Abstract
Infection of C57BL/6 mice by the intracerebral route with the Daniels (DA) strain of Theiler's murine encephalomyelitis virus (TMEV) resulted in acute behavioral seizures in approximately 50% of the mice. By titration, the viral dose correlated with the percentage of mice developing seizures; however, neuropathological changes were similar over the dose range, and viral clearance from the brains occurred uniformly by day 14 postinfection (p.i.). Other TMEV strains and mutants (GDVII, WW, BeAn 8386 [BeAn], DApBL2M, H101) induced seizures in C57BL/6 mice to various degrees. The BeAn strain and DApBL2M mutant were similar to the DA strain in the percentages of mice developing seizures and neuropathological changes and in the extent of infected cells. The GDVII and WW strains caused 100% mortality by days 5 and 6 p.i., respectively, at which time neuropathological changes and neuronal infection were extensive. The H101 mutant induced seizures and caused 100% mortality by day 7 p.i.; however, only minor neuropathological changes and few infected cells were observed. Thus, in H101 mutant infections, it appears that elevated levels of cytokines, rather than neuronal cell death, play the dominant role in seizure induction.
INTRODUCTION
Different neurotropic viruses cause encephalitis, and many patients with viral encephalitis experience seizures during acute infection (reviewed in reference 4). Compared to the general population, these individuals have a 20% greater risk of experiencing seizures. In addition, 4 to 20% of recovered viral encephalitis patients go on to develop epilepsy, which has been estimated to affect 8 persons per 1,000 in the general population (reviewed in reference 4).
Infection of C57BL/6 mice with the Daniels (DA) strain of Theiler's murine encephalomyelitis virus (TMEV) induces behavioral seizures in about 50% of the infected mice between days 3 and 10 postinfection (p.i.) (8). The seizures occurred at a frequency of one per mouse per 2-h observation period and typically lasted for 1 to 2 min (8, 19). The seizures were afebrile and limbic in nature (8). No mouse had seizures before day 3 p.i., and the seizures peaked on day 6 p.i. The majority of seizures were assigned a Racine scale seizure score of 3 (forelimb clonus) or above (score of 4, rearing; score of 5, rearing and falling) (8, 16). Mice experiencing seizures were impaired in both motor function and coordination (8). Extended observation, including video-electroencephalography (video-EEG), showed that mice experiencing acute behavioral seizures had an asymptomatic latent period followed by the development of spontaneous seizures or epilepsy several months later (18, 19).
Our earlier studies concentrated on innate immune contributions to the development of seizures (5, 7, 9). We previously found that tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) and concomitant inflammatory changes in the brain contribute to the development of acute seizures (5, 7). Further, we have shown the importance of complement component 3 (C3) activation in the central nervous system (CNS) for the development of acute seizures (9). C3 activation within the brain in response to viral infection may help drive inflammation and cytokine production, ultimately leading to seizures.
Here we investigate viral contributions to the development of acute seizures. TMEV is a nonenveloped, positive-sense, single-stranded RNA virus of the family Picornaviridae. This virus is a naturally occurring enteric pathogen of the mouse (20, 21). The strains of TMEV are divided into two groups based on their neurovirulence following intracerebral (i.c.) inoculation of mice (6). The less neurovirulent strain group is the Theiler's original (TO) group and includes the TO, DA, BeAn 8386 (BeAn), and WW strains of TMEV. Susceptible strains of mice (SJL/J) survive the acute polioencephalomyelitis that occurs at 1 to 2 weeks p.i. following i.c. inoculation and subsequently develop an inflammatory demyelinating disease with viral persistence around 4 weeks p.i. (6). Although highly homologous at both the nucleotide and amino acid levels and highly similar with respect to the CNS disease produced, there are differences between the individual strains of the TO group that can be seen upon direct comparison of neuropathological changes following infection (27). C57BL/6 mice infected i.c. with strains from the TO group develop acute disease; however, the virus is subsequently cleared. Therefore, C57BL/6 mice are resistant to the demyelinating disease (13). The highly neurovirulent group of TMEV strains is the GDVII group, which includes the GDVII and FA strains; i.c. infection of SJL/J mice results in a fatal acute polioencephalomyelitis (6). The neuropathological changes observed in SJL/J mice during acute infection are distinct for the GDVII and DA strains (22). C57BL/6 mice infected i.c. with GDVII also succumb to fatal acute polioencephalomyelitis (8). In addition to differences in neurovirulence, neuropathology, ability to induce acute versus chronic disease, and ability to persist, strains of the TO and GDVII groups also differ in the size of the plaques that they form on cell monolayers during viral plaque assays (11). Strains of the GDVII group form large plaques, while strains of the TO group form small plaques (11).
Our current study investigated both the contribution of the initial viral dose to the development of seizures and the ability of other TMEV strains or mutants to induce acute seizures. The contribution of the viral dose was determined for the DA strain. Also, infections with the DA, GDVII, WW, and BeAn strains and DApBL2M and H101 mutants of TMEV were compared. The DA strain was isolated in 1948 from the brain of a mouse in the Harvard colony that displayed spontaneous paralysis (2). The WW strain was isolated in the late 1970s during the course of inoculating multiple sclerosis brain homogenates (human cadaver source) into newborn mice (ICR strain) (26), and it forms small plaques (10). The BeAn strain was isolated from a feral mouse in Belem, Brazil, in 1957 (15, 17). The GDVII strain was isolated in the late 1930s from the brain of a sick mouse 11 days p.i. (21). Viral isolates were initially passaged through mouse brains and later adapted to cell culture and subjected to plaque purification (10).
The DApBL2M and H101 mutants of TMEV were generated previously in our laboratory. The DApBL2M mutant of the DA strain was created through standard molecular biological manipulations of the full-length infectious cDNA clone of the DA strain (24, 25). The DApBL2M mutant encodes VP1 loop II of the GDVII strain plus a point mutation (S171R) in VP2 puff B on the background of the DA strain (24). The H101 mutant was inadvertently created as a result of a transcription error by the T7 polymerase while a modified full-length infectious cDNA clone of the DA strain was in use as the template (23). The H101 mutant encodes a point mutation (T101I) in VP1 loop II, as was expected from the modified full-length infectious cDNA clone; however, there are also several nucleotide substitutions in the 5′ untranslated region as well as additional amino acid substitutions in the capsid protein-coding region (23). VP1 loop II and VP2 puff B are critical components of the receptor binding site (24). The DApBL2M and H101 mutants both form small plaques on cell monolayers (23, 24).
From the virus titration, we found that the percentage of C57BL/6 mice that developed seizures correlated to the initial viral dose of the DA strain. However, neuropathological changes did not differ, and the viral-antigen-positive cells were uniformly cleared from the brain by day 14 p.i. over the dose range examined.
From the viral strain comparison, we found that other strains and mutants of TMEV induced seizures to various degrees. The BeAn strain and DApBL2M mutant were found to be comparable to the DA strain with respect to the percentages of mice displaying seizures, neuropathological changes, and viral clearance. The GDVII and WW strains induced seizures but caused 100% mortality by day 6 p.i., with extensive neuropathological changes and viral-antigen-positive cells in the brain. The H101 mutant induced seizures and caused 100% mortality by day 7 p.i. in the absence of both neuropathological changes and virus-infected cells in the brain. Therefore, it appears that multiple pathways, including neuronal cell destruction (GDVII) and cytokine activity (H101), may lead to the development of seizures following viral infection and that these pathways need not be mutually exclusive but can act in concert.
MATERIALS AND METHODS
Animals.
C57BL/6 male mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The care and use of the mice were performed in accordance with the guidelines prepared by the committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council.
Infection.
On day 0, mice (5 to 6 weeks old) were anesthetized with isoflurane (inhalation) and infected i.c. with 3 × 103, 3 × 104, 3 × 105, or 3 × 106 PFU of the DA strain for virus titration or were subjected to mock infection with 20 μl of phosphate-buffered saline (PBS). The site of injection was in the postparietal cortex of the right cerebral hemisphere to a depth of 2 mm (posterior [caudal] and medial of the right eye at approximately bregma −2 mm and interaural +8 mm) (5). The needle had a William's collar to limit penetration of the tip to 2 mm. The DA strain was propagated as previously described (28).
On day 0, mice (5 to 6 weeks old) were infected as described above with 3 × 105 PFU of the DA, WW (from Howard Lipton, University of Illinois, Chicago), BeAn (American Type Culture Collection, Manassas, VA), DApBL2M, and H101 strains and mutants or with 1 × 103 PFU of the GDVII strain (for viral strain comparisons). The WW, BeAn, DApBL2M, GDVII, and H101 strains and mutants were propagated in BHK-21 cells as previously described for strain DA (28).
Observations.
To monitor seizure activity, mice were observed continuously for 2 h (randomly between the hours of 9:00 a.m. and 5:00 p.m) each day p.i. through day 14 p.i. Seizure activity was graded using the Racine scale as follows: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; and stage 5, rearing and falling (1, 16).
Histology.
Infected or mock-infected mice were euthanized with isoflurane on days 5, 6, 7, and 14 p.i. in order to allow observation of the neurological manifestations (neuronal cell death and inflammation) of the seizures. Animals were perfused with PBS followed by a buffered 4% paraformaldehyde solution. Brains were harvested and fixed in 4% paraformaldehyde, divided into five coronal slabs per brain, and embedded in paraffin. Multiple 4-μm-thick tissue sections, containing sections from all five coronal slabs per brain, were cut, mounted on slides, and stained via various methods as described below. The tissue section of only one of the five coronal slabs represented per slide contained the hippocampal/dentate gyral regions of the brain.
Luxol fast blue-stained slides were used to enumerate perivascular cuffs (PVC), comprised of infiltrating mononuclear cells, and to evaluate neuronal cell loss in the hippocampus in a blinded fashion using one slide per brain (n = 4 to 6 brains per group for the viral titration experiment; n = 1 to 11 brains per group for the viral strain comparison experiment). PVC were enumerated and summed for each of the two hippocampi present in a brain and each of the two dentate gyri present in a brain. Neuronal cell loss in the pyramidal cell layer of the hippocampus, from CA1 to CA3, was scored as follows: score 0, no damage; score 1, 10 to 29% cell loss (10 to 29% of the pyramidal cell layer from CA1 to CA3 was missing); score 2, 30 to 59% cell loss; and score 3, >60% cell loss (5). An undamaged (intact) control brain was assigned a score of 0 with no cell loss (and thus 100% of the cells present). A score was given for each of the two hippocampi present in a brain, and then the scores were summed; thus, the highest possible score for cell loss per brain was 6 (i.e., the sum of the highest score, 3, for each of two regions of the brain). The cytoarchitecture of the hippocampus was referenced to Fig. 43 to 49 in The Mouse Brain in Stereotaxic Coordinates (3).
Immunohistochemistry.
Viral-antigen-positive cells were detected on paraffin sections using TMEV hyperimmune rabbit serum, as previously described (24, 28). The slides were labeled using the avidin-biotin peroxidase complex technique with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO)-0.01% hydrogen peroxide (Sigma)-PBS. Specificity of antibody binding was confirmed by parallel staining minus the hyperimmune serum.
Enumeration of viral-antigen-positive cells was performed in a blinded fashion by using one slide per brain and by evaluating tissue sections from all five coronal slabs represented per slide (n = 4 to 6 brains per group for the viral titration experiment; n = 1 to 11 brains per group for the viral strain comparison experiment). Viral-antigen-positive cells were enumerated and summed from all five coronal slabs represented per slide for the following brain regions in C57BL/6 mice: frontal lobe, septum, caudoputamen, hippocampus, thalamus, hypothalamus, midbrain, and cortex.
Statistical analysis.
The StatView program (SAS Institute Inc., Cary, NC) was used for all statistical analyses. Analysis of variance (ANOVA), followed when necessary by the Fisher's Protected Least Significant Difference (PLSD) post hoc test, was performed to determine group differences for continuous data (viral-antigen-positive cells, PVC). The chi-square test was utilized for nominal data (i.e., the presence or absence of seizures). Finally, the unpaired two-group Mann-Whitney U test was performed for all nonparametric analyses (neuronal cell loss).
RESULTS
Viral titration.
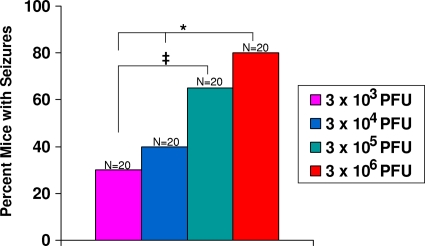
To investigate the contribution of the initial viral dose to the development of seizures, C57BL/6 mice were infected with 3 × 103, 3 × 104, 3 × 105, or 3 × 106 PFU of the DA strain and observed for the development of seizures through day 14 p.i. The percentage of mice that developed seizures increased as the viral dose increased: 30% at 3 × 103 PFU, 40% at 3 × 104 PFU, 65% at 3 × 105 PFU, and 80% at 3 × 106 PFU (Fig. 1). Therefore, the viral dose directly correlated with the percentage of mice having seizures. However, the frequency (one per mouse per 2 h observation period) and duration (1 to 2 min) of the seizures were not impacted by the dose of virus.
Fig. 1.
Seizure (Racine scale stage 3 to 5) frequency. Mice were infected with increasing amounts of the Daniels (DA) strain and observed for the development of seizures through day 14 p.i. ‡, P < 0.05; *, P < 0.01 (chi square). The total numbers of mice infected (N) are shown over the individual bars of the graph. Percent mice with seizures (y axis) is calculated as follows: (number of mice with seizures/total number of mice infected) × 100.
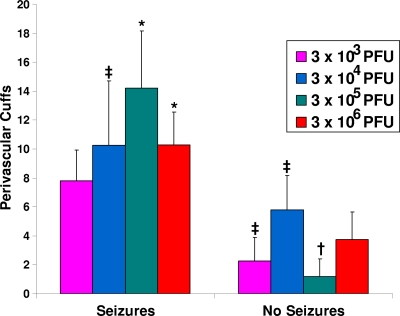
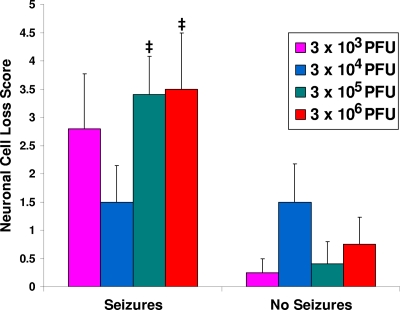
PVC and neuronal cell loss (markers of inflammation and cell death) were evaluated in the hippocampi in the brains to determine the effect of viral dose on neuropathology. The dose of DA did not affect the numbers of PVC (Fig. 2) or the extent of neuronal cell loss (Fig. 3) in mice that developed seizures (left bars) or in those that did not develop seizures (right bars). There were significant differences between mice that had seizures and mice that did not have seizures, which is consistent with our previous observations (5). From this we conclude that inflammation and cell death in the brain were not affected by the viral dose in those mice that developed seizures. In general, the mice that developed seizures exhibited more extensive inflammation and cell death than the mice that did not develop seizures.
Fig. 2.
Perivascular cuffing within the hippocampus. Perivascular cuffs (PVC) were enumerated and the totals summed for each of the two hippocampi and dentate gyri present in a brain for mice sacrificed on day 14 p.i. The only significant differences seen were between some of the mice infected with the various amounts of the DA strain that had seizures and those that did not have seizures. Significant differences occurred between mice infected with 3 × 104 PFU that had seizures and mice infected with 3 × 103 and 3 × 105 PFU that did not have seizures (P < 0.05). Differences were noted between mice infected with 3 × 105 PFU that had seizures and mice infected with 3 × 103, 3 × 106 (P < 0.01), 3 × 104 (P < 0.05), and 3 × 105 (P < 0.001) PFU that did not have seizures. Differences were also seen between mice infected with 3 × 106 PFU that had seizures and mice infected with 3 × 103 (P < 0.05) and 3 × 105 (P < 0.01) PFU that did not have seizures. ‡, P < 0.05; *, P < 0.01; †, P < 0.001 (ANOVA, Fisher's PLSD post hoc test). Results represent the means + standard errors of the mean (SEM) of the results for groups with four to six mice per group.
Fig. 3.
Neuronal cell loss within the hippocampus. Neuronal cell loss was scored as described in Materials and Methods for mice sacrificed on day 14 p.i. The only significant differences seen were between some of the mice infected with the various amounts of the DA strain that had seizures and those that did not have seizures. Differences occurred between mice infected with 3 × 105 PFU that had seizures and mice infected with 3 × 103, 3 × 105, and 3 × 106 PFU that did not have seizures. Differences were also noted between mice infected with 3 × 106 PFU that had seizures and mice infected with 3 × 105 PFU that did not have seizures. ‡, P < 0.05 (Mann-Whitney U test). Results represent means + standard errors of the means (SEM) of data from groups with four to six mice per group.
Viral-antigen-positive cells were enumerated in the brains of mice infected with the DA strain to determine the effect of viral dose on viral clearance at day 14 p.i. (Table 1). No viral-antigen-positive cells were detected in mice infected with 3 × 103 or 3 × 104 PFU. A few viral-antigen-positive cells were present in brains of mice infected with 3 × 105 PFU and mice infected with 3 × 106 PFU that had seizures. However, even when viral-antigen-positive cells were detected, they were rare and there were no significant differences among the groups (Table 1). Therefore, DA viral-antigen-positive cells are essentially cleared from the brain by day 14 p.i. irrespective of the initial viral dose.
Table 1.
Viral clearance by day 14 p.i. from brains of DA-infected mice after virus titrationa
| Initial viral dose (PFU) | Mouse group | No. of viral-antigen-positive cells detected per mouse | Mean (± SEM) |
|---|---|---|---|
| 3 × 103 | Seizures | 0, 0, 0, 0, 0 | 0 (0) |
| No seizures | 0, 0, 0, 0 | 0 (0) | |
| 3 × 104 | Seizures | 0, 0, 0, 0, 0 | 0 (0) |
| No seizures | 0, 0, 0, 0, 0, 0 | 0 (0) | |
| 3 × 105 | Seizures | 18, 3, 3, 0, 0 | 4.8 (3.37) |
| No seizures | 31, 0, 0, 0, 0 | 6.2 (6.2) | |
| 3 × 106 | Seizures | 35, 1, 1, 0, 0, 0 | 6.17 (5.77) |
| No seizures | 0, 0, 0, 0 | 0 (0) |
DA, Daniels; p.i., postinfection; SEM, standard error of the mean.
Viral strain comparison.
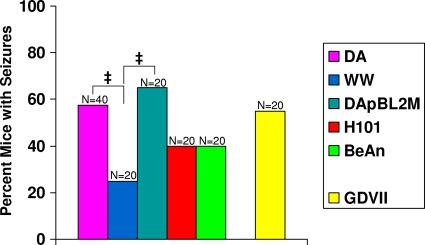
To investigate the ability of other TMEVs to induce acute seizures, mice were infected with the various TMEVs and observed for the development of seizures through day 14 p.i. The percentages of mice that developed seizures were 25% for the WW strain, 40% for both the BeAn strain and the H101 mutant, 55% for the GDVII strain, 57.5% for the DA strain, and 65% for the DApBL2M mutant (Fig. 4). The percentage of mice having seizures following infection with the WW strain was low and significantly less than the percentage of mice having seizures following infection with either the DA strain or the DApBL2M mutant (P < 0.05). This could have been due to the death of the WW-infected mice prior to their having seizures. The TO group induced seizures at a rate ranging from 25 to 57.5%, whereas the GDVII strain induced seizures at a rate of 55% (Fig. 4); therefore, the percentage of mice having seizures did not correlate with the TMEV group classification (TO or GDVII) to which the viral strains belonged.
Fig. 4.
Seizure (Racine scale stage 3 to 5) frequency in mice infected with different TMEVs. Mice were infected with various TMEVs (see Materials and Methods) and observed for the development of seizures through day 14 p.i. ‡, P < 0.05 (chi square). Total numbers of mice infected (N) are shown over the individual bars of the graph. Percent mice with seizures (y axis) is calculated as follows: (number of mice with seizures/total number of mice infected) × 100.
PVC numbers and neuronal cell loss (measurements for inflammation and neuronal cell death) were evaluated by examining the hippocampus of mice infected with the DA, WW, BeAn, and GDVII strains and the DApBL2M and H101 mutants on days 5, 6, 7, and 14 p.i. to determine the effect of viral strain on neuropathology. The numbers of PVC for mice that developed seizures and those that did not are given in Tables 2 and 3, respectively. Notably, the mice infected with the H101 mutant that developed seizures had significantly fewer PVC than the mice infected with the DA (P < 0.01) and WW (P < 0.05) strains at day 6 p.i. and significantly fewer PVC than the mice infected with the DApBL2M mutant (P < 0.0001) and the DA (P < 0.0001) and BeAn (P < 0.01) strains at day 7 p.i. (Table 2). There were no significant differences found in the numbers of PVC for the mice that did not develop seizures following infection with the various TMEVs (Table 3).
Table 2.
Perivascular cuffing within the hippocampus of mice with seizures following infection with different TMEVsa
| Viral strain | Day of sacrifice | No. of perivascular cuffs detected per mouse | Mean (± SEM)b |
|---|---|---|---|
| GDVII | 5 | 0, 0, 15, 5, 9, 10, 3, 12, 2, 11, 4 | 6.46 (1.56) |
| 6 | ND | ||
| 7 | ND | ||
| 14 | ND | ||
| WW | 5 | 0 | 0 |
| 6 | 9, 13, 4, 2 | 7 (2.48) | |
| 7 | ND | ||
| 14 | ND | ||
| H101 | 5 | 0, 0 | 0 (0) |
| 6 | 0, 0, 1 | 0.33 (0.33) | |
| 7 | 0, 0, 0 | 0 (0) | |
| 14 | ND | ||
| DA | 5 | ND | |
| 6 | 11, 4, 15 | 10 (3.22) | |
| 7 | 14, 16, 24, 11, 7, 14, 10, 12 | 13.5 (1.79) | |
| 14 | 10, 7, 8, 6, 1, 3, 7 | 6 (1.16) | |
| BeAn | 5 | ND | |
| 6 | ND | ||
| 7 | 19, 9, 6, 9 | 10.75 (2.84) | |
| 14 | 6, 11, 8, 7 | 8 (1.08) | |
| DApBL2M | 5 | ND | |
| 6 | ND | ||
| 7 | 12, 17, 15, 15 | 14.75 (1.03) | |
| 14 | 8, 4, 8, 4 | 6 (1.16) |
DA, Daniels; ND, not determined; SEM, standard error of the mean; TMEV, Theiler's murine encephalomyelitis virus.
ANOVA and Fisher's PLSD post hoc tests are described in the text.
Table 3.
Perivascular cuffing within the hippocampus of mice without seizures following infection with different TMEVsa
| Viral strain | Day of sacrifice | No. of perivascular cuffs detected per mouse | Mean (± SEM)b |
|---|---|---|---|
| GDVII | 5 | 4, 23, 7, 5, 0, 16, 7 | 8.86 (2.99) |
| 6 | ND | ||
| 7 | ND | ||
| 14 | ND | ||
| WW | 5 | 19, 26, 2, 4 | 12.75 (5.82) |
| 6 | 0, 0, 0, 8, 6, 9, 3, 10, 9 | 5 (1.42) | |
| 7 | ND | ||
| 14 | ND | ||
| H101 | 5 | 0, 0, 0, 0, 0 | 0 (0) |
| 6 | 0, 0, 0, 0, 3 | 0.6 (0.6) | |
| 7 | 0 | 0 | |
| 14 | ND | ||
| DA | 5 | ND | |
| 6 | 7, 0 | 3.5 (3.5) | |
| 7 | 5, 11, 10, 1, 0, 0, 0 | 3.86 (1.84) | |
| 14 | 0, 4, 0, 0, 6, 0 | 1.67 (1.09) | |
| BeAn | 5 | ND | |
| 6 | ND | ||
| 7 | 0, 0, 0, 7 | 1.75 (1.75) | |
| 14 | 3, 1, 3, 0, 5, 0, 4 | 2.29 (0.75) | |
| DApBL2M | 5 | ND | |
| 6 | ND | ||
| 7 | 0, 0, 3, 1 | 1 (0.71) | |
| 14 | 0, 0 | 0 (0) |
DA, Daniels; ND, not determined; SEM, standard error of the mean; TMEV, Theiler's murine encephalomyelitis virus.
ANOVA and Fisher's PLSD post hoc tests are described in the text.
The extent of neuronal cell loss for mice that developed seizures and for those that did not develop seizures is given in Tables 4 and 5, respectively. As was seen with the PVC, mice infected with the H101 mutant that developed seizures had significantly less neuronal cell damage than mice infected with the WW strain that had seizures at day 6 p.i. (P < 0.05) and significantly less neuronal cell damage than the mice infected with the BeAn and DA strains that had seizures at day 7 p.i. (P < 0.05) (Table 4). Likewise, the mice infected with the H101 mutant that did not develop seizures had significantly less neuronal cell damage at day 5 p.i. than the mice infected with the GDVII strain that did not develop seizures (P < 0.05) and significantly less neuronal cell damage at day 6 p.i. than the mice infected with the WW strain that did not develop seizures (P < 0.05) (Table 5). There were no significant differences between the mice infected with the various TMEVs that had seizures (Table 4) and those that did not have seizures (Table 5) on day 14 p.i. From these data, we conclude that there were differences in the results with respect to neuropathology: in general, the neuropathology was greatly reduced in the hippocampus of mice infected with the H101 strain at days 5, 6, and 7 p.i. compared to the results seen with other TMEVs.
Table 4.
Neuronal cell loss within the hippocampus of mice with seizures following infection with different TMEVsa
| Viral strain | Day of sacrifice | Score of neuronal cell loss per mouse | Mean (± SEM)b |
|---|---|---|---|
| GDVII | 5 | 0, 4, 2, 4, 4, 3, 3, 4, 5, 4, 5 | 3.46 (0.43) |
| 6 | ND | ||
| 7 | ND | ||
| 14 | ND | ||
| WW | 5 | 0 | 0 |
| 6 | 5, 6, 3, 3 | 4.25 (0.75) | |
| 7 | ND | ||
| 14 | ND | ||
| H101 | 5 | 0, 1 | 0.5 (0.5) |
| 6 | 0, 0, 0 | 0 (0) | |
| 7 | 0, 0, 0 | 0 (0) | |
| 14 | ND | ||
| DA | 5 | ND | |
| 6 | 0, 0, 0 | 0 (0) | |
| 7 | 4, 3, 1, 1, 0, 2, 1, 1 | 1.63 (0.46) | |
| 14 | 6, 5, 1, 3, 4, 0, 1 | 2.86 (0.86) | |
| BeAn | 5 | ND | |
| 6 | ND | ||
| 7 | 2, 2, 2, 2, | 2 (0) | |
| 14 | 6, 5, 5, 6 | 5.5 (0.29) | |
| DApBL2M | 5 | ND | |
| 6 | ND | ||
| 7 | 0, 1, 0, 1, | 0.5 (0.29) | |
| 14 | 6, 2, 1, 3 | 3 (1.08) |
DA, Daniels; ND, not determined; SEM, standard error of the mean; TMEV, Theiler's murine encephalomyelitis virus.
Mann-Whitney U tests are described in the text.
Table 5.
Neuronal cell loss within the hippocampus of mice without seizures following infection with different TMEVsa
| Viral strain | Day of sacrifice | Score of neuronal cell loss per mouse | Mean (± SEM)b |
|---|---|---|---|
| GDVII | 5 | 3, 3, 4, 4, 0, 3, 4 | 3 (0.54) |
| 6 | ND | ||
| 7 | ND | ||
| 14 | ND | ||
| WW | 5 | 5, 1, 0, 2 | 2 (1.08) |
| 6 | 0, 0, 0, 2, 1, 2, 6, 1, 0.5 | 1.39 (0.63) | |
| 7 | ND | ||
| 14 | ND | ||
| H101 | 5 | 0, 0, 0, 0, 1 | 0.2 (0.2) |
| 6 | 0, 0, 0, 0, 0 | 0 (0) | |
| 7 | 0 | 0 | |
| 14 | ND | ||
| DA | 5 | ND | |
| 6 | 0, 0 | 0 (0) | |
| 7 | 0, 3, 1.5, 0, 0, 0, 0 | 0.64 (0.45) | |
| 14 | 0, 5, 0, 0, 2, 0 | 1.17 (0.83) | |
| BeAn | 5 | ND | |
| 6 | ND | ||
| 7 | 0, 0, 0, 0 | 0 (0) | |
| 14 | 0.5, 0, 5, 0, 0, 0, 6 | 1.64 (1.00) | |
| DApBL2M | 5 | ND | |
| 6 | ND | ||
| 7 | 0, 0, 0, 0 | 0 (0) | |
| 14 | 0, 0 | 0 (0) |
DA, Daniels; ND, not determined; SEM, standard error of the mean; TMEV, Theiler's murine encephalomyelitis virus.
Mann-Whitney U tests are described in the text.
Viral-antigen-positive cells were enumerated in mice that were infected with the various TMEVs and were sacrificed on days 5, 6, 7, and 14 p.i. to determine the location of infected cells and the extent of viral clearance. The numbers of viral-antigen-positive cells for mice that developed seizures and those that did not are given in Tables 6 and 7, respectively. As with the neuropathology experiments, the mice infected with the H101 mutant that developed seizures had significantly fewer viral-antigen-positive cells than the mice infected with the GDVII strain that had seizures at day 5 p.i. (P < 0.001), significantly fewer viral-antigen-positive cells than the mice infected with the WW strain that had seizures at day 6 p.i. (P < 0.0001), and significantly fewer viral-antigen-positive cells than the mice infected with either the DA or BeAn strains (both with seizures) at day 7 p.i. (P < 0.05) (Table 6). Also, the mice infected with the H101 mutant that did not develop seizures had significantly fewer viral-antigen-positive cells at day 5 p.i. than the mice infected with the GDVII strain that did not develop seizures (P < 0.0001) and significantly fewer viral-antigen-positive cells at day 6 p.i. than the mice infected with the WW strain that did not develop seizures (P < 0.01) (Table 7). There were no significant differences between the mice infected with the various TMEVs that had seizures (Table 6) and those that did not (Table 7) for those mice sacrificed on day 14 p.i. Therefore, it appears that the H101 mutant was unable to infect cells in any of the various brain regions examined.
Table 6.
Viral clearance from brains of mice with seizures following infection with different TMEVsa
| Viral strain | Day of sacrifice | No. of viral antigen-positive cells per mouse | Mean (± SEM)b |
|---|---|---|---|
| GDVII | 5 | 1,428, 1,442, 1,350, 1,540, 1,240, 1,145, 1,980, 2,180, 2,485, 2,700, 3,485 | 1,906.82 (222.57) |
| 6 | ND | ||
| 7 | ND | ||
| 14 | ND | ||
| WW | 5 | 425 | 425 |
| 6 | 655, 650, 695, 920 | 730 (64.13) | |
| 7 | ND | ||
| 14 | ND | ||
| H101 | 5 | 0, 0 | 0 (0) |
| 6 | 0, 0, 0 | 0 (0) | |
| 7 | 0, 0, 0 | 0 (0) | |
| 14 | ND | ||
| DA | 5 | ND | |
| 6 | 150, 83, 365 | 199.33 (85.06) | |
| 7 | 251, 1,318, 819, 0, 0, 45, 305, 222, 366 | 369.56 (145.30) | |
| 14 | 0, 0, 0, 0, 27, 1, 23 | 7.29 (4.60) | |
| BeAn | 5 | ND | |
| 6 | ND | ||
| 7 | 530, 493, 325, 246 | 398.5 (67.63) | |
| 14 | 0, 0, 0, 13 | 3.25 (3.25) | |
| DApBL2M | 5 | ND | |
| 6 | ND | ||
| 7 | 507, 163, 117, 22 | 202.25 (105.74) | |
| 14 | 0, 0, 0, 0 | 0 (0) |
DA, Daniels; ND, not determined; SEM, standard error of the mean; TMEV, Theiler's murine encephalomyelitis virus.
ANOVA and Fisher's PLSD post hoc tests are described in the text.
Table 7.
Viral clearance from brains of mice without seizures following infection with different TMEVsa
| Viral strain | Day of sacrifice | No. of viral antigen-positive cells per mouse | Mean (± SEM)b |
|---|---|---|---|
| GDVII | 5 | 1,555, 1,390, 880, 1,540, 1,675, 1,180, 2,060, 2,830 | 1,638.75 (209.70) |
| 6 | ND | ||
| 7 | ND | ||
| 14 | ND | ||
| WW | 5 | 710, 570, 300, 270 | 462.5 (106.57) |
| 6 | 174, 328, 310, 430, 850, 201, 800, 220, 110 | 380.33 (89.78) | |
| 7 | ND | ||
| 14 | ND | ||
| H101 | 5 | 0, 0, 0, 0, 0 | 0 (0) |
| 6 | 0, 0, 0, 0, 0 | 0 (0) | |
| 7 | 0 | 0 | |
| 14 | ND | ||
| DA | 5 | ND | |
| 6 | 14, 47 | 30.5 (16.5) | |
| 7 | 0, 33, 0, 7, 174, 30, 2 | 35.14 (23.74) | |
| 14 | 0, 0, 0, 0, 0, 5 | 0.83 (0.83) | |
| BeAn | 5 | ND | |
| 6 | ND | ||
| 7 | 15, 0, 0, 0 | 3.75 (3.75) | |
| 14 | 0, 0, 0, 0, 0, 0, 2 | 0.29 (0.29) | |
| DApBL2M | 5 | ND | |
| 6 | ND | ||
| 7 | 0, 0, 0, 0 | 0 (0) | |
| 14 | 0, 0 | 0 (0) |
DA, Daniels; ND, not determined; SEM, standard error of the mean; TMEV, Theiler's murine encephalomyelitis virus.
ANOVA and Fisher's PLSD post hoc tests are described in the text.
The distribution of the viral-antigen-positive cells throughout the brains of infected mice differed depending on the strain or mutant on days 5, 6, and 7 p.i. In both DA and BeAn infection, the majority of the viral-antigen-positive cells were located within cortex and hippocampus, with a few viral-antigen-positive cells present in the frontal lobe, caudoputamen, septum, thalamus, and hypothalamus. No viral-antigen-positive cells were found in the midbrain. DApBL2M infection was similar to DA and BeAn in that the majority of the antigen-positive cells were located within the cortex and hippocampus. However, the only other region of the brain to have a few viral-antigen-positive cells was the frontal lobe. In GDVII infection, there was a more uniform distribution of viral-antigen-positive cells within various regions of the brain that could be ranked as follows: cortex > hippocampus ≅ frontal lobe > thalamus and hypothalamus (combined) > midbrain > septum ≅ caudoputamen. The distribution of viral-antigen-positive cells in WW infection was similar to that of GDVII infection in that it was more uniform and had a similar ranking: cortex > hippocampus ≅ frontal lobe > caudoputamen > thalamus and hypothalamus (combined) ≅ septum > midbrain. Finally, no viral-antigen-positive cells were found within any of the various brain regions examined in H101 infection, and yet 40% of these mice had seizures.
DISCUSSION
We found that the initial viral dose directly correlated with the percentage of mice having seizures following infection with DA (Fig. 1). However, the neuropathology (PVC and neuronal cell loss) was the same over the titration range examined in mice that either developed seizures or did not (Fig. 2 and 3). Therefore, once seizures develop in DA-infected mice, the neuropathology is consistent irrespective of the viral dose. The neuropathology in DA-infected mice that do not develop seizures is markedly reduced in comparison.
In the viral strain comparison, a much lower titer (1 × 103 PFU) was used for the GDVII strain, since this strain belongs to the highly neurovirulent subgroup of TMEV. A titer of 3 × 105 PFU killed all of the C57BL/6 mice around day 3 p.i., the first day that seizures are observed to occur (data not shown). In demyelination-susceptible SJL/J mice, infection with 1 × 103 PFU of the GDVII strain resulted in death by day 6 p.i. whereas infection with 1 × 105 PFU of the GDVII strain resulted in death by day 4 p.i. (23). In addition, the titration experiment performed using the DA strain demonstrated that the initial viral dose affected the percentage of mice having seizures (Fig. 1) but not the numbers of PVC (Fig. 2), extent of neuronal cell loss (Fig. 3), or numbers (Table 1) or localization (data not shown) of viral-antigen-positive cells. Therefore, we surmise that the titer of the GDVII strain would also affect the percentage of mice having seizures but not the numbers of PVC, extent of neuronal cell loss, or numbers or localization of viral-antigen-positive cells. Previously, we demonstrated that 51% of C57BL/6 mice developed seizures when infected with the GDVII strain (2 × 103 PFU) and that the mortality rate of the GDVII-infected mice was 100% (8). Here, 55% of mice developed seizures when infected with the GDVII strain (1 × 103 PFU), with 100% mortality. The GDVII-infected mice survived through day 5 p.i., whereas the mice infected with the WW strain and the H101 mutant, used at a titer of 3 × 105 PFU, also survived only through days 6 and 7 p.i., respectively. This is the rationale for comparing GDVII-infected mice to WW-infected and H101-infected mice for numbers of PVC, extent of neuronal cell loss, and numbers and localization of viral-antigen-positive cells for day 5 p.i., even though there is a difference in the titer of the initial viral dose. The numbers of PVC (Tables 2 and 3), extent of neuronal cell loss (Tables 4 and 5), and numbers of viral-antigen-positive cells (Tables 6 and 7) were all found to be relatively high for GDVII-infected mice whether or not seizures were observed by day 5 p.i., the day through which these mice survived.
The WW strain is in the TO group since it causes an inflammatory demyelinating disease, with viral persistence, in susceptible SJL/J mice (12, 14). However, for resistant C57BL/6 mice, the WW strain is more like the GDVII strain in that all the C57BL/6 mice die by day 6 p.i. (GDVII by day 5 p.i.). The early death of WW-infected C57BL/6 mice was surprising, since the WW strain is able to persistently infect susceptible SJL/J mice and produces small plaques (10). The numbers of PVC (Tables 2 and 3), extent of neuronal cell loss (Tables 4 and 5), and numbers of viral-antigen-positive cells (Tables 6 and 7) were all found to be relatively high for WW-infected mice that did or did not have seizures by day 6 p.i., the day through which these mice survived.
The H101 mutant does not infect neurons within the gray matter of the brain and does not cause neuronal apoptosis but does induce T cell infiltration of the meninges (meningitis) (23). In SJL/J mice, infection with less than 1 × 102 PFU of the H101 mutant induced hydrocephalus accompanied by meningitis and macrocephaly, without viral persistence or demyelination, following resolution of the acute disease. Infection with more than 1 × 102 PFU of the H101 mutant resulted in acute fatal meningitis with macrocephaly and death within 3 to 5 days. Infection of C57BL/6 mice with 2 × 102 PFU of the H101 mutant resulted in hydrocephalus at 1 month p.i. (23). Infection of C57BL/6 mice with 3 × 105 PFU of the H101 mutant resulted in death by day 7 p.i. (this study). Infection with the H101 mutant induced minimal amounts of both PVC numbers (Tables 2 and 3) and neuronal cell loss (Tables 4 and 5) in the hippocampus of C57BL/6 mice, and no viral-antigen-positive cells were found (Tables 6 and 7), in the hippocampus or elsewhere in the brains of C57BL/6 mice, through day 7 p.i., the day through which these mice survived.
GDVII-, WW-, and H101-infected mice all died early after infection, at the time of peak seizure activity, whether the infected mice developed seizures or not. The deaths of GDVII- and WW-infected mice may have been due to the great extent of both inflammation (PVC) and neuronal cell loss in the hippocampus of the brain (polioencephalomyelitis) as well as viral load. However, the death of H101-infected mice must be caused by other factors, such as the concomitant production of cytokines by resident CNS cells.
In susceptible SJL/J mice, the TMEVs of the TO group initially infect neurons in the gray matter of the brain (acute phase) followed by infection of glial cells and macrophages accompanied by inflammatory demyelinating lesions in the white matter of the spinal cord (chronic phase) (24). TMEVs in the GDVII group infect neurons in the gray matter of the CNS, and death usually results in 10 days. The DApBL2M mutant initially infected neurons in the gray matter of the brain (acute phase) followed by the persistence of inflammatory gray matter lesions, without demyelination, in the brain (chronic phase). This was most likely due to continued infection (persistence of virus) of gray matter neurons in the brain (24). We found that brains of C57BL/6 mice that had been infected with the DApBL2M mutant and that exhibited seizures had viral-antigen-positive cells at day 7 p.i. but no detectable viral-antigen-positive cells by day 14 p.i. (Table 6).
Differences in neuropathology during the acute phase in SJL/J mice have been described for the DA strain of the TO group, the GDVII strain of the GDVII group, and the DApBL2M mutant (22). The DA strain induced high numbers of infiltrating T cells (perivascular inflammation) in SJL/J mice but low levels of neuronal apoptosis, with no correlation between the two. The GDVII strain induced low numbers of infiltrating T cells in SJL/J mice but high levels of neuronal apoptosis, and there was a negative correlation between apoptosis and perivascular inflammation. The DApBL2M mutant induced high numbers of infiltrating T cells and high levels of neuronal apoptosis, and there was a positive correlation between apoptosis and perivascular inflammation (22). Here we found that in C57BL/6 mice the DApBL2M mutant induced the highest number of PVC, for those TMEVs compared, in infected mice developing seizures by day 7 p.i. (Table 2). However, the neuronal cell loss score was low for the DApBL2M-infected mice that developed seizures at day 7 p.i. (Table 4). Also, there were no significant difference between DApBL2M- and DA-infected mice with respect to PVC numbers, neuronal cell loss score, or viral-antigen-positive cell numbers at days 7 and 14 p.i. The GDVII-infected mice were not compared to either the DA- or DApBL2M-infected mice, as all of the GDVII-infected mice died by day 5 p.i.
The neuropathology of BeAn-infected C57BL/6 mice is overall very similar to that of DA-infected C57BL/6 mice at days 7 and 14 p.i. (Tables 2 to 5). Likewise, the numbers of viral-antigen-positive cells in the brains of BeAn-infected C57BL/6 mice are comparable to those of DA-infected C57BL/6 mice at days 7 and 14 p.i. (Tables 6 and 7). In both DA and BeAn infections, viral-antigen-positive cells are essentially cleared from the brains of mice by day 14 p.i.
The fact that mice infected with the H101 mutant experience seizures in the absence of neuropathology or viral-antigen-positive cells suggests that cytokines are involved in seizure induction, which is consistent with our previous observations (5). A cytokine storm altering neuronal connectivity is likely to be important in the development of seizures in H101 infection. However, in GDVII infection, extensive neuropathology and the presence of viral-antigen-positive cells early after infection are likely to be important in the development of seizures. Therefore, multiple pathways may lead to the development of acute seizures following virus infection. The multiple pathways need not be mutually exclusive, and the contribution of each pathway could depend on the virus.
In summary, we found that the percentage of C57BL/6 mice that developed seizures following infection with the DA strain directly correlated to the initial viral dose. In addition, neuropathology did not vary in relation to the viral dose, and the viral-antigen-positive cells were gone or rare in brains by day 14 p.i. We also found that other TMEVs were able to induce seizures in mice to various degrees. Infections with the DA and BeAn strains and the DApBL2M mutant were found to be similar in the percentages of mice having seizures, neuropathology, and viral clearance. The GDVII and WW strains induced seizures and caused 100% mortality by day 6 p.i., in conjunction with extensive neuropathology and the presence of viral-antigen-positive cells. Therefore, with the exception of the H101 mutant, mice that develop seizures have more viral-antigen-positive cells present in the brain during the peak of seizure activity and have greater inflammation and neuronal cell destruction than mice that do not develop seizures. The H101 mutant induced seizures and caused 100% mortality by day 7 p.i. in the absence of marked neuropathology and viral-antigen-positive cells, suggesting that cytokines play a role in the development of seizures. We are currently examining the role of cytokines, particularly TNF-α and IL-6, in H101 infection, as well as infection with the various other TMEVs.
ACKNOWLEDGMENTS
We thank Daniel J. Doty, Jordan T. Sim, and Braden T. McElreath for excellent technical assistance. We thank Howard Lipton (University of Illinois, Chicago) for the WW strain of TMEV. We acknowledge Kathleen Borick for the outstanding preparation of the manuscript.
This work was supported by funding from the NIH (grant 1R01NS065714-01A1), the Margolis Foundation, CURE, and the Emma Mary Deland Foundation.
Disclosure of conflict of interest: we confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors have no conflict of interest to disclose.
Footnotes
Published ahead of print on 15 June 2011.
REFERENCES
- 1. Benkovic S. A., O'Callaghan J. P., Miller D. B. 2004. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 1024:59–76 [DOI] [PubMed] [Google Scholar]
- 2. Daniels J. B., Pappenheimer A. M., Richardson S. 1952. Observations on encephalomyelitis of mice (DA strain). J. Exp. Med. 96:517–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franklin K. B. J., Paxinos G. 1997. The mouse brain in stereotaxic coordinates. Academic Press, San Diego, CA [Google Scholar]
- 4. Getts D. R., Balcar V. J., Matsumoto I., Müller M., King N. J. C. 2008. Viruses and the immune system: their roles in seizure cascade development. J. Neurochem. 104:1167–1176 [DOI] [PubMed] [Google Scholar]
- 5. Kirkman N. J., Libbey J. E., Wilcox K. S., White H. S., Fujinami R. S. 2010. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia 51:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Libbey J. E., Fujinami R. S. 2003. Viral demyelinating disease in experimental animals, p. 125–133 In Herndon R. M. (ed.), Multiple sclerosis: immunology, pathology and pathophysiology. Demos, New York, NY [Google Scholar]
- 7. Libbey J. E., Kennett N. J., White H. S., Wilcox K. S., Fujinami R. S. 2011. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J. Virol. 85:6913–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Libbey J. E., et al. 2008. Seizures following picornavirus infection. Epilepsia 49:1066–1074 [DOI] [PubMed] [Google Scholar]
- 9. Libbey J. E., Kirkman N. J., Wilcox K. S., White H. S., Fujinami R. S. 2010. Role for complement in the development of seizures following acute viral infection. J. Virol. 84:6452–6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipton H. L. 1978. Characterization of the TO strains of Theiler's mouse encephalomyelitis viruses. Infect. Immun. 20:869–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lipton H. L. 1980. Persistent Theiler's murine encephalomyelitis virus infection in mice depends on plaque size. J. Gen. Virol. 46:169–177 [DOI] [PubMed] [Google Scholar]
- 12. Lipton H. L., Dal Canto M. C. 1978. The production of chronic CNS demyelination after prolonged latency: a common property of the TO strains of Theiler's viruses. Neurology 28:370 [Google Scholar]
- 13. Lipton H. L., Dal Canto M. C. 1979. Susceptibility of inbred mice to chronic central nervous system infection by Theiler's murine encephalomyelitis virus. Infect. Immun. 26:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipton H. L., Dal Canto M. C. 1979. The TO strains of Theiler's viruses cause “slow virus-like” infections in mice. Ann. Neurol. 6:25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipton H. L., Melvold R. 1984. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J. Immunol. 132:1821–1825 [PubMed] [Google Scholar]
- 16. Racine R. J. 1972. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 32:281–294 [DOI] [PubMed] [Google Scholar]
- 17. Rozhon E. J., Kratochvil J. D., Lipton H. L. 1983. Analysis of genetic variation in Theiler's virus during persistent infection in the mouse central nervous system. Virology 128:16–32 [DOI] [PubMed] [Google Scholar]
- 18. Stewart K.-A. A., Wilcox K. S., Fujinami R. S., White H. S. 2008. A novel model of infection-induced epilepsy: chronic seizures and neuronal cell loss in Theiler's virus infected C57BL/6 mice. Epilepsia 49(Suppl. 7):323–324 [Google Scholar]
- 19. Stewart K.-A. A., Wilcox K. S., Fujinami R. S., White H. S. 2010. Theiler's virus infection chronically alters seizure susceptibility. Epilepsia 51:1418–1428 [DOI] [PubMed] [Google Scholar]
- 20. Theiler M. 1937. Spontaneous encephalomyelitis of mice, a new virus disease. J. Exp. Med. 65:705–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Theiler M., Gard S. 1940. Encephalomyelitis of mice. I. Characteristics and pathogenesis of the virus. J. Exp. Med. 72:49–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsunoda I., Libbey J. E., Fujinami R. S. 2007. TGF-β1 suppresses T cell infiltration and VP2 puff B mutation enhances apoptosis in acute polioencephalitis induced by Theiler's virus. J. Neuroimmunol. 190:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsunoda I., McCright I. J., Kuang L.-Q., Zurbriggen A., Fujinami R. S. 1997. Hydrocephalus in mice infected with a Theiler's murine encephalomyelitis virus variant. J. Neuropathol. Exp. Neurol. 56:1302–1313 [DOI] [PubMed] [Google Scholar]
- 24. Tsunoda I., et al. 2001. Prolonged gray matter disease without demyelination caused by Theiler's murine encephalomyelitis virus with a mutation in VP2 puff B. J. Virol. 75:7494–7505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wada Y., McCright I. J., Whitby F. G., Tsunoda I., Fujinami R. S. 1998. Replacement of loop II of VP1 of the DA strain with loop II of the GDVII strain of Theiler's murine encephalomyelitis virus alters neurovirulence, viral persistence, and demyelination. J. Virol. 72:7557–7562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wroblewska Z., et al. 1977. Virus-specific intracytoplasmic inclusions in mouse brain produced by a newly isolated strain of Theiler virus. I. Virologic and morphologic studies. Lab. Invest. 37:595–602 [PubMed] [Google Scholar]
- 27. Zoecklein L. J., et al. 2003. Direct comparison of demyelinating disease induced by the Daniels strain and BeAn strain of Theiler's murine encephalomyelitis virus. Brain Pathol. 13:291–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zurbriggen A., Fujinami R. S. 1989. A neutralization-resistant Theiler's virus variant produces an altered disease pattern in the mouse central nervous system. J. Virol. 63:1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]