaturing conditions for Western blot analyses with chicken serum against NDV or mouse serum against RV. Chicken or mouse serum binding was detected with horseradish peroxidase (HRP)-conjugated rabbit anti-chicken IgG or goat anti-mouse IgG, respectively (Sigma, St. Louis, MO).
Immunoelectron microscopy.
Purified virus particles were bound to 200-mesh Formvar–carbon-coated nickel grids (Electron Microscopy Sciences, Hatfield, PA). For immunolabeling, grids were blocked in PBS containing 2% globulin-free BSA (Sigma-Aldrich, St. Louis, MO) and incubated with mouse anti-NDV polyclonal IgG or mouse anti-RV polyclonal IgG. Grids were then washed in blocking solution and incubated in goat anti-mouse IgG conjugated to 10-nm gold beads (Sigma). The grids received a final wash, followed by negative staining with 1% phosphotungstic acid. They were examined under a model H7500 transmission electron microscope (Hitachi High Technologies, Schaumburg, IL) at 80 kV. All images were obtained by using an XR100 digital camera system (Advanced Microscopy Techniques, Danvers, MA).
Quantification of interferon induction and inhibition of viral replication.
A549 cells were incubated with rL or rL-RVG at an MOI of 5 for 1 h at 37°C; the viruses were then removed by washing five times with PBS. After the cells were incubated for an additional 24 h, the cell supernatants were harvested, UV treated for 2 h, and then mixed with mouse serum against NDV at a dilution of 1:20 and incubated for 1 h at 37°C. The complete neutralization of residual infectious NDV in the supernatant-serum mixtures was confirmed by inoculation of chicken eggs. The antiviral activity of the cell supernatants was then detected in A549 cells and quantified as described previously (38). The antiviral activity was quantified in terms of an inhibition unit (IhU), where one IhU was defined as the final dilution at which 50% inhibition of rVSV-EGFP infection occurred. For the interferon inhibition test, type I interferon was generated in A549 cells by treating them with poly(I:C) as described previously (30), and the antiviral activity in the supernatants was quantified in IhUs in A549 cells. Serial dilutions of the type I interferon generated were used to treat A549 cells for 24 h. The pretreated A549 cells were then infected with 100 50% tissue culture infective doses (TCID50) of rL or rL-RVG in the presence of trypsin. Virus replication in A549 cells was detected at 48 h postinoculation by using IFA with chicken serum against NDV.
Pathogenicity in poultry and mice.
To determine the pathogenicity of rL-RVG in poultry, the mean death time (MDT), the intracerebral pathogenicity index (ICPI), and the intravenous pathogenicity index (IVPI) were determined in embryonated SPF chicken eggs or in SPF chickens as described previously (25). To assess the pathogenicity of recombinant viruses in mice, groups of 19 3-week-old BALB/c mice were inoculated intramuscularly (i.m.) and intracerebrally (i.c.) with 2 × 108 50% egg infective doses (EID50) of the virus in a volume of 0.03 ml. Three mice from each group were killed on days 3, 5, and 7 postinoculation, and organs, including brain, lung, liver, spleen, kidney, and heart were collected and homogenized in 0.5 ml PBS for virus titration in embryonated SPF chicken eggs by inoculation of the allantoic cavity (3). The remaining 10 mice were observed daily for 3 weeks for signs of disease, weight loss, or death.
Immunization studies.
For mouse immunization studies, 4-week-old female BALB/c mice were divided into four groups of 10 animals each. Three groups were inoculated i.m. in the gastrocnemius muscle with 100 μl of 108.3 EID50, 107. 3 EID50, or 106.3 EID50 of rL-RVG. The fourth group was inoculated i.m. with 100 μl of 108.3 EID50 of rL. All mice were bled from the retro-orbital sinus under isoflurane inhalation anesthesia for the serologic assay. For cat immunization assays, outbred cats (0.5 to ∼2 years old) were divided into two groups of five animals each. One group was inoculated i.m. in the quadriceps muscle with 1 ml of 109.8 EID50 of rL-RVG, and the other group was inoculated with 1 ml of 106 focus-forming units (FFU) of ERA. For dog immunization, 3-month-old beagle dogs were arranged in four groups of 10 animals each. Three groups were inoculated i.m. in the quadriceps muscle with 1 ml of 109.8 EID50, 109.3 EID50, or 108.3 EID50 of rL-RVG, respectively. The fourth group was inoculated with 1 ml of 106 FFU of ERA by the same route. At 3 and 60 weeks after the initial vaccination, the cats and dogs received second and third vaccine doses, respectively. They were bled from the vein of the front leg prior to vaccination and at different times postvaccination for serological assessment. All cats and dogs used in this study had no record of prior rabies vaccination.
Challenge test.
The mouse challenge was carried out at 3 weeks postimmunization. Mice were injected i.m. in the thigh muscle of the hind leg with 50 50% minimum lethal doses (MLD50) of RV (strain GX/09, 100 μl). Mice were observed for 4 weeks for clinical signs of rabies. Any mouse that showed definitive clinical signs of rabies, such as paralysis, tremors, or spasms, was euthanized by CO2 intoxication. The survival rates obtained with the different vaccination groups were compared. The challenge in dogs was carried out 60 weeks after the second dose of vaccination by injecting 2 × 0.5 ml containing 105.8 MLD50 of GX/09 into the bilateral masseter muscle, 0.5 ml per side. The dogs were observed for 12 weeks, and any animal showing definitive rabid signs was euthanized with a barbiturate solution administered intravenously. All dogs that survived the challenge at the end of the observation period were also euthanized, and their brain tissue checked for rabies virus antigen by using a direct immunofluorescence (IF) test (39).
Serologic tests.
Animal sera were tested for RV neutralizing antibodies (VNA) by using the rapid fluorescent focus inhibition test (RFFIT) as described elsewhere (55); titers are expressed in international units per milliliter of serum (IU/ml) with a WHO standard as a reference. The hemagglutination inhibition (HI) antibody to NDV was tested as previously described (25).
Laboratory facility.
All experiments related to the rabies virus GX/09 were conducted in a biosafety level 3 (BSL-3) facility at the Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences. All animal studies were approved by the Review Board of Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
RESULTS
Generation of recombinant NDV viruses expressing the RVG gene.
To generate recombinant NDV expressing RVG, an infectious recombinant genomic cDNA clone of NDV La Sota was constructed by inserting the RVG gene between the P and M genes (Fig. 1 A). The resultant recombinant virus, rL-RVG, was rescued from the full-length genomic cDNA clone as described in Materials and Methods, and the presence of the RVG gene was confirmed by using reverse transcription (RT)-PCR. Expression of the G protein by rL-RVG was detected by immunostaining infected BHK-21 cells 36 h postinfection. As expected, cells infected with rL were not stained by mouse serum against RV but were stained by chicken serum against NDV (Fig. 1B). In contrast, cells infected with rL-RVG were stained by both the mouse antiserum to RV and the chicken antiserum to NDV (Fig. 1B). RVG expression by the recombinant viruses was also confirmed by Western blot analysis with polyclonal mouse antiserum to RV (Fig. 1C).
Fig. 1.
Generation of recombinant NDV expressing the RVG gene. (A) Schematic representation of the rL genome with the PmeI site introduced between the P and M genes and RVG inserted at the PmeI site. (B) Immunofluorescence analysis of RVG protein expression. Confluent BHK-21 cells were infected with rL or rL-RVG at an MOI of 0.5. The infected cells were fixed and probed with chicken serum against NDV and mouse serum against RV and then incubated with an FITC-conjugated rabbit anti-chicken antibody or a TRITC-conjugated goat anti-mouse antibody. Cell nuclei were stained with DAPI. Cells were analyzed by using a confocal laser microscope. (C) Western blot analyses of recombinant NDV expressing RVG. Lysates of BHK-21 cells infected with rL or rL-RVG were incubated with chicken serum against NDV, mouse serum against RV, or an anti-β-actin monoclonal antibody. Binding was visualized with 3, 3-diaminobenzidine reagent after incubation with peroxidase-conjugated secondary antibodies. The locations of marker proteins are indicated on the left, and the antiserum or antibody used is indicated on the right. PMM, protein molecular marker. (D) Growth properties of recombinant viruses in embryonated eggs. rL and rL-RVG (0.1 ml of 100 EID50) were inoculated into the allantoic cavities of 10-day-old embryonated eggs, and the allantoic fluid of six eggs from each group was harvested at the time points indicated and pooled for EID50 determination in eggs. The data shown are the means of the results of three experiments; the error bars indicate the standard deviations (SD).
The growth properties of rL and rL-RVG in eggs were examined. As shown in Fig. 1D, rL-RVG and rL grew to similar levels and reached peak titers of 9.8 log EID50/ml at 72 h postinoculation. This titer was approximately one-fifth of a log lower than that of rL. The stability of the RVG gene within rL-RVG was assessed by serially passaging the virus in SPF chicken eggs for 10 passages. After 10 passages, the RVG gene was assessed by using RT-PCR and immunofluorescence and found to be stably maintained and expressed (data not shown).
RVG expression enables the NDV vector to spread from cell to cell and alters its replication in cell culture in the absence of trypsin.
Progeny rabies virions that bud from infected cells are spread from cell to cell in cell culture, presumably as they do in animals. Rabies viruses can spread to contiguous or to noncontiguous cells which are surrounded by the interstitial space (14). To investigate whether RVG expression alters the cell spread of the NDV vector, BHK-21 and NA cells were infected with rL or rL-RVG, each at an MOI of 0.05. The infected cells were detected at different times postinoculation by using indirect IFA with chicken serum against NDV. As a low-pathogenicity NDV strain, rL infected individual cells but did not spread to adjacent BHK-21 or NA cells in the absence of TPCK trypsin (1 mg/ml). Therefore, the percentage of infected cells at 96 h postinfection was almost identical to that at 24 h postinfection (Fig. 2 A and B). However, rL-RVG acquired the ability to spread from cell to cell in the absence of TPCK trypsin and spread from the initial infected cell to adjacent cells, forming larger and larger plaques. These plaques gradually fused together, and all of the cells in the observation field were infected at 96 h postinoculation (Fig. 2A and B). To further confirm that the cell-to-cell spread was specifically caused by the RVG that was displayed on the cell surface, we added diluted anti-NDV mouse serum (at 12.5 times the neutralization titer), anti-RV mouse serum (at 22.5 times the neutralization titer), or 10-times-diluted naïve mouse serum to the culture medium at 1 h postinoculation with rL-RVG at an MOI of 0.05. The spread of the infection from cell to cell in BHK-21 (Fig. 2C, panel I) and NA cells (Fig. 2C, panel II) was completely blocked by the presence of the anti-RV mouse serum. However, the anti-NDV mouse and naïve mouse sera had no effect on the spread of infection. These results suggest that the expression of RVG dramatically altered the cell-to-cell spread in vitro.
Fig. 2.
Cell-to-cell spread of recombinant NDV and vector in BHK-21 cells. (A) Monolayers of BHK-21 cells were infected with either rL or rL-RVG at an MOI of 0.05. After five washes 1 h postinfection, the infected cells were incubated at 37°C. The infected cells were then examined at the indicated hours postinfection by using IFA with chicken serum against NDV as described in Materials and Methods. (B) The percentages of infected cells after infection with rL and rL-RVG at different times were calculated based on the results of the IFA. (C) Cell-to-cell spread inhibition assay for recombinant virus in BHK-21 and NA cells. Monolayers of BHK-21 (I) and NA cells (II) were infected with either rL or rL-RVG at an MOI of 0.05. After five washes 1 h postinfection, the cells were incubated with culture medium containing 100-fold-diluted mouse serum against NDV, mouse serum against RV, or naïve mouse serum. Infected cells were examined 48 h postinfection by using IFA with chicken serum against NDV.
To investigate whether cell-to-cell spread alters the replication of the NDV vector in cell culture, the one-step and multiple-step growth kinetics of rL and rL-RVG in BHK-21 cells were compared in the presence and absence of TPCK trypsin (1 mg/ml). In the multistep growth assay, BHK-21 cells were infected with egg-propagated rL or rL-RVG at an MOI of 0.01. In the presence of TPCK trypsin, the growth kinetics of rL-RVG were almost identical to those of rL in BHK-21 cells. The titers of rL and rL-RVG in the cell supernatant gradually increased and peaked at 107.5 EID50/ml and 107.2 EID50/ml, respectively, at 96 h postinoculation (Fig. 3 A). In the absence of TPCK trypsin, rL and rL-RVG showed different growth kinetics in cell culture. As expected, the titers of rL increased slowly during the 5-day observation period after inoculation. However, the titers of rL-RVG increased gradually after inoculation, reaching a peak titer of 107.6 EID50/ml at 96 h postinoculation (Fig. 3B). In the one-step growth assay, BHK-21 cells were infected with rL or L-RVG at an MOI of 5. The viruses in the cell supernatant were collected at different times after inoculation and titrated in chicken eggs. The mean titers of rL and rL-RVG gradually increased and reached peak titers (∼107.0 to 107.3 EID50/ml) at 72 h postinoculation (Fig. 3C and D). No significant differences in peak titers were found between rL and rL-RVG in the presence (Fig. 3C) or absence (Fig. 3D) of TPCK trypsin. These results show that the insertion and expression of the RVG gene significantly affected the growth of the NDV vector in cell culture in the absence of trypsin.
Fig. 3.
Kinetics of infectious virus replication in BHK-21 cells. Monolayers of BHK-21 cells were infected with either egg-propagated rL or rL-RVG at an MOI of 0.01 (A, B) or an MOI of 5 (C, D), respectively. After five washes 1 h postinfection, the cells were incubated with 100-fold-diluted mouse serum against NDV for 30 min to neutralize the residual viruses in the supernatants. After replacement of the medium with fresh medium, the infected cells were incubated at 37°C in the presence (A, C) or absence (B, D) of TPCK trypsin (1 μg/ml). The culture supernatants were collected at different times, and their virus titers were determined as numbers of EID50 in 10-day-old embryonated eggs. The data shown are the mean titers plus SD of five independent experiments. Significant differences between rL and rL-RVG were assessed by using the t test. *, P < 0.05; **, P < 0.01.
RVG is incorporated into the surface of NDV particles but does not alter trypsin-dependent infectivity in mammalian cell culture.
To investigate whether RVG was incorporated into recombinant NDV particles, virions were purified from the allantoic fluid of SPF chicken embryos inoculated with rL or rL-RVG and subjected to SDS-PAGE and Western blotting. SDS-PAGE clearly separated the purified virions of rL and rL-RVG into the major NDV structural proteins, including HN, N, M, and F1 (Fig. 4 A, panel I). The RVG proteins were probed with the anti-RV mouse serum in the purified virions of rL-RVG but not in those of rL (Fig. 4A, panel II). Partially purified virions of rL and rL-RVG were also observed by electron microscopy by using negative staining. Both virions showed typical paramyxovirus morphology, with densely arrayed spikes on their envelopes. Immunogold staining revealed that the polyclonal antibody to NDV bound to whole viral particles of rL and rL-RVG (Fig. 4B), whereas the polyclonal antibody to RV bound only to the viral particles of rL-RVG (Fig. 4B). These results indicate that the RVG that was incorporated into the surface of the recombinant NDV particles did not change the morphology of NDV.
Fig. 4.
RVG incorporation into recombinant virus particles. (A) SDS-PAGE of recombinant and vector virus particles. rL and rL-RVG were propagated in eggs and purified by differential centrifugation and sedimentation through 40%-to-60% (wt/vol) sucrose gradients. Viral proteins were analyzed by using SDS-PAGE with 10% gels under reducing conditions (I) and were subjected to Western blot analyses with mouse serum against RV (II). (B) Electron microscopy of recombinant and vector virus particles. rL or rL-RVG propagated in eggs was prepared and partially purified by centrifugation through 20% sucrose. Viruses were stained with IgG purified from mouse naïve serum (ab, antibody), mouse serum against NDV, or mouse serum against RV and with goat anti-mouse IgG conjugated with colloidal gold and then negatively stained.
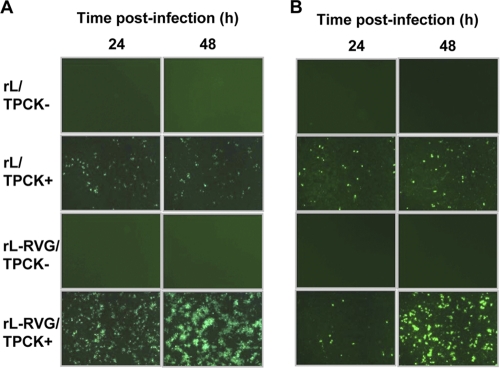
To further investigate whether RVG incorporation influences the trypsin-dependent infectivity of the NDV vector in mammalian cells, rL and rL-RVG were propagated in BHK-21 cells in the presence or absence of trypsin. Cell supernatants were collected 24 h postinoculation and then used to infect BHK-21 and NA cells in the absence of trypsin. The infections were detected by using an IFA with chicken serum against NDV. Both the rL and rL-RVG that were produced in BHK-21 cells in the presence of trypsin were able to infect BHK-21 (Fig. 5 A) and NA cells (Fig. 5B). In contrast, the rL and rL-RVG that were generated in BHK-21 cells in the absence of trypsin could not infect BHK-21 (Fig. 5A) or NA cells (Fig. 5B), indicating that the RVG incorporation into viral particles did not alter the trypsin-dependent infectivity of the NDV vector in mammalian cells.
Fig. 5.
Analysis of the trypsin-dependent infectivity of recombinant and vector viruses in BHK-21 and NA cells. rL and rL-RVG, propagated in BHK-21 cells in the presence (TPCK+) or absence (TPCK−) of trypsin, were used to infect BHK-21 (A) or NA cells (B) at 200 EID50. The rL and rL-RVG infections were examined by using IFA with chicken serum against NDV at 24 and 48 h postinfection, respectively.
To examine whether RVG incorporation altered the neutralization and infectivity characteristics of the recombinant NDV, 104 EID50 of rL and rL-RVG, propagated in chicken eggs, was mixed with 2-fold serially diluted mouse serum against NDV, mouse serum against RV, or naïve mouse serum. After a 1-h incubation at 37°C, the residual infectious viruses in the virus-serum mixtures were evaluated by inoculation into chicken eggs. The incubation with the anti-NDV serum completely prevented the infection of rL and rL-RVG. The mean neutralization titers for rL and rL-RVG were 896 and 768, respectively (Fig. 6). However, the anti-RV and naïve mouse sera showed no significant inhibition of the infection of rL and rL-RVG (Fig. 6). These results indicate that the recombinant rL-RVG has the same sensitivity to the NDV neutralization antibody as the parent vector virus. The incorporation of RVG into the viral envelope did not change the sensitivity of the NDV vector to the NDV or RV neutralization antibody.
Fig. 6.
Neutralization assay for the infectivity of recombinant and vector viruses. rL and rL-RVG (104 EID50) were mixed with serially diluted mouse serum against NDV, mouse serum against RV, or naïve mouse serum and incubated for 1 h at 37°C. The residual infectious viruses were quantified by titration in chicken eggs. The neutralization titers of antisera were defined as the dilution for 50% inhibition of infection. The data shown are the mean titers plus SD of five independent experiments.
RVG expression does not significantly influence the effect of the NDV vector on the innate immune responses of mammalian cells.
To investigate whether RVG expression alters the ability of the NDV vector to stimulate type I interferon responses in mammalian cells, human A549 cells were infected with rL and rL-RVG at an MOI of 5. At 24 h postinfection, the antiviral activity in the supernatants was quantified by using a replication inhibition assay as described in Materials and Methods. The levels of interferon activity stimulated by rL and rL-RVG were 1,228.8 IhU and 1,433.6 IhU, respectively. In contrast, there was no detectable antiviral activity in the supernatant of mock-infected A549 cells. The results show that rL-RVG and rL induce type I interferon responses in mammalian cells similarly (Fig. 7 A). To further compare the sensitivities of rL and rL-RVG to the interferon of mammalian cells, A549 cells were pretreated with type I interferon that was generated in A549 cells. The minimal amounts of interferon that inhibited replication of rL and rL-RVG by 50% were 1.6 IhU and 1.4 IhU, respectively; rL and rL-RVG thus showed similar levels of sensitivity to mammalian type I interferon (Fig. 7B). These results suggest that RVG expression does not significantly influence the effect of the NDV vector on the innate immune responses of mammalian cells.
Fig. 7.
Interferon induction and inhibition in a human cell line. (A) Type I interferon induction in A549 cells after infection with rL and rL-RVG. The inhibition units (IhU) were defined as the final dilution to give 50% inhibition of infection. (B) The levels of sensitivity of rL and rL-RVG to type I interferon in A549 cells. The minimal IhUs to inhibit 50% infection of rL and rL-RVG were determined in A549 cells. The data shown are the means plus SD of five independent experiments.
Expression of the RVG gene does not increase the virulence of the NDV vector in poultry or mice.
To investigate whether the expression of the RVG gene alters the pathogenicity of the NDV vector, we compared the biological character and pathogenicity of rL-RVG with those of its vector in poultry and mice. The MDT, ICPI, and IVPI tests are internationally accepted methods for assessing the pathogenicity of NDV strains in poultry (22). NDV strains are categorized into three groups on the basis of their MDTs: velogenic (<60 h), mesogenic (60 to 90 h), and lentogenic (>90 h) (22). The MDT values for rL and rL-RVG were both greater than 120 h (Fig. 8 A), indicating that these two viruses are lentogenic. After receiving rL and rL-RVG, all chickens remained healthy during the observation period. The ICPI values for rL and rL-RVG were 0.4 and 0, respectively (Fig. 8A); the IVPI values for rL and rL-RVG were both 0 (Fig. 8A). These data show that rL-RVG and rL are of low pathogenicity to SPF chickens and embryos, suggesting that the insertion of the RVG genes did not increase the virulence of the NDV vector.
Fig. 8.
Pathogenicity evaluation in chickens and mice. (A) Pathogenicity assay in SPF eggs and chickens. The MDT, ICPI, and IVPI were determined as described in Materials and Methods. (B) Weight changes of mice inoculated with recombinant and vector viruses. Groups of five mice were inoculated i.m. in the thigh muscle of a hind leg (I) or i.c. (II) with 108.3 EID50 (in 30 μl) of rL or rL-RVG and observed and weighed daily for 14 days. All mice survived for the duration of the experiment. Body weight changes for each group are shown as ratios of the body weight at day 0, which was set as 100.
To investigate the replication and pathogenicity of the recombinant virus in mammalian animals, mice were inoculated i.c. and i.m. with rL and rL-RVG, respectively. All of the mice survived after inoculation. There were no differences between rL infection and rL-RVG infection groups in body weight changes after intramuscular (Fig. 8B, panel I) or intracerebral (Fig. 8B, panel II) administration. Lung, heart, spleen, kidney, and brain tissues, collected at 3, 5, and 7 days after inoculation, showed no evidence of virus after inoculation in eggs. These data suggest that the expression of RVG does not change the pathogenicity of vector virus in mice and that both rL and rL-RVG have limited replication in specific major organs of mammalian animals.
Recombinant virus induces a dose-dependent immune response and protective efficacy against rabies in mice.
Three groups of 10 mice were intramuscularly inoculated in the thigh muscle of the hind leg with 108.3 EID50, 107.3 EID50, and 106.3 EID50 of rL-RV, respectively; a fourth group of 10 mice was inoculated with 108.3 EID50 of rL as a control. Three weeks after inoculation, sera were collected for NDV HI antibody and RV VNA assays. Meanwhile, the mice were challenged with 50 MLD50 of RV GX/09. Significant NDV HI antibody was detected in all mice (Fig. 9 A, panel I), but RV VNA was only detected in the blood of mice inoculated with rL-RV and not in the blood of those inoculated with rL (Fig. 9A, panel II). Both the NDV HI antibody and RV VNA showed significant dose-dependent responses. The mean titers of RV VNA for the 108.3 EID50, 107.3 EID50, and 106.3 EID50 groups were 12 IU, 6 IU, and 1.3 IU, respectively. Inoculation with 108.3 EID50 and 107.3 EID50 elicited considerably higher levels of NDV HI antibody (Fig. 9A, panel I) and RV VNA (Fig. 9A, panel II) than did inoculation with 106.3 EID50. As shown in Fig. 9B, all mice inoculated with 108.3 EID50 or 107.3 EID50 of rL-RVG survived the challenge with RV GX/09 and showed no signs of rabies. In the group that received 106.3 EID50, 6 of the 10 mice survived the challenge; 4 mice died within 12 days of exposure to RV GX/09. All mice in the rL-inoculated group died within 12 days of exposure to RV GX/09. These mice showed severe neurologic signs, including hunching and hind-leg paralysis. Rabies virus antigens were detected in the brains of all of the mice that died during the challenge. These results demonstrate that rL-RVG is highly immunogenic and efficacious against rabies in mice.
Fig. 9.
Immunogenicity and protective efficacy in mice. (A) Immunization assay. Groups of 10 4-week-old mice were injected with 108.3, 107.3, and 106.3 EID50 of rL-RVG and with 108.3 EID50 of rL as a control. Three weeks after vaccination, blood samples were collected to detect HI antibodies to NDV (I) and VNA to RV (II) as described in Materials and Methods. The data shown are means plus SD. Significant differences between groups were assessed by using the t test. **, P < 0.01. (B) Mouse challenge. Mice were challenged i.m. with 50 MLD50 of the RV street virus GX/09 at 3 weeks postvaccination and observed for 3 weeks. Percentages of mice surviving on different days postchallenge were recorded.
Antibody responses induced by the recombinant NDV in dogs and cats.
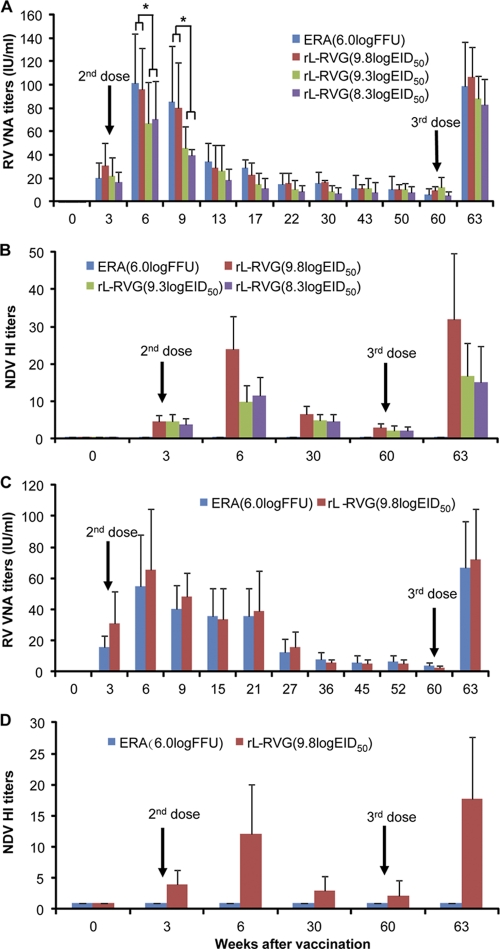
Dogs and cats represent two sizeable animal populations that need to be vaccinated for rabies control. Therefore, we further evaluated the immunogenicity of rL-RVG in these animals by using the immunization schedules detailed in Materials and Methods. Three weeks after the first dose, RV VNA was detected in all four groups of dogs (Fig. 10 A). In the ERA-vaccinated dogs and the dogs vaccinated with 109.8 EID50, 109.3 EID50, and 108.3 EID50 of rL-RV, the mean titers of RV VNA were 20.1 IU, 30.7 IU, 21.6, and 16.8 IU, respectively. Three weeks after the second dose, the mean titers of RV VNA for the four groups listed above increased to 101.3 IU, 96 IU, 67.2 IU, and 70.4 IU, respectively. After that, the RV VNA gradually declined. At 22 weeks postvaccination, the mean titers of RV VNA for the four groups were 85.3 IU, 80.4 IU, 46.2 IU, and 40.6 IU, respectively. There were no significant differences in RV VNA titers among all four vaccinated groups at 22 weeks postvaccination. From this point, the VNA to RV in all four groups of dogs remained relatively stable. At 60 weeks postvaccination, the mean titers of RV VNA in the four groups were 5.8 IU, 9.8 IU, 12.4 IU, and 5.5 IU, respectively. After receiving the third dose at 60 weeks postvaccination, all four groups of dogs showed substantial reboost responses to RV VNA. The titers of RV VNA in all four groups rose sharply to 98.6 IU, 101.8 IU, 88.6 IU, and 82.8 IU, respectively, at 3 weeks after the third dose. In the experiment with cats, all animals seroconverted to RV VNA following vaccination (Fig. 10C). The mean titers of RV VNA for the rL-RVG group at 3, 6, and 60 weeks postvaccination were 30.8 IU, 65.2 IU, and 2.4 IU, respectively. For ERA-vaccinated cats, the mean titers of RV VNA at 3, 6, and 60 weeks postvaccination were 15.8 IU, 54.8 IU, and 3.7 IU, respectively. After receiving the third dose, all cats experienced sizeable reboost responses to RV VNA. The RV VNA titers in the rL-RVG and ERA groups rose sharply to 67 IU and 72 IU, respectively. No significant differences were seen between the rL-RVG and ERA groups in RV VNA titers.
Fig. 10.
Immunogenicity evaluation in dogs and cats. Three-month-old beagle dogs were placed in four groups (6 or 10 each). Three groups were administered 1 ml of 109.8 EID50 (n = 6), 109.3 EID50 (n = 10), and 108.3 EID50 (n = 10) of rL-RVG, respectively. A fourth group was inoculated with 1 ml of 106 FFU of ERA (n = 6). Two groups of five cats were given three doses of 109.8 EID50 of rL-RVG or 106.0 FFU of ERA. At 3 and 60 weeks after the initial vaccination, the dogs and cats received second and third doses. The serum samples from dogs (A, B) and cats (C, D) were collected at different times after vaccination and the RV VNA titers (A, C) and NDV HI antibody titers (B, D) were detected. The antibody titers for each group of animals are indicated as the means plus SD. Statistically significant differences were determined by using the t test. *, P < 0.05.
Meanwhile, moderate levels of NDV HI antibodies were detected at 3 weeks after the first dose in all dogs (Fig. 10B) and cats (Fig. 10D) vaccinated with rL-RVG or rL, which increased slightly at 3 weeks after the second dose. For dogs, the mean NDV HI antibody titers in the groups vaccinated with 109.8 EID50, 109.3 EID50, and 108.3 EID50 of rL-RVG were 4.7, 4.6, and 3.8, respectively, at 3 weeks after the first dose and 24, 9.9, and 11.6, respectively, at 3 weeks after the second dose, and they gradually decreased to 2.8, 2.2, and 2.0, respectively, at 60 weeks postvaccination. After receiving the third dose at 60 weeks postvaccination, all dogs in the rL-RVG-vaccinated groups experienced reboost responses to the NDV HI antibody. The mean titers for the three groups increased to 32, 16.8, and 15.2, respectively (Fig. 10B). For cats, the mean NDV HI antibody titers in the rL-RVG-vaccinated groups were 3.9, 12.1, and 2.2 at 3, 6, and 60 weeks postvaccination. After receiving the third dose at 57 weeks after the second dose, all cats in the rL-RV-vaccinated groups had reboost responses to the NDV HI antibody. The mean NDV HI antibody titer increased to 17.7 at 3 weeks postvaccination (Fig. 10D). Of note, none of the dogs or cats vaccinated with ERA showed any detectable NDV HI antibody (Fig. 10B and D). These results demonstrate that rL-RVG is an immunogenic candidate rabies vaccine for dogs and cats.
No abnormal clinical signs were observed during the course of the study. These results suggest that the recombinant NDV rL-RVG is safe for dogs and cats, even after repeat inoculation with high dosages.
Immunization protects dogs from street RV challenge.
To determine the protective efficacy of rL-RVG in dogs against rabies, we conducted challenge tests at 57 weeks after the second immunization doses. Five dogs immunized with 108.3 EID50 of rL-RVG, three dogs immunized with ERA, three dogs inoculated with 109.8 EID50 of NDV vector rL, and three dogs inoculated with PBS were randomly selected from each group, and their individual RV VNA titers determined (Fig. 11 A). All dogs were i.m. challenged with 2 × 105.5 MLD50 of RV GX/09 as described in Materials and Methods. All animals that were vaccinated with rL-RVG and ERA survived the challenge and showed no clinical signs of rabies during the 12-week observation period. On the other hand, all six dogs inoculated with rL or PBS developed similar clinical signs of rabies 8 to 12 days postchallenge. Five of these dogs died within 3 weeks of the challenge, and one dog was euthanized at 21 days postvaccination (Fig. 11B). Rabies virus antigen was detected in the brains of all of the dead or euthanized dogs. All of the mice that were intracerebrally inoculated with brain tissue from each of the dead or euthanized dogs also developed signs of rabies and died within 12 days of inoculation. Rabies virus antigen was also detected in the brains of these mice. These results demonstrate that rL-RVG provides efficient and long-lasting protection against rabies in dogs.
Fig. 11.
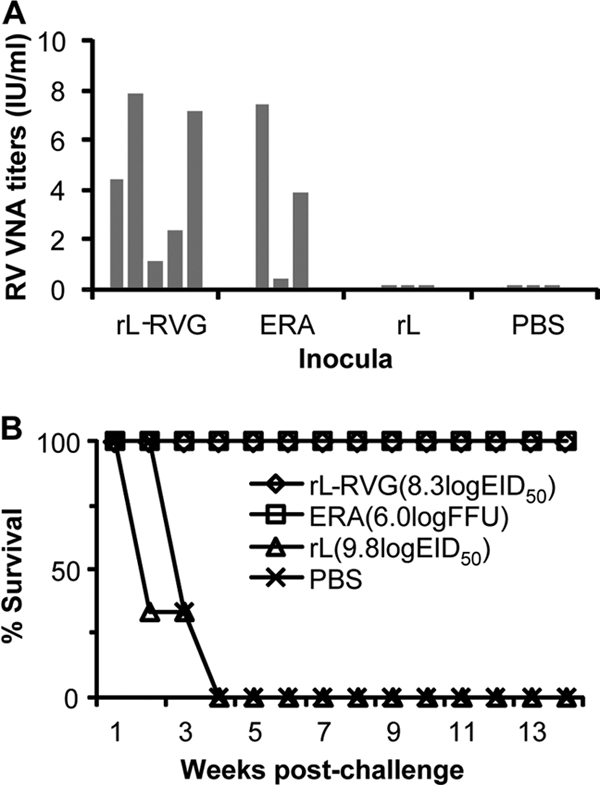
Challenge study in dogs. Dogs received 108.3 EID50 of rL-RVG (n = 5), 106.0 FFU of ERA (n = 3), 109.8 EID50 of rL (n = 3), or PBS (n = 3) and were then challenged with 2 × 105.5 MLD50 of rabies street virus GX/09 at 57 weeks after the second dose. The RV VNA titer for each animal was determined before challenge (A), and each bar shows the RV VNA titer of an individual dog. After viral challenge, the dogs were observed daily for clinical signs of rabies for 12 weeks. The percentages of dogs surviving in the different groups at different times postchallenge are recorded (B).
DISCUSSION
We generated a recombinant NDV that expresses rabies virus glycoprotein, rL-RVG, by using reverse genetics and evaluated its potential as a novel vectored vaccine against rabies in animals. The expression of RVG enabled rL-RVG to acquire the RV-like ability to spread from cell to cell. RVG was incorporated into the surface of the rL-RVG viral particles and did not alter the trypsin-dependent infectivity of the vector in mammalian cells. rL-RVG and rL showed similar levels of sensitivity to VNAs against NDV and similar levels of resistance to VNAs against RV. Animal studies demonstrated that rL-RVG is safe in poultry, mice, cats, and dogs, even at multiple doses as high as 109.8 EID50 in the latter two species. Vaccination with rL-RVG induced significant RV VNA responses and provided complete protection from RV street virus challenge. Most importantly, rL-RVG induced strong and long-lasting protective immune responses to RV in dogs and cats. The vaccinated dogs were completely protected from RV street virus challenge after 1 year, displaying no signs of disease or death. This is the first study to demonstrate that intramuscular immunization with an NDV-vectored vaccine can induce long-lasting systemic protective immunity against rabies in animals, and this vaccine may also have potential use in high-risk human individuals to control rabies infections.
NDV acquires its envelope from the host cell plasma membrane and incorporates foreign glycoproteins at the envelope (8, 15, 18, 44). The ability of foreign proteins to function as new receptor binding proteins has also been demonstrated in other paramyxoviruses (11). RVG mediates both receptor binding and penetration (13, 24) and is an important virulence factor for the virus (20, 56). The introduction of the RVG gene does not increase the virulence of the NDV vector. Recombinant rL-RVG grew to slightly lower titers than rL in embryonated eggs and in cell culture in the presence of trypsin. This may be due to the effects of foreign gene insertion in the NDV genome (36, 44). Considering this, it was remarkable to find that rL-RVG grows to a much higher titer than rL in BHK-21 cells in the absence of trypsin. As the virions of rL-RVG generated from BHK-21 cells in the absence of trypsin lack the ability to reinfect BHK-21 cells and the infectivity of rL-RVG could be inhibited only by VNAs to NDV and not VNAs to RV, it is more likely that the change in the growth property of rL-RVG was the result of RVG expression enabling the vector to spread from cell to cell. The RVG protein on the cell surface mediates fusion with adjacent cells, resulting in the transportation of the viral genome and transcription complex of NDV from one cell to the next. This hypothesis is supported by our finding that the spread of rL-RVG could be inhibited by the presence of VNAs to RV but not by VNAs to NDV.
A previous study showed that progeny rabies virions that bud from infected cells can spread to contiguous or to noncontiguous cells which are surrounded by the interstitial space (14). In the case of direct spread from cell to cell, the pathogenic RV can spread despite the presence of rabies VNA, whereas the spread of nonpathogenic RV is prevented in the presence of rabies VNA (14, 21). In our study, rL-NDV was generated with the RVG gene from a nonpathogenic RV, the ERA strain, which may explain why the cell-to-cell spread of rL-RVG could be completely prevented by the presence of VNAs to RV.
Our result that rL-RVG is highly sensitive to VNA to NDV but resistant to rabies VNA differs from the results of studies with recombinant human parainfluenza virus type 3 (HPIV-3) expressing the GP of Ebola virus (11, 65). In those studies, HPIV-3 VNA completely neutralized the HPIV-3 vector alone and weakly neutralized the infectivity of the recombinant HPIV-3 expressing Ebola virus GP on the virion envelope. The virions containing both the HN/F glycoproteins of HPIV-3 and the GP of Ebola virus were more efficiently neutralized by Ebola VNAs than by HPIV-3 VNAs despite the greater amount of HPIV-3 glycoproteins (11, 65). The reasons for these observations are not fully understood. One possibility is that the Ebola virus GP incorporated on the surface of the HPIV-3 particle could mask the neutralization epitopes of the HN and F glycoproteins (53).
NDV is a strong inducer of the interferon response in mammalian cells and is highly sensitive to the interferon induced in such cells (4, 5). The V protein encoded by NDV functions as an alpha interferon antagonist and is usually less efficient in mammalian cells (29, 49). In mammals, the localized release of limited amounts of progeny virus at the primary infection site is dependent not only on trypsinlike proteases but also on the innate immunity of the host cells (8, 18). In fact, the results of a previous study suggest that the F protein cleavage site does not have the same impact on the virulence of NDV in primates as it does in poultry (17). Our results confirm that rL-RVG and rL induce similar levels of and show similar levels of sensitivity to interferon in mammalian cells. Therefore, rL-RVG replication should still be limited by the innate immune response in mammalian hosts. This may partially explain why rL and rL-RVG could not be detected in mice 2 days after infection, even though RVG expression enables the NDV vector to spread from cell to cell in vivo.
Our results also show that RVG expression by NDV is not associated with disease in poultry, mice, cats, or dogs. The results of the MDT, IVPI, and ICPI assays demonstrated that recombinant rL-RVG retained the nonvirulent property of rL to poultry; there were no differences between rL and rL-RVG in virus replication or tissue distribution in mice after infection, which further confirms that RVG expression in vivo did not confer RV-specific pathogenicity to the NDV vector. This is not the first time that a lack of correlation between cell pathological effect (CPE) in vitro and virulence in vivo has been observed. Previous studies have reported that the increased CPE in vitro was accompanied by a decrease in the replication of recombinant HPIV-3 expressing Ebola virus GP compared to that of the HPIV-3 vector, and this difference was not associated with increased virulence in guinea pigs or nonhuman primates (11, 65). For RSV, increasing the expression of the fusion (F) glycoprotein by repositioning its gene in the genome resulted in increased CPE in vitro, but no change in virulence was observed in mice (35). Also, RSV with a deletion in its M-2 regulatory protein showed a considerable increase in CPE in vitro but was highly attenuated in chimpanzees (6). The precise mechanism for the attenuation of recombinant virus in vivo requires further investigation.
The development of an immune response against one particular vector would probably make that vector ineffective for subsequent vaccination in the same individual. The prevalence of immunity to a vector, such as vaccinia virus or adenovirus type 5 vector virus, would restrict the replication of the recombinant virus vaccine, resulting in a reduced immune response to the expressed foreign antigen (31, 42, 54, 66). This does not seem to be a problem for the NDV vector, as most of the rabies vaccine target population, including cats and dogs, is seronegative for the NDV vector. Furthermore, the second dose induced sizeable boost immune responses, as has been documented with several other virus-vectored vaccines (9, 12, 23, 25, 59). For HPIV-3 and goat poxvirus-vectored vaccines, even interference from preexisting immunity against the vector virus could be completely overcome by two doses of vaccine (9, 12). Our results here also showed that a second dose of rL-RVG, received 3 weeks after the first dose, induced significant boost responses in cats and dogs. When a third dose was received 3 weeks after the second vaccination, it did not induce boost responses to either RVG or NDV, indicating that the second vaccination induced a sufficient immune response to prevent the replication of rL-RVG in the animals (data not shown). However, when the third dose was received 1 year later, it induced significant immune responses to both RVG and NDV, again indicating that the immunity to the NDV vector would not be strong enough to restrict the replication of recombinant viruses 1 year later. Moreover, when we used rL-RVG to immunize dogs that had received traditional live rabies vaccine or inactivated rabies vaccine 1 year earlier or used traditional live rabies vaccine or inactivated rabies vaccine to immunize the dogs that had received rL-RVG 1 year earlier, the RV VNAs always induced significant boost responses in all animals tested (data not shown).
rL-RVG grows to titers similar to those of rL in chicken eggs, which indicates that its manufacture is technically and economically feasible in developing countries. A dosage as low as 108.3 EID50, i.e., almost a 30-fold dilution of 1 ml of egg allantoic fluid, induced an acceptable and durable neutralization antibody response in dogs and provided complete protection from RV street virus challenge after 1 year. This is in contrast to some other vector systems, such as those based on replication-deficient adenoviruses, which require costly helper cell lines and high-dose immunizations.
NDV-based vaccines are generally administered via the respiratory tract when used to vaccinate against respiratory diseases of poultry (3). In nonhuman primates, the potency of an NDV-vectored vaccine via the respiratory tract route has been demonstrated (8, 15, 16, 18). Potency in bovines via both the respiratory tract and intramuscular routes has also been demonstrated (32, 34). It is interesting that the intratracheal but not the intranasal route is essential for the induction of humoral immunity in nonhuman primates (17). This phenomenon might be explained by the fact that NDV replicates poorly in the upper respiratory tract of mammals due to the lower temperature there compared with this site in birds (17). Although the intratracheal route is more likely to induce mucosal immune responses, in this study, we only tested the NDV-vectored vaccine via the intramuscular route, since this route should be more practicable than the intratracheal route for mass application in dogs and cats.
ACKNOWLEDGMENTS
We thank Susan Watson for editing the manuscript and Bernard Moss for providing the modified vaccinia virus strain Ankara expressing the T7 RNA polymerase.
This work was supported by the Chinese National S&T Plan (grant 2009ZX10004-214), by a grant from the Chinese Ministry of Agriculture (no. 200803014) and by the GHI program of Emory University.
Footnotes
Published ahead of print on 1 June 2011.
REFERENCES
- 1. Anonymous. 2009. Human vaccinia infection after contact with a raccoon rabies vaccine bait—Pennsylvania, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:1204–1207 [PubMed] [Google Scholar]
- 2. Alexander D. J. 1989. Newcastle disease. American Association for Avian Pathologists, Kennett Square, PA [Google Scholar]
- 3. Alexander D. J. 1997. Newcastle disease and other avian paramyxoviridae infections. Iowa State University Press, Ames, IA [Google Scholar]
- 4. Blach-Olszewska Z. 1970. Interferon induction by Newcastle disease virus in mice. Arch. Immunol. Ther. Exp. (Warsz.). 18:418–441 [PubMed] [Google Scholar]
- 5. Brehm G., Kirchner H. 1986. Analysis of the interferons induced in mice in vivo and in macrophages in vitro by Newcastle disease virus and by polyinosinic-polycytidylic acid. J. Interferon Res. 6:21–28 [DOI] [PubMed] [Google Scholar]
- 6. Buchholz U. J., et al. 2005. Deletion of M2 gene open reading frames 1 and 2 of human metapneumovirus: effects on RNA synthesis, attenuation, and immunogenicity. J. Virol. 79:6588–6597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bukreyev A., Collins P. L. 2008. Newcastle disease virus as a vaccine vector for humans. Curr. Opin. Mol. Ther. 10:46–55 [PubMed] [Google Scholar]
- 8. Bukreyev A., et al. 2005. Recombinant Newcastle disease virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J. Virol. 79:13275–13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bukreyev A., et al. 2007. Successful topical respiratory tract immunization of primates against Ebola virus. J. Virol. 81:6379–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bukreyev A., Skiadopoulos M. H., Murphy B. R., Collins P. L. 2006. Nonsegmented negative-strand viruses as vaccine vectors. J. Virol. 80:10293–10306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bukreyev A., et al. 2006. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J. Virol. 80:2267–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen W., et al. 2010. A goat poxvirus-vectored peste-des-petits-ruminants vaccine induces long-lasting neutralization antibody to high levels in goats and sheep. Vaccine 28:4742–4750 [DOI] [PubMed] [Google Scholar]
- 13. Dietzschold B., Schnell M., Koprowski H. 2005. Pathogenesis of rabies. Curr. Top. Microbiol. Immunol. 292:45–56 [DOI] [PubMed] [Google Scholar]
- 14. Dietzschold B., et al. 1985. Differences in cell-to-cell spread of pathogenic and apathogenic rabies virus in vivo and in vitro. J. Virol. 56:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DiNapoli J. M., et al. 2007. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc. Natl. Acad. Sci. U. S. A. 104:9788–9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DiNapoli J. M., et al. 2010. Newcastle disease virus-vectored vaccines expressing the hemagglutinin or neuraminidase protein of H5N1 highly pathogenic avian influenza virus protect against virus challenge in monkeys. J. Virol. 84:1489–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DiNapoli J. M., et al. 2009. Delivery to the lower respiratory tract is required for effective immunization with Newcastle disease virus-vectored vaccines intended for humans. Vaccine 27:1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DiNapoli J. M., et al. 2007. Immunization of primates with a Newcastle disease virus-vectored vaccine via the respiratory tract induces a high titer of serum neutralizing antibodies against highly pathogenic avian influenza virus. J. Virol. 81:11560–11568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Faber M., et al. 2009. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc. Natl. Acad. Sci. U. S. A. 106:11300–11305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faber M., et al. 2004. Identification of viral genomic elements responsible for rabies virus neuroinvasiveness. Proc. Natl. Acad. Sci. U. S. A. 101:16328–16332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flamand A., Raux H., Gaudin Y., Ruigrok R. W. 1993. Mechanisms of rabies virus neutralization. Virology 194:302–313 [DOI] [PubMed] [Google Scholar]
- 22. Fu Z. F. 1997. Rabies and rabies research: past, present and future. Vaccine 15:S20–S24 [DOI] [PubMed] [Google Scholar]
- 23. Gao W., et al. 2006. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 80:1959–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaudin Y., Ruigrok R. W., Knossow M., Flamand A. 1993. Low-pH conformational changes of rabies virus glycoprotein and their role in membrane fusion. J. Virol. 67:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ge J., et al. 2007. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J. Virol. 81:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ge J., et al. 2010. Generation and evaluation of a Newcastle disease virus-based H9 avian influenza live vaccine. Avian Dis. 54:294–296 [DOI] [PubMed] [Google Scholar]
- 27. Hanlon C. A., et al. 1998. First North American field release of a vaccinia-rabies glycoprotein recombinant virus. J. Wildl. Dis. 34:228–239 [DOI] [PubMed] [Google Scholar]
- 28. Huang Z., Elankumaran S., Yunus A. S., Samal S. K. 2004. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J. Virol. 78:10054–10063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang Z., Krishnamurthy S., Panda A., Samal S. K. 2003. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J. Virol. 77:8676–8685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiao P., et al. 2008. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 82:1146–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanesa-thasan N., et al. 2000. Safety and immunogenicity of NYVAC-JEV and ALVAC-JEV attenuated recombinant Japanese encephalitis virus-poxvirus vaccines in vaccinia-nonimmune and vaccinia-immune humans. Vaccine 19:483–491 [DOI] [PubMed] [Google Scholar]
- 32. Khattar S. K., Collins P. L., Samal S. K. 2010. Immunization of cattle with recombinant Newcastle disease virus expressing bovine herpesvirus-1 (BHV-1) glycoprotein D induces mucosal and serum antibody responses and provides partial protection against BHV-1. Vaccine 28:3159–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knobel D. L., et al. 2005. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 83:360–368 [PMC free article] [PubMed] [Google Scholar]
- 34. Kortekaas J., et al. 2010. Intramuscular inoculation of calves with an experimental Newcastle disease virus-based vector vaccine elicits neutralizing antibodies against Rift Valley fever virus. Vaccine 28:2271–2276 [DOI] [PubMed] [Google Scholar]
- 35. Krempl C., Murphy B. R., Collins P. L. 2002. Recombinant respiratory syncytial virus with the G and F genes shifted to the promoter-proximal positions. J. Virol. 76:11931–11942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krishnamurthy S., Huang Z., Samal S. K. 2000. Recovery of a virulent strain of Newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology 278:168–182 [DOI] [PubMed] [Google Scholar]
- 37. Lembo T., et al. 2010. The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS Negl. Trop. Dis. 4:e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Z., et al. 2006. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J. Virol. 80:11115–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lodmell D. L., Bell J. F., Moore G. J., Raymond G. H. 1969. Comparative study of abortive and nonabortive rabies in mice. J. Infect. Dis. 119:569–580 [DOI] [PubMed] [Google Scholar]
- 40. Lodmell D. L., Ewalt L. C., Parnell M. J., Rupprecht C. E., Hanlon C. A. 2006. One-time intradermal DNA vaccination in ear pinnae one year prior to infection protects dogs against rabies virus. Vaccine 24:412–416 [DOI] [PubMed] [Google Scholar]
- 41. Martinez-Sobrido L., et al. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J. Virol. 80:1130–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCoy K., et al. 2007. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 81:6594–6604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mehta S. R., et al. 2009. Early versus delayed invasive intervention in acute coronary syndromes. N. Engl. J. Med. 360:2165–2175 [DOI] [PubMed] [Google Scholar]
- 44. Nakaya T., et al. 2001. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 75:11868–11873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakaya Y., et al. 2004. Induction of cellular immune responses to simian immunodeficiency virus gag by two recombinant negative-strand RNA virus vectors. J. Virol. 78:9366–9375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neumann G., Whitt M. A., Kawaoka Y. 2002. A decade after the generation of a negative-sense RNA virus from cloned cDNA—what have we learned? J. Gen. Virol. 83:2635–2662 [DOI] [PubMed] [Google Scholar]
- 47. Osinubi M. O., et al. 2009. Enhancing comparative rabies DNA vaccine effectiveness through glycoprotein gene modifications. Vaccine 27:7214–7218 [DOI] [PubMed] [Google Scholar]
- 48. Panda A., Huang Z., Elankumaran S., Rockemann D. D., Samal S. K. 2004. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb. Pathog. 36:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park M. S., Garcia-Sastre A., Cros J. F., Basler C. F., Palese P. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522–9532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park M. S., Steel J., Garcia-Sastre A., Swayne D., Palese P. 2006. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc. Natl. Acad. Sci. U. S. A. 103:8203–8208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peeters B. P., de Leeuw O. S., Koch G., Gielkens A. L. 1999. Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 73:5001–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reed L. J., Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 53. Reynard O., et al. 2009. Ebolavirus glycoprotein GP masks both its own epitopes and the presence of cellular surface proteins. J. Virol. 83:9596–9601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharpe S., et al. 2001. Induction of simian immunodeficiency virus (SIV)-specific CTL in rhesus macaques by vaccination with modified vaccinia virus Ankara expressing SIV transgenes: influence of pre-existing anti-vector immunity. J. Gen. Virol. 82:2215–2223 [DOI] [PubMed] [Google Scholar]
- 55. Smith J. S., Yager P. A., Baer G. M. 1973. A rapid reproducible test for determining rabies neutralizing antibody. Bull. World Health Organ. 48:535–541 [PMC free article] [PubMed] [Google Scholar]
- 56. Tao L., et al. 2010. Molecular basis of neurovirulence of flury rabies virus vaccine strains: importance of the polymerase and the glycoprotein R333Q mutation. J. Virol. 84:8926–8936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Veits J., et al. 2006. Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc. Natl. Acad. Sci. U. S. A. 103:8197–8202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wandeler A. I., Capt S., Kappeler A., Hauser R. 1988. Oral immunization of wildlife against rabies: concept and first field experiments. Rev. Infect. Dis. 10(Suppl. 4):S649–S653 [DOI] [PubMed] [Google Scholar]
- 59. Weingartl H. M., et al. 2006. Recombinant Nipah virus vaccines protect pigs against challenge. J. Virol. 80:7929–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilde H., et al. 2005. Rabies control in South and Southeast Asia. Vaccine 23:2284–2289 [DOI] [PubMed] [Google Scholar]
- 61. World Health Organization 2005. WHO expert consultation on rabies. First report. WHO Technical Report Series 931. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 62. Reference deleted.
- 63. Wu X., Rupprecht C. E. 2008. Glycoprotein gene relocation in rabies virus. Virus Res. 131:95–99 [DOI] [PubMed] [Google Scholar]
- 64. Wyatt L. S., Moss B., Rozenblatt S. 1995. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology 210:202–205 [DOI] [PubMed] [Google Scholar]
- 65. Yang L., et al. 2008. A paramyxovirus-vectored intranasal vaccine against Ebola virus is immunogenic in vector-immune animals. Virology 377:255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang Z. Y., et al. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao L., et al. 2010. Expression of MIP-1alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J. Virol. 84:9642–9648 [DOI] [PMC free article] [PubMed] [Google Scholar]