Abstract
The cytolethal distending toxin (Cdt), expressed by the periodontal pathogen Aggregatibacter actinomycetemcomitans, inhibits the proliferation of cultured epithelial cells by arresting the cell cycle. The gingival epithelium is an early line of defense against microbial assault. When damaged, bacteria collectively gain entry into underlying connective tissue where microbial products can affect infiltrating inflammatory cells, leading to the destruction of the attachment apparatus. Histological evaluation of rat and healthy human gingival tissue exposed ex vivo to the Cdt for 36 and 18 hours, respectively, revealed extensive detachment of the keratinized outer layer and distention of spinous and basal cells in the oral epithelium. Treated human tissue also exhibited disruption of rete pegs and dissolution of cell junctions. Cells in the connective tissue appeared unaffected. Primary gingival epithelial cells, but not gingival fibroblasts, isolated from the same healthy human tissue were cell-cycle-arrested when treated with the toxin. These findings provide new evidence that the Cdt severely damages the oral epithelium, ex vivo, by specifically targeting epithelial cells, in situ. The Cdt shows preferential targeting of the epithelium as opposed to connective tissue in animal and human gingival explant models.
Abbreviations: cytolethal distending toxin (Cdt), connective tissue (CT), 4′,6-diamidino-2-phenylindole (DAPI), human gingival epithelial cells (HGEC), human gingival explants (HGX), human gingival fibroblasts (HGF), junctional epithelium (JE), oral epithelium (OE), rete pegs (RP), sulcular epithelium (SE)
Keywords: Aggregatibacter actinomycetemcomitans, cytolethal distending toxin, gingival epithelial cells, gingival fibroblasts, gingival explants
Introduction
The end-point of periodontal diseases is the destruction of the tissues that support and anchor the teeth. This process is initiated by a persistent polymicrobial infection (Armitage, 2010) and sustained by interactions between the microbial antagonists and host immune system (Taubman et al., 2005). The gingival epithelium is an early line of defense against these micro-organisms and functions as part of a signaling network that alerts inflammatory cells to microbial assault (Handfield et al., 2005). Damage to the epithelial barrier allows pathogens, opportunistic bacterial species, and/or their products to more easily reach and interact with the underlying connective tissue (CT) and infiltrating inflammatory cells.
Gingival epithelium consists of oral (OE), sulcular (SE), and junctional epithelium (JE) (Bosshardt and Lang, 2005). The basal cell layers of all 3 types of gingival epithelia are composed of rapidly proliferating cells that migrate toward the outer surface of the tissue. The gingival epithelial surface is under constant assault by biofilm-forming bacteria, including the Gram-negative bacterium, Aggregatibacter actinomycetemcomitans. The cytolethal distending toxin (Cdt) produced by this bacterium potentially enhances its ability to interact with, damage, and modulate gene expression of epithelial cells (Henderson et al., 2010). The Cdt is a secreted heterotrimer that damages the DNA of susceptible cells, leading to cell-cycle-mediated growth arrest or, in some cases, apoptosis (Sugai et al., 1998; Mayer et al., 1999). The rapid growth arrest of Cdt-treated immortalized oral epithelial cell lines, co-cultured with periodontal ligament fibroblasts (Kang et al., 2005; Kanno et al., 2005), prompted us to advance the hypothesis that this putative virulence factor affects the integrity of human gingival epithelial cells (HGEC) in situ.
In this report, we assessed the ability of the Cdt to alter the histology of gingival tissue from rats and healthy human subjects. Our positive findings support the development of ex vivo models for assessing the molecular effects of the Cdt on HGEC in situ. Establishing that the Cdt of A. actinomycetemcomitans affects the integrity of the gingival epithelium justifies targeting this toxin in therapeutic modalities designed to reduce the severity of some forms of periodontal disease.
Materials & Methods
Sources of Gingival Tissue and Primary Cells
The upper jaws of 6 one-month-old Sprague-Dawley rats were dissected immediately following death by decapitation of isoflurane-sedated animals. All procedures were carried out in accordance with the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee.
Human gingival explants (HGX) were obtained during routine crown-lengthening surgeries conducted on 18 physically healthy adults in the University of Pennsylvania School of Dental Medicine Graduate Periodontics Clinic. Upon presentation for surgery, all individuals were required to have clinically healthy periodontal tissues with no plaque or calculus present on their teeth. The marginal gingiva and/or keratinized gingiva distal to terminal maxillary teeth was used as the source of tissue for all toxin experiments. Thus, explants were composed of OE and underlying CT. For comparison with untreated healthy tissue, HGX were collected during osseous surgeries conducted on two adults diagnosed with periodontal disease. Microbiological sampling of these patients was not done prior to surgery. The protocol for tissue procurement received Institutional Review Board approval, and all donors provided informed consent.
HGEC and fibroblasts (HGF) were isolated from clinically healthy human tissue as described previously (Oda and Watson, 1990; Kanno et al., 2005) and were grown in serum-free keratinocyte medium and Medium 199 supplemented with Earle’s salts and 10% fetal bovine serum (FBS), respectively.
Protein Isolation and Toxin Reconstitution
Recombinant Cdt proteins were isolated by affinity chromatography as described previously (Cao et al., 2005). Heterotrimers were reconstituted, in a refolding buffer, from wild-type subunits (Mao and DiRienzo, 2002) or CdtBH160A (DiRienzo et al., 2009).
Histology and Immunostaining
Freshly excised rat jaws were partitioned, and molars with adjacent tissue were exposed to various concentrations of wild-type (CdtABC) or mutated (CdtABH160AC) toxin for 0 to 48 hrs in DMEM containing 5% FBS and 1% Antibiotic-Antimycotic solution (Invitrogen, Carlsbad, CA, USA) at 37°C with 5% CO2. Samples were fixed in 4% buffered formalin and decalcified in an acidic Decal Stat High Speed Bone Decalcifier (Decal Chemical Corporation, Tallman, NY, USA) following the manufacturer’s instructions.
Following excision, HGX were placed in F12 medium, supplemented with 5% FBS and 1% Antibiotic-Antimycotic solution. The tissue was cut into 5-mm pieces and treated, in various experiments, with 0-10 µg/mL CdtABC or CdtABH160AC. The toxin-treated samples were incubated at 37°C, with 5% CO2, for various periods of time ranging from 0 to 36 hrs, then fixed in 4% buffered formalin.
Following incubation, rat and human explants were processed for paraffin sections by the Tissue Processing Service at the School of Dental Medicine. Sections were stained with hematoxylin and eosin or labeled with pan-keratin Ab3 mouse monoclonal antibody (Lab Vision Products, Fremont, CA, USA), followed by anti-mouse biotinylated antibody and HRP-conjugated streptavidin for enzymatic detection with 3,3′ diaminobenzidine (DAB) (R&D Systems, Minneapolis, MN, USA) or goat anti-mouse Alexa Fluor 488 (Invitrogen) for fluorescent microscopy. Other sections were labeled with rabbit anti-CdtB IgG (Cao et al., 2008) and goat anti-rabbit IgG Alexa Fluor 594 (Invitrogen).
Isolated primary cells were grown in 8-well chamber slides and incubated with either mouse anti-human epithelial cell adhesion molecule (Ep-Cam EBA-1) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-fibroblast CD90/Thy-1 antigen (Ab-1) antibody (Oncogene Research Products, La Jolla, CA, USA), followed by Alexa Fluor 488 goat anti-mouse IgG.
Cell nuclei, in all samples, were visualized with DAPI. Specimens were viewed with a Nikon Eclipse 80i fluorescence microscope. Bright-field and fluorescent digital images were recorded with Spot Advanced 4.6 software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA).
Cell Cycle Analysis
Cell cultures were untreated (no toxin) or treated with 10 µg/mL CdtABC or CdtABH160AC for 18-96 hrs as described previously (Cao et al., 2005). Propidium-iodide-stained nuclei were examined on a FACSCalibur four-color dual-laser flow cytometer (BD Biosciences, Bedford, MA, USA) at the Abramson Cancer Center Flow Cytometry and Cell Sorting Resource Laboratory of the University of Pennsylvania. Data from 30,000 events were analyzed with ModFit LT 3.2 (Verity Software House, NH Topsham, ME, USA).
Results
Effects of the Cdt on Rat Gingival Tissue
Excised rat jaw sections were incubated with increasing concentrations of CdtABC for up to 48 hrs. Untreated tissue stained with hematoxylin-eosin typically exhibited tearing of the sulcular (SE) and junctional (JE) epithelia (Fig. 1A). These alterations were likely an artifact of sectioning due to soft tissue attached to the tooth. Minor peeling of the keratinized outer layer of the OE was also commonly observed in untreated tissue. Tissue exposed to the 5 µg/mL CdtABC for 36 hrs had an overall swollen appearance with a pronounced thickening of the epithelial layers, extensive disruption of the keratinized layer, and distention of HGEC in the basal layer (Fig. 1B). These effects were magnified in tissue treated with 10 µg/mL of the toxin (Fig. 1C).
Figure 1.

Effects of Cdt on the histology of gingival tissue from the upper jaws of rats. Specimens were incubated with (A) 0, (B) 5, and (C) 10 µg/mL of CdtABC or (D) 10 µg/mL of CdtABH160AC in tissue culture medium at 37°C in an atmosphere containing 5% CO2 for 36 hrs. After incubation, the tissue was decalcified, and representative sections were stained with hematoxylin and eosin for histological evaluation. Enlarged areas, designated by the red outline, are shown in the next panel or inset. JE, junctional epithelium; SE, sulcular epithelium; OE, oral epithelium; RP, rete pegs; CT, connective tissue. Magnification bars = 100 µm. All treatments were performed on pieces of tissue cut from the same rat jaw. Experiments were repeated on tissue from 6 rats. Stained sections from a representative experiment on a single rat are shown.
Histidine 160 in the catalytic site of the CdtB subunit is essential for the cell cycle arrest activity of the Cdt (Elwellet al., 2001). Mutated subunit CdtBH160A forms a heterotrimer with wild-type CdtA and CdtC that fails to inhibit the proliferation of susceptible cells (DiRienzo et al., 2009). Tissue exposed to 10 µg/mL of toxin reconstituted with CdtABH160AC appeared histologically similar to untreated tissue (Fig. 1D). These results provided the first evidence that an explant model could be used to study the activities of the Cdt on HGEC in situ.
Stability of Human Gingival Tissue ex vivo
In light of the findings with rat tissue, we hypothesized that the Cdt would induce structural changes within HGX. To test this hypothesis, it was first necessary to verify that HGX derived from healthy gingiva could be established and maintained in tissue culture medium for up to 24 hrs. Sections stained with hematoxylin-eosin and labeled with a pan-keratin monoclonal antibody contained morphologically intact OE, rete pegs (RP), and CT (Fig. 2A). HGEC and cell junctions appeared normal. The only evident change in the integrity of the tissue was a slight mechanical separation of the keratinized surface layer by 18 hrs of incubation. No further deterioration of the tissue was observed by 24 hrs. Explants derived from patients diagnosed with periodontitis consistently displayed a highly disrupted keratinized layer as well as distended cells and dissolution of cell junctions in the basal cell layer of the OE (Fig. 2B).
Figure 2.
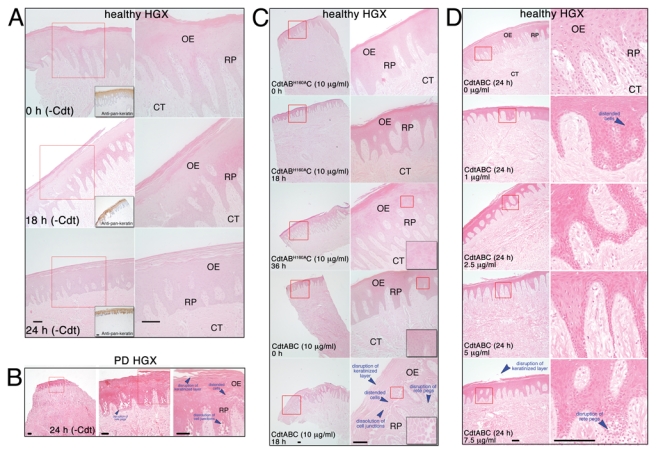
Effects of Cdt on the integrity of human gingival tissue. (A) Stability of untreated (no toxin) HGX maintained ex vivo. Explants from systemically and periodontally healthy patients were incubated for 0 hr, 18 hrs, and 24 hrs in tissue culture medium at 37°C in an atmosphere containing 5% CO2. After incubation, the tissue was fixed and embedded in paraffin, and representative sections were stained with hematoxylin and eosin. Other sections were incubated with pan-keratin Ab3 mouse monoclonal antibody (1:200), followed by anti-mouse biotinylated antibody and HRP-conjugated high-sensitivity streptavidin for the enzymatic detection of epithelial cells (insets). (B) Representative explant from a patient diagnosed with periodontal disease (PD). Sections were stained with hematoxylin and eosin after 24 hrs of incubation without toxin. (C) Human gingival explants were exposed to 10 µg/mL of CdtABH160AC for 0, 18, and 36 hrs or to 10 µg/mL of CdtABC for 0 and 18 hrs. Insets show enlarged areas designated by the red outline. (D) Dose response of HGX to the Cdt. Explants were treated with 0, 1.0, 2.5, 5.0, and 7.5 µg/mL of CdtABC for 24 hrs. Specimens were processed and sections stained as described above. Red outlines designate areas enlarged in the next panel. Abbreviations are the same as those described in the legend for Fig. 1. Magnification bars = 100 µm. All treatments in an experiment were performed on pieces of tissue cut from the same explant obtained from a single patient. Experiments were performed a minimum of 3 times on explants from different patients.
Effects of the Cdt on Human Gingival Tissue
The histologic appearance of HGX incubated, for up to 36 hrs, with 10 µg/mL of the mutated toxin CdtABH160AC (Fig. 2C) was identical to that of explants incubated for 0 hr with CdtABC. Tissue exposed to 10 µg/mL CdtABC for 18 hrs exhibited prominent histologic alterations. There was more pronounced separation of the keratinized surface layer, extensive disruption of the epithelial layers, and marked loss of structural integrity of the RP. The epithelial layers appeared to be swollen or thickened, and the HGEC were dramatically distended, with an apparent loss of the cell junctions. The morphological changes were reminiscent of the histology of HGX from periodontitis patients (Fig. 2B).
HGX exposed to lower concentrations of the toxin (2.5 and 5.0 µg/mL) for 24 hrs exhibited detectable peeling of the keratinized outer layer and distention of spinous and basal HGEC (Fig. 2D). Changes in the integrity of the RP and cell junctions were not as evident in comparison with those of tissue exposed to higher concentrations of CdtABC.
Co-localization of the Cdt and Epithelial Cells
To confirm that the Cdt targets HGEC as opposed to CT stromal cells, we labeled sections from toxin-treated tissue with anti-CdtB IgG. CdtB was observed primarily in the epithelial layers (red fluorescence in Fig. 3A). HGEC were localized by co-labeling with a pan-keratin antibody (green fluorescence in Fig. 3A). Significantly less CdtB was detected on the keratinized outer surface layer of the gingival epithelium as well as in the underlying CT. Similar results were obtained when the tissue was treated with CdtABH160AC(Fig. 3B). However, in this case no change in tissue morphology was observed, as evidenced by the appearance of more well-delineated RP and non-distended HGEC. Wild-type toxin appeared to penetrate the CT to a greater extent than CdtABH160AC. Anti-CdtB IgG did not bind to tissue that was not exposed to the Cdt (Fig. 3C).
Figure 3.

Localization of CdtB in human tissue treated with Cdt. Human gingival explants were incubated with (A) 10 µg/mL of CdtABC, (B) 10 µg/mL of CdtABH160AC, and (C) no toxin for 19 hrs. The specimens were then co-labeled with pan-keratin Ab3 mouse antibody (1:200) and rabbit anti-CdtB antibody (1:50,000), followed by goat anti-mouse Alexa Fluor 488 (1:400; green fluorescence) and goat anti-rabbit IgG Alexa Fluor 594 conjugate (1:400; red fluorescence). Cell nuclei were visualized with DAPI (blue fluorescence). The arrows in (A) show the location of CdtB in the various tissue layers. The inset in (C) is a tissue section stained only with the goat anti-mouse Alexa Fluor 488 conjugate (1:400) and DAPI. Abbreviations are the same as in the legend for Fig. 1. GS, gingival surface. Magnification bar = 100 µm. Experiments were performed 3 times on explants from different patients.
Effect of the Cdt on Primary Gingival Epithelial Cells and Fibroblasts
To further support observations that the Cdt was primarily affecting epithelial and not CT stromal cells, we isolated primary HGEC and HGF from the same type of tissue as that used to generate HGX. Cultured primary HGEC had an epithelioid morphology, bound Ep-CAM antibody, and failed to bind an antibody that recognizes CD90/Thy-1 (Fig. 4A). Cultured HGF exhibited a morphology typical of fibroblasts and bound the CD90/Thy-1 (Ab-1) antibody. We previously showed that the Ab-1 marker is expressed by oral fibroblasts but not by immortalized labial epithelial cells (Kang et al., 2005).
Figure 4.
Effect of Cdt on cultured human primary HGEC and HGF. (A) HGEC incubated with mouse anti-human Ep-Cam (EBA-1; 1:100) antibody and HGF labeled with anti-fibroblast CD90/Thy-1 antigen (Ab-1; 1:500) antibody. Both preparations were then labeled with Alexa Fluor 488 goat anti-mouse IgG conjugate (1:400; green fluorescence). Nuclei were stained with DAPI (blue fluorescence). The arrows show the location of Ep-CAM on the cell surface. The inset shows cells stained only with the goat anti-mouse Alexa Fluor 488 conjugate (1:400) and DAPI. (B) Flow cytometry of HGEC untreated and treated with 2.5 µg/mL of CdtABC for 36 hrs. The heavy black line designates the propidium iodide profile. The inset shows HGEC treated with 10 µg/mL of CdtABH160AC for 36 hrs. Values represent percent of the total cell population in diploid G1, diploid G2, and diploid S. (C) HGF were untreated and treated with 10 µg/mL of CdtABC for 36 hrs. The inset shows HGF exposed to CdtABC for 96 hrs. Cell cycle experiments were performed a minimum of 3 times.
HGEC exposed to the CdtABC for 18-36 hrs were arrested at the G2/M interphase of the cell cycle, as indicated by a significant increase in the number of cells with a 4n DNA content. Cells treated for 36 hrs are shown in Fig. 4B. CdtABH160AC had no effect on the cell cycle (inset). In contrast, there was no increase in the number of cells having a 4n DNA content when HGF were treated with CdtABC for 36 and 96 hrs (Fig. 4B and inset, respectively). These results were consistent with those of previous studies in which primary periodontal ligament fibroblasts were found to be resistant to the cell-cycle-inhibitory activity of the Cdt (Kang et al., 2005; Kanno et al., 2005).
Discussion
A. actinomycetemcomitans is a well-studied periodontal pathogen found in periodontal lesions from individuals with disease (Christersson et al., 1987) and has been shown to attach to (Fine et al., 2006) and invade (Meyer et al., 1996; Lepine et al., 1998) oral epithelial cells. This is the only oral bacterium that expresses a Cdt (Henderson et al., 2010). Anecdotal evidence indicates that the A. actinomycetemcomitans Cdt potentially plays a role in events associated with plaque-induced periodontal diseases. This toxin inhibits the proliferation of oral epithelial cell lines (DiRienzo et al., 2002; Kanno et al., 2005) and T-lymphocytes (Shenker et al., 1999) and is expressed by non-oral pathogens associated with diseases that involve an epithelial or mucosal layer (Ge et al., 2008). The spread of cdt genes is most likely due to horizontal gene transfer (Doungudomdacha et al., 2007). Thus, a major objective is to link the biological activity of Cdt, a plausible virulence factor, directly with the manifestations of periodontitis.
Based on the sensitivity of epithelioid cells to the Cdt in vitro, we proposed that the effects of the toxin on oral epithelial cells could be characterized in situ. Initially, we found that Cdt-induced tissue damage is detectable, by histological methods, in rat gingiva. HGX were shown to survive ex vivo for up to 4 days without evidence of degenerative changes or de-differentiation of cells (Powell, 1967). Intense proliferative activity in the basal HGEC was detected for up to 48 hrs (Powell, 1971). This stability favored the use of HGX over a less authentic co-culture model (Odioso et al., 1995). In our hands, HGX maintained their morphological integrity for up to 36 hrs ex vivo. Cdt-induced histological changes were detected by 18 hrs of incubation, well within the window of tissue health reported previously (Powell, 1967). Indications that tissue damage was directly related to the toxin included distention of cells, dissolution of cell junctions, and presence of CdtB specifically in spinous and basal layers of CdtABC-treated tissue. No tissue damage was observed when tissue was treated with toxin made with biologically inactive CdtBH160A. Relatively more wild-type than mutated toxin was found in the CT layer, which could be the result of increased basal epithelial cell layer damage. Stromal cells within the CT appeared unaffected by the Cdt. This observation is consistent with the minimal amount of CdtB detected in the CT and the failure of the toxin to affect cell-cycle progression of cultured primary HGF. The latter result supported earlier findings that even though the Cdt bound to primary HPLF, the cells did not incur DNA-damage (Kanget al., 2005; Kanno et al., 2005).
A potential limitation of this study is that experiments with human tissue were conducted on explants containing keratinized OE. However, the effect of the Cdt on SE and JE can be studied more effectively in the rat model, where intact gingiva is collected together with adjacent alveolar bone and teeth. Pronounced Cdt-induced effects were found in the basal cell layer of HGX, suggesting that proliferating cells are extremely susceptible to the toxin. Since the Cdt is actively secreted by the bacterium, it likely permeates the enlarged interstitial spaces of JE and into non-keratinized forms of gingival epithelium to affect their basal cell layers. Distention of HGEC and dissolution of cell junctions could promote more widespread penetration of the toxin throughout the tissue. Damaged epithelial cells can signal the recruitment of inflammatory cells, increasing the extent of tissue damage due to the production of inflammatory mediators. This extensive perturbation of the tissue allows intact bacteria access to the CT. where host cell and bacterial products, such as lipopolysaccharide and peptidoglycan, can stimulate bone resorption.
In summary, the rat and human tissue models have potential for studying the details of molecular interactions of the Cdt with HGEC in situ. We clearly demonstrated that gingival tissue exposed, ex vivo, to the toxin exhibited severe structural damage to the epithelial layers. This localized damage supports the possibility that Cdt-induced alterations in gingival epithelial structure could play a part early in the pathogenesis of periodontitis in the presence of A. actinomycetemcomitans. Comparison of the histology of Cdt-treated healthy HGX and untreated explants from several individuals with periodontal disease indicates that morphologically similar tissue damage can occur in vivo.
Acknowledgments
We thank Drs. Leszek Kubin and Denys Volgin, School of Veterinary Medicine, for help with obtaining rat tissue; Dr. Edward Macarak, Department of Anatomy and Cell Biology, for advice on tissue staining; and Dr. Sunday Akintoye, Department of Oral Medicine, for the use of a fluorescence microscope.
Footnotes
This work was supported by USPHS Research Grant DE012593 from the National Institute of Dental and Craniofacial Research . Portions of this work were performed as partial fulfillment of the requirements for a Master’s Degree in Oral Biology (MH).
The authors have no conflicting financial interests.
References
- Armitage GC. (2010). Comparison of the microbiological features of chronic and aggressive periodontitis. Periodontol 2000 53:70-88 [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Lang NP. (2005). The junctional epithelium: from health to disease. J Dent Res 84:9-20 [DOI] [PubMed] [Google Scholar]
- Cao L, Volgina A, Huang CM, Korostoff J, DiRienzo JM. (2005). Characterization of point mutations in the cdtA gene of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans . Mol Microbiol 58:1303-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Bandelac G, Volgina A, Korostoff J, DiRienzo JM. (2008). Role of aromatic amino acids in receptor binding activity and subunit assembly of the cytolethal distending toxin of Aggregatibacter actinomycetemcomitans . Infect Immun 76:2812-2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christersson LA, Albini B, Zambon JJ, Wikesjö UM, Genco RJ. (1987). Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electron microscopic studies. J Periodontol 58:529-539 [DOI] [PubMed] [Google Scholar]
- DiRienzo JM, Song M, Wan LS, Ellen RP. (2002). Kinetics of KB and HEp-2 cell responses to an invasive, cytolethal distending toxin-producing strain of Actinobacillus actinomycetemcomitans . Oral Microbiol Immunol 17:245-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo JM, Cao L, Volgina A, Bandelac G, Korostoff J. (2009). Functional and structural characterization of chimeras of a bacterial genotoxin and human type I DNAse. FEMS Microbiol Lett 291:222-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doungudomdacha S, Volgina A, DiRienzo JM. (2007). Evidence that the cytolethal distending toxin locus was once part of a genomic island in the periodontal pathogen Aggregatibacter (Actinobacillus) actinomycetemcomitans strain Y4. J Med Microbiol 56(Pt 11):1519-1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell CK, Chao K, Patel K, Dreyfus L. (2001). Escherichia coli CdtBmediates cytolethal distending toxin cell cycle arrest. Infect Immun 69:3418-3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine DH, Kaplan JB, Kachlany SC, Schreiner HC. (2006). How we got attached to Actinobacillus actinomycetemcomitans: a model for infectious diseases. Periodontol 2000 42:114-157 [DOI] [PubMed] [Google Scholar]
- Ge Z, Schauer DB, Fox JG. (2008). In vivo virulence properties of bacterial cytolethal-distending toxin. Cell Microbiol 10:1599-1607 [DOI] [PubMed] [Google Scholar]
- Handfield M, Mans JJ, Zheng G, Lopez MC, Mao S, Progulske-Fox A, et al. (2005). Distinct transcriptional profiles characterize oral epithelium-microbiota interactions. Cell Microbiol 7:811-823 [DOI] [PubMed] [Google Scholar]
- Henderson B, Ward JM, Ready D. (2010). Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen. Periodontol 2000 54:78-105 [DOI] [PubMed] [Google Scholar]
- Kang P, Korostoff J, Volgina A, Grzesik W, DiRienzo JM. (2005). Differential effect of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans on co-cultures of human oral cells. J Med Microbiol 54(Pt 8):785-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno F, Korostoff J, Volgina A, DiRienzo JM. (2005). Resistance of human periodontal ligament fibroblasts to the cytolethal distending toxin of Actinobacillus actinomycetemcomitans . J Periodontol 76:1189-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine G, Caudry S, DiRienzo JM, Ellen RP. (1998). Epithelial cell invasion by Actinobacillus actinomycetemcomitans strains from restriction fragment-length polymorphism groups associated with juvenile periodontitis or carrier status. Oral Microbiol Immunol 13:341-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, DiRienzo JM. (2002). Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell Microbiol 4:245-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bueno LC, Hansen EJ, DiRienzo JM. (1999). Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans . Infect Immun 67:1227-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DH, Lippmann JE, Fives-Taylor PM. (1996). Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun 64:2988-2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda D, Watson E. (1990). Human oral epithelial cell culture I. Improved conditions for reproducible culture in serum-free medium. In Vitro Cell Dev Biol 26:589-595 [DOI] [PubMed] [Google Scholar]
- Odioso LL, Doyle MJ, Quinn KW, Bartel RL, Zimber MP, Stevens-Burns D. (1995). Development and characterization of an in vitro gingival epithelial model. J Periodontal Res 30:210-219 [DOI] [PubMed] [Google Scholar]
- Powell RN. (1967). Gingival tissue physiology in vitro. I. Gingival organ culture. J Periodontal Res 2:290-296 [DOI] [PubMed] [Google Scholar]
- Powell RN. (1971). Gingival tissue physiology in vitro. 3. Cell proliferation in gingival epithelium. J Periodontal Res 6:38-44 [DOI] [PubMed] [Google Scholar]
- Shenker BJ, McKay T, Datar S, Miller M, Chowhan R, Demuth D. (1999). Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol 162:4773-4780 [PubMed] [Google Scholar]
- Sugai M, Kawamoto T, Peres SY, Ueno Y, Komatsuzawa H, Fujiwara T, et al. (1998). The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun 66:5008-5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman MA, Valverde P, Han X, Kawai T. (2005). Immune response: the key to bone resorption in periodontal disease. J Periodontol 76(11 Suppl):2033S-2041S [DOI] [PubMed] [Google Scholar]



