Abstract
Apoptosis is an important antivirus defense by virtue of its impact on virus multiplication and pathogenesis. To define molecular mechanisms by which viruses are detected and the apoptotic response is initiated, we examined the antiviral role of host inhibitor-of-apoptosis (IAP) proteins in insect cells. We report here that the principal IAPs, DIAP1 and SfIAP, of the model insects Drosophila melanogaster and Spodoptera frugiperda, respectively, are rapidly depleted and thereby inactivated upon infection with the apoptosis-inducing baculovirus Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV). Virus-induced loss of these host IAPs triggered caspase activation and apoptotic death. Elevation of IAP levels by ectopic expression repressed caspase activation. Loss of host IAP in both species was triggered by AcMNPV DNA replication. By using selected inhibitors, we found that virus-induced IAP depletion was mediated in part by the proteasome but not by caspase cleavage. Consistent with this conclusion, mutagenic disruption of the SfIAP RING motif, which acts as an E3 ubiquitin ligase, stabilized SfIAP during infection. Importantly, SfIAP was also stabilized upon the removal of its 99-residue N-terminal leader, which serves as a critical determinant of IAP turnover. These data indicated that a host pathway initiated by virus DNA replication and acting through instability motifs embedded within IAP triggers IAP depletion and thereby causes apoptosis. Taken together, the results of our study suggest that host modulation of cellular IAP levels is a conserved mechanism by which insects mount an apoptotic antiviral response. Thus, host IAPs may function as critical sentinels of virus invasion in insects.
INTRODUCTION
Apoptosis is an antiviral response in animals that has a profound influence on the multiplication and pathogenicity of many viruses (reviewed in references 2, 4, 9, and 10). The signal-induced pathways by which host cells detect virus invasion and initiate apoptosis are therefore critical to the outcome of an infection and its associated disease. By virtue of their central position in regulating apoptosis, the host cell's inhibitor-of-apoptosis (IAP) proteins may function as strategic determinants of virus-induced apoptosis. Indeed, IAPs also determine cell fate during tumorigenesis, stress, and pathogen invasion (14, 30, 37, 39, 44). As expected for a protein with the authority to regulate cell death, the function and stability of intracellular IAPs are subject to multiple levels of control (reviewed in references 6, 14, and 49). However, the molecular mechanisms that govern IAP activity and thus cell fate during virus infection are poorly understood.
The IAPs are a highly conserved family of survival factors that regulate developmental and stress-induced apoptosis, inflammation, the cell cycle, and other signaled events (14, 36, 37, 39, 44). In insects, these short-lived proteins govern activation of the caspases, which execute the proteolytic cleavages responsible for apoptosis (16, 24, 37). The IAPs possess hallmark baculovirus IAP repeat (BIR) domains (∼80 residues), consisting of a conserved Zn2+-coordinating arrangement of Cys and His residues (CCHC), which are required for function (30). The antiapoptotic activity of some, but not all, IAPs is derived from the ability of the BIRs to bind and neutralize caspases (16, 36, 37, 44). The BIRs also interact with proapoptotic factors that dissociate the IAP-caspase complex, thereby liberating active caspases to execute apoptosis (30).
The importance of IAPs as central regulators of apoptosis is most readily apparent in insects, where an individual IAP is required to repress constitutive apoptotic signaling. In Drosophila melanogaster (order Diptera) and Spodoptera frugiperda (order Lepidoptera), two distantly related species that are susceptible to virus-induced apoptosis, ablation of the principal IAP, designated DIAP1 or SfIAP, is sufficient to trigger apoptosis (22, 33, 50, 52). Thus, the intracellular level of these IAPs determines cell fate. DIAP1 and SfIAP both possess two BIRs, a C-terminal RING, and an N-terminal leader (Fig. 1). Their stability is governed by multiple factors, including the E3-ubiquitin ligase activity of the RING, residues embedded within their N-terminal leader, and proapoptotic factors (Reaper, Grim, HID, Sickle, and Jafrac2) that interact with the BIRs (8, 11, 45, 51). Phosphorylation mediated by Drosophila DmIKKε and Hippo can also affect DIAP1 stability and thus function (15, 25). The multiple levels of IAP control argue that these proteins are responsible for governing apoptosis triggered by diverse signals. However, the pro- or antiapoptotic roles of cellular IAPs during virus infection are poorly understood.
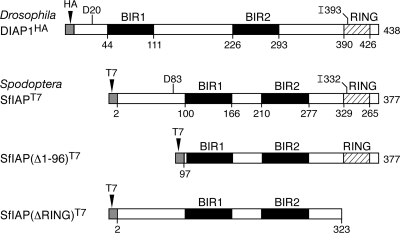
Fig. 1.
Structural organization of Drosophila and Spodoptera IAPs. DIAP1 (438 residues) and SfIAP (377 residues) contain two BIRs (black box) and a C-terminal RING domain (crosshatched box); the boundary of each domain is indicated. DIAP1 and SfIAP were engineered with the indicated N-terminal T7 and HA epitope tags (shaded box) for overexpression; neither tag affected the stability or function of these IAPs (data not shown). SfIAP(Δ1-96)T7 and SfIAP(ΔRING)T7 lack 96 residues of the N-terminal leader or the C-terminal RING (residues 324 to 377), respectively. Residues Asp20 (D20) and Ile393 (I393) of DIAP1 and Asp83 (D83) and Ile 332 (I332) of SfIAP, which were replaced with Ala, are indicated.
The large DNA baculoviruses can trigger rapid and widespread apoptosis of insect cells, including those from Drosophila and Spodoptera (reviewed in references 10 and 12). Recent studies have indicated that biochemical events associated with replication of the ∼130-kb double-stranded DNA genome of the prototype baculovirus Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) are required to trigger apoptosis through activation of the intrinsic caspase pathway (26, 27, 41, 42, 54). Consequently, AcMNPV and other baculoviruses encode their own apoptotic suppressors, including viral IAPs and caspase substrate inhibitors like P35 and P49, to block premature cell death and thereby expedite virus multiplication (10, 12). Nonetheless, these virus-encoded inhibitors function downstream from the replication event(s) that triggers apoptosis. Thus, it is likely that the host cell initiates apoptosis in response to virus replication. The biochemical nature of the first signaling events and the host antiviral components involved are unknown.
To define the invertebrate host mechanisms that sense infection and initiate apoptosis, we have examined the antiviral role of cellular IAPs during baculovirus infection of model systems from Drosophila and Spodoptera. When expressed ectopically, DIAP1 and SfIAP are sufficient to prevent AcMNPV-induced apoptosis (8, 27). However, when present at their normal intracellular levels, the same IAPs fail to block apoptosis. To explain this conundrum, we investigated the activity and fate of cellular IAPs during infection. We report here that both DIAP1 and SfIAP are rapidly depleted and thereby inactivated upon infection with AcMNPV. Virus DNA replication was sufficient to trigger IAP loss in both insect species. IAP degradation involved the proteasome and was facilitated by the IAP RING and residues embedded within the N-terminal IAP leader. Our finding that cellular IAPs of two diverse insect species are actively depleted in a virus-specific manner suggests that host modulation of IAP levels is a conserved mechanism by which insects mount an apoptotic antiviral response. Moreover, insects appear to use the same core machinery to control virus invasion as they do for executing developmental and stress-induced apoptosis.
MATERIALS AND METHODS
Cells.
S. frugiperda IPLB-SF21 cells (48) and D. melanogaster DL-1 cells (40) were maintained at 27°C in TC100 growth medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone) or Schneider's growth medium (Invitrogen)–15% FBS, respectively. Stably transfected, hygromycin-resistant DL-1 cell lines were generated and characterized previously (43). The DL-1 pMT-LacZ and pMT-DIAP1HA cell lines express lacZ or diap1HA (with an N-terminal hemagglutinin [HA] epitope) under the control of the Drosophila metallothionein (MT) promoter, which was induced with 500 μM CuSO4 in Schneider's medium–15% FBS. These cell lines were maintained in 300 μg hygromycin B (Invitrogen) per ml unless inoculated with virus.
Viruses.
Wild-type L-1 strain AcMNPV and AcMNPV recombinants wt/lacZ (p35+ iap− polyhedrin gene [polh]− lacZ+), vP35 (pIE1prm p35/Δ35K/lacZ p35+ polh− lacZ+), vOpIAP (pIE1prm Op-iap3HA/Δ35K/lacZ Op-IAP3HA p35− polh− lacZ+), and vΔp35/lacZ (vΔ35K/lacZ p49− p35− iap− polh− lacZ+) were described previously (18, 27, 28). The early ie-1 promoter directs expression of p35 and Op-IAP3HA from the polh locus of vP35 and vOpIAP, respectively. For inoculation, extracellular budded virus in TC100–10% FBS was added to SF21 or DL-1 monolayers. After 1 h at room temperature, the inoculum was replaced with serum-supplemented medium and cells were incubated at 27°C. Inoculations were conducted with a multiplicity of infection (MOI) of 10 PFU per cell (wt/lacZ) or 20 PFU per cell (vP35 and vOpIAP). We used the minimum dose (∼20 PFU per cell) of vΔp35/lacZ that was sufficient to cause >80% apoptosis by 24 h.
Plasmids.
Insect expression vector pIE1prm/hr5/PA, in which the constitutively active AcMNPV ie-1 promoter (prm) directs gene expression, was used here (7). Plasmids pIE1hr-P35 and pIE1hr-P49, which produce AcMNPV caspase inhibitor P35 and Spodoptera littoralis nucleopolyhedrovirus caspase inhibitor P49, respectively, were described previously (7, 54). SfIAP expression plasmids pIE1prm/hr5/sfiapT7/PA (formerly designated pIE1prm/hr5/M1-SfIAPT7/PA), pIE1prm/hr5/sfiap(Δ1-96)T7/PA (pIE1prm/hr5/M4-SfIAPT7/PA), and pIE1prm/hr5/sfiap(I332A)T7/PA, in which the ie-1 promoter directs the expression of N-terminally T7-tagged proteins, were described previously (8). pIE1prm/hr5/sfiap(D83A)T7/PA was generated by standard PCR-based mutagenesis of pIE1prm/hr5/sfiapT7/PA. pIE1prm/hr5/sfiap(ΔRING)T7/PA was generated using the approach described for pIE1prm/hr5/M4-SfIAPΔRT7/PA (8). pBluescript K/S(+) (Invitrogen)-based plasmids containing portions of the enhanced green fluorescence protein gene (egfp) or AcMNPV genes p47 and p143 for use as templates for in vitro transcription reactions were described previously (41, 42). For the same purpose, nucleotides 517 to 1359 of Sfiap (GenBank accession number AX213188) were cloned into plasmid pBluescript K/S(+). All plasmids and mutations thereof were verified by nucleotide sequencing.
Plasmid transfections.
SF21 monolayers (106 cells per 35-mm plate) were overlaid with TC100 medium containing CsCl-purified plasmid that was mixed previously with cationic liposomes consisting of N-[1-(2,3 dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl sulfate-l-α-phosphatidylethanolamine, dioleoyl (C18:1, [cis]-9); (DOTAP-DOPE; 10 μl), as described previously (26). For preparation of SF21 cell extract (see below), 3.2 × 107 cells in monolayer were transfected with 100 μg plasmid and 150 μl DOTAP-DOPE.
Small-molecule inhibitors.
The pancaspase inhibitor benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD-fmk; Calbiochem) was dissolved in dimethyl sulfoxide (DMSO) and diluted in Schneider's growth medium–15% FBS to a final concentration of 150 μM. The proteasome inhibitor lactacystin (Calbiochem) was dissolved in DMSO and diluted in Schneider's growth medium–15% FBS to a final concentration of 16 μM. DL-1 monolayers were overlaid with Schneider's growth medium–15% FBS containing the drugs and incubated at 27°C for the remainder of the experiment.
RNA silencing.
Single-stranded RNA was synthesized by using in vitro transcription reactions (Ampliscribe T3 and T7 kits; Epicentre) with linearized pBluescript K/S+ plasmids as a template. Complementary RNAs were heated to 65°C and cooled by 1°C/min to generate double-stranded RNA (dsRNA). SF21 and DL-1 cells were transfected with a dsRNA-liposome mixture as described previously (42). Visual inspection revealed that the dsRNAs used here, with the exception of Sfiap-specific dsRNA, had no or minor effects on cell viability.
Cell survival assays.
Viable, nonapoptotic DL-1 cells were counted following infection with AcMNPV recombinant vOpIAP by using a phase-contrast microscope (Axiovert 135TV; Zeiss) with a Microfire camera (Optronics) and Pictureframe software (Optronics) as described previously (20, 43). The mean values ± standard deviations of surviving cells were determined from six nonoverlapping fields of view from triplicate infections and normalized to that of uninfected cells transfected with control egfp dsRNA. For survival assays using β-galactosidase production as a measure of viability, plasmid-transfected SF21 monolayers were collected 48 h after infection with AcMNPV recombinant vΔp35/lacZ, washed, and lysed in Tropix buffer (Galacto-Light Plus kit; Applied Biosystems). Intracellular β-galactosidase activity was measured according to the manufacturer's instructions and is reported as the average activity values ± the standard deviations obtained from triplicate infections.
Immunoblot analyses.
Intact cells and apoptotic bodies were collected by centrifugation, lysed in ≥1% sodium dodecyl sulfate (SDS) and ≥1% β-mercaptoethanol, and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to Immobilon-P polyvinylidene difluoride (Millipore) or nitrocellulose (Osmonics, Inc.) membranes that were then incubated with the following antiserum diluted as indicated in parentheses: polyclonal α-IE1 (1:10,000) (34), polyclonal anti-DIAP1 (1:1,000) (43), polyclonal anti-DrICE (1:1,000) (27), monoclonal anti-actin (1:5,000) (BD Biosciences), affinity-purified polyclonal anti-SfIAP (1:1,000) (8), polyclonal anti-Sf-caspase-1 (1:1,000) (26), or monoclonal anti-T7 (1:10,000) (Novagen). Signal development was conducted by using alkaline phosphatase-conjugated goat anti-rabbit or goat anti-mouse (for anti-actin and anti-T7) immunoglobulin G (Jackson ImmunoResearch Laboratories, Inc.) with the CDP-Star Chemiluminescent Detection System (Roche).
Caspase assays.
Infected cells were dislodged, collected, and lysed in caspase activity buffer consisting of 10 mM HEPES (pH 7.0), 2 mM EDTA, 5 mM dithiothreitol (DTT), 0.1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate; Pierce}, and 1× protease inhibitor (Roche). The lysates were clarified by centrifugation, and caspase activity was measured by using the substrate N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-AMC) (Sigma) as described previously (26). Values are reported as the average rates ± standard deviations of fluorescent product accumulation in relative light units (RLU) for triplicate samples.
Spodoptera cell extracts.
SF21 cells grown in suspension or transfected with expression vector plasmids 24 h earlier were collected by centrifugation, suspended in 20 mM HEPES (pH 7.5)–10 mM KCl–1.5 mM MgCl2–1 mM DTT–1× complete protease inhibitor (Roche Diagnostics), and subjected to Dounce homogenization. After clarification by centrifugation (10 min, 1,000 × g), the extracts were used immediately. Reaction mixtures containing cell extract were mixed with or without purified recombinant proteins at the indicated concentrations and incubated at 27°C. Samples were withdrawn at the indicated intervals and either assayed for caspase activity or subjected to immunoblot analysis.
Protein production.
Recombinant P49-His6, D94A-mutated P49-His6, P35-His6, and D87A-mutated P35-His6 were isolated from Escherichia coli strain BL-21(DE3) by Ni2+ affinity chromatography (Qiagen) as described previously (3, 13). Purified proteins were dialyzed overnight into 50 mM HEPES (pH 7.6)–150 mM NaCl–1 mM DTT–10% glycerol. Protein purity was determined by SDS-PAGE, followed by staining with Biosafe Coomassie stain (Bio-Rad) according to the manufacturer's directions. Protein concentrations were measured by using Bio-Rad protein assay reagent.
RESULTS
Baculovirus infection depletes DIAP1.
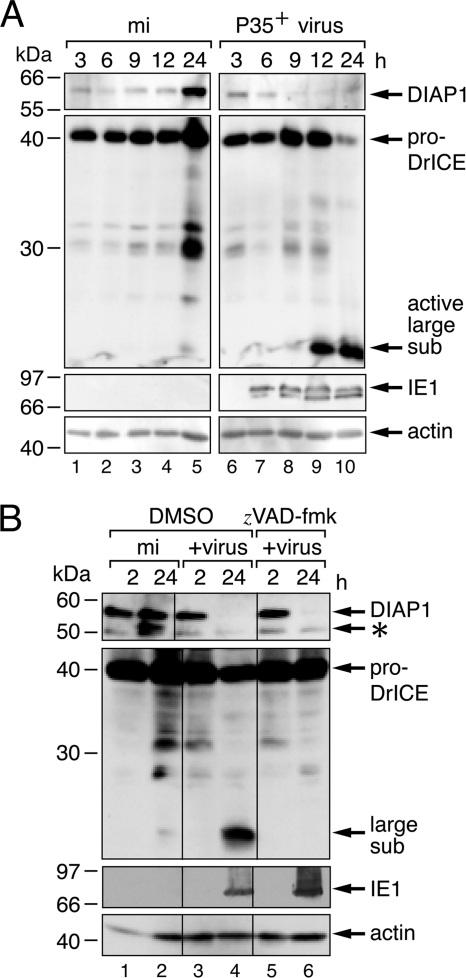
In Drosophila, inactivation or loss of DIAP1 releases caspase inhibition and leads to apoptosis (22, 33, 43). Thus, DIAP1 is a critical regulator of Drosophila cell survival. Here, we tested the role of DIAP1 in cell survival during baculovirus infection. To this end, we first monitored the fate of DIAP1 in Drosophila DL-1 cells inoculated with an AcMNPV recombinant that encodes P35 (P35+), a potent caspase inhibitor that functions downstream of DIAP1 to block apoptosis and cytolysis in Drosophila (17, 27). In mock-infected cells, endogenous DIAP1 was readily detected by immunoblot assays using affinity-purified antiserum (Fig. 2A, lanes 1 to 5). However, in P35+-infected cells, DIAP1 declined to low levels (lanes 6 to 10). The most significant loss of DIAP1 (6 to 9 h after infection) occurred after the appearance of the AcMNPV transactivator IE1 (lane 7), which is required for virus DNA replication (41, 42). Notably, DIAP1 depletion began prior to proteolytic processing of effector caspase DrICE to its active large and small subunits (Fig. 2A, lanes 8 to 10). To verify that DIAP1 depletion was not due to caspase-mediated cleavage, we monitored DIAP1 levels in the presence of the cell-permeating pancaspase inhibitor zVAD-fmk. Virus-induced loss of DIAP1 was comparable with or without zVAD-fmk (Fig. 2B, lanes 4 and 6). The inhibition of proDrICE processing to its active subunits verified that zVAD-fmk was effective at blocking initiator and effector caspase activity in DL-1 cells (compare lanes 4 and 6). We concluded that virus-induced depletion of DIAP1 is triggered by events prior to and independent of Drosophila caspase activation.
Fig. 2.
AcMNPV-induced depletion of DIAP1. (A) Levels of endogenous DIAP1 and DrICE. Drosophila DL-1 monolayers were mock infected (mi) or inoculated with P35+ AcMNPV recombinant vP35 (MOI, 20). SDS cell lysates prepared at the indicated times (hours) thereafter were subjected to immunoblot analysis by using anti-DIAP1 (top), anti-DrICE (middle-top), anti-IE1 (middle-bottom), or anti-actin (bottom) antibody; samples were analyzed in the same immunoblot assays but are separated here for presentation. Our anti-DrICE antibody recognizes the indicated proform (pro-DrICE) and active large subunit of DrICE (27). Actin levels demonstrated comparable protein loading. Protein molecular size standards (in kilodaltons) are indicated on the left. (B) Effect of caspase inhibitor zVAD-fmk. DL-1 cell lysates were prepared at the indicated times (hours) after mock infection or inoculation (+virus) with a P35-deficient AcMNPV recombinant (MOI, 20) in the presence of the vehicle (DMSO) or zVAD-fmk (150 μM) and subjected to immunoblot analysis as described for panel A. The abundance of the internal initiation product of DIAP1 (asterisk) was variable (11). It was unaffected by caspase inhibitors but was not produced upon M38A substitution (data not shown), suggesting that Met38 is the internal start site.
Overproduced DIAP1 prevents virus-induced apoptosis.
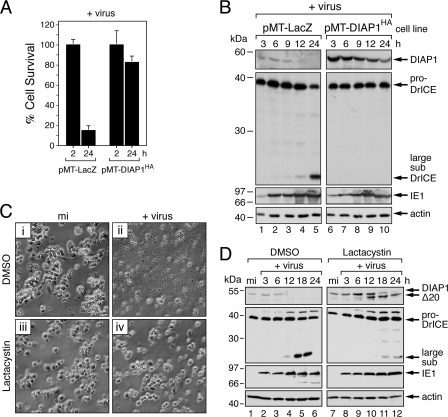
In most of our experiments, AcMNPV reduced endogenous DIAP1 to below the level of immunoblot assay detection. Thus, to test the premise that DIAP1 depletion is responsible for virus-induced apoptosis, we raised the intracellular level of DIAP1 above that lost during infection. To this end, we used a cloned DL-1 cell line in which epitope-tagged DIAP1HA was expressed from the MT promoter (27, 43). As indicated by cell viability assays (Fig. 3A), DIAP1HA-producing cells failed to undergo apoptosis when inoculated with a P35-deficient mutant of AcMNPV. Conversely, ∼85% of the control cells that expressed only lacZ underwent apoptosis within 24 h. Immunoblot analysis confirmed that DIAP1HA levels were higher than those of endogenous DIAP1 (Fig. 3B). Although DIAP1HA levels declined during infection, they did not drop below the limit of detection (lanes 6 to 10). Confirming that the elevated levels of DIAP1HA were sufficient to prevent virus-induced apoptosis, processing of proDrICE was blocked in these cells (lanes 6 to 10), but not in lacZ control cells (lanes 1 to 5). The early appearance of transactivator IE1 verified that each cell line was infected (Fig. 3B, lanes 6 to 10). These findings supported the conclusion that DIAP1 depletion was responsible for virus-induced apoptosis.
Fig. 3.
Suppression of virus-induced apoptosis by elevation of DIAP1 levels. (A) Cell survival. Drosophila DL-1 cell lines stably transfected with MT promoter-directed diap1HA (pMT-DIAP1HA) or control lacZ (pMT-LacZ) were treated with CuSO4 and inoculated 24 h later (+virus) with P35-deficient AcMNPV (MOI, 20). Intact, nonapoptotic cells were quantified 2 h and 24 h after infection. The values shown represent the average percent cell survival (± the standard deviation) for three independent plates and normalized to that for 2 h after infection. (B) Overexpressed DIAP1. SDS lysates of stably transfected cells described for panel A were prepared at the indicated times (hours) after infection and subjected to immunoblot analysis by using anti-DIAP1 (top), anti-DrICE (middle-top), anti-IE1 (middle-bottom), or anti-actin (bottom) antibody; all lysates were analyzed on the same blot. Protein molecular size standards are indicated on the left. (C) Effect of lactacystin. Parental DL-1 cells were mock infected (mi) (panels i and iii) or inoculated (+virus) with P35-deficient AcMNPV (MOI, 20) (ii and iv), treated with the DMSO vehicle (panels i and ii) or 16 μM lactacystin (iii and iv), and photographed 24 h later (magnification, ×500). (D) Endogenous DIAP1. SDS lysates of DL-1 cells described for panel C were prepared at the indicated times (hours) after infection and subjected to immunoblot analysis as described for panel A. The size of DIAP1Δ20 is consistent with cleavage at Asp20.
Proteasome inhibition prevents DIAP1 depletion and virus-induced apoptosis.
The instability of DIAP1 (half-life of 30 to 40 min) is determined in part by RING-dependent ubiquitination and proteasome degradation (51, 53). In an attempt to increase DIAP1 longevity and thereby affect sensitivity to virus-induced apoptosis, we treated DL-1 cells with the irreversible proteasome inhibitor lactacystin. When added 1 h after infection, lactacystin prevented the apoptotic cytolysis of DL-1 cells triggered by P35-deficient AcMNPV (Fig. 3C, compare panels ii and iv) but had little, if any, effect on normal cell morphology (compare panels i and iii). Immunoblot assays indicated that lactacystin reduced the turnover of DIAP1 in infected cells (Fig. 3D, lanes 7 to 12). Full-length DIAP1 persisted through 18 h after infection and was gradually replaced by a DIAP1 truncation (DIAP1Δ20) with a size expected for cleavage at Asp20 within the caspase cleavage site DQVD20↓N (11). Indeed, only full-length DIAP1, not DIAP1Δ20, was detected in caspase-inhibited, lactacystin-treated cells (J. K. Mitchell and P. D. Friesen, unpublished data). The presence of low-level constitutive caspase (DrICE) activity in DL-1 cells (27) accounts for the Asp20 cleavage of DIAP1, which is also observed in uninfected cells and accumulates upon proteasome inhibition (data not shown). Lactacystin also reduced virus-induced DrICE processing (Fig. 3D, compare lanes 4 to 6 and lanes 10 to 12) and reduced intracellular caspase activity. The drug had no effect on the appearance of early IE1, which suggested that early virus replicative events were normal. Although we cannot yet rule out the possibility that lactacystin is a direct inhibitor of viral DNA replication, our data are most consistent with the conclusion that proteasome activity, which contributes to DIAP1 loss, is required for baculovirus-induced apoptosis in Drosophila cells.
DIAP1 depletion requires baculovirus DNA replication.
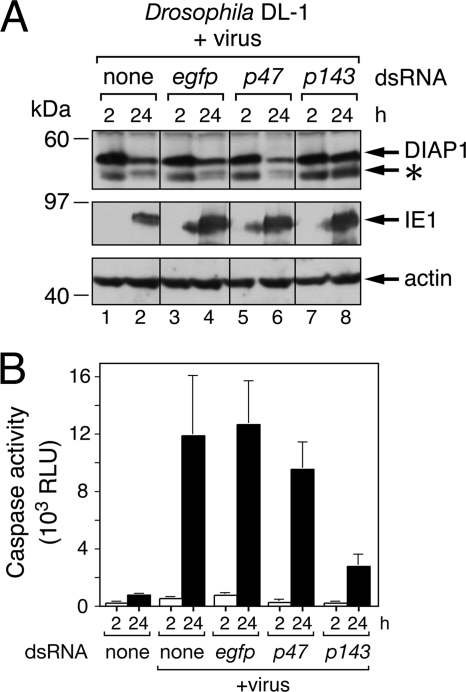
We predicted that if a host response mediates DIAP1 depletion, then a virus-specific event should initiate the process. Because AcMNPV DNA replication triggers apoptosis (41), we next determined if this virus-specific event affects DIAP1 loss. To this end, we inhibited AcMNPV DNA replication by RNA interference (RNAi)-mediated ablation of P143, an essential DNA helicase that, upon silencing, blocks viral DNA synthesis (41). In DL-1 cells treated with p143-specific dsRNA, endogenous DIAP1 levels were unchanged during infection with P35-deficient AcMNPV (Fig. 4A, lanes 7 and 8, top panel). Conversely, DIAP1 was depleted upon silencing of p47 (lanes 5 and 6), an AcMNPV early gene (see below) required for virus late multiplicative events but not DNA replication (41). Control egfp-specific dsRNA had no effect on DIAP1 loss (lanes 3 to 4) compared to untreated cells (lanes 1 to 2). Transactivator IE1 was produced in all infections (Fig. 4A, middle panel). To confirm that silencing of p143 blocked virus-induced apoptosis, we measured intracellular caspase activity, which determines apoptosis. Using DEVD-AMC as the substrate (Fig. 4B), caspase activity increased dramatically in infected cells not silenced or silenced for egfp or p47. In contrast, only low levels of caspase activity were detected in infected cells silenced for p143 (Fig. 4B). Thus, ablation of this helicase diminished virus-induced apoptosis, as expected (41). We concluded that virus DNA replication contributes to DIAP1 depletion and thus apoptosis.
Fig. 4.
AcMNPV DNA replication-induced DIAP1 depletion. (A) Effect of RNAi on endogenous DIAP1. DL-1 cells were transfected with no dsRNA (none) or with egfp- or virus p47- or p143-specific dsRNA and inoculated (+virus) 24 h later with P35-deficient (P35−) AcMNPV (MOI, 20). SDS lysates prepared at the indicated times (hours) after infection were subjected to immunoblot analysis by using anti-DIAP1 (top), anti-IE1 (middle), or anti-actin (bottom) antibody; actin levels indicated comparable protein loading. Protein molecular size standards are indicated on the left. The asterisk denotes the DIAP1 internal initiation product. (B) Intracellular caspase activity. Extracts of DL-1 cells transfected with the indicated dsRNAs were prepared 24 h after mock infection or infection with P35-deficient AcMNPV (MOI, 20) and assayed for caspase activity using DEVD-AMC as a substrate. Values are reported as the average rates ± standard deviations of caspase activity in RLU determined from triplicate infections.
AcMNPV causes depletion of host Spodoptera SfIAP.
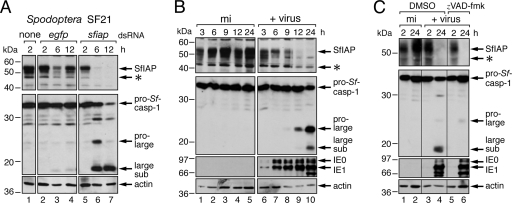
To determine whether IAP depletion is a general mechanism by which insects counter infection, we determined the effect of infection on cellular IAP in S. frugiperda, a species that is permissive for AcMNPV but only distantly related to Drosophila. SfIAP (Fig. 1) is the principal cellular IAP of Spodoptera (21, 33). In cultured Spodoptera SF21 cells, SfIAP was detected as a 53-kDa protein by using immunoblot assays with affinity-purified anti-SfIAP antibody (Fig. 5A, lane 1). To verify the identity of SfIAP and confirm its role in suppressing apoptosis, we ablated SfIAP by RNAi. Transfection of SF21 cells with sfiap-specific dsRNA caused rapid loss of SfIAP (lanes 5 to 7). This ablation led to immediate activation of the principal Spodoptera effector caspase Sf-caspase-1 (1, 26, 54), which was cleaved into its active subunits (lanes 5 to 7). Thus, SfIAP loss preceded caspase activation.
Fig. 5.
Correlation of SfIAP depletion and caspase activation. (A) Endogenous SfIAP during RNAi. Suspended Spodoptera SF21 cells were mixed with no dsRNA (none) or with egfp- or sfiap-specific dsRNA for 4 h, plated, and harvested at the indicated times (hours) thereafter. SDS cell lysates were subjected to immunoblot analysis by using anti-SfIAP (top), anti-Sf-caspase-1 (middle), or anti-actin (bottom) antibody. Anti-Sf-caspase-1 recognizes the indicated proform, pro-large, and large subunits of Sf-caspase-1 (26). (B) Endogenous SfIAP during infection. SF21 monolayers were mock infected (mi) or inoculated (+virus) with P35+ AcMNPV recombinant wt/lacZ (MOI, 10). SDS cell lysates prepared at the indicated times (hours) thereafter were subjected to immunoblot analysis by using anti-SfIAP (top), anti-Sf-caspase-1 (top-middle), anti-IE1 (bottom-middle), or anti-actin (bottom) antibody; asterisks denote possible internal initiation products of SfIAP. Samples were analyzed in the same immunoblot assay for both panels. (C) Effect of caspase inhibitor zVAD-fmk. SF21 SDS cell lysates were prepared at the indicated times (hours) after mock infection or inoculation (+virus) with a P35-deficient (P35−) AcMNPV recombinant (MOI, 20) in the presence of the vehicle (DMSO) or zVAD-fmk (150 μM) and subjected to immunoblot analysis as described for panel B.
Upon infection with P35-encoding AcMNPV, endogenous SfIAP was depleted to levels below the limit of detection (Fig. 5B, lanes 6 to 10). The disappearance of SfIAP was preceded by the appearance of transcriptional activators IE1 and IE0 (Fig. 5B, lanes 6 to 10) and was accompanied by proteolytic processing of pro-Sf-caspase-1 (lanes 9 and 10). Virus-produced P35 blocks all effector caspase activity but not the initiator caspase that activates pro-Sf-caspase-1 (26, 54). Thus, to inhibit the Spodoptera initiator caspase, we used zVAD-fmk. This cell-permeating drug simultaneously inhibits initiator and effector caspases in Spodoptera (31), as indicated by the lack of processing of pro-Sf-caspase-1 in treated cells (Fig. 5C, compare lanes 4 and 6). When triggered by an AcMNPV P35 deletion mutant, the loss of endogenous SfIAP was unaffected by zVAD-fmk (Fig. 5C). This finding was confirmed by using P49, which is a baculovirus-encoded inhibitor of the Spodoptera initiator caspase (54). The depletion of endogenous SfIAP through early (6 to 12 h) and late (24 h) times in cells infected with a P49-encoding AcMNPV recombinant was indistinguishable from that in cells infected with P35-encoding AcMNPV (data not shown). We concluded that AcMNPV-induced depletion of SfIAP is not caspase mediated.
AcMNPV DNA replication is required for SfIAP depletion.
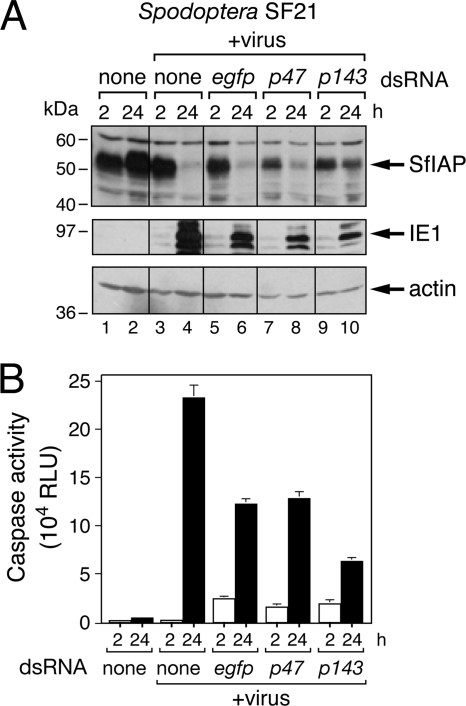
Because baculovirus DNA replication also triggers apoptosis in Spodoptera cells (41), we determined whether it does so by contributing to SfIAP depletion. When virus DNA synthesis was blocked by RNAi ablation of helicase P143, an AcMNPV replicative factor, SfIAP levels were relatively unchanged over a 24-h period (Fig. 6A, lanes 9 and 10). Because virus DNA replication is also required for virus late gene expression, we tested the effect of RNAi ablation of the nonreplicative expression factor P47, which is a subunit of the late RNA polymerase complex required for late gene expression but not virus DNA replication. Ablation of P47 blocks late gene expression but not virus DNA synthesis in SF21 cells (41). However, P47 ablation failed to affect virus-induced SfIAP depletion, which was comparable to that of untreated or control egfp dsRNA-treated cells (Fig. 6A, lanes 3 to 8). Caspase assays (Fig. 6B) confirmed that infected cells ablated for helicase P143 exhibited the lowest level of caspase activation, indicating diminished apoptosis. We concluded that virus DNA replication is required for SfIAP depletion. Our finding that virus DNA replication contributes to cellular IAP loss in two divergent arthropods supports the hypothesis that IAP depletion is an antiviral response in insects.
Fig. 6.
AcMNPV DNA replication-induced SfIAP depletion. (A) Effect of RNAi on endogenous SfIAP. SF21 cells were transfected with no dsRNA (none) or with egfp-, p47-, or p143-specific dsRNA and inoculated (+virus) 24 h later with P35-deficient recombinant AcMNPV (MOI, ∼20). SDS cell lysates prepared at the indicated times (hours) after infection were subjected to immunoblot analysis by using anti-SfIAP (top), anti-IE1 (middle), or anti-actin (bottom) antibody. Samples were analyzed in the same immunoblot assay. (B) Intracellular caspase activity. Extracts of SF21 cells transfected 24 h previously with the indicated dsRNAs were prepared 2 h and 24 h after mock infection or infection with P35-deficient vΔp35/lacZ (MOI, ∼20) and assayed for caspase activity as described in the legend to Fig. 4B. RLU values represent the average caspase activity for triplicate infections ± the standard deviation.
The SfIAP leader and RING domain mediate turnover.
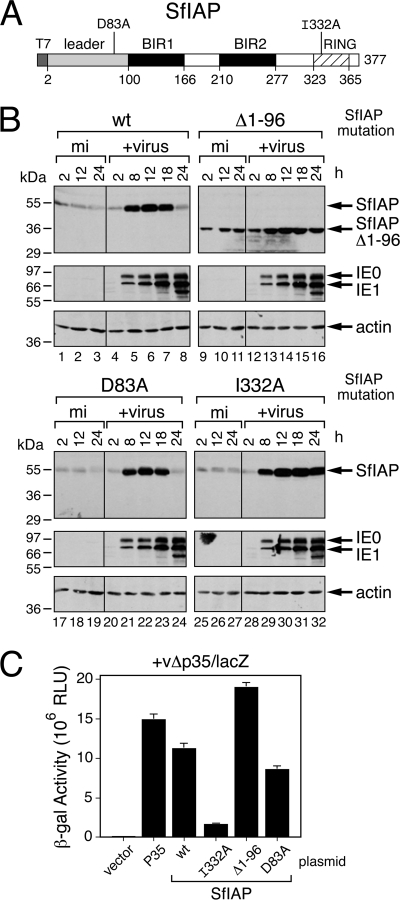
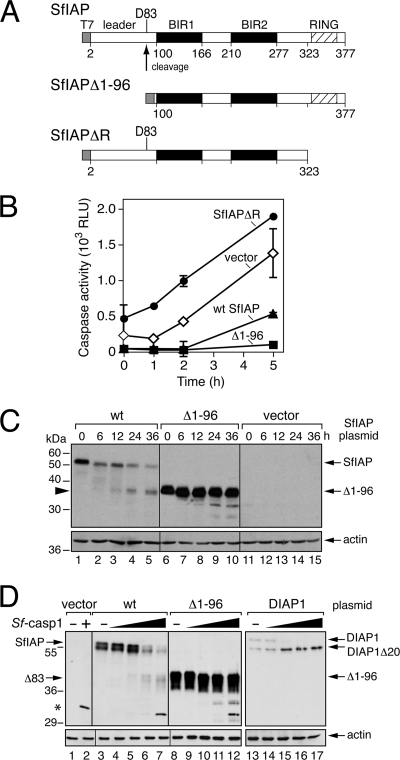
We postulated that the rapid loss of the dipteran and lepidopteran IAPs was due to the activity of IAP regulatory motifs that respond to degradative signals triggered by virus infection. In previous studies, we showed that the 99-residue SfIAP leader, which precedes BIR1 (Fig. 7A), contributes to SfIAP turnover but does not affect antiapoptotic function (8). To test the leader's role in virus-induced SfIAP depletion, we compared the fates of wild-type and leaderless SfIAPT7 after infection with P35-deficient AcMNPV. To this end, we transfected SF21 cells with a plasmid vector that used the ie-1 promoter for expression and subsequently infected them with AcMNPV recombinant vΔp35/lacZ to stimulate SfIAP expression and to trigger apoptosis. Because each SfIAP protein was translated from the same AUG start codon within the same 5′ noncoding mRNA leader, the transcriptional and translational context of each construct was identical to that of the others. Thus, the rate of synthesis of each construct was identical and any loss was a reliable indication of protein instability (8). Early (8 h) after infection, the level of each SfIAPT7 construct was boosted comparably due to IE1-mediated transactivation of the expression vector (Fig. 7B). After its initial increase, leaderless SfIAP(Δ1-96)T7 was present at stable levels throughout infection (Fig. 7B, lanes 12 to 16). In contrast, full-length SfIAPT7 was depleted to low levels by 24 h (lanes 4 to 8). Loss of SfIAPT7 was not detected until later due its relative abundance early in infection. We concluded that unlike leaderless SfIAP, leader-containing SfIAPT7 was unstable during infection. These findings were consistent with the decreased stability of leader-containing SfIAP compared to leaderless SfIAP in uninfected cells, as indicated by their measured half-lives of 33 min and 152 min, respectively (8).
Fig. 7.
Role of SfIAP motifs in virus-induced depletion. (A) SfIAP motifs. Amino acid substitutions D83A, within the 99-residue N-terminal leader (shaded box), and I332A, within the RING (crosshatched), are shown relative to the requisite BIRs (black boxes). (B) Transient expression of SfIAP. SF21 cells transfected 24 h earlier with plasmids encoding wild-type (wt), leaderless (Δ1-96), D83A-substituted, or I332A-substituted SfIAPT7 were mock infected (mi) or inoculated (+virus) with AcMNPV recombinant vΔp35/lacZ (MOI, ∼20). SDS cell lysates prepared at the indicated times (hours) after infection were subjected to immunoblot analysis by using anti-SfIAP (top), anti-IE1 (middle), or anti-actin (bottom) antibody. (C) Cell survival. SF21 monolayers transfected 24 h earlier with the vector alone, P35-encoding plasmid, or the SfIAPT7-encoding plasmids described for panel A were infected with vΔp35/lacZ (MOI, ∼20). Cell extracts prepared 48 h later were assayed for β-galactosidase; the activity of this reporter, directed by the very late polyhedrin promoter of vΔp35/lacZ, is directly proportional to suppression of virus-induced apoptosis. The RLU values shown represent the average β-galactosidase activities of triplicate infections ± the standard deviations.
Leaderless SfIAP(Δ1-96)T7 was also a potent inhibitor of virus-induced apoptosis, as demonstrated by its capacity to support β-galactosidase production by AcMNPV recombinant vΔp35/lacZ (Fig. 7C). This P35-deficient virus possesses a very late polyhedrin promoter-directed lacZ reporter, which is active only when apoptosis is suppressed (8, 18, 20). The antiapoptotic potency of SfIAP(Δ1-96)T7 was comparable to that of caspase inhibitor P35 (Fig. 7C). Although less stable, leader-containing wild-type SfIAPT7 also suppressed apoptosis. We attributed this activity to the elevated level of SfIAPT7 compared to endogenous SfIAP from 8 to 12 h after infection (Fig. 7B).
Previous studies (45) suggested that caspase cleavage at DKTD83↓N within the SfIAP leader contributes to protein turnover as a result of the exposure of Asn84, which promotes N-end rule ubiquitination and degradation. We therefore tested the effect of eliminating the required cleavage residue (Asp83) by alanine substitution. D83A-mutated SfIAPT7 [SfIAP(D83A)T7] was resistant to cleavage by purified Sf-caspase-1, which readily cleaved wild-type SfIAPT7 (data not shown). However, virus-induced loss of SfIAP(D83A)T7 was comparable to that of wild-type SfIAPT7 by 24 h after infection (Fig. 7B, lanes 20 to 24). In contrast, RING-mutated SfIAP(I332A)T7, which has a relatively long half-life in uninfected cells (8), was abundant through 24 h (Fig. 7B, lanes 28 to 32). The RING activity of this construct was disabled by Ala substitution of Ile332, which is a conserved residue required for the E3 ubiquitin ligase activity of the RING (8). Thus, loss of RING function had a greater stabilizing effect on SfIAP than did loss of the capacity for caspase cleavage of the leader. Despite its abundance, SfIAP(I332A)T7 was a poor inhibitor of virus-induced apoptosis compared to SfIAP(D83A)T7 or wild-type SfIAPT7 (Fig. 7C). We concluded that the RING contributes to SfIAP turnover during infection and is required for SfIAP antiapoptotic activity. In contrast, caspase-mediated cleavage at Asp83 contributes minimally to SfIAP turnover and is not required for antiapoptotic activity during infection.
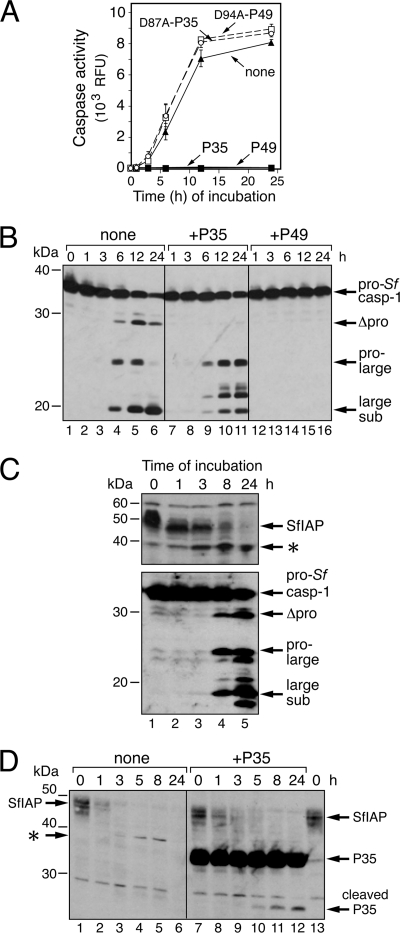
IAP is depleted in Spodoptera cell extracts.
To investigate the mechanisms that govern IAP stability, we developed a cell-free system in which IAP turnover triggers caspase activation. This advantageous system has helped define IAP instability motifs and will be useful in identifying critical protein interactions. SF21 cell-free (1,000 × g) extracts exhibited a rapid and dramatic (>350-fold) increase in caspase activity (Fig. 8A) when incubated at 27°C in the absence of exogenously added components, including ATP. Endogenous pro-Sf-caspase-1 was processed to active subunits with comparable kinetics (Fig. 8B). Caspase inhibitors P49 and P35, but not their loss-of-function cleavage-defective forms (3), neutralized this activity (Fig. 8A). When added prior to incubation, P49, but not P35, blocked pro-Sf-caspase-1 processing (Fig. 8B). Thus, in a pattern identical to that of infected cells, the initiator caspase that activates Sf-caspase-1 is P49 sensitive but P35 resistant (26, 54). Importantly, caspase activation was preceded by a rapid loss of SfIAP (Fig. 8C). Exogenously added P35 had no effect on the disappearance of SfIAP (Fig. 8D, compare lanes 1 to 6 and lanes 7 to 12). Indicative of caspase activity, P35 cleavage was detected only after SfIAP depletion. Likewise, exogenously added P49 failed to affect the spontaneous loss of SfIAP in vitro (data not shown), suggesting that the P49- and P35-inhibitable initiator and effector caspases are not involved. Thus, in vitro loss of SfIAP also precedes caspase activation and is caspase independent. This finding provided important confirmation of the same conclusion reached using infected cells.
Fig. 8.
SfIAP depletion in cell extracts. (A) Caspase activity. SF21 cell extract prepared by Dounce homogenization was mixed with buffer (none), 1 μM P35-His6 (P35), 1 μM D87A-mutated P35-His6 (D87A-P35), 0.1 μM P49-His6 (P49), or 0.1 μM D94A-mutated P49-His6 (D94A-P49). At the indicated times (hours) during incubation at 27°C, samples were removed and assayed for caspase activity by using DEVD-AMC as the substrate. The RLU values shown represent the average caspase activities of triplicate samples ± the standard deviations. (B) Effects of P35 and P49 on Sf-caspase-1 processing. SF21 cell extract was mixed with buffer alone, P35-His6, or P49-His6 as indicated in the legend to panel A, sampled at the indicated times (hours) thereafter, and subjected to immunoblot analysis by using anti-Sf-caspase-1 antibody. Arrows indicate the fully processed large subunit, the intermediate pro-large subunit, and the uncleaved proform of Sf-caspase-1. (C) Processing of pro-Sf-caspase-1. Equal samples of SF21 cell extract in the absence of exogenously added protein were removed at the indicated times (hours) during 27°C incubation and subjected to immunoblot analysis by using anti-SfIAP (top) or anti-Sf-caspase-1 (bottom) antibody. A possible Asp83-cleaved SfIAP product (asterisk) is indicated (see below). (D) P35-resistant depletion of endogenous SfIAP. SF21 cell extract was mixed with buffer alone or with P35-His6 (1 μM), sampled at the indicated times (hours), and sub- jected to immunoblot analysis by using anti-SfIAP. Lanes 1 and 13 contain identical samples. The asterisk denotes a potential Asp83 cleavage of endogenous SfIAP (lanes 3 to 5) that is not generated upon P35 inhibition of caspase activity (lanes 9 to 11). Because His6-tagged SfIAP was used as the antigen, our anti-SfIAP serum also detects the His6 tag of full-length and caspase-cleaved P35-His6 (arrows).
The SfIAP leader contributes to in vitro depletion.
Our cell-free system fails to support de novo protein synthesis (data not shown). Thus, the spontaneous loss of SfIAP was not due to new synthesis of IAP antagonists. To define the determinants of SfIAP's in vitro instability, we compared the turnover of SfIAP with that of mutations thereof (Fig. 9A). To this end, we transfected SF21 cells with SfIAP expression plasmids and subsequently prepared cell extracts. Upon overexpression, full-length SfIAPHA delayed in vitro caspase activation and lowered caspase activity compared to that of control (vector-only) extracts (Fig. 9B). Without its N-terminal leader, SfIAP(Δ1-96)HA was more potent at delaying caspase activation and reducing caspase activity. In contrast to the functional SfIAPs, overproduction of RINGless SfIAP(ΔR) increased caspase activity over that of control extracts (Fig. 9B). This behavior was predictable because RINGless SfIAP activates caspases by inhibiting endogenous SfIAP (8).
Fig. 9.
Cell-free depletion of overexpressed SfIAP. (A) SfIAP constructs. Wild-type, leaderless (Δ1-96), and RINGless (ΔR) SfIAPT7 constructs are shown with the caspase cleavage site at Asp83. (B) Caspase activity. Cell extracts prepared from SF21 cells transfected 24 h earlier with the vector or plasmids encoding wild-type (wt), leaderless (Δ1-96), or RINGless (ΔR) SfIAPHA were sampled at the indicated times (hours) after incubation at 27°C and assayed for caspase activity; RLU values represent the average caspase activities of triplicate samples ± the standard deviations. (C) SfIAP levels. Samples of cell extract prepared as described for panel B were subjected to immunoblot analysis by using anti-SfIAP (top) or anti-actin (bottom) antibody. The cleavage fragment of wild-type SfIAP (arrowhead) is indicated. Protein molecular size standards (in kilodaltons) are on the left. (D) SfIAP cleavage by Sf-caspase-1. Extracts prepared from SF21 cells transfected with plasmid encoding the indicated SfIAPHA constructs or Drosophila DIAP1 were mixed with increasing amounts of purified Sf-caspase-1-His6, incubated for 30 min, and subjected to immunoblot analysis by using anti-SfIAP (lanes 1 to 12), anti-DIAP1 (lanes 13 to 17), or anti-actin (bottom) antibody. Cleavage products and a cross-reactive fragment of Sf-caspase-1 (asterisk) are indicated.
The higher anti-caspase potency of leaderless SfIAP was explained in part by increased levels of SfIAP(Δ1-96) in the extract (Fig. 9C, compare lanes 6 to 10 with lanes 1 to 5). SfIAP(Δ1-96) also persisted through the incubation, confirming the relative stability of leaderless SfIAP. Nonetheless, even with increased SfIAP(Δ1-96), caspase activity increased significantly after 6 h of incubation (data not shown). Because exogenously added inhibitor P35 or P49 blocked all caspase activity throughout the incubation period (Fig. 8), we concluded that SfIAP's in vitro ability to block caspase activity is limited or transient compared to that of the irreversible substrate inhibitors P35 and P49. The late-appearing caspase activity cleaved wild-type SfIAP but not leaderless SfIAP(Δ1-96) (Fig. 9C, lanes 3 to 5). SfIAP was cleaved to generate the same fragment (Δ83) when extracts (0 h) were treated with purified Sf-caspase-1 (Fig. 9D). Likewise, Sf-caspase-1 cleaved the Drosophila DIAP1 leader at Asp20 to generate DIAP1Δ20 (Fig. 9D). We concluded that SfIAP is sensitive to cleavage by endogenous Sf-caspase-1 at Asp83 but only late during incubation, after SfIAP potency was lost. These data indicated that intracellular SfIAP is available for caspase cleavage within its leader. However, our collective data demonstrated that the observed depletion of endogenous SfIAP in infected cells (Fig. 5) and in cell extracts after caspase inhibition (Fig. 8) is not caspase mediated. Thus, caspase cleavage of the IAP leader appears to regulate IAP function during normal homeostasis of Spodoptera cells (45) rather than virus infection.
DISCUSSION
By using cell lines from distinct orders of insects (Lepidoptera and Diptera), we report here that baculovirus infection triggers rapid depletion of invertebrate IAPs. This loss initiates caspase-mediated apoptosis, which, in the absence of virus-encoded apoptosis inhibitors, limits virus multiplication. Our study indicated that loss of cellular IAP occurred in response to virus DNA replication and preceded caspase activation. In particular, distinct domains embedded within SfIAP contributed to its loss and suggested that a host pathway triggered by virus-specific events mediates depletion. Our findings provide further evidence that intracellular levels of IAP govern invertebrate apoptosis and strengthen the hypothesis that inactivation of cellular IAPs is a conserved mechanism by which insects respond to virus infection.
Virus-induced apoptosis by IAP depletion.
Upon infection, AcMNPV caused early and rapid loss of endogenous DIAP1 and SfIAP in Drosophila DL-1 and Spodoptera SF21 cells, respectively (Fig. 2 and 5). We found that depletion occurred prior to activation of host caspases and was unaffected by broad-spectrum caspase inhibitors. Moreover, when their intracellular level was increased above that depleted during infection, both DIAP1 and SfIAP suppressed virus-induced apoptosis (Fig. 3A and 7C). Thus, we concluded that the loss of IAP triggered by virus events caused apoptotic death.
The finding that DNA and RNA viruses trigger apoptotic suicide by depletion of cellular IAP in diverse insect species (this report and reference 43) is significant because it suggests for the first time that invertebrates use the same regulatory factors (IAPs) and apoptotic machinery for an antivirus response as they do for developmental and stress-related apoptosis. It is well established that IAPs govern invertebrate cell fate (22, 33, 38, 50). However, the critical role for IAP in determining the antiviral response was not necessarily anticipated. In vertebrates, for example, several antimicrobial responses cause caspase activation and cell suicide by pathways in which IAP involvement is minimal or indirect (reviewed in references 19 and 35). Here, we found that virus-triggered depletion of host SfIAP required the same protein motifs that govern SfIAP turnover during normal homeostasis of cells (see below). Thus, baculovirus-induced apoptosis may differ from other forms of programmed cell death only in the signaling pathway that mediates IAP destruction. The search for the upstream virus-specific signals that govern insect IAP depletion is under way.
The role of IAP motifs in regulating turnover.
Mutational analyses indicated that SfIAP's 99-residue leader is a principal determinant of protein loss during infection (Fig. 7B). The leader also contributed to the instability of SfIAP in cell extracts (Fig. 9) and the relatively short half-life (∼30 min) of SfIAP under normal, nonapoptotic conditions (8). Due to the critical role of IAP in determining insect cell fate, the mechanism by which the leader regulates IAP stability is of significant interest. A caspase cleavage site(s) located within the leader proximal to BIR1 is conserved among insect IAPs, suggesting that caspases contribute to IAP instability (45). However, we found no evidence that caspases deplete intracellular DIAP1 or SfIAP. Indeed, virus-induced IAP loss was unaffected by caspase inhibitors (Fig. 2B and 5B) and disruption of the consensus cleavage site (DKTD83↓N) within the SfIAP leader had no effect on its depletion (Fig. 7B). In support of these findings, we found that the rapid depletion of endogenous SfIAP in cell extracts was also caspase independent (Fig. 8). Thus, the role of caspase-mediated instability of insect IAPs remains unclear in the context of infection. In recent experiments, we have discovered that residues near the N terminus of the leader are the principal determinants of SfIAP turnover (R. Vandergaast and P. Friesen, unpublished data). This instability element contributes to caspase-independent SfIAP depletion and is currently under investigation.
Our study indicated that the C-terminal RING, which has E3 ubiquitin ligase activity, also contributes to IAP instability. The DIAP1 RING catalyzes ubiquitination and proteosome-mediated turnover of DIAP1 and bound ligands (reviewed in reference 37). Treatment with lactacystin stabilized endogenous DIAP1 and suppressed virus-induced apoptosis (Fig. 3). This finding confirmed the role of the proteasome in DIAP1 turnover during infection. Likewise, I332A-mutated SfIAP, which lacks the Ile residue required for RING E3 ubiquitin ligase activity (8), was resistant to virus-induced depletion (Fig. 7B). RING-defective SfIAP also failed to block virus-induced apoptosis (Fig. 7C), confirming the RING's requirement for antiapoptotic activity (8). Collectively, our results indicate that multiple domains influence IAP stability. Thus, invertebrate IAPs may have evolved instability motifs to respond to diverse signaling pathways wherein IAP turnover expedites an immediate apoptotic response, such as that during pathogen invasion.
Mechanism of virus-induced IAP depletion.
AcMNPV DNA replication is required for virus-induced apoptosis in Drosophila DL-1 and Spodoptera SF21 cells (41). By silencing AcMNPV genes essential for viral DNA synthesis, we have shown here that virus DNA replication is also required for intracellular depletion of DIAP1 and SfIAP (Fig. 4 and 6). Thus, it is likely that virus DNA replication activates a pathway that triggers apoptosis by IAP turnover. Given that DNA replication by viruses induces a DNA damage response in vertebrates (29), it is attractive to hypothesize that invertebrates interpret the same as DNA damage, which is a known apoptotic signal in insects, including Drosophila (reviewed in reference 47). Consistent with this possibility, the DNA-damaging agent etoposide induced rapid loss of DIAP1 and triggered widespread apoptosis in Drosophila DL-1 cells (J. Mitchell and P. Friesen, unpublished data). In Drosophila, DNA damage can activate the tumor suppressor DmP53, which upregulates proapoptotic factors like Reaper (5, 32). Reaper and other DIAP1 antagonists can cause IAP depletion by direct binding through the BIRs (reviewed in references 16 and 37). Although it is unclear whether Reaper-like factors contribute to baculovirus-induced apoptosis, it seems likely that host pathways contributing to a DNA damage response are involved.
Our findings also suggested that new synthesis of proapoptotic factors is not required for virus-induced IAP depletion. Endogenous SfIAP was depleted rapidly in Spodoptera cell extracts (Fig. 8) without de novo protein synthesis. The in vitro turnover of SfIAP triggered caspase activation and was governed in part by the SfIAP leader (Fig. 9). It is relevant in this regard that AcMNPV DNA replication is also required for host translation arrest (41), a hallmark of baculovirus infection (46). In view of the short half-lives of DIAP1 and SfIAP, viral inhibition of host protein synthesis would block replenishment of the short-lived IAP pool, thereby depleting IAP. Despite this possibility, our studies indicated that cellular IAP disappears up to 6 h before host translation arrest begins (data not shown). Therefore, it is unlikely that virus-induced IAP depletion is entirely due to general inhibition of host protein synthesis. Rather, a posttranslational modification mediated by factors activated by the DNA damage response could contribute to IAP loss. Indeed, phosphorylation affects DIAP1 stability (15, 25). SfIAP is also phosphorylated (Vandergaast and Friesen, unpublished).
Cellular IAPs as innate sentinels of virus infection.
Our studies of apoptosis caused by baculoviruses and nodaviruses (43) suggest a critical role for cellular IAPs in combating virus infections in dipteran and lepidopteran insects. We predict that, by virtue of their central position in controlling apoptosis, the IAPs play key regulatory roles in the antiviral response of many insect species. Studies of reovirus infections have revealed that inactivation of cellular IAP, including XIAP, cIAP-1, and survivin, also occur in mammals (23). Thus, the antiviral role of IAPs may extend to vertebrates as well. Our studies here indicated that caspase-mediated apoptosis is the direct result of cellular IAP destruction that was mediated by multiple domains embedded within the IAP itself. The presence of such instability elements suggests that insect IAPs are responsive to signal-induced pathways that are triggered by virus-specific replicative events. Identification of these invertebrate pathways is now a priority.
ACKNOWLEDGMENTS
We thank Erik Settles, Duy Tran, and Jonathan Mitchell for generation of stable cell lines and helpful discussions.
This work was supported in part by Public Health Service grants AI25557 and AI40482 from the National Institute of Allergy and Infectious Diseases (P.D.F.) and NIH predoctoral traineeship T32 GM07215 (R.V. and R.J.C.).
Footnotes
Published ahead of print on 8 June 2011.
REFERENCES
- 1. Ahmad M., et al. 1997. Spodoptera frugiperda caspase-1, a novel insect death protease that cleaves the nuclear immunophilin FKBP46, is the target of the baculovirus antiapoptotic protein P35. J. Biol. Chem. 272:1421–1424 [DOI] [PubMed] [Google Scholar]
- 2. Benedict C. A., Norris P. S., Ware C. F. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013–1018 [DOI] [PubMed] [Google Scholar]
- 3. Bertin J., et al. 1996. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J. Virol. 70:6251–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Best S. M. 2008. Viral subversion of apoptotic enzymes: escape from death row. Annu. Rev. Microbiol. 62:171–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brodsky M. H., et al. 2004. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol. Cell. Biol. 24:1219–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broemer M., Meier P. 2009. Ubiquitin-mediated regulation of apoptosis. Trends Cell Biol. 19:130–140 [DOI] [PubMed] [Google Scholar]
- 7. Cartier J. L., Hershberger P. A., Friesen P. D. 1994. Suppression of apoptosis in insect cells stably transfected with baculovirus p35: dominant interference by N-terminal sequences p351–76. J. Virol. 68:7728–7737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cerio R. J., Vandergaast R., Friesen P. D. 2010. Host insect inhibitor-of-apoptosis SfIAP functionally replaces baculovirus IAP but is differentially regulated by its N-terminal leader. J. Virol. 84:11448–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarke P., Tyler K. L. 2009. Apoptosis in animal models of virus-induced disease. Nat. Rev. Microbiol. 7:144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clem R. J. 2007. Baculoviruses and apoptosis: a diversity of genes and responses. Curr. Drug Targets 8:1069–1074 [DOI] [PubMed] [Google Scholar]
- 11. Ditzel M., et al. 2003. Degradation of DIAP1 by the N-end rule pathway is essential for regulating apoptosis. Nat. Cell Biol. 5:467–473 [DOI] [PubMed] [Google Scholar]
- 12. Friesen P. D. 2007. Insect viruses, p. 707–736.In Knipe D. M.(ed.), Fields virology, 5th ed., vol. 1 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 13. Guy M. P., Friesen P. D. 2008. Reactive-site cleavage residues confer target specificity to baculovirus P49, a dimeric member of the P35 family of caspase inhibitors. J. Virol. 82:7504–7514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gyrd-Hansen M., Meier P. 2010. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat. Rev. Cancer 10:561–574 [DOI] [PubMed] [Google Scholar]
- 15. Harvey K. F., Pfleger C. M., Hariharan I. K. 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114:457–467 [DOI] [PubMed] [Google Scholar]
- 16. Hay B. A., Guo M. 2006. Caspase-dependent cell death in Drosophila. Annu. Rev. Cell Dev. Biol. 22:623–650 [DOI] [PubMed] [Google Scholar]
- 17. Hay B. A., Wolff T., Rubin G. M. 1994. Expression of baculovirus P35 prevents cell death in Drosophila. Development 120:2121–2129 [DOI] [PubMed] [Google Scholar]
- 18. Hershberger P. A., Dickson J. A., Friesen P. D. 1992. Site-specific mutagenesis of the 35-kilodalton protein gene encoded by Autographa californica nuclear polyhedrosis virus: cell line-specific effects on virus replication. J. Virol. 66:5525–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hornung V., Latz E. 2010. Intracellular DNA recognition. Nat. Rev. Immunol. 10:123–130 [DOI] [PubMed] [Google Scholar]
- 20. Hozak R. R., Manji G. A., Friesen P. D. 2000. The BIR motifs mediate dominant interference and oligomerization of inhibitor of apoptosis Op-IAP. Mol. Cell. Biol. 20:1877–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang Q., et al. 2000. Evolutionary conservation of apoptosis mechanisms: lepidopteran and baculoviral inhibitor of apoptosis proteins are inhibitors of mammalian caspase-9. Proc. Natl. Acad. Sci. U. S. A. 97:1427–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Igaki T., Yamamoto-Goto Y., Tokushige N., Kanda H., Miura M. 2002. Down-regulation of DIAP1 triggers a novel Drosophila cell death pathway mediated by Dark and DRONC. J. Biol. Chem. 277:23103–23106 [DOI] [PubMed] [Google Scholar]
- 23. Kominsky D. J., Bickel R. J., Tyler K. L. 2002. Reovirus-induced apoptosis requires mitochondrial release of Smac/DIABLO and involves reduction of cellular inhibitor of apoptosis protein levels. J. Virol. 76:11414–11424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar S. 2007. Caspase function in programmed cell death. Cell Death Differ. 14:32–43 [DOI] [PubMed] [Google Scholar]
- 25. Kuranaga E., et al. 2006. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell 126:583–596 [DOI] [PubMed] [Google Scholar]
- 26. LaCount D. J., Hanson S. F., Schneider C. L., Friesen P. D. 2000. Caspase inhibitor P35 and inhibitor of apoptosis Op-IAP block in vivo proteolytic activation of an effector caspase at different steps. J. Biol. Chem. 275:15657–15664 [DOI] [PubMed] [Google Scholar]
- 27. Lannan E., Vandergaast R., Friesen P. D. 2007. Baculovirus caspase inhibitors P49 and P35 block virus-induced apoptosis downstream of effector caspase DrICE activation in Drosophila melanogaster cells. J. Virol. 81:9319–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee H. H., Miller L. K. 1978. Isolation of genotypic variants of Autographa californica nuclear polyhedrosis virus. J. Virol. 27:754–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lilley C. E., Schwartz R. A., Weitzman M. D. 2007. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 15:119–126 [DOI] [PubMed] [Google Scholar]
- 30. Mace P. D., Shirley S., Day C. L. 2010. Assembling the building blocks: structure and function of inhibitor of apoptosis proteins. Cell Death Differ. 17:46–53 [DOI] [PubMed] [Google Scholar]
- 31. Manji G. A., Friesen P. D. 2001. Apoptosis in motion. An apical, P35-insensitive caspase mediates programmed cell death in insect cells. J. Biol. Chem. 276:16704–16710 [DOI] [PubMed] [Google Scholar]
- 32. Moon N. S., et al. 2008. E2F and p53 induce apoptosis independently during Drosophila development but intersect in the context of DNA damage. PLoS Genet. 4:e1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muro I., Hay B. A., Clem R. J. 2002. The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J. Biol. Chem. 277:49644–49650 [DOI] [PubMed] [Google Scholar]
- 34. Olson V. A., Wetter J. A., Friesen P. D. 2001. Oligomerization mediated by a helix-loop-helix-like domain of baculovirus IE1 is required for early promoter transactivation. J. Virol. 75:6042–6051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Neill L. A., Bowie A. G. 2010. Sensing and signaling in antiviral innate immunity. Curr. Biol. 20:R328–R333 [DOI] [PubMed] [Google Scholar]
- 36. O'Riordan M. X., Bauler L. D., Scott F. L., Duckett C. S. 2008. Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation. Dev. Cell 15:497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orme M., Meier P. 2009. Inhibitor of apoptosis proteins in Drosophila: gatekeepers of death. Apoptosis 14:950–960 [DOI] [PubMed] [Google Scholar]
- 38. Pridgeon J. W., et al. 2008. Topically applied AaeIAP1 double-stranded RNA kills female adults of Aedes aegypti. J. Med. Entomol. 45:414–420 [DOI] [PubMed] [Google Scholar]
- 39. Rumble J. M., Duckett C. S. 2008. Diverse functions within the IAP family. J. Cell Sci. 121:3505–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneider I. 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27:353–365 [PubMed] [Google Scholar]
- 41. Schultz K. L., Friesen P. D. 2009. Baculovirus DNA replication-specific expression factors trigger apoptosis and shutoff of host protein synthesis during infection. J. Virol. 83:11123–11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schultz K. L., Wetter J. A., Fiore D. C., Friesen P. D. 2009. Transactivator IE1 is required for baculovirus early replication events that trigger apoptosis in permissive and nonpermissive cells. J. Virol. 83:262–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Settles E. W., Friesen P. D. 2008. Flock house virus induces apoptosis by depletion of Drosophila inhibitor-of-apoptosis protein DIAP1. J. Virol. 82:1378–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Srinivasula S. M., Ashwell J. D. 2008. IAPs: what's in a name? Mol. Cell 30:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tenev T., Ditzel M., Zachariou A., Meier P. 2007. The antiapoptotic activity of insect IAPs requires activation by an evolutionarily conserved mechanism. Cell Death Differ. 14:1191–1201 [DOI] [PubMed] [Google Scholar]
- 46. Thiem S. M. 2009. Baculovirus genes affecting host function. In Vitro Cell. Dev. Biol. Anim. 45:111–126 [DOI] [PubMed] [Google Scholar]
- 47. van den Heuvel S., Dyson N. J. 2008. Conserved functions of the pRB and E2F families. Nat. Rev. Mol. Cell Biol. 9:713–724 [DOI] [PubMed] [Google Scholar]
- 48. Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. 1977. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 13:213–217 [DOI] [PubMed] [Google Scholar]
- 49. Vaux D. L., Silke J. 2005. IAPs, RINGs and ubiquitylation. Nat. Rev. Mol. Cell Biol. 6:287–297 [DOI] [PubMed] [Google Scholar]
- 50. Wang S. L., Hawkins C. J., Yoo S. J., Muller H. A., Hay B. A. 1999. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98:453–463 [DOI] [PubMed] [Google Scholar]
- 51. Wilson R., et al. 2002. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell Biol. 4:445–450 [DOI] [PubMed] [Google Scholar]
- 52. Yin V. P., Thummel C. S. 2004. A balance between the diap1 death inhibitor and reaper and hid death inducers controls steroid-triggered cell death in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 101:8022–8027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoo S. J., et al. 2002. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 4:416–424 [DOI] [PubMed] [Google Scholar]
- 54. Zoog S. J., Schiller J. J., Wetter J. A., Chejanovsky N., Friesen P. D. 2002. Baculovirus apoptotic suppressor P49 is a substrate inhibitor of initiator caspases resistant to P35 in vivo. EMBO J. 21:5130–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]