Abstract
Casp8p41, a novel protein generated when HIV-1 protease cleaves caspase 8, independently causes NF-κB activation, proinflammatory cytokine production, and cell death. Here we investigate the mechanism by which Casp8p41 induces cell death. Immunogold staining and electron microscopy demonstrate that Casp8p41 localizes to mitochondria of activated primary CD4 T cells, suggesting mitochondrial involvement. Therefore, we assessed the dependency of Casp8p41-induced death on Bax/Bak and caspase 9. In wild-type (WT) mouse embryonic fibroblast (MEF) cells, Casp8p41 causes rapid mitochondrial depolarization (P < 0.001), yet Casp8p41 expression in Bax/Bak double-knockout (DKO) MEF cells does not. Similarly, caspase 9-deficient T cells (JMR cells), which express Casp8p41, undergo minimal cell death, whereas reconstituting these cells with caspase 9 (F9 cells) restores Casp8p41 cytotoxicity (P < 0.01). The infection of caspase 9-deficient cells with a green fluorescent protein (GFP) HIV-1 reporter virus results in cell death in 32% of infected GFP-positive cells, while the restoration of caspase 9 expression in these cells restores infected-cell killing to 68% (P < 0.05), with similar levels of viral replication between infections. Our data demonstrate that Casp8p41 requires Bax/Bak to induce mitochondrial depolarization, which leads to caspase 9 activation following either Casp8p41 expression or HIV-1 infection. This understanding allows the design of strategies to interrupt this form of death of HIV-1-infected cells.
INTRODUCTION
There are numerous factors that promote CD4 T cell loss during chronic human immunodeficiency virus type 1 (HIV-1) disease, the majority of which are not unique to HIV-1 (12). Indeed, the effects of enhanced immune activation (2), bacterial translocation (56), and the heightened production of proapoptotic ligands (57) are seen in a variety of other disease states. A recently described pathway of CD4 death that is unique to HIV-1 is one whereby HIV-1 protease, which is active within the cytosolic compartment of HIV-1-infected T cells (24–26), cleaves the host protein caspase 8, creating a novel cleavage fragment termed Casp8p41 (44). This fragment is missing the catalytic cysteine at position 360 that is responsible for the catalytic activity of caspase 8. Nevertheless, Casp8p41 independently induces NF-κB activation, resulting in enhanced HIV-1 replication (3) and enhanced proinflammatory cytokine production (58), while simultaneously inducing apoptotic death in the cells where it is expressed (43). The abrogation of Casp8p41 production in pseudotyped HIV-1 infections significantly reduces T cell death (43). Mutations in HIV-1 protease that selectively alter the ability of the protease to cleave caspase 8 also reduce HIV-1-induced cell death compared to wild-type (WT) protease (42). Since Casp8p41 is unique to HIV-1-infected cells (44) and is a host cellular protein, which would not be subject to mutational escape, it is an attractive candidate for therapeutic targeting.
Little is known concerning the molecular mechanisms by which Casp8p41 achieves its biological effects. In a cell-free system, changes associated with apoptosis occur only in the presence of mitochondria, suggesting that Casp8p41-induced death requires mitochondria, but the molecular signals involved remain unknown (1). Apoptosis can occur through multiple signals, including the mitochondrial pathway or the death receptor pathway. The death receptor pathway may involve or bypass mitochondria; however, multiple redundancies are present in the regulation of apoptosis such that cross talk exists between the intrinsic and extrinsic pathways. This redundancy allows mitochondria to be a central regulator of apoptosis (23). More often, death receptor signaling involves mitochondrial coordination, which is a requisite for other apoptotic stimuli, including genotoxic stress (6), growth factor withdrawal (14), and chemotherapeutic agents (55). The release of inner mitochondrial proteins, such as cytochrome c and smac/DIABLO, is regulated by Bcl-2 family members (67). Bcl-2 antagonist/killer (Bak) and Bcl-2-associated X protein (Bax) are two proapoptotic Bcl-2 family members. These proteins undergo a conformational change, exposing their amino termini, which allows them to homo- or hetero-oligomerize, form a pore, and release cytochrome c from the mitochondria (52, 64). The released cytochrome c couples with APAF-1 and inactive procaspase 9 to form the apoptosome, which results in the activation of caspase 9. Active caspase 9 cleaves caspases 3 and 7, resulting in the phenotypic changes associated with apoptosis (59). The phenotypic changes of apoptosis in the mitochondria include swelling, a loss of transmembrane potential, and the release of cytochrome c into the cytosol (29), and these events are controlled by Bax and/or Bak.
The goal of the current study was to determine whether Casp8p41-induced apoptosis is dependent upon mitochondrial depolarization and to identify whether Bax and/or Bak is involved, with a consequent downstream involvement of caspase 9.
MATERIALS AND METHODS
Cell culture.
Jurkat and HeLa cells were obtained from the ATCC (Manassas, VA). JMR and F9 cells (48) were obtained from Scott Kaufmann (Rochester, MN), and MEF WT, Bax and Bak single-knockout (KO), as well as Bax/Bak double-knockout (DKO) cells (64) were obtained from Zheng Dong (Augusta, GA). Primary CD4 T cells were obtained from donated blood in accordance with Institutional Review Board (IRB) protocol 1039-03, isolated by using the Stem Cell Technology RosetteSep kit, activated for 24 h with 1 μg/ml phytohemagglutinin (PHA), washed in medium, and incubated for 48 h with 50 units/ml interleukin-2 (IL-2). Jurkat and primary CD4 T cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 2 mM glutamine. JMR and F9 cells were cultured in RPMI 1640 medium supplemented with 15% fetal bovine serum and 2 mM glutamine. HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 2 mM glutamine. MEF wild-type and Bax/Bak DKO cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 2 mM sodium pyruvate, 1% (minimal essential medium [MEM]) nonessential amino acids, 5 units/ml penicillin, and 5 μg/ml streptomycin.
Plasmid construction.
Casp8p41 in pEGFP and pcDNA3 were previously described (3, 44). The Casp8p41ΔDED constructs were made by PCR using primers 5′-CGGATCCATGGAAAGGGACTT-3′ and 5′-CTAGATTAAAACACTTTGGGTTTTCCAGCAAGG-3′, cut with BamHI and XbaI, and inserted into the designated sites in vectors. All constructs were confirmed by DNA sequence analysis and tested for expression prior to experimental use.
Transfection and infections.
Primary CD4 T cells were transfected by using Amaxa (Lonza, Basel, Switzerland) according to the manufacturer's protocol. Jurkat, JMR, and F9 cells were transfected with 2 μg of DNA per 105 cells using a square-wave electroporator (BXT, San Diego, CA) at 320 V and 280 V, respectively, for 10 ms. MEF cells were transfected by using Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol.
HIV-1 infections were performed with a single-cycle reporter virus, HIV-1gfp, which has a 426-nucleotide (nt) deletion in env, has the enhanced green fluorescent protein (GFP) (EGFP) gene in place of nef, and is also vpr negative. pHIV-1gfp was derived from pHIV-1luc (34) as follows. The EGFP gene was amplified from peGFP-N1 (Clontech) by using Phusion DNA polymerase (Finnzymes) with primers 5′-ATATAGCGGCCGCTATGGTGAGCAAGGGCG-3′ and 5′-ATATACTCGAGTTACTTGTACAGCTCGTC-3′. The amplicon was cleaved with NotI and XhoI and then inserted into these sites in HIV-1luc, and the correct plasmid was verified by sequencing. HIV-1gfp was prepared in 293T cells by cotransfection with a vesicular stomatitis virus glycoprotein G (VSV-G) expression plasmid (3 and 1 μg of DNA, respectively). JMR, F9, and Jurkat cells were infected with 1,000 ng of p24 (measured by p24 enzyme-linked immunosorbent assay [ELISA]) per 110 cells for 3 h. Cells were then washed 3 times and incubated in fresh medium.
Western blots.
Ten million cells were lysed in 100 μl of lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Triton X-100 [TX-100], 2 μg/ml aprotinin, 10 μg/ml leupeptin, 2 μg/ml pepstatin, and1 mM phenylmethylsulfonyl fluoride [PMSF]) for 10 min on ice. Cells were then centrifuged at 400 × g for 5 min at 4°C. For cell fractionation, the lysate was then further centrifuged at 15,000 × g for 5 min at 4°C, resulting in a mitochondrial enriched pellet and cytosolic supernatant.
For Western blot analysis, 10 μg to 100 μg of cell lysate was run on 10 to 15% polyacrylamide gels and then transferred onto polyvinylidene difluoride (PVDF) membranes for 2 h at 1,200 mA in transfer buffer (24 mM Tris, 192 mM glycine). The membranes were then blocked in Tris-buffered saline-Tween (TBST) (20 mM Tris, 150 mM NaCl, 0.05% Tween 20) with 2% bovine serum albumin (BSA) (Sigma, St. Louis, MO) for more than 1 h at room temperature or overnight at 4°C. Membranes were blotted with the following primary antibodies: anti-hemagglutinin (HA) peroxidase 3F10 (Roche, St. Louis, MO), anti-HSP70 (generous gift from David Toft) (41), anti-actin (Sigma, St. Louis, MO), and anti-caspase 9 (Medical & Biological Laboratories, Woburn, MA). Membranes were then washed three times with TBST, and a horseradish peroxidase-linked secondary antibody was used when necessary. All blots were developed by using a detection kit from Thermo Fisher Scientific (Waltham, MA).
Flow cytometry.
Cell death was measured by using light scatter, with the sizes of the cells depicting alive/dead phenotypes (15); propidium iodide (PI) (Sigma, St. Louis, MO) uptake into dead cells only; low tetramethylrhodamine ethyl ester (TMRE; Invitrogen, Carlsbad, CA) expression indicating a depolarization of the mitochondria; or terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) (Roche, St. Louis, MO) positivity indicating DNA fragmentation. TUNEL staining was done according to the manufacturer's protocol. For PI staining, 1 μg/ml of PI was added to 500,000 collected cells and analyzed immediately. TMRE staining was preformed on 500,000 collected cells by the addition of 15 nmol TMRE to 1 ml of 2% fetal bovine serum in phosphate-buffered saline (PBS), and the mixture was incubated at room temperature for 30 min. Cells were then washed one time with PBS and analyzed immediately. Intracellular Casp8p41 expression was determined by flow cytometry as previously described (42). Flows were run on a FACScan flow cytometer (BD Biosciences, San Diego, CA). All gating was first set to control samples and then applied to samples of interest.
Microscopy.
For immunogold staining, transfected primary CD4 T cells were fixed 3 h posttransfection in 4% paraformaldehyde and 0.2% glutaraldehyde for 1 h, centrifuged, and then embedded in 10% gelatin, infiltrated with 2.3 M sucrose, for 3 h. The cells were then frozen in liquid nitrogen, and 50- to 60-nm cryosections were cut. Grids were incubated with 20 mM glycine plus 2% fetal bovine serum in PBS for 15 min, blocked with 10% fetal bovine serum in PBS for 20 min, and incubated with anti-Casp8p41 (43) or anti-HA (Upstate clone DW2) diluted with blocking solution at a 1:200 dilution at 4°C overnight. After six 3-min washes with 2% fetal bovine serum in PBS, grids were incubated with goat anti-mouse or goat anti-rabbit IgG plus IgM 10-nm gold (Amersham, Piscataway, NJ) diluted with blocking solution at a 1:30 dilution for 2 h at room temperature. Samples were postfixed with 1% glutaraldehyde for 10 min, contrast stained, embedded with 0.4% uranyl and 2% methylcellulose, and air dried. Samples were examined by using a Jeol ExII transmission electron microscope (Jeol, Tokyo, Japan). The density of dots on the mitochondria was calculated by using Image J software.
For fluorescence microscopy, HeLa cells were transfected overnight with GFP, GFP-Casp8p41, or GFP-Casp8p41ΔDED and then stained with 100 nM Mitotracker Red (Invitrogen, Carlsbad, CA) and incubated at 37°C for 45 min. Cells were then fixed with 2% paraformaldehyde for 1 h at room temperature. Slides were prepared with 4′,6-diamidino-2-phenylindole (DAPI) containing Vectashield (Vector Laboratories, Burlingame, CA). Laser scanning confocal microscopy was performed by using an LSM 510 confocal laser scanning microscope (Carl Zeiss MicroImagin, Oberkochn, Germany).
Statistical analysis.
For the immunogold experiments, multiple mitochondria and nonmitochondrial areas were chosen at random and compared. Positive staining was quantified as the number of discrete immunogold dots per 100 mm2 of mitochondrial, or nonmitochondrial, area for each image. In instances when zero dots were seen in a space, a value of 0.9 was used as the numerator. Mean values from three independent images were compared by routine analysis of variance (ANOVA). A P value of <0.05 was considered statistically significant.
For flow cytometric data, the proportion of transfected cells positive for a cell death marker (TUNEL, etc.) was determined as follows: (number of double-positive cells/(number of double-positive cells + number of transfected cells that are marker negative) × 100. Mean values from three independent experiments were compared by the Student t test. A P value of <0.05 was considered statistically significant.
RESULTS
Casp8p41 localizes to the mitochondria.
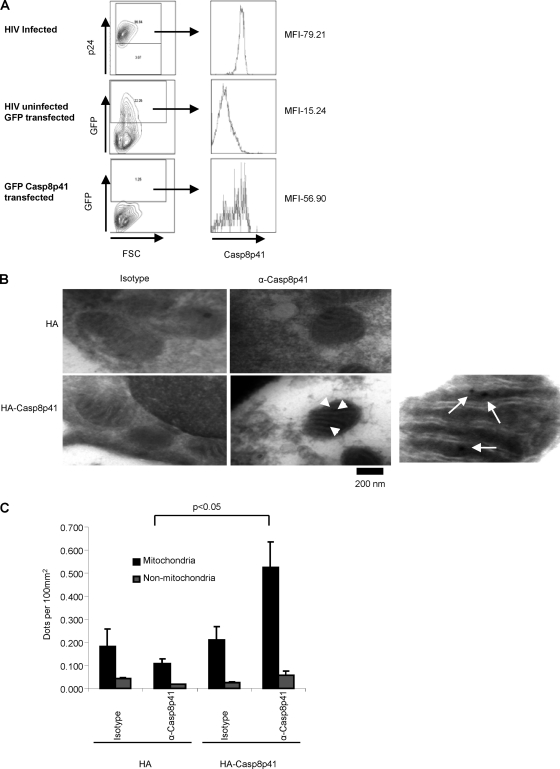
We have previously observed that GFP-Casp8p41 colocalizes with Mitotracker staining in HeLa cells (1); however, the expression levels of transfected protein might not mimic levels found in infected cells, the large GFP tag might alter the subcellular localization of Casp8p41, or the results may not reflect the situation in primary CD4 T cells. Therefore, Jurkat cells were transfected with GFP-Casp8p41 or infected with HIV-1 IIIb, and cells were compared for intracellular Casp8p41, specifically in the GFP-positive (GFP+) or p24+ populations (Fig. 1A). This analysis revealed that the level of transfected protein expression was comparable to Casp8p41 expression levels in HIV-1-infected CD4 T cells. Next, HA-Casp8p41 or the HA control vector was expressed in primary CD4 T cells and stained with either anti-Casp8p41 or isotype control antibodies, followed by a gold-labeled secondary antibody. Consistent with our prior observations, the expression of Casp8p41 was rapidly associated with changes characteristic of apoptosis, in this case manifesting as mitochondrial swelling and a loss of cristae (1, 43, 44). To minimize this effect, we analyzed Casp8p41 localization 3 h after transfection, which resulted in low levels of protein expression, yet at this early time point normal mitochondrial architecture was maintained. The Casp8p41-expressing cells stained with anti-Casp8p41 antibody showed Casp8p41 localized to mitochondria (Fig. 1B). The specificity of the staining was determined by counting the number of gold dots under each condition in the total mitochondrial area, versus the total nonmitochondrial area, for several randomly selected cells; these results are presented in Fig. 1C. Note that Casp8p41-expressing cells also showed Casp8p41 staining in nonmitochondrial areas, possibly representing translated protein in transit toward the mitochondria.
Fig. 1.
Casp8p41 localizes to the mitochondria. (A) Jurkat T cells were transfected with GFP or GFP-Casp8p41 or infected with HIV-1 IIIb. The HIV-1-infected cells were stained with P24 (fluorescein isothiocyanate [FITC]), and all cells were stained for Casp8p41. Shown are the amounts of Casp8p41 in the GFP+ transfected cells or the P24+ HIV-1-infected cells. MFI, mean fluorescence intensity; FSC, forward scatter. (B) Electron microscopy of primary CD4 T cells transfected with an empty HA vector (top) or HA-Casp8p41 (bottom), stained with IgG3 control antibody (left) or Casp8p41 antibody (right), and analyzed at 3 h posttransfection. The rightmost panel is an enlarged image of Casp8p41 stained with Casp8p41 antibody to better distinguish gold particles. Arrows indicate where gold particles can be seen. (C) Quantitative analysis of numbers of gold particles found in the indicated areas.
Casp8p41ΔDED has impaired induction of apoptosis.
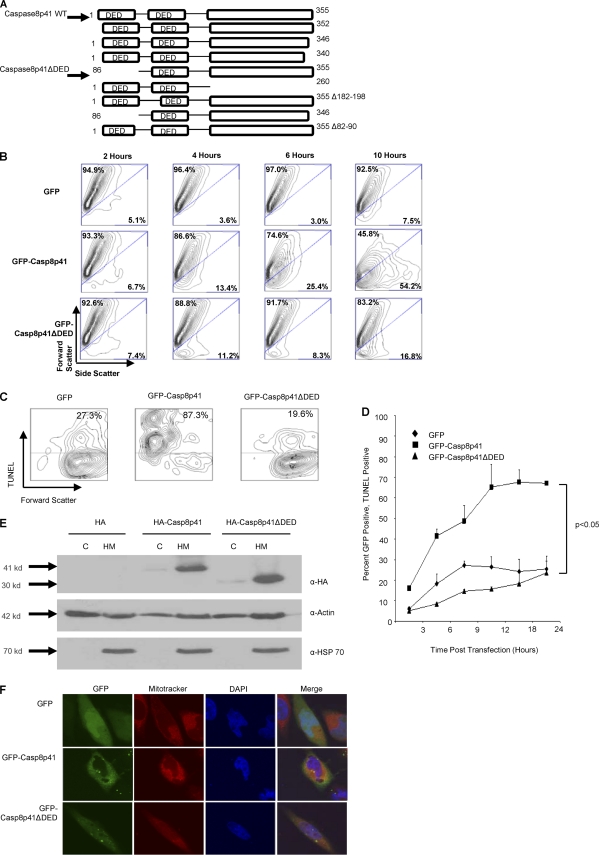
In order to study the molecular mechanism by which Casp8p41 initiates cell death, we sought a Casp8p41 mutant that induces less apoptosis than parental Casp8p41. Since Casp8p41 does not contain a catalytic active site, we employed a bidirectional serial truncation approach (Fig. 2A). Each truncation mutant was confirmed by sequencing and screened for its ability to be expressed and to cause cell death. Of the mutants created, only one, which contains an N-terminal deletion of the first of two tandem death effector domains (DEDs), caused less cell death than did the parental Casp8p41, as measured by light scatter in primary CD4 T cells and TUNEL staining in Jurkat cells (P < 0.05) (Fig. 2B, C, and D). Importantly, this mutant, Casp8p41ΔDED, localizes to the heavy membrane fraction and colocalizes with Mitotracker, a stain for mitochondria, demonstrating that Casp8p41ΔDED's reduced apoptosis induction is not due to an altered subcellular localization (Fig. 2E and F).
Fig. 2.
Casp8p41ΔDED induces less cell death than Casp8p41. (A) Pictorial representation of all Casp8p41 mutants that were constructed. (B) Primary CD4 T cells were transfected with empty vector, GFP-Casp8p41, or GFP-Casp8p41ΔDED. The viability of the GFP-positive population was measured via light scatter over the course of 10 h. (C) Jurkat cells were transfected with empty vector, GFP-Casp8p41, or GFP-Casp8p41ΔDED, and the cell death of the GFP-positive population was measured via TUNEL staining at 12 h posttransfection. (D) Cell death of GFP-positive cells was measured via TUNEL staining over a 24-h time course of Jurkat cells expressing empty vector, GFP-Casp8p41, or GFP-Casp8p41ΔDED. Error bars represent standard errors of the means. (E) Transfected Jurkat cells were fractionated into heavy membrane (HM) or cytosolic (C) fractions and analyzed for HA. Actin served as a loading control, and HSP70 was used to assess the purity of the fractions. (F) Confocal microscopy of empty-vector-, GFP-Casp8p41-, and GFP-Casp8p41ΔDED-transfected HeLa cells costained with Mitotracker and DAPI for visualization of the mitochondria and nucleus, respectively.
Casp8p41 does not induce apoptosis in Bax/Bak knockout MEF cells.
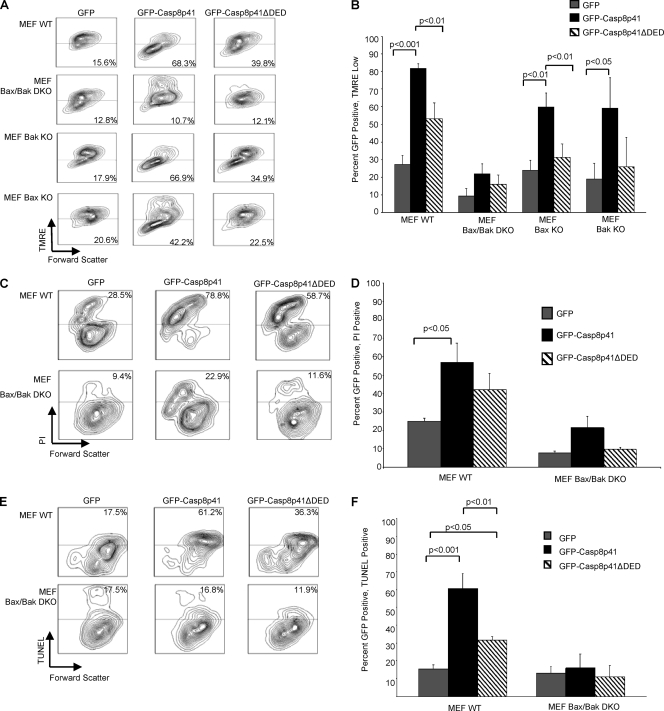
Apoptotic stimuli that involve mitochondrial signaling often require Bax and/or Bak (67). Therefore, we opted to test whether Casp8p41-induced cell death was dependent upon Bax/Bak by utilizing knockout (KO) mouse embryonic fibroblast (MEF) cells deficient in Bax and Bak. Wild-type (WT), Bak single-KO, Bax single-KO, or Bax/Bak double-knockout (DKO) MEF cells were transfected with GFP, GFP-Casp8p41, or GFP-Casp8p41ΔDED, and the mitochondrial membrane potential was measured by using TMRE (Fig. 3A and B). GFP-Casp8p41 expression caused a decrease in the mitochondrial transmembrane potential in MEF WT, Bak KO, and Bax KO cells that was not seen in GFP-transfected cells (P < 0.001, P < 0.01, and P < 0.05, respectively) or DED (P < 0.01, P < 0.01, and P > 0.05, respectively). Conversely, GFP, GFP-Casp8p41, or GFP-Casp8p41ΔDED failed to cause a mitochondrial transmembrane potential loss, as measured by TMRE staining, in Bax/Bak DKO MEF cells, suggesting that Casp8p41 requires either Bak or Bax to induce death, since the absence of both but not either one alone hinders the pathway. Similarly, Casp8p41 failed to cause cell membrane permeability, measured via PI positivity, or cell death, measured by TUNEL, in Bax/Bak DKO MEF cells (Fig. 3C, D, E, and F). It is noteworthy that GFP-Casp8p41ΔDED did cause lesser decreases in mitochondrial transmembrane potential, PI positivity, and cell death, consistent with its reduced cytotoxicity compared to that of Casp8p41.
Fig. 3.
Casp8p41 requires Bak/Bax to depolarize mitochondria and induce cell death. (A) WT mouse embryonic fibroblast (MEF) cells, MEF Bax/Bak DKO cells, Bax single-KO cells, or Bak single-KO cells were transfected with empty vector, GFP-Casp8p41, or GFP-Casp8p41ΔDED. GFP-positive cells were analyzed for mitochondrial depolarization by TMRE staining. (B) Pooled results from three independent experiments, with P values shown only for significant comparisons. (C) Cell membrane permeability was measured in GFP-positive cells by PI staining. (D) Pooled results from three independent experiments, with P values shown only for significant comparisons. (E) Cell death was measured in GFP-positive cells by TUNEL. (F) Pooled results from three independent experiments, with P values shown only for significant comparisons.
Casp8p41-induced cell death utilizes caspase 9.
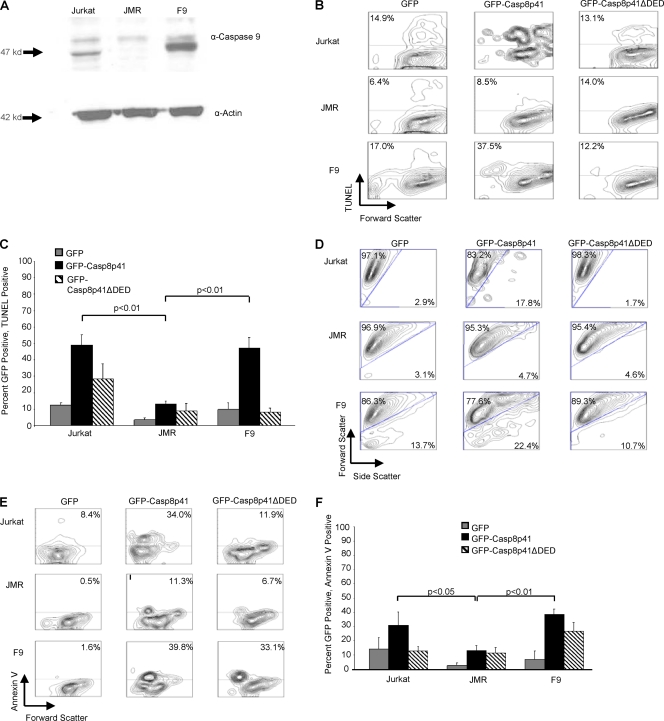
Having demonstrated a requirement of Bax/Bak for Casp8p41 to induce cell death, we next assessed whether caspase 9 was required. Following the Bax/Bak-dependent release of cytochrome c from mitochondria, cytochrome c complexes with APAF-1 and recruits procaspase 9, which, in the presence of ATP, causes the proteolytic activation of caspase 9 (31). Therefore, a dependence upon caspase 9 for apoptosis is synonymous with mitochondrial dependence (48). For these experiments, we used Jurkat T cells, a Jurkat-derivative cell line deficient in caspase 9 (JMR cells) (48), or JMR cells reconstituted with FLAG-tagged caspase 9 (F9 cells), verified via Western blotting (Fig. 4A).
Fig. 4.
Casp8p41 requires caspase 9 to induce apoptosis. (A) Western blotting for caspase 9 in Jurkat, JMR, and F9 cell lines with actin as a loading control. F9 cells were reconstituted with Flag-caspase 9. (B) Jurkat, JMR, and F9 cells were transfected with the GFP empty vector, GFP-Casp8p41, or GFP-Casp8p41ΔDED and stained by TUNEL at 10 h posttransfection. Shown are the proportions of GFP+ cells that are TUNEL positive. (C) Pooled results from three independent experiments showing percentages of GFP-positive cells that are also TUNEL positive, with P values shown only for significant comparisons. (D) Light scatter of GFP-positive populations in Jurkat, JMR, and F9 cells analyzed at 10 h posttransfection. (E) Annexin V staining of GFP-positive Jurkat, JMR, or F9 cells at 12 h posttransfection. (F) Graphic representation with standard deviations from three independent experiments showing percentages of GFP-positive cells that are annexin V positive, with P values shown only for significant comparisons.
GFP, GFP-Casp8p41, or GFP-Casp8p41ΔDED was transfected into the three cell types, and apoptosis was assessed specifically in the GFP-positive populations. When caspase 9-deficient JMR cells were transfected with GFP-Casp8p41, a reduction of TUNEL staining was seen at 10 h posttransfection compared to caspase 9-containing Jurkat cells (P < 0.01) (Fig. 4B and C). A reconstitution of the caspase 9-deficient JMR cells with FLAG-caspase 9 (F9 cells) restores GFP-Casp8p41-induced apoptosis (P < 0.01). The same effects were seen by light scatter and annexin V staining (P < 0.05 for JMR versus Jurkat cells and P < 0.01 for JMR versus F9 cells) (Fig. 4D, E, and F).
Pseudotyped HIV-1-induced cell death requires caspase 9.
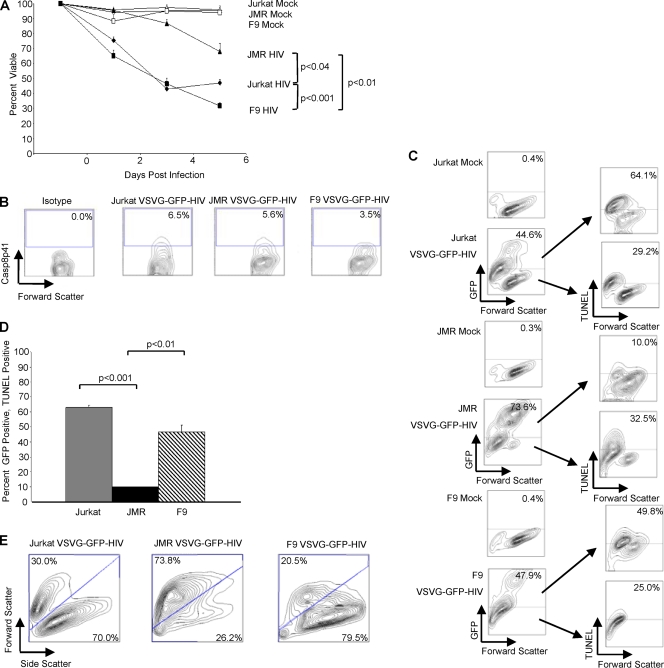
Casp8p41 is produced only in HIV-1-infected cells and is cytotoxic to those cells in which it is expressed (44). Indeed, the expression of HIV-1 protease in the presence of an HIV-1 protease inhibitor blocks both Casp8p41 production and protease-induced death (44). Since Casp8p41 requires caspase 9 to induce death, we hypothesized that HIV-1-induced cell death would be attenuated in caspase 9-deficient cells that are infected with HIV-1. Jurkat cells, caspase 9-deficient JMR cells, and caspase 9-deficient cells reconstituted with FLAG-caspase 9 (F9 cells) were infected with a pseudotyped HIV-1 reporter virus (HIV-1gfp, which is env and vpr minus), and cell viability was measured in the infected (GFP-positive) and uninfected (GFP-negative) cells over the course of 6 days postinfection. Starting at 2 days postinfection, the viabilities of Jurkat cells and the reconstituted F9 cells decreased, whereas the caspase 9-deficient JMR cells and the mock-infected cells remained more viable (Fig. 5A). Not surprisingly, the caspase 9-deficient cells underwent small degrees of HIV-1-induced cell death, likely due to other HIV-1 proteins that do not require caspase 9 to cause death. However, the total number of dead cells in the infected JMR culture was significantly lower (P < 0.001) than that in the infected Jurkat and F9 cell cultures. Differences in viability are not due to differences in levels of infection; supernatant p24 levels from infected caspase 9-deficient cell culture were comparable to those of Jurkat and reconstituted FLAG-caspase 9-infected cultures (126.56 ng, 170.13 ng, and 211.56 ng p24 per ml of supernatant for Jurkat, JMR, and F9 cells, respectively [P > 0.05]); and Casp8p41 expression levels were similar across cell lines (Fig. 5B). The numbers of GFP-positive cells were comparable in Jurkat and FLAG-caspase 9-reconstituted F9 cells, while the caspase 9-deficient JMR cells had higher numbers of GFP-positive cells, indicating more productively infected cells (Fig. 5C), consistent with previously reported observations that the inhibition of HIV-1-induced cell death enhances productive HIV-1 infection (8). Indeed, the HIV-1-infected Jurkat and FLAG-caspase 9-reconstituted F9 cells had a higher percentage of GFP-positive cells that were TUNEL positive than did caspase 9-deficient JMR cells, indicating that productive HIV-1 infection causes apoptotic cell death in the presence, but not in the absence, of caspase 9 (Fig. 5C and D). Light scatter analysis of the infected cell populations confirmed the changes in cell death seen with trypan blue and TUNEL stainings (Fig. 5E). Rates of cell death in the GFP-negative (uninfected) cells were comparable in the presence or absence of caspase 9, suggesting that the bystander mechanism(s) of cell death is not dependent upon caspase 9 (Fig. 5C).
Fig. 5.
HIV-1-induced apoptosis is reduced in caspase 9-deficient cells. (A) Jurkat, JMR, and F9 cells were infected with pseudotyped GFP–HIV-1 or mock infected, and viability was measured over 6 days. (B) Casp8p41 expression levels in GFP+ cells at 4 days postinfection. (C) On day 6, 1 million cells from mock-infected and pseudotyped GFP–HIV-1-infected cells were stained by TUNEL. Analysis shows the TUNEL positivity for both GFP-positive and GFP-negative cells. (D) Graphic representation with standard deviations for GFP-positive cells from the GFP–HIV-1-infected cells that were also TUNEL positive, with P values shown only for significant comparisons. (E) Light scatter on the GFP-positive population of pseudotyped GFP–HIV-1-infected cells.
DISCUSSION
While there are numerous mechanisms by which CD4 T cell numbers might be reduced during HIV-1 disease, few of these mechanisms are HIV-1 specific (12). In the case of apoptosis induced by HIV-1 proteins, it has been impossible to discern the relevance of those stimuli in vivo because nothing distinguishes a dying cell that was stimulated to die, for example, by Tat from one stimulated to die by Fas ligand. However, the identification of Casp8p41 and its characteristic of being HIV-1 specific allows measurements of its presence relative to those of other mechanisms of cell death, in turn allowing an assessment of its contribution to T cell death in vivo (44). Indeed, demonstrations of Casp8p41 in peripheral blood mononuclear cells (PBMC) and lymph nodes of HIV-1-infected patients (44), its correlation with viral replication (3), and its ability to predict CD4 T cell changes in patients altogether suggest its pathophysiological relevance (13). Therefore, it is important to gain a further understanding of the mechanism by which Casp8p41 induces CD4 T cell death. In the present study we have demonstrated that Casp8p41-induced cell death, and, indeed, HIV-1-induced cell death, is dependent on the linked events of Bax/Bak-dependent mitochondrial depolarization and the subsequent activation of caspase 9. These findings coupled with our immunoelectron microscopy and subcellular fractionation data provide an insight into potential molecular targets for Casp8p41.
The mechanisms by which Bax and Bak are activated are complex but can include proapoptotic BH3-only proteins, including Bad, Bim, or Noxa, directly binding antiapoptotic proteins, including Bcl-xL, Mcl-1, or Bcl-2, allowing activated Bax and Bak to oligomerize and release cytochrome c from the mitochondria (66). Another theory suggested a direct activation of Bax/Bak via binding with BH3 proteins such as tBID (62). No caspase has been shown to interact directly with Bax or Bak; however, caspase interactions with other Bcl-2 family members promote Bax/Bak-dependent apoptosis (59). For example, Bcl-2 and Mcl-1 are cleaved by caspase 3, which derepresses their antiapoptotic effect and promotes Bax/Bak-dependent death (7, 65). Caspase 8 causes apoptosis by cleaving BID to tBID, which later activates Bax and Bak directly (27, 35). Since Casp8p41 does not contain the catalytic site of caspase 8 (44), it is unlikely to act through BID activation; however, it may act upon other Bcl-2 family members in a noncatalytic manner to derepress Bax and/or Bak. If true, then the discovery of which Bcl-2 homolog interacts with Casp8p41 will have profound implications for our understanding of HIV-1 pathogenesis and will lend important therapeutic insights.
Controversy still exists as to whether infected-cell killing or uninfected-cell killing predominates during the progression of HIV-1 to AIDS (16, 20, 37). Favoring the uninfected cell death argument is a plethora of data that indicate that HIV-1 can induce bystander death, and a single paper more than a decade old concluded that uninfected-cell death predominates based on in situ hybridization (ISH) data that inexplicably found no infected cells dying (17). Conversely, data supporting the importance of infected-cell killing, particularly in lymphoid tissues, have emerged from human and macaque experiments, as follows. Gut biopsy specimens from HIV-1-infected patients in the chronic phase of disease uniformly demonstrated a proinflammatory infiltration associated with an absence of CD4 T cells (10) in both the large and small bowels (11, 28, 60). In acute infection of rhesus macaques with simian immunodeficiency virus (SIV), within days of infection, a similarly dramatic depletion of gastrointestinal (GI) tract CD4 T cells was observed (61). Phenotypic assessments of which gut CD4 T cells are lost during HIV-1/SIV infection of individuals (5, 21, 33, 39, 45, 50, 51, 54) confirmed the preferential depletion of CD4 T cells and indicated that the majority of such cells express CCR5 and are therefore permissive to infection (30, 46). The suggestion that the majority of these cells, which are later depleted, are infected was confirmed by ISH for HIV-1 and SIV during the acute phase of disease (22, 32, 39, 53) as well as by flow cytometric sorting of defined T cell subsets followed by quantitative PCR assays for viral DNA (4, 16). The results of such studies confirm the overwhelming number of CD4 T cells in the gut that are directly infected and demonstrate that gut CD4 T cells are 10-fold more frequently infected than CD4 T cells in the peripheral blood (36, 40), with up to 60% of gut CD4 T cells being directly infected (9). Conversely, in long-term-nonprogressor populations, gut viral burden is limited, and CD4 T cell numbers are preserved (49). Taken together, these data confirm that in those studies that have examined mechanisms of CD4 depletion in the gut, there is a massive infection of CD4+ T cells in the gut and that days to weeks later, these cells are lost, implying that mechanisms of infected-cell death are responsible. In either case, the question arises as to why a virus would kill its host cell (19). Teleologically, it appears that as the virus has used up the host machinery, apoptosis is initiated, which activates NF-κB in a caspase-dependent manner. This has the effect of (i) directly stimulating viral replication, and (ii) driving a proinflammatory cytokine cascade, which serves to activate a population of virus-permissive target cells. Both effects have been specifically demonstrated for Casp8p41 (3, 58). Furthermore, HIV-1 proteins such as Nef activate the T cell to increase viral production but can downregulate antiapoptotic proteins such as Bcl-2 and Bcl-xL, which leaves the cell vulnerable to other host-driven apoptotic stimuli (12, 63).
Our data also extend our understanding of caspase 8 biology. For several years conflicting reports variably identified caspase 8 as either a cytosolic protein or a mitochondrial protein (38, 47). In our experience, full-length caspase 8 expression adopts a cytoplasmic or plasma membrane-associated distribution (1), yet Casp8p41 is more often mitochondrial. These observations may be rationalized by a recent report that the mitochondrial protein cardiolipin serves as a recruiting and anchoring protein for caspase 8. This allows caspase 8 to be embedded in the mitochondrial membrane and further oligomerize, whereupon it cleaves BID to tBID, which in turn activates Bax (18). These data coupled with our own suggest that the cleavage of caspase 8, either by autoactivation or by HIV-1 protease, results in a change in the localization of caspase 8 from the cytosol to the mitochondria.
The ultimate goal of eradicating HIV-1 infection will require both the suppression of viral replication and the removal of latently infected viral reservoirs through the selective induction of cell death. An increased understanding of how HIV-1 causes infected-cell death through Casp8p41 may lead to novel strategies to augment this death of all infected cells, including those destined to become reservoirs.
ACKNOWLEDGMENTS
We thank Scott Kaufmann and Zheng Dong for the gift of cell lines.
This work was supported by a grant from the National Institutes of Health (grant R01 AI40384).
Footnotes
Published ahead of print on 8 June 2011.
REFERENCES
- 1. Algeciras-Schimnich A., et al. 2007. Analysis of HIV protease killing through caspase 8 reveals a novel interaction between caspase 8 and mitochondria. Open Virol. J. 1:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bellone G., et al. 2000. Production and pro-apoptotic activity of soluble CD95 ligand in pancreatic carcinoma. Clin. Cancer Res. 6:2448–2455 [PubMed] [Google Scholar]
- 3. Bren G. D., et al. 2008. Infected cell killing by HIV-1 protease promotes NF-kappaB dependent HIV-1 replication. PLoS One 3:e2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenchley J. M., et al. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 78:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenchley J. M., et al. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Q., Gong B., Almasan A. 2000. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 7:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng E. H., et al. 1997. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278:1966–1968 [DOI] [PubMed] [Google Scholar]
- 8. Chinnaiyan A. M., Woffendin C., Dixit V. M., Nabel G. J. 1997. The inhibition of pro-apoptotic ICE-like proteases enhances HIV replication. Nat. Med. 3:333–337 [DOI] [PubMed] [Google Scholar]
- 9. Chun T. W., et al. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188 [DOI] [PubMed] [Google Scholar]
- 10. Clayton F., et al. 1992. Rectal mucosal pathology varies with human immunodeficiency virus antigen content and disease stage. Gastroenterology 103:919–933 [DOI] [PubMed] [Google Scholar]
- 11. Clayton F., Snow G., Reka S., Kotler D. P. 1997. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin. Exp. Immunol. 107:288–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummins N., Badley A. D. 2010. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis. 1:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cummins N. W., et al. 2010. Intracellular Casp8p41 content is inversely associated with CD4 T cell count. J. Infect. Dis. 202:386–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Decaudin D., et al. 1997. Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res. 57:62–67 [PubMed] [Google Scholar]
- 15. Dive C., et al. 1992. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim. Biophys. Acta 1133:275–285 [DOI] [PubMed] [Google Scholar]
- 16. Douek D. C., Picker L. J., Koup R. A. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265–304 [DOI] [PubMed] [Google Scholar]
- 17. Finkel T. H., et al. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129–134 [DOI] [PubMed] [Google Scholar]
- 18. Gonzalvez F., et al. 2008. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J. Cell Biol. 183:681–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gougeon M. L. 2003. Apoptosis as an HIV strategy to escape immune attack. Nat. Rev. Immunol. 3:392–404 [DOI] [PubMed] [Google Scholar]
- 20. Grossman Z., Meier-Schellersheim M., Sousa A. E., Victorino R. M., Paul W. E. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319–323 [DOI] [PubMed] [Google Scholar]
- 21. Guadalupe M., et al. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heise C., Vogel P., Miller C. J., Halsted C. H., Dandekar S. 1993. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am. J. Pathol. 142:1759–1771 [PMC free article] [PubMed] [Google Scholar]
- 23. Hengartner M. O. 2000. The biochemistry of apoptosis. Nature 407:770–776 [DOI] [PubMed] [Google Scholar]
- 24. Kaplan A. H., Manchester M., Swanstrom R. 1994. The activity of the protease of human immunodeficiency virus type 1 is initiated at the membrane of infected cells before the release of viral proteins and is required for release to occur with maximum efficiency. J. Virol. 68:6782–6786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaplan A. H., Swanstrom R. 1991. The HIV-1 gag precursor is processed via two pathways: implications for cytotoxicity. Biomed. Biochim. Acta 50:647–653 [PubMed] [Google Scholar]
- 26. Kaplan A. H., Swanstrom R. 1991. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc. Natl. Acad. Sci. U. S. A. 88:4528–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim H., et al. 2009. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell 36:487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kotler D. P., Reka S., Clayton F. 1993. Intestinal mucosal inflammation associated with human immunodeficiency virus infection. Dig. Dis. Sci. 38:1119–1127 [DOI] [PubMed] [Google Scholar]
- 29. Kroemer G., Galluzzi L., Brenner C. 2007. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87:99–163 [DOI] [PubMed] [Google Scholar]
- 30. Lapenta C., et al. 1999. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur. J. Immunol. 29:1202–1208 [DOI] [PubMed] [Google Scholar]
- 31. Li P., et al. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489 [DOI] [PubMed] [Google Scholar]
- 32. Li Q., et al. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 33. Lim S. G., et al. 1993. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin. Exp. Immunol. 92:448–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Llano M., et al. 2006. An essential role for LEDGF/p75 in HIV integration. Science 314:461–464 [DOI] [PubMed] [Google Scholar]
- 35. Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490 [DOI] [PubMed] [Google Scholar]
- 36. Mattapallil J. J., et al. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 37. McCune J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974–979 [DOI] [PubMed] [Google Scholar]
- 38. Medema J. P., et al. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mehandru S., et al. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehandru S., et al. 2007. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 81:599–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nair S. C., et al. 1996. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones 1:237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Natesampillai S., Nie Z., Cummins N. W., Bren G. D., Jocmans D. 2010. Patients with discordant responses to antiretroviral therapy have impaired killing of HIV-infected T cells. PLoS Pathog. 6:e1001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nie Z., Bren G. D., Rizza S. A., Badley A. D. 2008. HIV protease cleavage of procaspase 8 is necessary for death of HIV-infected cells. Open Virol. J. 2:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nie Z., et al. 2007. Human immunodeficiency virus type 1 protease cleaves procaspase 8 in vivo. J. Virol. 81:6947–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Picker L. J., et al. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poles M. A., Elliott J., Taing P., Anton P. A., Chen I. S. 2001. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 75:8390–8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qin Z. H., et al. 2001. Pro-caspase-8 is predominantly localized in mitochondria and released into cytoplasm upon apoptotic stimulation. J. Biol. Chem. 276:8079–8086 [DOI] [PubMed] [Google Scholar]
- 48. Samraj A. K., Keil E., Ueffing N., Schulze-Osthoff K., Schmitz I. 2006. Loss of caspase-9 provides genetic evidence for the type I/II concept of CD95-mediated apoptosis. J. Biol. Chem. 281:29652–29659 [DOI] [PubMed] [Google Scholar]
- 49. Sankaran S., et al. 2005. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 102:9860–9865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schneider T., et al. 1995. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut 37:524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shacklett B. L., et al. 2003. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J. Virol. 77:5621–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sharpe J. C., Arnoult D., Youle R. J. 2004. Control of mitochondrial permeability by Bcl-2 family members. Biochim. Biophys. Acta 1644:107–113 [DOI] [PubMed] [Google Scholar]
- 53. Smith P. D., Fox C. H., Masur H., Winter H. S., Alling D. W. 1994. Quantitative analysis of mononuclear cells expressing human immunodeficiency virus type 1 RNA in esophageal mucosa. J. Exp. Med. 180:1541–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smit-McBride Z., Mattapallil J. J., McChesney M., Ferrick D., Dandekar S. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646–6656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Somervaille T. C., Linch D. C., Khwaja A. 2001. Growth factor withdrawal from primary human erythroid progenitors induces apoptosis through a pathway involving glycogen synthase kinase-3 and Bax. Blood 98:1374–1381 [DOI] [PubMed] [Google Scholar]
- 56. Strehlau J., et al. 1997. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc. Natl. Acad. Sci. U. S. A. 94:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tancrede C. H., Andremont A. O. 1985. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J. Infect. Dis. 152:99–103 [DOI] [PubMed] [Google Scholar]
- 58. Taylor J. A., et al. 2010. Casp8p41 expression in primary T cells induces a proinflammatory response. AIDS 24:1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taylor R. C., Cullen S. P., Martin S. J. 2008. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 9:231–241 [DOI] [PubMed] [Google Scholar]
- 60. Ullrich R., Zeitz M., Riecken E. O. 1992. Enteric immunologic abnormalities in human immunodeficiency virus infection. Semin. Liver Dis. 12:167–174 [DOI] [PubMed] [Google Scholar]
- 61. Veazey R. S., et al. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431 [DOI] [PubMed] [Google Scholar]
- 62. Walensky L. D., et al. 2006. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell 24:199–210 [DOI] [PubMed] [Google Scholar]
- 63. Wang J. K., Kiyokawa E., Verdin E., Trono D. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. U. S. A. 97:394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wei M. C., et al. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weng C., Li Y., Xu D., Shi Y., Tang H. 2005. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J. Biol. Chem. 280:10491–10500 [DOI] [PubMed] [Google Scholar]
- 66. Willis S. N., et al. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315:856–859 [DOI] [PubMed] [Google Scholar]
- 67. Youle R. J., Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9:47–59 [DOI] [PubMed] [Google Scholar]