Abstract
Vaccine-induced memory T cells localized at mucosal sites can provide rapid protection from viral infection. All-trans-retinoic acid (ATRA) has been shown to act physiologically to induce the expression of gut-homing receptors on lymphocytes. We tested whether the administration of exogenous ATRA during a systemic vaccination of mice could enhance the generation of mucosal CD8+ T cell immunity, which might represent a strategy for establishing better protection from viral infection via mucosal routes. ATRA induced the expression of CCR9 and α4β7 on both mouse and human CD8+ T cells activated in vitro. The administration of ATRA to mice during in vivo priming with a replication-defective recombinant adenovirus vector expressing the lymphocytic choriomeningitis virus glycoprotein (LCMVgp) (Ad5gp) increased numbers of both effector and memory T cells in intestinal mucosal tissues and showed higher frequencies of systemic central memory-like T cells that exhibited enhanced proliferation during boosting immunization with recombinant modified vaccinia virus Ankara expressing LCMVgp (MVAgp). Mice that received ATRA during Ad5gp vaccination were more resistant to intravaginal challenge by recombinant vaccinia virus expressing LCMVgp (VVgp), reflecting in part stronger T cell recall responses in situ. Thus, ATRA appears to be useful as an adjuvant during vaccination to increase memory T cell responses and protection from viral infection at mucosal sites and may facilitate the development of more effective vaccines against mucosally transmitted pathogens such as HIV.
INTRODUCTION
Mucosal immunity provides a first line of defense against environmental pathogens. Virus-specific CD8+ T cells localized at mucosal sites can recognize antigens (Ags) and become rapidly activated to eliminate infected cells through direct cytolysis and inhibit virus replication by cytokine secretion, while systemic responses can reduce or eliminate virus that evades mucosal control. A vaccine designed to prevent viral transmission via mucosal sites would optimally induce both systemic and mucosal T cell immunity (2, 42). Current approaches to systemic vaccination generally induce limited mucosal responses. To promote mucosal T cell responses, vaccine strategies being investigated include mucosal delivery systems or the use of adjuvants that program responding T cells to home to mucosal sites (22). The mucosal delivery of vaccines to mimic the natural pathway of pathogen transmission has the potential to preferentially induce mucosal responses but can be compromised by a limited induction of systemic responses and the possibility of tolerance induction (22, 42). The addition of adjuvants such as cholera toxin to vaccines to instruct T cells primed systemically to migrate to mucosal sites might bypass these obstacles, but such strategies can be associated with toxicities that must be resolved prior to general use with human vaccines (23, 44).
The administration of all-trans-retinoic acid (ATRA), the major metabolic derivative of vitamin A, has a proven record of safety in the clinical treatment of acne (5, 15, 30) and some hematological malignancies (62). ATRA signals through its broadly expressed nuclear retinoic acid receptors (RARα, -β, and -γ) and can have pleiotropic effects on the development and differentiation of numerous cell types, including innate and adaptive immune cells (4, 12, 13, 27, 38–41, 57, 59). Dendritic cells (DCs) from gut-associated tissues, including mesenteric lymph nodes (LN) (MLN), lamina propria (LP), and Peyer's patches (PP), express the enzymes necessary to convert vitamin A into ATRA (25, 27, 59). ATRA can enhance T cell proliferation (12) and upregulate the expression of gut-homing receptors on T cells in vitro (25, 28, 40, 57). CD4+ and CD8+ T cells primed in vitro in the presence of ATRA or DCs isolated from intestinal sites express high levels of the integrin α4β7 and the chemokine receptor CCR9, which, upon binding to MAdCAM-1 and CCL25, respectively, mediate the migration of T cells to gut mucosa (25, 28, 53).
Based on these safety and activity profiles of ATRA, we reasoned that the provision of ATRA during vaccination might augment the ability of T cell-based viral vaccines to promote the gut/mucosal homing of CD8 T cells in order to provide increased protection from mucosal viral challenge. We found that antigen-specific T cells primed in vivo by vaccination in the presence of ATRA exhibited increased accumulation at mucosa-associated sites. Surprisingly, the provision of ATRA during T cell priming in vivo also resulted in the formation of more vaccine-specific central memory-like CD8+ T cells in systemic sites, and these cells were able to proliferate better during recall responses and provided enhanced protection from viral challenge via vaginal mucosal sites. These data indicate that ATRA may be useful as an adjuvant for vaccines against mucosally transmitted viruses.
MATERIALS AND METHODS
Mice and infection.
Approximately 6- to 8-week-old female C57BL/6J (B6) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Thy1.1+ P14 mice were provided by Kaja Murali-Krishna at the University of Washington (UW) (Seattle, WA). All mice were handled at the Fred Hutchinson Cancer Research Center (FHCRC) (Seattle, WA) according to the protocols approved by the IACUC. Recombinant Ad5 expressing the lymphocytic choriomeningitis virus glycoprotein (LCMVgp) (Ad5gp) and modified vaccinia virus Ankara (MVA)-LCMVgp (MVAgp) were provided by Julie McEralth (FHCRC), and vaccinia virus (VV) LCMVgp (VVgp) was provided by K. Murali-Krishna (UW). Mice were immunized intramuscularly (i.m.) in the quadriceps with Ad5gp (5 × 108 PFU) or intraperitoneally (i.p.) with MVAgp (1 × 107PFU). Intravaginal (i.vag.) infection with VVgp (6 × 106 PFU or 3 × 107 PFU) was performed on anesthetized mice approximately 4 to 6 days after receiving 3 mg of medroxy-progesterone (Sicor Pharmaceuticals Inc., Irvine, CA) i.m. for menstrual synchronization.
Reagents.
All-trans-retinoic acid (ATRA) (Sigma-Aldrich, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) at 40 mg/ml and stored as aliquots in the dark at −80°C. A working solution of ATRA (3 mg/ml) was prepared by dilution into soybean oil, and an equal volume of DMSO mixed in soybean oil was used as a vehicle control. One dose of ATRA at 300 μg/100 μl or vehicle was delivered through i.p. injection into mice on day −1 and day +1 during Ad5gp vaccination. FTY-720 (Cayman Chemical, Ann Arbor, MI) was dissolved in ethanol at 20 mg/ml and stored as aliquots at −80°C. A working solution of FTY-720 (200 μg/ml) was prepared by dilution into 50% ethanol plus 50% phosphate-buffered saline (PBS), and a daily dose of FTY-720 at 20 μg/100 μl or control dilution buffer was delivered i.p. into mice for 4 days. Fluorochrome-conjugated anti-mouse monoclonal antibodies (Abs) CD4 (RM4-5), CD8α (53-6.7), CD11c (HL3), CD80 (16-10A1), CD86 (GL1), CD83 (Michel-17), 2B4 (CD244), α4β7 (DATK32), Thy1.1 (OX-7), Thy1.2 (53-2.1), tumor necrosis factor (TNF) (MP6-XT22), gamma interferon (IFN-γ) (XMG1.2), and interleukin-2 (IL-2) (JES6-5H4) were purchased from BD Pharmingen; granzyme B (16G6), CD62L (MEL-14), I-A/I-E (M5/114.15.2), CCR7 (4B12), CCR9 (CW-1.2), and CD103 (2E7) were purchased from eBiosciences; CD44 (IM7) was purchased from BioLegend; and KLRG1 (2F1) was purchased from Southern Biotech (Birmingham, AL). The immune-monitoring laboratory at FHCRC provided the gp33/Db tetramer. The recombinant human cytokines IL-2, IL-7, IL-15, IL-1β, TNF-α, and IL-6 were purchased from R&D Systems Inc. (Minneapolis, MN); prostaglandin E2 (PGE2) was purchased from MP Biomedicals (Solon, OH); and poly(I:C) was purchased from InvivoGen (San Diego, CA). The Melan-A26–35 (A*0201-restricted) variant peptide (ELAGIGILTV) and the allophycocyanin (APC)-conjugated pentamer were obtained from ProImmune (Oxford, United Kingdom); anti-human CD8 (RPA-T8) was obtained from BD Pharmingen; and anti-human CCR9 (BL/CCR9), anti-human CD27 (O323), anti-human α4 integrin (9F10), and anti-human β7 integrin (FIB504) antibodies were obtained from BioLegend.
In vitro stimulation of mouse DCs and T cells.
DCs were isolated from naïve mice by Pan DC MicroBeads (Miltenyi Biotec Inc., Auburn, CA), cultured at 5 × 105 cells/well with 1:10 serial dilutions of ATRA for 22 h or 44 h, washed extensively before pulsing with 1 μM LCMVgp33 peptide (KAVYNFATM) for 1 h, and washed twice before irradiation at 1,000 Gy. Naïve splenic P14 cells were negatively selected for CD8α+ cells, labeled with CFSE (carboxyfluorescein succinimidyl ester; Sigma), and cultured at 1 × 106 cells/well with Ag-pulsed DCs for approximately 4 to 6 days. Alternatively, naïve P14 cells were cultured with congenic splenocytes to reach ∼4% CD8+ cells in the presence of 1 μM gp33 peptide and serial dilutions of ATRA or DMSO for 2 days before being replaced with medium containing ATRA or DMSO for approximately 2 to 4 days.
Human T cell stimulation.
Peripheral blood mononuclear cells (PBMC) from healthy HLA-A*0201+ blood donors were harvested by leukapheresis following informed consent and cryopreserved. Human monocyte-derived DCs were differentiated and then matured with classic maturation mix (CMM) (including 10 ng/ml IL-1β, 10 ng/ml TNF-α, 1 μg/ml PGE2, and 10 ng/ml IL-6) or 200 μg/ml poly(I:C) (Toll-like receptor 3 [TLR3] adjuvant) overnight as described previously (21). Naïve CD8+ T cells were isolated by negative selection and stimulated for 3 days with anti-CD3/CD28 beads (Invitrogen Dynal, Oslo, Norway) or primed with autologous DCs pulsed with Melan-A peptide (10 μg/ml). Anti-CD3/CD28-stimulated T cells were cultured in medium containing 50 IU/ml recombinant human IL-2 (rhIL-2) together with 100 nM ATRA or DMSO as a vehicle control starting on day 2, Melan-A-primed T cells were cultured in medium containing 5 ng/ml rhIL-7 and 5 ng/ml rhIL-15 together with 100 nM ATRA or the DMSO control starting on day 3 following stimulation/priming, and the medium was replenished every 2 days for the duration of culture for 7 to 9 days. Cells were harvested and stained for human CD8, CD27, CCR9, α4 integrin, β7 integrin, and the HLA-A*0201/Melan-A pentamer for fluorescence-activated cell sorter (FACS) analysis.
Lymphocyte preparation.
Spleen, inguinal LN (iLN), genital-associated LN, mesenteric LN, and Peyer's patches (PP) were mechanically disrupted and filtered through a 40-μm nylon mesh. After the removal of PP and fat tissues, flushed small intestines were cut into small pieces and treated with 1 mM dithioerythritol (DTE) (CalBioChem, San Diego, CA) for 20 min twice. Cells in the supernatant were combined as crude intraepithelial lymphocytes (IEL). The remaining tissues were incubated with 1.3 mM EDTA for 30 min, digested with 100 IU/ml collagenase IV (Gibco, Invitrogen, Grand Island, NY) for 1 h, and passed through a cell strainer as crude LP. Vagina (cervical and vaginal tissues), ovaries, and genital tract (fallopian tubes, uterus, and ovaries) were digested with collagenase IV. Crude preparations were purified through 44% to 67% Percoll (GE Healthcare, Piscataway, NJ) before analysis on an LSRII or FACs-Canto II instrument (BD Biosciences, San Jose, CA). Flow data were analyzed with FlowJo8.8 (Tree Star Inc., Ashland, OR). Graphs were made with Prism5 software (GraphPad Software, La Jolla, CA), with the paired or unpaired Student t test and log-rank test of survival curves for statistical analysis. In addition to P values as indicated, statistical significance of differences are also shown in Fig. 5.
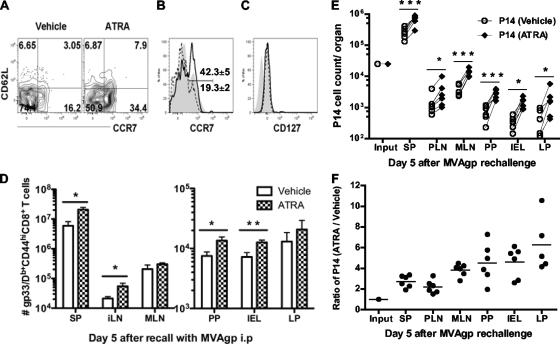
Fig. 5.
ATRA increases the frequency of central memory T cells in spleen, which exhibit greater proliferation during the recall response. (A to C) Naïve B6 mice were given ATRA or vehicle during immunization with Ad5gp, and memory cells from the spleen were screened on day 38 for flow plots of CD62L and CCR7 expression (A) and histogram overlaps of CCR7 expression (B) on gated gp33/Db tetramer-positive CD44hi CD8α+ cells and histogram overlap of CD127 expression (C) on gated CD62L+/− CCR7+ gp33/Db tetramer-positive CD44hi CD8α+ cells from vehicle-treated (V) (dashed line) or ATRA-treated (A) (solid line) mice. Filled gray areas in panels B and C represent total numbers of CD8+ T cells in spleen. Numbers in panel B represent the mean frequencies of CCR7+ cells. (D) Mice were boosted on day 38 with MVAgp and analyzed 5 days later for the total numbers of gp33-specific CD44hi CD8+ T cells in various tissues. (E and F) To compare directly the effects of ATRA and vehicle on systemic memory cells, memory P14 cells were sorted from spleens of both groups on day 35, cotransferred into naïve mice followed by immunization with MVAgp, and evaluated on day 5 postboosting for the numbers of each type of donor cell in various tissues (E) and the relative ratio of P14 cells (ATRA-treated donor cells/vehicle control) (F). The lines between dots in panel E connect each individual mouse to the results with P14 cells derived from the ATRA-treated and vehicle donors. Paired (E) and unpaired (D) Student t tests and one-way ANOVA (F) were performed on the data. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Adoptive transfer of P14 cells.
Naïve splenic Thy1.1+ P14 cells were negatively selected for CD8α+ T cells, and 1.5 × 103 CD8+ P14 cells were adoptively transferred into congenic Thy1.2+ mice 2 days before immunization in the presence of ATRA or vehicle. Five weeks later, splenic memory P14 cells (CD44hi Thy1.1+ CD8+) were sorted by FACS analysis and mixed at a 1:1 ratio (25,000 cells each) for secondary transfer into naïve Thy1.2+ mice followed by MVAgp immunization. Ex vivo-cultured cells (Thy1.1+ Thy1.2− P14 cells exposed to ATRA and Thy1.1+ Thy1.2+ P14 cells exposed to DMSO) were also negatively selected for CD8α+ live T cells, either cotransferred at a 1:1 ratio (1 × 106 cells each) or transferred separately (2.5 × 106 or 5 × 106 cells) into naïve congenic Thy1.2+ mice, followed by influenza virus challenge.
Intracellular staining.
Lymphocytes were cultured with 1 μM LCMVgp33 peptide in the presence of 1 μg/ml GolgiPlug (BD Pharmingen, San Diego, CA) at 37°C for 5 h, washed, and stained for surface markers before fixation in 2% paraformaldehyde. Cell membranes were permeabilized in Cytofix/Cytoperm, washed with 1× Cytoperm/Cytowash buffer (BD Pharmingen, San Diego, CA), and stained with fluorochrome-conjugated Abs for FACS analysis.
Virus plaque assay.
Tissues were harvested from euthanized mice, homogenized, and centrifuged at 2,000 rpm for 10 min. The supernatants were 1:10 serially diluted in Dulbecco's modified Eagle's medium (DMEM) and added onto BSC-40 monolayers for 1 h at 37°C. The cells were then cultured in complete DMEM containing 5% fetal calf serum (FCS) for 2 days before staining with 0.1% crystal violet solution for counting viral PFU.
RESULTS
ATRA modulates DC maturation in vitro and enhances priming of T cells with increased expression of mucosa-homing markers.
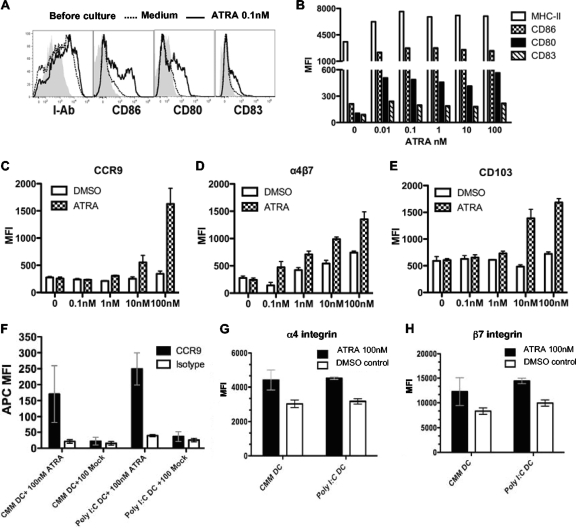
ATRA can potentially have effects on both antigen-presenting cells and T cells. To explore the direct effect of ATRA on mouse DCs, DCs isolated from naïve C57BL/6 mice were cultured with ATRA for 2 days and monitored for the expression of surface markers. ATRA at a wide range of doses induced modest but statistically significant upregulations of maturation markers, including major histocompatibility complex class II (MHC-II), CD86, CD80, and CD83, on splenic DCs (Fig. 1A and B). However, the preculture of DCs with low doses of ATRA (<1 nM) had little impact on T cell activation (data not shown). Therefore, to examine the combined effect of ATRA during activation on mouse T cells and DCs, we cultured naïve LCMVgp33-specific T cell receptor (TCR) transgenic CD8+ T cells from P14 mice with gp33 peptide-pulsed splenic DCs in the presence of ATRA or DMSO as a vehicle control. At ATRA doses of ≥10 nM, levels of CCR9, α4β7, and CD103 were increased on responding P14 cells (Fig. 1C, D, and E), whereas cell proliferation was not significantly altered compared to that of control P14 cells (data not shown). Consistent with data from previous in vitro studies (25, 28, 53), our data indicate that providing ATRA during in vitro immune responses can enhance the expression of mucosal homing/adhesion molecules on Ag-specific T cells primed with DCs from lymphoid organs other than mucosa-associated tissues.
Fig. 1.
ATRA enhances expression of mucosal homing markers on T cells primed in vitro. (A and B) Naïve mouse splenic DCs were cultured with ATRA for 44 h, and gated live DCs were assessed for histograms (A) and mean fluorescent intensities (MFI) (B) of surface MHC-II, CD86, CD80, and CD83. Gray area, before culture; dashed line, medium alone; solid line, 0.1 nM ATRA. (C to E) Assay of the effect of ATRA on mouse T cells. Naïve gp33-specific TCR transgenic P14 cells were diluted into naïve B6 splenocytes to reach 4% of the total amount of CD8+ T cells; cultured in gp33 peptide, titrated ATRA, or the DMSO control for 2 days; and then replaced with medium containing ATRA or DMSO. Gated live tetramer-positive CD8+ T cells were analyzed on day 4 for MFI of CCR9 (C), α4β7 (D), and CD103 (E). (F to H) Assay of the effect of ATRA on human T cells. Naïve human CD8+ T cells isolated from PBMC of healthy HLA-A*0201 donors were primed in the presence of ATRA or the DMSO control with Melan-A peptide-pulsed human monocyte-derived DCs that had been matured with classic maturation mix (CMM) or poly(I:C) (TLR3 ligand), and gated live Melan-A pentamer-positive CD27+ CD8+ T cells were analyzed on day 9 for MFI of CCR9 (F), α4 (G), and β7 integrin (H) on anti-α4 and anti-β7 dual-positive CD8+ T cells. Data represent means ± standard errors of the means (SEM) of data from two repeated experiments with triplicates/sample/experiment.
As ATRA has also been shown to induce human DC maturation and enhance DC Ag presentation and the secretion of cytokines, including TNF and IL-12 (13, 38), we wanted to determine if ATRA had similar effects on human antigen-specific T cells during in vitro activation. We have previously shown that healthy HLA-A*0201+ humans contain an HLA-A*0201-restricted Melan-A-specific naïve CD8+ T cell population that constitutes ∼0.1 to 0.5% of total CD8+ T cells, making it possible to track the priming of a human antigen-specific T cell response in vitro (21). ATRA has been shown to induce mucosal homing molecules on human T cells during activation via allogeneic tissue-derived DCs (11), suggesting that this might also occur in response to cognate antigen. Therefore, we cultured CD8+ T cells isolated from the peripheral blood of healthy HLA-A*0201+ donors and stimulated these cells in vitro with either anti-CD3/CD28 beads or Melan-A-pulsed autologous human monocyte-derived DCs matured with classic cytokine maturation mix (CMM) (which includes IL-1β, TNF-α, PGE2, and IL-6) or poly(I:C) (TLR3 ligand). In the presence of ATRA (100 nM), we observed significantly increased expression levels of CCR9 and the α4 and β7 integrin chains on both Melan-A peptide-activated (Fig. 1F, G, and H) and CD3/CD28-activated (see Fig. S1A, S1B, S1C, and S1D in the supplemental material) human CD8+ T cells. Due to the absence of a commercially available antibody that recognizes the heterodimer of human α4β7, two separate Abs were used to evaluate the expression of the integrin chains α4 and β7. Although the costaining of anti-α4 and anti-β7 Abs on responding CD8+ T cells does not necessarily mean that these molecules exist exclusively as a heterodimer, since both integrins can combine with other molecules, the expression of both chains of the integrin were enhanced by exposure to ATRA (Fig. 1G and H and see Fig. S1C and S1D in the supplemental material). Thus, our data suggest that ATRA has an effect in vitro on responding human T cells similar to that observed with mouse T cells, making findings with in vivo murine models assessing ATRA as an adjuvant for the promotion of mucosal responses potentially translatable to studies with similar vaccination strategies for humans.
T cells activated in the presence of ATRA home preferentially to mucosal sites.
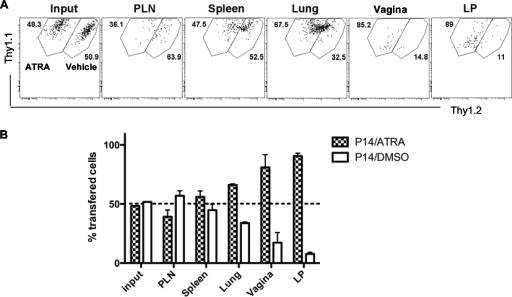
To provide a barrier to virus infection via mucosal surfaces, activated T cells must home to these sites. To determine if the ATRA-induced upregulation of homing/adhesion molecules on ex vivo-activated T cells had any impact on their migration properties in vivo, congenically marked P14 cells were activated by gp33 peptide-pulsed splenocytes in the presence (Thy1.1+ Thy1.2−) or absence (Thy1.1+ Thy1.2+) of 100 nM ATRA for 4 days, followed by the cotransfer of equal numbers of purified CD8+ Thy1.1+ Thy1.2− P14 cells and CD8+ Thy1.1+ Thy1.2+ P14 cells into Thy1.2+ recipient mice that had been intramuscularly immunized 1 day earlier with an “empty” Ad5 viral vector to induce inflammation. On day 2.5 posttransfer, we observed increased numbers of Thy1.1+ Thy1.2− P14 cells in mucosal sites, including the small intestine lamina propria, vaginal tissues, and, to a lesser extent, lung tissues, compared to those of cotransferred Thy1.1+ Thy1.2+ cells activated in the absence of ATRA (Fig. 2), indicating a mucosal homing advantage for ATRA-modulated T cells.
Fig. 2.
T cells activated in vitro in the presence of ATRA migrate preferentially to mucosal tissues. Naïve P14 cells were stimulated with gp33-41 peptide in the presence of 100 nM ATRA (for Thy1.1+ 1.2− P14 cells) or DMSO (for Thy1.1+ 1.2+ P14 cells) for 4 days and negatively selected for CD8+ T cells followed by 1:1 cotransfer (1 × 106 P14 cells each) into Thy1.2+ mice on day 1 after intramuscular immunization with an empty Ad5 viral vector (to induce inflammation in the host). The tissue migration of P14 cells treated with ATRA versus P14 cells treated with DMSO was examined on gated Thy1.1+ CD8+ T cells, shown as representative flow plots (A) and bar graphs (B), on day 2.5 posttransfer. Data represent results from 6 mice and are shown as means ± SEM.
ATRA provision during systemic priming promotes T cell migration to mucosal tissues.
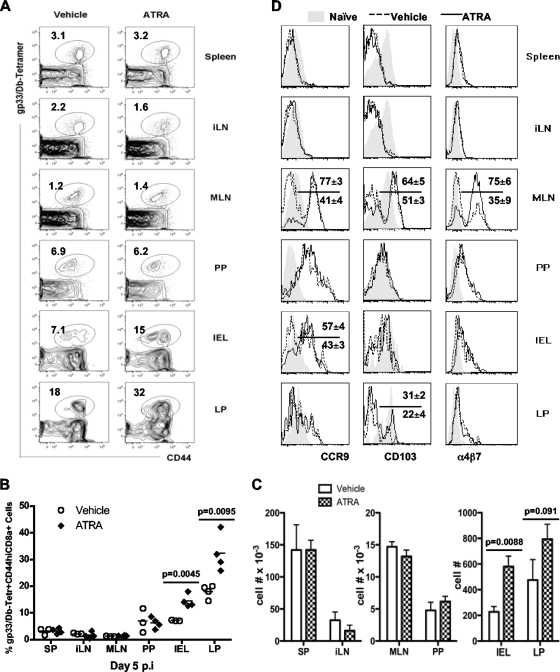
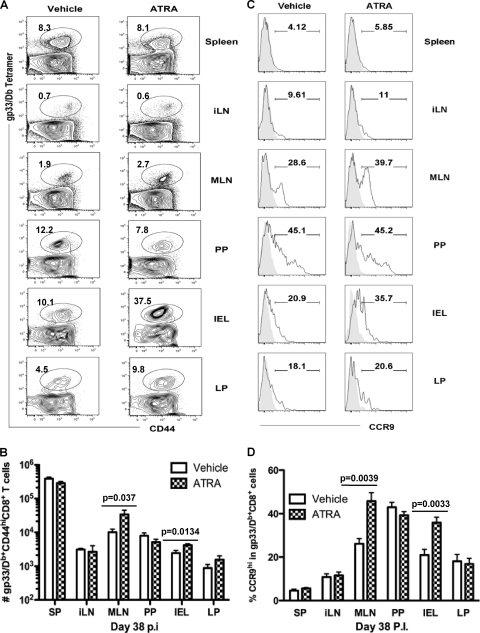
We next sought to understand if the provision of ATRA during vaccination in vivo could enhance endogenous CD8+ T cell responses at mucosal sites; 300 μg of ATRA or the vehicle control was administered i.p. to naïve mice on day −1 and day +1 during intramuscular priming on day 0 with a replication-deficient recombinant adenoviral vector expressing the LCMVgp33 epitope as a model antigen (Ad5gp). The kinetics of gp33-specific CD8+ T cells reached a peak at days 7 to 8 postimmunization with Ad5gp, followed by a contraction phase, depicting a typical acute course of a T cell response. Effector cells from various tissues on day 5 postinfection (p.i.) and day 7 p.i. were evaluated (Fig. 3 and data not shown). ATRA provision during priming did not significantly alter the phenotype of vaccine-induced CD8+ T cells in the spleen and inguinal lymph node (iLN), as reflected by similar levels of expression of CD27, 2B4, CD62L, CCR7, and KLRG1 on gp33-specific CD44hi CD8+ T cells from ATRA-treated and control mice (data not shown). ATRA had no apparent effect on the function of CD8+ T cells isolated from the spleen and iLN, as reflected by similar levels of production of the cytokines IFN-γ, TNF, and IL-2 and expression of granzyme B in control cells (data not shown). ATRA provision during T cell priming in vivo did not significantly alter the magnitude of vaccine-induced CD8+ T cells in systemic sites such as the spleen and iLN. However, ATRA administration during priming did result in an altered tissue distribution of effector CD8+ T cells, with higher frequencies (Fig. 3A and B) and a concomitant increase in absolute numbers (Fig. 3C) of gp33-specific CD8+ T cells detected in the small intestinal epithelium (IEL) (14.5% ± 2.4% versus 7.1% ± 0.2%; P = 0.0045) and LP (32.3% ± 7.1% versus 18% ± 2.5%; P = 0.0095).
Fig. 3.
ATRA administration during priming increases the frequency of effector CD8+ T cells at mucosal sites. Naïve B6 mice were immunized with Ad5gp and received ATRA or vehicle on day −1 and day +1. Responses were assessed by gating on CD8+ T cells in tissues on day 5 for staining of the gp33 tetramer versus CD44 expression (A), measuring frequencies (B) and cell numbers (C) of gp33-specifc cells, and analyzing CCR9, CD103, and α4β7 expressions (D). Numbers in panel A represent mean values of gated cells. In panel D, the dashed line (vehicle treated) and solid line (ATRA treated) represent gp33/Db tetramer-positive CD44hi CD8α+ T cells, and the filled area indicates CD44low CD8α+ T cells derived from the same tissues. Numbers in D represent means ± SEM of gated cells in ATRA-treated (above the line) and vehicle-treated (below the line) mice. Data represent results from one of two repeated experiments with 4 mice/group/experiment.
We next examined the impact of ATRA during priming on the expressions of CCR9, CD103, and α4β7, surface molecules known to be involved in the homing/localization of lymphocytes to mucosal sites (6, 8, 32, 49, 58). The expressions of CCR9, CD103, and α4β7 on responding T cells in the spleen and iLN were not significantly different between ATRA-treated and control mice. However, the provision of ATRA during vaccination/priming did result in altered expression levels of these molecules on responding T cells in mucosal sites, as shown by the increases in the percentages of CCR9hi effector CD8+ T cells in MLN (77% ± 3% versus 41% ± 4%; P = 0.002) and IEL (57% ± 4% versus 43% ± 3%; P = 0.033) from ATRA-treated mice compared to those in controls (Fig. 3D). In addition, more responding T cells in ATRA-treated mice expressed CD103 in the MLN (64% ± 5% versus 51% ± 3%; P = 0.041) and LP (31% ± 2% versus 22% ± 4%; P = 0.038), whereas the α4β7 expression level was increased on responding T cells from MLN in ATRA-treated mice (75% ± 6% versus 35% ± 9%; P = 0.001) (Fig. 3D).
ATRA provision during priming promotes accumulation of effector memory T cells at mucosal sites.
To determine if the provision of ATRA during T cell priming impacted the number of resident memory T cells at mucosal sites, we quantitated mucosal memory T cells in mice given ATRA or the vehicle control during Ad5gp immunization. On day 38 p.i., the frequencies of gp33-specific memory CD8+ T cells in spleen and iLN were similar between ATRA-treated and control mice (Fig. 4A). However, more gp33-specific CD44hi memory CD8+ T cells were detected in MLN (P = 0.037) and IEL (P = 0.0134) from ATRA-treated mice (Fig. 4A and B). Moreover, T cells isolated from these sites were predominantly CD62L−, suggesting that these were primarily effector memory T (TEM) cells (data not shown). While the expressions of CCR9 and α4β7 on most memory CD8+ T cells in spleen and iLN had returned to levels similar to those observed for naïve cells, a higher proportion of CCR9high memory cells in gut-associated tissues was found for mice that had received ATRA during T cell priming (39.7% versus 28.6% for MLN [P = 0.039] and 35.7% versus 20.9% for IEL [P = 0.0033]) (Fig. 4C and D). We also observed less-pronounced increases in the expression levels of α4β7 and CD103 in ATRA-treated mice (data not shown). These results suggest that exposure to ATRA during T cell priming may “program” memory CD8+ T cells to be retained at mucosal sites in part due to the regulation of expression of homing/adhesion molecules, although other undefined factors also likely contribute to the observed mucosal accumulation of T cells.
Fig. 4.
ATRA increases numbers of memory CD8+ T cells at mucosal sites in primed mice. Tissues were harvested from C57BL/6 mice on day 38 post-Ad5gp vaccination in the presence of vehicle or ATRA and analyzed for CD44 and tetramer binding of the gated CD8+ T cells (A), total numbers of gp33-specifc memory CD8+ T cells (B), histograms of CCR9 expression (C), and frequencies of CCR9hi cells in gated gp33/Db+ CD44hi CD8+ T cells. The filled area in panel C indicates CD44low CD8α+ T cells from the same tissues. Numbers in panels A and C represent mean values for 3 to 5 mice in each group from one of two repeated experiments.
ATRA provision during priming results in more systemic central memory T cells with greater proliferative potential upon the recall response.
To determine if ATRA impacted memory T cell differentiation in sites other than mucosal tissues, we analyzed the phenotype of splenic memory CD8+ T cells at 5 weeks post-Ad5gp vaccination in the presence of ATRA or the vehicle control. The frequencies of both CD62L+ CCR7+ (7.9% ± 2.1% versus 3.1% ± 1.6%; P = 0.024) and CD62L− CCR7+ (34.4% ± 3.7% versus 16.2% ± 2.2%; P = 0.001) memory cells were increased in ATRA-treated mice (Fig. 5A). Furthermore, these CD62L+/− CCR7+ memory T cells also expressed CD127, a marker for central memory T cells (Fig. 5B and C). Thus, ATRA during T cell priming results in a skewing of memory populations to CD62L+/− CCR7+ CD127+ central memory-like cells (42.3% ± 5.1% versus 19.3% ± 2.3%; P = 0.001) (Fig. 5B and C).
Since CCR7 has been shown to be a marker for central memory T cells, which typically exhibit increased proliferative recall responses to antigen (46, 47), we next examined if this increase in numbers of CCR7+ cells in ATRA-treated mice resulted in better T cell recall responses during boosting. Therefore, mice given ATRA or the vehicle control during T cell priming were boosted with modified vaccinia virus Ankara expressing the same LCMVgp antigen (MVAgp) on day 38, when memory was established. We observed an increase in total numbers (Fig. 5D) of gp33-specific CD44hi CD8+ T cells in the spleen (P = 0.034), iLN (P = 0.031), PP (P = 0.038), and IEL (P = 0.012) from mice that received ATRA during initial T cell priming (compared to controls), indicating enhanced secondary T cell responses in both systemic and mucosal sites.
The differences in the abilities of memory T cells to respond in mice that had received ATRA versus the control during priming could be impacted by different rates of Ag clearance or other factors such as CD4+ T cell help. Therefore, we next sought to directly compare the recall responses of the induced memory CD8+ T cells primed in the presence of ATRA with those in the absence of ATRA. Memory T cells were generated by the adoptive transfer of 1,500 congenically marked naïve P14 cells into naïve recipient mice followed by priming with Ad5gp in the presence of ATRA or the vehicle control. Five weeks later, memory P14 cells were sorted from spleens of mice from each group and cotransferred into naïve mice at a 1:1 ratio (25,000 cells each), followed by i.p. immunization with MVAgp. The Thy1 congenic marker was used to differentiate memory P14 cells isolated from mice that received ATRA during priming (Thy1.1/1.1) from those isolated from control mice (Thy1.1/1.2) and from endogenous host responses (Thy1.2/1.2). On day 5 after boosting immunization, memory P14 cells from mice that received ATRA during priming mounted enhanced recall responses to antigen, as evidenced by the higher number of Thy1.1/1.1 P14 cells than control memory cells in all tissues examined (Fig. 5E). Moreover, the ratio of ATRA-primed P14 cells to control P14 cells appeared highest in gut-associated tissues (P = 0.0009 by one-way analysis of variance [ANOVA]) (Fig. 5F), suggesting that systemic memory cells primed in the presence of ATRA not only have a greater proliferative potential but also may be imprinted for enhanced migration to mucosal tissues during recall responses.
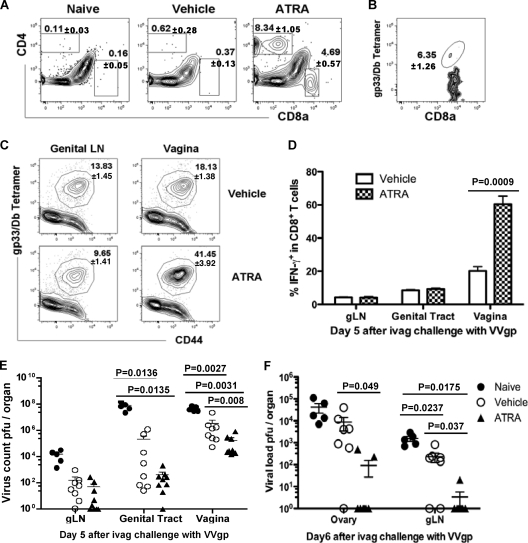
ATRA provision during priming leads to enhanced T cell responses and better protection from vaginal viral challenge.
Since ATRA-modulated in vitro-activated P14 cells migrated preferentially to mucosal sites, including intestinal and cervical/vaginal tissues after adoptive transfer (Fig. 2A and B), and more CD4+ (8.3% ± 1.1% versus 0.6% ± 0.3%; P = 0.002) and CD8+ (4.7% ± 0.6% versus 0.4% ± 0.1%; P = 0.003) infiltrating T cells that included Ag-specific CD8+ T cells were detected in the vagina of ATRA-treated mice following Ad5gp vaccination (Fig. 6A and B), we questioned whether T cell recall responses would be altered in those mice during vaginal viral infection. Mice were treated with ATRA or vehicle during Ad5gp vaccination, and 8 weeks later they were challenged intravaginally with a replication-competent recombinant vaccinia virus expressing the LCMVgp antigen (VVgp). On day 5 postchallenge, gp33-specific CD8+ T cell recall responses were significantly increased in the vagina of mice that received ATRA during initial priming compared to control mice (41.5% ± 3.9% versus 18.1% ± 1.4%; P = 0.0045) (Fig. 6C). The enhanced CD8+ T cell responses at vaginal sites were also reflected by IFN-γ production (P = 0.0009) following in vitro restimulation, whereas enhanced T cell responses were not observed at other sites, including the genital LN (gLN), genital tract, spleen, and peripheral LN (Fig. 6D and data not shown). Furthermore, the enhanced T cell responses in the vaginal tissues also resulted in an increased protection of the host from viral infection, as we observed decreased viral loads in the vagina of mice that had received ATRA during initial T cell priming compared to those of control-treated (P = 0.008) and naïve (P = 0.0031) mice on day 5 postchallenge with 6 × 106 PFU VVgp (Fig. 6E). As no virus was detected in the ovaries of any immunized mice after this challenge, we next challenged mice with a higher viral dose to determine if the increased mucosal immunity in mice that received ATRA during vaccination could limit the virus from spreading from the vagina to the adjacent genital tract. Therefore, naïve mice and mice immunized in the presence or absence of ATRA were challenged with VVgp (3 × 107 PFU) and examined on day 6 for viral clearance from the ovaries and draining gLN. This dose of vaccinia virus resulted in detectable virus in these sites, with less virus being found in all immunized mice than in naïve mice. Furthermore, mice that had received ATRA during initial vaccination had significantly reduced viral titers in the ovaries (P = 0.049) and gLN (P = 0.037) than control immunized mice (Fig. 6F), suggesting that the increased mucosal T cell responses in these mice could contribute to reducing the viral spread.
Fig. 6.
ATRA provision during vaccination leads to enhanced T cell infiltration and stronger recall responses and protection from vaginal viral challenge. (A and B) Naïve B6 mice received ATRA or vehicle during immunization with Ad5gp and were assessed on day 5 for frequencies of CD4+ and CD8+ T cells in the vagina (A) and frequencies of gp33/Db tetramer-positive CD8α+ T cells in gated CD8+ cells in vaginal tissues (B) of ATRA-treated mice. (C to E) B6 mice immunized with Ad5gp in the presence of ATRA or vehicle were treated 8 weeks later with progesterone to synchronize mucosal surfaces for 5 days, followed by intravaginal infection with VVgp at 6 × 106 PFU. T cell responses were assessed on day 5 postchallenge for flow plots of the gp33 tetramer versus CD44 (C), bar graph of the frequency of IFN-γ-producing cells in gated CD8α+ T cells (D), and tissue viral loads pooled from two separate experiments (E). (F) An increased dose of VVgp of 3 × 107 PFU was used to challenge naïve or vaccinated mice treated with ATRA or vehicle, and viral loads in ovaries and gLN on day 6 p.i. are shown as means ± SEM.
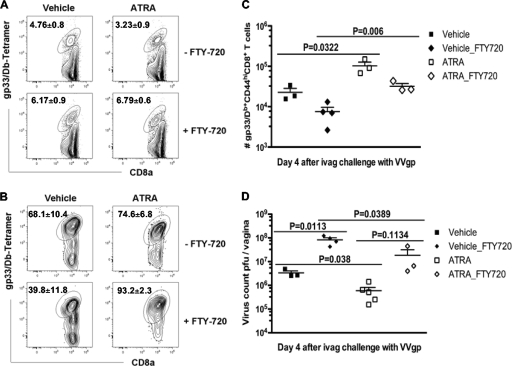
ATRA-treated immunized mice contain more mucosal memory T cells responding in situ during vaginal viral challenge.
The limited amount of resident T cells in vaginal tissues made it technically not feasible to acquire sufficient numbers of antigen-specific memory cells from vaginal mucosal tissues to assess the responses of resident T cells before viral challenge from ATRA- and vehicle-treated immunized mice. Therefore, to determine if the enhanced T cell recall response in the vagina of ATRA-treated mice specifically reflected, at least in part, increased effector activity and expansion of the mucosa-resident memory T cells rather than recruited cells, we administered FTY-720, an immune-suppressive drug, for 4 days beginning immediately after challenge with VVgp to mice that had previously received ATRA or the vehicle control during Ad5gp immunization. The phosphorylation of FTY-720 in vivo inhibits the S1P/S1P1-dependent egress of lymphocytes from lymph nodes (9), thereby preventing the homing of effector T cells to peripheral lesions and making effector activities observed at mucosal surfaces a reflection of resident mucosal cells, but has no direct impact on T cell activation or viral replication (43). The blocking of lymphocyte egress from lymph nodes by FTY-720 was confirmed by the observed ∼2-fold increase in numbers of gp33-specific CD8+ T cells detected in gLN after challenge compared to those of untreated groups, regardless of prior ATRA administration (vehicle, P = 0.034; ATRA, P = 0.021) (Fig. 7A). Conversely, FTY-720 reduced the number and frequency of gp33-specific CD8+ T cells detected in the vagina of all challenged mice, providing further evidence that egress from lymph nodes was blocked and that the observed mucosal responses reflected largely a local expansion of preexisting resident memory cells (Fig. 7B and C). We observed significantly increased numbers of gp33-specific CD8+ T cells in the vagina of challenged mice that had received ATRA during initial vaccination compared to control immunized mice (93.2% ± 2.3% versus 39.8% ± 11.8%; P = 0.006) (Fig. 7B and C). The responding T cells in the vagina appeared more activated if the mice received FTY-720 treatment, showing an increased frequency of KLRG1+ cells (vehicle groups, 44.6% ± 4.1% versus 33.3% ± 0.7 [P = 0.019]; ATRA groups, 55.8% ± 5.6% versus 37.6% ± 1.3% [P = 0.021]) (see Fig S2 in the supplemental material). Furthermore, the greater mucosal T cell response in Ad5gp/ATRA-immunized mice in the presence of FTY-720 resulted in lower viral loads in vaginal tissues (Fig. 7D), although these viral loads were higher than those when lymphoid egress was not blocked with FTY-720, permitting recruited T cells to participate in viral control. Thus, these data indicate that both mucosa-resident and recruited memory T cells contribute to the recall response to viral challenge and that the enhanced mucosa-resident memory CD8+ T cell responses generated in mice that received ATRA during initial vaccination contribute to better protection from viral exposure in the vagina.
Fig. 7.
ATRA-treated mice contain more mucosal memory CD8α+ T cells responding in situ during vaginal challenge. Mice immunized with Ad5gp in the presence of ATRA or vehicle were infected with VVgp intravaginally followed by FTY-720 treatment for 4 days. (A to C) T cell responses were assessed on day 4 postchallenge for representative flow plots of gp33-specific cells in gated CD8α+ T cells in genital LN (A) and vagina (B) and total numbers of gp33-specific CD8α+ T cells in vagina (C). (D) Viral loads in vaginal tissues. Data shown are means ± SEM of data from 3 to 5 mice in each group.
DISCUSSION
One goal for designing protective vaccines against mucosally transmitted viruses is to induce memory T cells in mucosa-associated tissues that are able to provide rapid and robust responses early during infection (42, 50). Knowledge of the mechanisms by which the tissue tropism of T cells is determined has made it increasingly possible to intervene to direct the tissue localization of activated T cells for therapeutic applications (1, 24, 26, 34). In contrast to skin homing via the expression of CCR4 and ligands for E-/P-selectins, the mucosal homing of T cells to gut mucosa is dependent mainly on their expression of CCR9 and α4β7, which can be modulated by DC-derived ATRA during T cell priming (24, 34). In this study we evaluated the effect of providing exogenous ATRA during systemic vaccination and found increased numbers of effector and memory CD8+ T cells in mucosa-associated tissues that provided enhanced protection against mucosal viral challenges as well as increased central memory-like T (TCM) cells in systemic sites that preferentially migrate to mucosal sites upon boosting.
ATRA administration can influence T cell responses both indirectly, by effects on DC maturation/function, and directly, by effects on T cells during activation. In addition to the clear in vitro effect of ATRA on DC maturation, DCs can take up and deliver exogenous ATRA to T cells during Ag presentation (48), and the observed in vivo activity leading to enhanced mucosal responses likely reflects in part such a delivery of exogenous ATRA to responding T cells by nonmucosal DCs during systemic priming. The enhanced expression of CCR9 and CD103 was more evident on activated T cells found in mucosa-associated tissues, suggesting that the regulation of these molecules may be more dependent than α4β7 on the mucosal microenvironment, such as stromal cells, DCs, and local ATRA concentrations (1, 17). In both ATRA- and vehicle-treated mice, CCR9hi effector and memory T cells were detected in PP, where the epithelium is known to express the receptor ligand CCL25 (7), and from MLN, which are the draining lymph node for the CCL25-producing IEL/LP. ATRA-treated mice contained more T cells expressing high levels of CCR9 in these sites at both phases of the immune response, which may reflect both enhanced mucosal homing of systemic effector T cells and retention of memory cells. Since only early effector T cells that express high levels of α4β7 can infiltrate into intestinal tissues (36), it is possible that in the presence of exogenous ATRA, early effector cells primed at systemic sites and recruited to MLN of ATRA-treated mice further upregulate or better maintain the expression of CCR9 and CD103, leading to the accumulation/retention of more activated T cells, which become memory cells.
Although originally implicated in gut homing, ATRA also increased the accumulation of in vitro-activated and vaccine-induced T cells at other mucosal sites such as the vagina. The mechanisms for preferential homing to these extraintestinal mucosa sites are not well described and may involve some of the molecules regulated by ATRA that also participate in lymphocyte localization to the intestine. It is unclear if CCR9 is involved in T cell homing to the vaginal mucosa. Previous studies have demonstrated that the lower genital tract and vaginal draining LN also express MAdCAM-1, the ligand for α4β7, as found in MLN, which could recruit α4β7+ lymphocytes to vaginal sites (29, 52). Although a recent study has suggested that the localization of B cells to the vaginal mucosa is independent of the expression of integrin β7 (16), the relative role of integrin β7, which is required for T cell homing to the intestine (32), in the infiltration of T cells into the vagina has not been fully defined. However, CD103 knockout mice, which lack the expression of αEβ7, do have decreased numbers of T cells in the vaginal epithelium (49). Our data did demonstrate that ATRA can induce CD103 expression on responding T cells, and CD103 has been found not only on intestinal IELs but also on a large fraction of T cells isolated from genitourinary and cervicovaginal epithelia and the lung (20, 45). CD103 has been shown to be dispensable for the entry of virus-activated T cells into the intestine (32), suggesting that it may be more involved in retaining the infiltrated lymphocytes at the mucosal surface of the intestine and vagina, where the epithelial cells express E-cadherin, the ligand for CD103 (6, 8, 18, 49, 51). It is also unknown if the systemic provision of ATRA during priming impacts the vaginal tissue expression of ligands for homing/adhesion molecules on T cells. Despite the undefined in vivo molecular basis of the observed effect of ATRA, the benefit of it as a mucosal adjuvant is clearly revealed in our T cell-based vaccination/viral challenge model, in which significantly stronger CD8+ T cell recall responses and enhanced protection from vaginal infection were detected in ATRA-treated hosts. It should be noted that viral protection might not be mediated solely by CD8+ T cells, as ATRA is also known to affect multiple cell types, including DCs/macrophages, CD4+ T cells, and antibody-producing B cells (24, 34, 39).
Details of the effects of ATRA provision during priming on memory T cell differentiation remain to be fully resolved, but ATRA treatment during systemic vaccination does induce more CCR7+ CD127+ TCM cells with variable CD62L expression levels in the spleen and more CD62L− effector memory (TEM) cells in mucosal tissues. This should benefit the vaccinated host, because TCM cells with high proliferative potential serve to replenish the memory pool during homeostasis and following infection, while TEM cells at mucosal surfaces provide immediate protection during mucosal challenge (31, 37, 46, 47). Although the total number of splenic memory T cells was not increased by ATRA treatment during vaccination, the higher frequency of TCM cells and the enhanced proliferation of memory T cells during boosting suggest that ATRA may also be promoting a more general improvement in vaccine efficacy, which awaits further investigation with systemic viral challenge models. T cell recall responses to infection in nonlymphoid tissues usually begin with an immediate response by tissue-resident memory cells followed by the infiltration of memory cells via the circulation (19, 63). Recent studies have shown that during viral challenge, local Ag-specific T cells are stimulated directly and expand in situ (60) and that memory cells from lymphoid organs are subsequently recruited and also proliferate within the peripheral tissues (61). This concerted recall response of local and recruited memory T cells was evident in vaginal tissues in our study, in which the enhanced protection of ATRA-treated mice was revealed to be due partly to more resident memory CD8+ T cells responding in situ and partly to memory cells recruited from lymph nodes. The importance of a rapid and strong CD8+ T cell response at mucosal portals of viral entry in combating the establishment of systemic and chronic virus infections has been emphasized for HIV vaccines (3, 14, 33, 50, 55), and our data suggest that ATRA should be further investigated for potential incorporation into the next generation of T cell-based HIV vaccines.
The systemic administration of ATRA could lead to retinoid toxicity. However, in our study ATRA needed to be delivered for only a short period during vaccination, and no clinical signs of toxicity were observed. ATRA is also known under some conditions to promote Treg conversion (4, 10, 41, 54), which could limit effector/memory T cell responses (56). Despite a small and only transient increase in levels of Treg cells observed early after priming at both systemic and mucosal sites in ATRA-treated mice (data not shown), ATRA-induced Treg cells were clearly not sufficient to prevent the increase of effector and memory CD8+ T cell responses detected at mucosal sites or reduce the improved magnitude and functional quality of memory CD8+ T cells at the systemic lymphoid organs compared to control immunized mice. This could reflect in part limited Treg cell induction in the context of the strong inflammatory response (64) with the adenoviral vaccine vector and the large CD8+ T cell response induced by viral vaccination.
In summary, we have evaluated the use of ATRA as an adjuvant during vaccination for enhancing T cell mucosal immunity. ATRA has a long history of use in humans, with an established safety record. Topical all-trans-retinoic acid (Tretinoin) was first used for acne therapy in 1962 and remains in use today (5, 15, 30), and it has also been administered orally for the clinical treatment of acute promyelocytic leukemia (62). Topical ATRA in conjunction with cholera toxin and a component of Freund's adjuvant has been recently shown to enhance mucosal antibody responses following transdermal immunization (35). Our results with a mouse model of vaccination and in vitro analysis of human T cells suggest that strategies to deliver ATRA topically, or potentially orally or systemically, at the time of parenteral vaccination should be evaluated for the development of vaccine regimens to establish enhanced protective mucosal immunity in humans.
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported in part by NIH/NCI 5 R01 CA033084 and by the Bill and Melinda Gates Foundation (grant 37902).
We are very grateful to Gregory Spies and Jennifer Vogt from the McElrath laboratory at the Fred Hutchinson Cancer Research Center for providing purified Ad5gp and MVAgp.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 8 June 2011.
REFERENCES
- 1. Agace W. W. 2006. Tissue-tropic effector T cells: generation and targeting opportunities. Nat. Rev. Immunol. 6:682–692 [DOI] [PubMed] [Google Scholar]
- 2. Belyakov I. M., Berzofsky J. A. 2004. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity 20:247–253 [DOI] [PubMed] [Google Scholar]
- 3. Belyakov I. M., et al. 2006. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood 107:3258–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benson M. J., Pino-Lagos K., Rosemblatt M., Noelle R. J. 2007. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204:1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger R., et al. 2007. Tretinoin gel microspheres 0.04% versus 0.1% in adolescents and adults with mild to moderate acne vulgaris: a 12-week, multicenter, randomized, double-blind, parallel-group, phase IV trial. Clin. Ther. 29:1086–1097 [DOI] [PubMed] [Google Scholar]
- 6. Berlin C., et al. 1995. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80:413–422 [DOI] [PubMed] [Google Scholar]
- 7. Campbell D. J., Butcher E. C. 2002. Intestinal attraction: CCL25 functions in effector lymphocyte recruitment to the small intestine. J. Clin. Invest. 110:1079–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cepek K. L., et al. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 372:190–193 [DOI] [PubMed] [Google Scholar]
- 9. Chiba K. 2005. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol. Ther. 108:308–319 [DOI] [PubMed] [Google Scholar]
- 10. Coombes J. L., et al. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eksteen B., et al. 2009. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology 137:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engedal N., Gjevik T., Blomhoff R., Blomhoff H. K. 2006. All-trans retinoic acid stimulates IL-2-mediated proliferation of human T lymphocytes: early induction of cyclin D3. J. Immunol. 177:2851–2861 [DOI] [PubMed] [Google Scholar]
- 13. Geissmann F., et al. 2003. Retinoids regulate survival and antigen presentation by immature dendritic cells. J. Exp. Med. 198:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Genescà M., et al. 2008. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 1:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gollnick H., et al. 2003. Management of acne: a report from a global alliance to improve outcomes in acne. J. Am. Acad. Dermatol. 49:S1–37 [DOI] [PubMed] [Google Scholar]
- 16. Goodsell A., et al. 2008. Beta7-integrin-independent enhancement of mucosal and systemic anti-HIV antibody responses following combined mucosal and systemic gene delivery. Immunology 123:378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hammerschmidt S. I., et al. 2008. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 205:2483–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heyday A., Theodoridis E., Ramsburg E., Shires J. 2001. Intraepithelial lymphocytes: exploring the third way in immunology. Nat. Immunol. 2:997–1003 [DOI] [PubMed] [Google Scholar]
- 19. Hikono H., et al. 2006. T-cell memory and recall responses to respiratory virus infections. Immunol. Rev. 211:119–132 [DOI] [PubMed] [Google Scholar]
- 20. Hladik F., Lentz G., Delpit E., McElroy A., McElrath M. J. 1999. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J. Immunol. 163:2306–2313 [PubMed] [Google Scholar]
- 21. Ho W. Y., Nguyen H. N., Wolfl M., Kuball J., Greenberg P. D. 2006. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naïve repertoire. J. Immunol. Methods 310:40–52 [DOI] [PubMed] [Google Scholar]
- 22. Holmgren J., Czerkinsky C. 2005. Mucosal immunity and vaccines. Nat. Med. 11:S45–53 [DOI] [PubMed] [Google Scholar]
- 23. Holmgren J., Harandi A. M., Czerkinsky C. 2005. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol. Lett. 97:181–188 [DOI] [PubMed] [Google Scholar]
- 24. Iwata M. 2009. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin. Immunol. 21:8–13 [DOI] [PubMed] [Google Scholar]
- 25. Iwata M., et al. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21:527–538 [DOI] [PubMed] [Google Scholar]
- 26. Johansson-Lindbom B., Agace W. W. 2004. Vitamin A helps gut T cells find their way in the dark. Nat. Med. 10:1300–1301 [DOI] [PubMed] [Google Scholar]
- 27. Johansson-Lindbom B., et al. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202:1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johansson-Lindbom B., et al. 2003. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelly K. A., Rank R. G. 1997. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect. Immun. 65:5198–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krautheim M., Gollnick H. P. M. 2004. Acne: topical treatment. Clin. Dermatol. 22:398–407 [DOI] [PubMed] [Google Scholar]
- 31. Lefrançois L., Marzo A. L. 2006. The descent of memory T-cell subsets. Nat. Rev. Immunol. 6:618–623 [DOI] [PubMed] [Google Scholar]
- 32. Lefrancois L., et al. 1999. The role of β integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 189:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Q., et al. 2009. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science 323:1726–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manicassamy S., Pulendran B. 2009. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin. Immunol. 21:22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin M. P., et al. 2010. Adjuvanted influenza vaccine administered intradermally elicits robust long-term immune responses that confer protection from lethal challenge. PLoS One 5:e10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masopust D., et al. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207:553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masopust D., Vezys V., Marzo A. L., Lefrançois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413–2417 [DOI] [PubMed] [Google Scholar]
- 38. Mohty M., et al. 2003. All-trans retinoic acid skews monocyte differentiation into interleukin-12-secreting dendritic-like cells. Br. J. Haematol. 122:829–836 [DOI] [PubMed] [Google Scholar]
- 39. Mora J. R., et al. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314:1157–1160 [DOI] [PubMed] [Google Scholar]
- 40. Mora J. R., et al. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424:88–93 [DOI] [PubMed] [Google Scholar]
- 41. Mucida D., et al. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317:256–260 [DOI] [PubMed] [Google Scholar]
- 42. Neutra M. R., Kozlowski P. A. 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6:148–158 [DOI] [PubMed] [Google Scholar]
- 43. Pinschewer D. D., et al. 2000. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J. Immunol. 164:5761–5770 [DOI] [PubMed] [Google Scholar]
- 44. Pizza M., et al. 2001. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19:2534–2541 [DOI] [PubMed] [Google Scholar]
- 45. Pudney J., Anderson D. J. 1995. Immunobiology of the human penile urethra. Am. J. Pathol. 147:155–165 [PMC free article] [PubMed] [Google Scholar]
- 46. Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 [DOI] [PubMed] [Google Scholar]
- 47. Sallusto F., Geginat J., Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763 [DOI] [PubMed] [Google Scholar]
- 48. Saurer L., McCullough K. C., Summerfield A. 2007. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J. Immunol. 79:3504–3514 [DOI] [PubMed] [Google Scholar]
- 49. Schon M. P., et al. 1999. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J. Immunol. 162:6641–6649 [PubMed] [Google Scholar]
- 50. Shattock R. J., et al. 2008. Improving defenses at the portal of HIV entry: mucosal and innate immunity. PLoS Med. 5:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smyth L. J. C., Kirby J. A., Cunningham A. C. 2007. Role of the mucosal integrin αΕ(CD103)β7 in tissue-restricted cytotoxicity. Clin. Exp. Immunol. 149:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soderberg K. A., Linehan M. M., Ruddle N. H., Iwasaki A. 2004. MAdCAM-1 expressing sacral lymph node in the lymphotoxin beta-deficient mouse provides a site for immune generation following vaginal herpes simplex virus-2 infection. J. Immunol. 173:1908–1913 [DOI] [PubMed] [Google Scholar]
- 53. Stenstad H., et al. 2006. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood 107:3447–3454 [DOI] [PubMed] [Google Scholar]
- 54. Sun C. M., et al. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suvas P. K., Dech H. M., Sambira F., Zeng J., Onami T. M. 2007. Systemic and mucosal infection program protective memory CD8 T cells in the vaginal mucosa. J. Immunol. 179:8122–8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suvas S., Kumaraguru U., Pack C. D., Lee S., Rouse B. T. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Svensson M., et al. 2008. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 1:38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Svensson M., et al. 2002. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uematsu S., et al. 2008. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 9:769–776 [DOI] [PubMed] [Google Scholar]
- 60. Wakim L. M., Waithman J., van Rooijen N., Heath W. R., Carbone F. R. 2008. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319:198–202 [DOI] [PubMed] [Google Scholar]
- 61. Wakim L. M., Gebhardt T., Heath W. R., Carbone F. R. 2008. Local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J. Immunol. 181:5837–5841 [DOI] [PubMed] [Google Scholar]
- 62. Wang Z. Y., Chen Z. 2008. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111:2505–2515 [DOI] [PubMed] [Google Scholar]
- 63. Woodland D. L., Kohlmeier J. E. 2009. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 9:153–161 [DOI] [PubMed] [Google Scholar]
- 64. Xiao S., et al. 2008. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 181:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.