Abstract
Using fMRI we investigated multimodal (visual and auditory) semantic and unimodal (visual only) phonological processing in reading disabled (RD) adolescents and non-impaired (NI) control participants. We found reduced activation for RD relative to NI in a number of left-hemisphere reading-related areas across all processing tasks regardless of task type (semantic vs. phonological) or modality (auditory vs. visual modality). Moreover, activation differences in these regions, which included the inferior frontal gyrus (IFG), the superior temporal gyrus (STG) and the occipitotemporal region (OT), was largely independent of in-scanner performance in our auditory semantic task. That is, although RD participants and NI participants differed in performance in visually presented conditions, they did not differ significantly in the auditory condition, yet similar patterns of reduced activation were observed in these regions across conditions. These findings indicate a neurobiological marker in RD that is independent of task, modality or performance. These findings are discussed in the context of current neurobiological models of RD.
Keywords: Reading disability, Dyslexia, Phonology, Semantics, Performance, fMRI
Introduction
The Neurobiology of RD
An increasing body of research suggests that the core deficit in developmental dyslexia (or reading disability; RD) lies within the language system, most prominently at the level of phonological processing and analysis (e.g., Wagner & Torgesen, 1987; see also Ramus, 2003 for a review). Moreover, a significant body of neuroimaging research has now established a common neurobiological characteristic of RD as a disruption across a number of critical left hemisphere (LH) reading-related sites. This disruption typically manifests as an under-activation relative to non impaired (NI) individuals and is primarily observed in both LH temporoparietal and LH occipitotemporal (OT) regions. Moreover, this relative under-activation is particularly pronounced during tasks that require printed word processing or make explicit demands on phonological processing or analysis, e.g., a rhyme task (e.g., Brunswick et al. 1999; Paulesu et al. 2001; Pugh et al. 2008; Salmelin et al. 1996; Shaywitz et al. 1998; 2002; Temple et al. 2001; see Sarkari et al. 2002 & Pugh et al. 2000a for reviews). This functional anomaly in LH regions has been observed consistently in children (Shaywitz et al. 2002) and adults (Salmelin et al. 1996; Shaywitz et al. 1998). Furthermore, this relative hypoactivation in LH posterior regions (notably the LH OT) seems to be stable across alphabetic languages (Paulesu et al. 2001) and is detectable as early as the end of kindergarten (Simos et al. 2002).
Several studies have also observed processing in RD readers that can be interpreted as compensatory. For example, Shaywitz et al. (1998, 2002) observed that during tasks that made explicit demands on phonological assembly (pseudoword and word-reading tasks), RD readers showed a disproportionately greater engagement of inferior frontal and prefrontal dorsolateral sites than non-impaired (NI) readers (see also Brunswick et al. 1999; for similar findings). Other studies have found similar potentially compensatory shifts to posterior right hemisphere (RH) regions in RD readers both in terms of activation increases (Shaywitz et al. 1998; Simos et al. 2000b, 2007) and functional connectivity (Pugh et al. 2000b; Rumsey et al. 1997).
Deficits beyond explicit phonological processing
The commonly observed LH relative under-activation described above is most pronounced for tasks that emphasize phonological processing of printed stimuli or for metalinguistic tasks that are phonological in nature, thus reflecting the core phonological processing deficit in RD. However, similar patterns of decreased activation for RD readers have been observed in print tasks that emphasize lexical or semantic processing (e.g., making a semantic relatedness judgment about two printed words), consistent with the idea that some phonological processing (in the form of assembly) is still required for all word reading tasks (see R. Frost 1998). For example, Shaywitz et al. (1998) looked at activation differences between NI and RD adults across a series of printed tasks that made variable demands on phonological processing, including a) a single letter rhyme and b) a pseudoword rhyme tasks as well as c) a semantic category judgment task that required participants to determine if two printed words were members of the same semantic category (e.g., CAT and DOG). RD readers showed reduced LH activation relative to NI readers during the pseudoword rhyme task, consistent with previous findings associated pseudoword processing (e.g., Brunswick et al. 1999; Horowitz 1998; Paulesu et al. 2001). Additionally, during the semantic judgment task RD adults had reduced activation in angular gyrus (AG) and more activation in bilateral inferior frontal gyrus (IFG) relative to NI adults. Moreover, Shaywitz et al. (2002) compared activation patterns for RD and NI children using a similar set of tasks, and again found less activation in LH AG (but not RH AG) during a semantic task for RD children relative to NI children as well as less activation in bilateral middle temporal gyrus (MTG) relative to NI children. Following up on this line of work, Pugh et al. (2000) found that NI but not RD adults showed correlated activation between the LH AG and the LH superior temporal gyrus (STG), for both a print pseudoword rhyme task and a print semantic category judgment task (tasks that require decoding or assembly), but not during a single letter rhyme task (in the single letter task connectivity did not differ between groups). Taken together these findings indicate differential functional cortical activation between RD and NI readers in response to visual linguistic processing in a variety of tasks, including those that do not require overt phonological processing beyond word decoding (e.g., semantic processing tasks).
Deficits in RD associated with processing of auditorily presented stimuli
Given that the core deficit in RD is typically proposed to reside within the phonological component of the language system, one question that arises is the extent to which reading difficulties associated with RD and the corresponding neurobiological dysfunction are circumscribed to printed language processing. Behaviorally, individuals with RD do not typically have difficulty processing spoken words unless the task is explicitly phonological (i.e., tests of phonological awareness such as elision and blending of phonemes and words, or rhyming of words or syllables), or for longer utterances or more complex tasks (such as syntactic processing or vocabulary knowledge; e.g., Scarborough 1991). However, there is some evidence indicating difficulty with processing of smaller units of speech or tones when the task is not explicitly phonological; for example, impairments have been observed when individuals with RD need to make temporal order judgments to rapidly presented tones (Tallal, 1980; Share et al. 2002); under circumstances where auditory stimuli must be extracted from noise (Chait et al. 2007); and for particular types of categorical perception (Bogliotti et al. 2008).
Consistent with these behavioral findings, neurobiological dysfunction during several lower-level auditory processing tasks has been observed in children and adults with RD. For example, Gaab et al. (2007) found that RD children exhibited comparable activation, in left prefrontal cortex during processing of rapid frequency changing and slow frequency changing non-linguistic (synthesized CVC-like) stimuli, whereas controls showed increased activation for stimuli with rapid frequency transitions. Temple et al. (2000) report similar findings for adults with RD compared to NI adults; preferential activation in left prefrontal cortex for rapid relative to slow changing transitions in NI but not for RD adults. Moreover, Ruff et al. (2003) observed deficits in categorical perception, such that RD adults failed to show neural response to deviant stimuli in a pre-attentive (pa-ta) oddball task; NI individuals exhibited increased activation to deviants in multiple language related LH regions (including the angular gyrus). Finally, Brier et al. (2003), using MEG, found differences in laterality (more LH activation for NI, more RH for RD) in a syllable discrimination task using a voice onset time series continuum. These findings suggest that, at least for some individuals with RD, there may be an underlying lower-level auditory processing difficulty and or phoneme discrimination deficit; however, it is unclear (particularly in the case of rapid auditory processing) how this difficulty is related to the more commonly observed phonological processing and decoding deficits observed in RD (see Ramus, 2003 and Tallal & Gabb, 2006 for reviews of this literature).
Neurobiological studies of spoken language processing in RD at the word and sentence level processing are surprisingly rare, especially considering the large number of studies on printed word processing in RD. Several early PET studies of adults with RD were consistent with findings from behavioral studies that limited spoken word processing dysfunction to tasks that were explicitly phonological. For example, Rumsey et al. (1994) examined adults with RD and NI controls across two tasks that required processing of auditorily presented linguistic information; a rhyme task and a syntactic processing task. Findings from this study revealed similar blood flow in both groups during the syntactic processing task, but reduced blood flow for individuals with RD (relative to NI) during the rhyme task in the LH temporoparietal and LH superior temporal regions. These findings are consistent with a deficit that is limited to explicit phonological processing for lexical-level stimuli that are presented auditorily (see also Rumsey et al. 1992 for a similar finding, and Rumsey, 1996 for a review).
A few findings from recent neuroimaging studies deviate somewhat from behavioral and early neuroimaging findings, in that they observe neurobiological dysfunction in individuals with RD that are associated with auditory word processing tasks that require lexical-level and/or semantic level processing, suggesting that deficits in spoken language processing may be more broad than previously conceived. For example, Booth et al. (2007) compared semantic processing in RD and NI children in both visual and auditory presentation modalities using a semantic association task, with variable association strength between the words (participants judged whether two words were related in meaning). Although the authors found relatively few statistically significant differences between the groups in overall activation in either modality, and no significant differences in the auditory modality, they did find that RD children showed weaker correlations between semantic association strength and activation in the LH MTG and LH inferior parietal lobule (regardless of modality). Moreover, Corina et al. (2001) examined both auditory phonological processing (with a rhyme task) and auditory lexical processing (with a lexical decision task) in RD boys and NI controls and found differential activation for children with RD in both tasks in a number of regions including reduced activation the left insula, left inferior temporal gyrus and bilateral inferior frontal gyrus, indicating dysfunction in RD for spoken word processing that is somewhat task independent. Thus a small number of neuroimaging studies have suggested that, at least some individuals, with RD, the deficit extends to higher–level language processing in the auditory domain.
Taken together, these findings from low-level auditory processing (rapid frequency discrimination & phoneme processing) and from spoken word processing (both for tasks that require explicit phonological processing and those that do not) suggest that individuals with RD are impaired at multiple levels of auditory processing. However, the relative dearth of spoken language studies and general auditory processing studies in RD limits the generality of these findings. Additional work is needed to determine the extent of any spoken language dysfunction. In particular, parametric manipulations of spoken word/language processing that mirror those conducted with print processing and direct comparisons of printed and spoken language processing are critical for determining which types and levels of processing are consistently associated with dysfunction in RD, and to determine the degree to which dysfunction in RD is modality independent.
The current paper presents work that extends previous work on neurobiological function and dysfunction in RD by directly comparing spoken and printed word processing. Furthermore, we examine both an overtly phonological and a semantic processing task in order to further define the extent of neurobiological dysfunction in RD.
Methods
Participants
Twenty-six adolescents (13 males and 13 females) ranging from 9.0 years in age to 19.0 years (M = 13.2 years,) participated in exchange for payment. Thirteen of these participants met our criteria for RD (4 females, 9 males), M age = 12.6, in that they scored below the 25th% on the Word Attack (WA) sub-test of the Woodcock Johnson (WJ) or scored below the 40th % with a prior diagnosis of RD (WA% M= 19.92)1. 13 participants were NI readers (9 males, 4 females) M age = 13.7, WA% M = 56. A one-way analysis of variance (ANOVA) indicated that the groups were significantly different on WA, F (1,24)= 39.97, p < .0001. Both groups had performance IQ (PIQ: Wechsler Abbreviated Scale of Intelligence, WASI)2 scores in the normal range NI (M = 107.8); RD (M=99.9) and the groups did not differ significantly on this measure, p >.1. Participants also differed significantly on their word reading skills (TOWRE sight reading), RD, M = 69.8; NI, M = 105; F (1,24) = 72.6, p <.0001. All participants reported normal or corrected-to-normal vision and no history of known neurological impairments and no diagnoses of ADHD. All 13 NI participants were right-handed, two of the 13 RD participants were left-handed handed, and the remaining 11 were right handed. The experiment was conducted with the understanding and the written consent or assent of each participant and all procedures were approved by the Yale University Institutional Review Board.
Task and Design
The current experiment examines functional activation observed during a rhyme task and during a semantic categorization task. In addition we examine modality in the semantic task by including both spoken and printed stimuli, and lexicality in the rhyme task by including both words and pronounceable pseudowords. Thus, the design is such that in one case we vary modality (auditory/visual) but match on stimulus type (words only); in the other task we hold modality constant (visual only) and vary stimulus type (word/pseudoword). This manipulation allows for a direct comparison of semantic and phonological processing in RD, as well as a direct comparison of auditorily and visually presented stimuli that do not require explicit phonological processing. Thus, this study contributes to the extant literature by examining the extent to which neurobiological dysfunction in RD is task and or modality specific. An additional goal of the current study was to examine the relationship between behavioral task performance and cortical functional activation. Bookheimer (2000) suggests that comparing impaired and non-impaired children on a task where performance does not differ is important for understanding the true extent of the neural dysfunction as performance differences/differences in effort alone can produce differences in neural activity between groups. In studies of RD this assertion is supported by the work of Flowers (1991) who found reduced cerebral blood flow in left superior temporal gyrus for RD adults relative to NI controls in an auditory letter detection task, but when in-scanner accuracy was used as a covariate these differences were no longer present. Moreover, work from our lab suggests a relationship between behavioral task performance and degree of LH dysfunction. For example, Pugh et al. 2008 found a reduction in LH dysfunction in RD adolescents (relative to NI controls) when stimuli were easier to process, either because of stimulus characteristics (high frequency, highly imageable words) or because they had been repeated (words presented multiple times) throughout the scanning session. Thus, with this factor in mind, we included a range of tasks encompassing both those where individuals with RD are typically more impaired behaviorally (e.g., phonological judgments with visual presentation) and tasks where they are typically less impaired behaviorally (non phonological judgments with auditory presentation), in order to attempt to examine the effect of performance on brain activation in RD and NI readers.
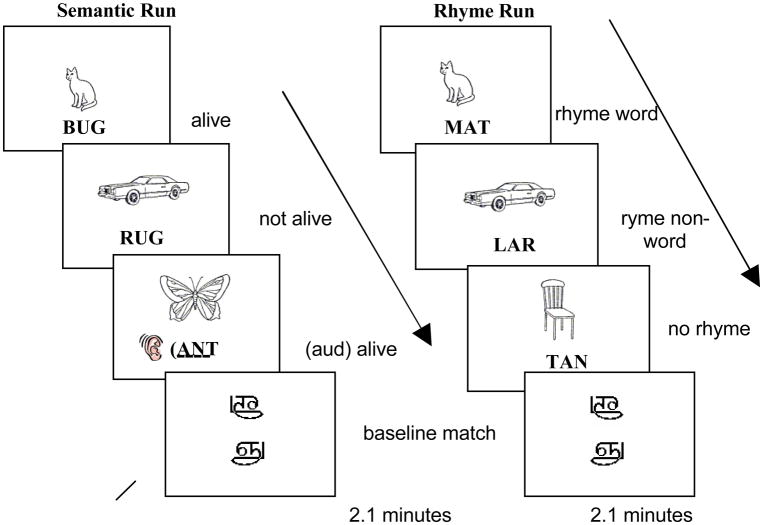
The two tasks (semantic and phonological) were presented in alternating runs. Across both tasks participants saw a picture line drawing and were then presented with a linguistic stimulus (auditory or visual word or pseudoword) and asked to make a judgment about the relationship between the picture and the linguistic token (Figure 1). In the semantic task the linguistic token was either a visually presented word or an auditorily presented word–allowing for an examination of modality. In this task, participants were required to make an animacy judgment about a picture and linguistic token – they were asked to press one button if both stimuli (the picture and the linguistic token) represented something that was alive (e.g., saw a picture of a CAT and heard or saw the word DOG), and another button if only one, or neither of the stimuli were alive (e.g., saw a picture of a CAT but heard or saw the word DESK). The rhyme task was unimodal, in that all stimuli were visually presented. In this task participants saw a picture and either a word token or a pseudoword token and were instructed to press one button if the name of the picture rhymed with the word or pseudoword (e.g., saw a picture of a DOG and saw the word WOG) and another button if the two stimuli did not rhyme (e.g., saw a picture of a DOG and saw the pseudoword WIG). Including both words and pseudowords in this task allowed for an examination of lexicality. In addition to the four activation conditions (semantic auditory, semantic visual, rhyme word, rhyme pseudoword) the design included a common baseline task that required participants to make a visual pattern match/mismatch decision to symbols from an unfamiliar orthography (Tamil). All visual stimuli throughout the experiment (visually presented words, pseudowords and Tamil characters) were accompanied by an auditory tone (matched in frequency and duration to the average of all auditorily presented words) in order to control for low-level auditory activations in our cross-modal comparisons.
Figure 1.
Task layout for both the semantic and phonological task blocks.
Participants completed 10 functional runs, 5 semantic and 5 rhyme, each containing 7 blocks (4 experimental and 2 baseline). Each of these blocks lasted for 18 seconds and contained 4 trials, each lasting 4.5 seconds for a total duration of 2.1 minutes per run. The picture appeared on the screen 1500 ms prior to the auditory or visual target item. The target and picture then remained on the screen for 2500 ms and participants had a total of 2800 ms to respond after the target came on screen. The Tamil trials had the same timing layout for consistency, one Tamil character would appear for 1500 ms followed by the second Tamil character below it – both remained on the screen for 2500 ms and the participant had a total of 2800 ms to respond (see Figure 1 for a schematic of both run types).
Stimuli
A list of monosyllabic high frequency words and pseudowords was used for the rhyme task and a list of monosyllabic high frequency words (half were presented visually and half presented auditorily) was used for the semantic task (avg. Kucera and Francis written frequency M= 253). All words and pseudowords were three to five letters in length. Words and pseudowords were presented in black Arial font on a white background. Pictures for both tasks were a set of black and white line drawings were obtained from the internet (selected for ease of identification). Line drawings and Tamil characters were also black on a white background. Participants were familiarized with the names of the line drawings before the experiment began in order to ensure that all participants would identify the objects correctly. All stimuli were forward projected to a large screen placed above the participant’s legs and participants viewed stimuli via prism mirrors that were adjusted individually. Volume for auditory stimuli was also adjusted individually.
fMRI Data Acquisition and Analysis
Functional imaging runs were 126 seconds long and consisted of 4, 18-second experimental blocks interleaved with 3, 18-second baseline blocks. After 6 initial warm-up images to obtain scanner equilibrium, a total of 63 full-brain functional images were acquired during each run, for up to 10 imaging runs (5 semantic runs and 5 rhyme runs), resulting in up to 90 images for each experimental condition and 270 images in the baseline condition. Order of activation block types was counterbalanced across runs.
Functional imaging was performed on a Siemens Sonata 1.5 Tesla scanner. Participants’ heads were immobilized within a circularly polarized head coil using a neck support, foam wedges and a restraining band drawn tightly around the forehead. Prior to functional imaging, 20 axial-oblique anatomic images (TE, 11 ms; TR, 420 ms; FOV, 200 mm; 6 mm slice thickness, no gap; 256 × 256 × 2 NEX) were prescribed parallel to the intercommissural line based on sagittal localizer images (TE, 7.7; TR, 500 ms; FOV, 240 mm; 23 slices, 5 mm slice thickness, no gap; 512 × 512 × 1 NEX). Activation images were collected using single shot, gradient echo, echo planar acquisitions (flip angle, 80 degrees; TE, 50 ms; TR, 2000 ms; FOV, 200 mm; 6 mm slice thickness, no gap; 64 × 64 × 1 NEX) at the same 20 slice locations used for anatomic images.
Functional images were first sinc-interpolated to correct for slice acquisition time, corrected for motion (Friston et al. 1995), and spatially smoothed with a Gaussian filter of size 3.125 mm FWHM. For each subject, an affine transformation to the standardized space defined by the Montreal Neurological Institute (MNI) was obtained, mapping between the subject-space T1 anatomic and the MNI-space “Colin” brain (available at http://www.bic.mni.mcgill.ca) using the BioImage Suite software package (www.bioimagesuite.org; Papademetris et al., 2003). Prior to across-subjects analysis, this transformation was applied to the single-subject activation maps, with trilinear interpolation, into 2 mm isotropic MNI space.
For each subject and voxel, linear regression was used to compare the mean signal during each experimental condition to the baseline condition, generating regression parameter estimates for each activation condition. First-, second- and third-order temporal trends, and run-to-run mean offsets were additionally included in the model. Across subjects, these values were entered into a mixed-model repeated measures ANOVA (Kirk, 1982; Woods, 1996; Holmes et al. 1998) with planned comparisons for main effects of group, task type, word type (for rhyme) and modality (for semantic) and their interactions, conducted on a voxel-wise basis.
Whole brain contrast maps are presented at a univariate threshold p <.01, FDR corrected, with an additional cluster threshold of 20 contiguous voxels. As per radiological convention, images of fMRI activations are oriented with the LH displayed toward the right and the RH displayed toward the left. Each column displays one multi-slice image from superior (top) to inferior (bottom) slices at MNI z coordinates: +28 +18 +12 −4 −20 −28.
To more fully describe task and stimulus-qualified reader group interactions, we conducted region of interest (ROI) analyses. Four LH regions were chosen that have been previously implicated in reading and reading dysfunction (c.f., Posner et al. 1999; Price, 2000; Pugh et al. 2000a, 2000b; Pugh et al. 2008): (1) LH fusiform gyrus/occipitotemporal OT; (2) LH superior temporal gyrus (STG); (3) LH inferior frontal gyrus (IFG); and (4) LH angular gyrus. ROI (1) was defined from coordinates form our recent study of good and poor readers (Pugh et al. 2008); ROIs (2–4) were defined strictly anatomically. Specifically, these ROIs were defined using the Talairach Demon atlas (Lancaster et al., 1999) and WFU-PickAtlas (Maldjian et al., 2003), with conversion to MNI space using the non-linear transform described by Brett (www.mrccbu.cam.ac.uk/Imaging/Common/mnispace.shtml). We defined (a) the LH inferior frontal gyrus: volume of 7118 voxels or 56944 mm3, created from the Inferior Frontal Gyrus atlas region and cropped to bounds within MNI z coordinates −16mm to 56mm, dilated by 1 voxel (2 mm); (b) the LH superior temporal gyrus: volume of 3760 voxels or 30080 mm3, created from the Superior Temporal Gyrus atlas region and cropped to include voxels at MNI z coordinates above 60mm, no dilation; (c) the angular gyrus: volume 1030 voxels or 8240 mm3, created from the atlas Angular Gyrus region with 1 voxel (2mm) dilation.
Results
Behavioral Data
Separate 5x2 mixed factor, analyses of variance (ANOVAs) were conducted for latency and accuracy. Experimental condition (baseline, semantic auditory, semantic visual, rhyme word, rhyme pseudoword) served as the within subject variable and skill (RD, NI) served as the between subjects variable. This analysis was followed up with post-hoc direct means comparisons for interactions.
Accuracy
The ANOVA revealed a main effect of task, F (4,96) = 21.53, p <.001, partial eta squared = .473; pair-wise comparisons revealed that accuracy in all tasks was significantly different than all other tasks, all p <. 05, with the highest accuracy for the baseline task, followed by the rhyme tasks (words > pseudowords), with the poorest performance in the category tasks (speech > print). There was also a main effect of reader group, F (1,24) = 12.32, p < .01, partial eta squared = .339; NI readers were generally more accurate, with consequent relative poor performance for RD readers, but this was qualified by a task by group interaction, F (4,96) = 3.89, p < .01, partial eta squared= .139. Follow-up pair-wise comparisons of accuracy in each task revealed that, relative to RD, NI readers were more accurate in the rhyme word, F (1,24)=18. 5, p <. 01; the rhyme pseudoword, F (1,24) = 13.4, p<.01, and marginally more accurate for the semantic-visual task, F(1,24) = 3.9, p=.059, critically there was no difference between the groups for the baseline task or the semantic-auditory task (all p >.05). See Table 1 for mean accuracy for each task broken down by group.
Table 1.
Mean in-scanner percent accuracy and reaction time (RT) with standard deviations in parentheses, by task and group
| Task | Group | Mean Accuracy | Mean RT |
|---|---|---|---|
| Baseline | NI | .96 (.03) | 978 (152) |
| RD | .91(.10) | 1025 (250) | |
| Total | .94 (.08) | 1002 (204) | |
|
| |||
| Semantic-Auditory | NI | .78 (.15) | 1424 (233) |
| RD | .73 (.11) | 1356(188) | |
| Total | .75 (.13) | 1390(210) | |
|
| |||
| Semantic-Print | NI | .79 (.13) | 1350(155) |
| RD | .69 (.13) | 1438(345) | |
| Total | .74 (.14) | 1385 (278) | |
|
| |||
| Rhyme-Word | NI | .91 (.05) | 1310(255) |
| RD | .71 (.16) | 1459(317) | |
| Total | .81 (.15) | 1385(280) | |
|
| |||
| Rhyme-Pseudoword | NI | .96 (.06) | 1332(226) |
| RD | .77 (.17) | 1505(246) | |
| Total | .87 (.16) | 1419(248) | |
Reaction Time
For reaction time analyses only data from correct trials were analyzed. The ANOVA revealed a main effect of task, F (4,96) = 31.19, p< .001, partial eta squared = .565, that was driven by faster reaction times for the baseline task relative to the experimental tasks. Reaction times did not differ between the experimental tasks (all p >.05). There were no other main effects or interactions. Table 1 shows mean RT for each task broken down by group.
fMRI data
Our tasks engaged a broad bi-hemispheric circuitry in general, and overall activation during all tasks/contrasts was reliably higher in a large number of areas for NI relative to RD participants. Areas that differed significantly between NI and RD included a number of LH sites that have been implicated in reading, including LH IFG, LH STG and LH OT/fusiform gyrus, as well as a few RH sites, particularly in the semantic conditions, including RH MFG/SFG and RH STG; RD readers also showed significantly less activation in several areas of the cerebellum (see Figures 2A–D).
Figure 2.
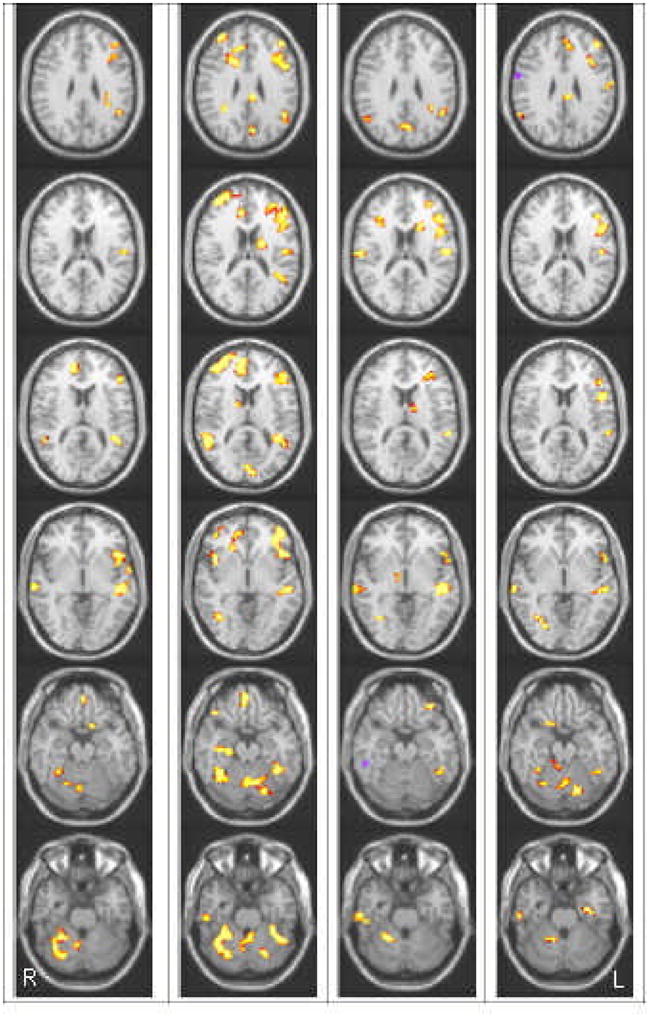
Omnibus group differences indicate regions where activation for NI is greater than RD (red/yellow) and where activation for RD is greater than NI (blue/purple) across the 4 tasks/stimuls types. (A) semantic task – auditory words (B) semantic task visual –words (C) rhyme task - words (D) rhyme task – pseudowords. Images are presented at a univariate threshold of p < .01, corrected for mapwise false discovery rate (FDR: Genovese, Lazar & Nichols, 2002), with a cluster filter of 20 min voxels. Each column displays one multi-slice image from superior (top) to inferior (bottom) slices at MNI z coordinates: +28 +18 +12 −4 −20 −28. As per radiological convention, images of fMRI activations are oriented with the LH displayed toward the right and the RH displayed toward the left.
Moreover, RD readers showed similar patterns of relative under-activation across all tasks/contrasts. To identify these areas, we completed a conjunction analysis (Hadjikhani & Roland, 1998), shown in Figure 3. Areas in yellow indicate areas that showed a significant effect of reader group in every one of the four tasks (semantic auditory, semantic visual, rhyme words & rhyme pseudowords). As can be seen in this map, NI routinely displayed significantly greater activation relative to RD in each of the four conditions for multiple LH reading related regions, including STG, IFG & OT. Only a few, very small sites showed a significant Group × Stimulus Type (modality, lexicality)3 interaction. Areas that showed a group by modality effect included a RH superior frontal site and the lentiform nucleus; areas that showed a group by lexicality effect included the LH MTG and RH IFG. For the Task (semantic > phonological) by group interaction, we found more numerous significant regions, yet they were again relatively small: these included the LH Insula/IFG the LH fusiform gyrus and the LH SFG. See Table 2 for the full list of areas for all task and stimulus type interactions with group. Moreover, very few areas showed increased activation for RD relative to NI for any of our tasks/conditions, and effects in this direction occurred only in the rhyme task. In this task, activation for pseudowords relative to baseline was greater for RD than NI in RH MTG and the RH precentral gyrus; words relative to baseline produced greater activation for RD relative to NI in a RH inferior temporal region. Table 3 lists the entire set of areas that showed greater activation for RD than NI.
Figure 3.
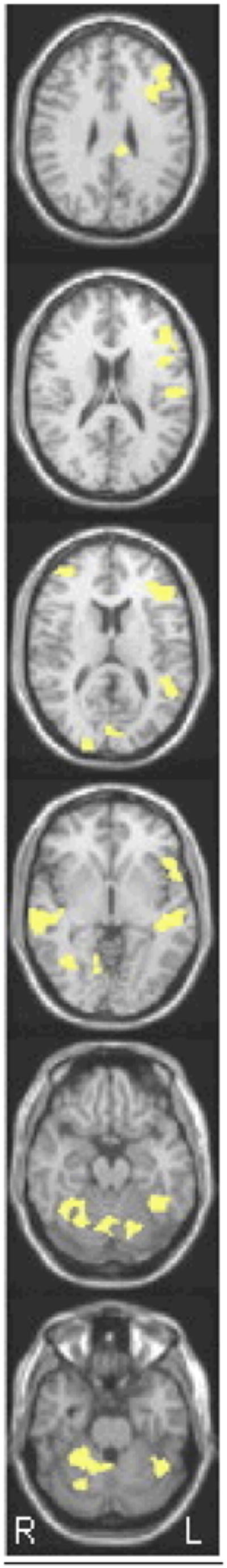
Intersect analysis showing voxels that showed a group effect (NI>RD) for all 4 tasks: sem-aud; sem-print; rhyme-word; rhyme-pseudoword. (conjoint threshold of p < .0001). The column displays one multi-slice image from superior (top) to inferior (bottom) slices at MNI z coordinates: +28 +18 +12 −4 −20 −28. As per radiological convention, images of fMRI activations are oriented with the LH displayed toward the right and the RH displayed toward the left.
Table 2.
Areas that showed task or stimulus condition by group interaction in the whole brain analysis. Because this was not a full factorial design, for appropriate comparisons, modality refers to the print vs. speech effect for the category task only; task includes only the printed word reading trials within the semantic and phonological tasks; and lexicality includes words and non-words in the phonology task only. X/Y/Z refer to coordinates of the peak activation in MNI space. P refers to the peak p-value within the region. Volume includes all voxels above threshold.
| Region | X | Y | Z | P | Volume (mm3) |
|---|---|---|---|---|---|
|
Modality (print>speech)
| |||||
| RH Superior Frontal Gyrus | 26 | 58 | 10 | .0056 | 840 |
| Lentiform Nucleus | 28 | −4 | 6 | .0027 | 168 |
|
Task (semantic>phon)
| |||||
| Caudate | −28 | −41 | 14 | .0012 | 512 |
| LH Fusiform Gyrus | −56 | −52 | −32 | .0006 | 504 |
| LH Middle Frontal Gyrus | −34 | −2 | 50 | .001 | 480 |
| RH Fusiform Gyrus | 49 | −57 | −30 | .0018 | 448 |
| RH Inferior Frontal Gyrus | 42 | 20 | −2 | .0014 | 448 |
| RH Orbital Gyrus | 4 | 51 | −22 | .0014 | 448 |
| LH Inferior Frontal Gyrus | −38 | 30 | 6 | .0005 | 440 |
| RH Middle Frontal Gyrus | 30 | 36 | −16 | .001 | 304 |
| LH Parahippocampal Gyrus | −34 | −34 | −28 | .0016 | 248 |
| Anterior Cingulate | −10 | 42 | 2 | .0026 | 224 |
| LHPostcentral gyrus | −30 | −30 | 32 | .0006 | 192 |
| RH Parahippocampal Gyrus | 14 | −26 | −13 | .0018 | 184 |
| LH Superior Frontal Gyrus | −22 | 30 | 52 | .0004 | 1032 |
| RH Precentral Gyrus | 42 | −16 | 62 | .0003 | 704 |
| LH Insula | −30 | −27 | 22 | .0009 | 176 |
|
Lexicality (words >nonwords)
| |||||
| LH Middle Frontal Gyrus | −40 | 18 | 50 | .0009 | 22 |
| RH Inferior Frontal Gyrus | 40 | 21 | −2 | .0006 | 108 |
Table 3.
Areas that showed greater activity for RD relative to NI in the whole brain analysis.
| Region | X | Y | Z | P | Volume (mm3) |
|---|---|---|---|---|---|
|
Rhyme task: – pseudowords-baseline
| |||||
| RH MTG | 65 | −7 | −12 | .000001 | 192 |
| RH Precentral gyrus | 61 | −4 | 28 | .000001 | 280 |
|
Rhyme task: words - baseline
| |||||
| RH Inferior temporal | 56 | −40 | −20 | .0001 | 168 |
Regions of Interest
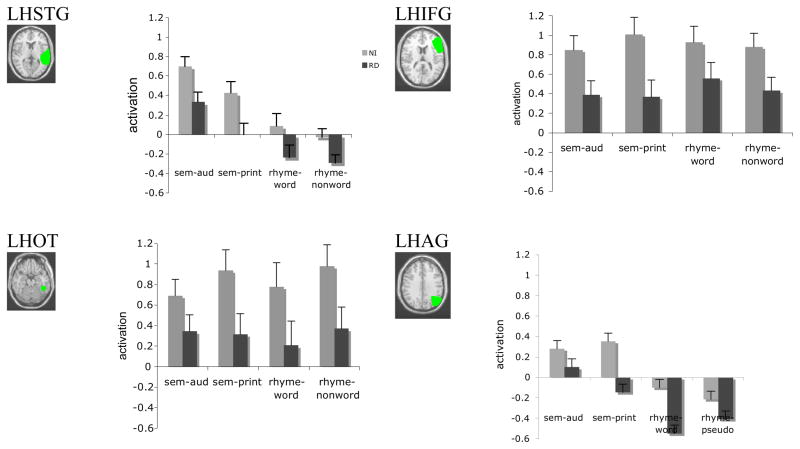
Figure 4 presents the activation levels for the four regions of interest outlined in the methods 1) LH IFG, 2) LH STG, 3) LH AG, and 4) LH OT. We focus here on LH regions as these regions are more consistently identified in the literature as showing relative under activation; though we acknowledge that we do see some RH relative under activation as well, consistent with a few studies of RD (e.g, Simos, et al. 2000). A mixed model repeated measures ANOVA revealed a main effect of group, F (1,24) = 32.5, p<.001, partial eta squared = .368, that was not modulated by region or task. Thus, we observed greater activation for NI relative to RD readers across all regions and tasks.
Figure 4.
Region of Interest location and mean activation values for the four regions of interest; LH STG, LHIFG; LOT and LAG are shown (error bars represent standard error around the mean).
Moreover, in some regions we observed a task or stimulus effect as evidenced by the interaction of region and task, F (9,216) = 11.9, p <. 001, partial eta squared = .332. In order to further examine this effect, we ran separate repeated measures ANOVAs for each region. As expected from the omnibus ANOVA, all regions showed a main effect of group (all F >1, all p < .05) with greater activation for NI relative to RD. Moreover, for both the angular gyrus and the STG there was a main effect of task, F (3,72) = 9.7 p <.001, partial eta squared = .228; F (3,72) = 19.8, p <.001, partial eta squared = .759, respectively, pair-wise comparisons revealed that the two print tasks did not differ from each other in either region (p >.05). Moreover, the two semantic tasks did not differ (p >.05) in either region. However, each of the phonological tasks was significantly different from each of the semantic tasks for both regions, all p< .01, suggesting that the LH STG and LH AG were sensitive to the differences in semantic relative to phonological processing. This finding is consistent with previous studies showing that these regions, particularly the AG, are sensitive to semantic processing (Fiebach et al. 2001; Price, Moore, Humphreys, & Wise, 1997; Rossell, Price, & Nobre, 2003; Simos et al. 2002). There were no task by group interactions (F >1) and the other two regions (LH OT and LH IFG) did not show any significant task effects. Additionally, the STG showed preferential activation for the auditory modality relative to the visual modality, p < .01, and neither of these task/stimuli effects were qualified by an interaction with group. There were no regions that showed significantly greater activity for either of the phonological tasks relative to the semantic tasks. Figure 4 shows mean activation for each region, group and task.
Discussion
The findings from the current study indicate that for this population of adolescents with RD, decreased activation relative to NI controls is not limited to print processing tasks; in our data we see reduced activation in multiple LH reading related regions in a spoken language processing task. The findings also demonstrate that LH dysfunction for RD is not limited to metalinguistic tasks that focus on phonology (rhyme), but is evident in printed and spoken language semantic processing tasks that do not require overt phonological processing or phonological assembly. Furthermore, in the semantic-auditory task NI and RD readers did not differ on mean in-scanner performance suggesting that the observed activation differences reflect a dysfunction in spoken language processing in our RD group that cannot be attributed to task performance alone. These findings are consistent with those of Hoeft et al. (2006), who found performance independent dysfunction in RD children in a visual rhyme task, and Papanicolaou et al. 2003 who report that differences between RD and NI were not due to task difficulty across a number of comparisons. Thus, these data are inconsistent with the suggestion that activation differences seen in RD are simply reflective of behavioral performance. However, the causes of this robust neurobiological anomaly in RD remain to be determined; the source of compromise at the level of neuroanatomy or neurochemistry is still a high priority for future research (e.g., Rae et al. 1998). Note that the performance on print tasks was not equated – children with RD often have primary difficulty in these tasks, thus we cannot rule out contributions of performance to activation differences for the print tasks.
Somewhat in contrast to the findings presented here, other work from our lab suggests that the degree of dysfunction in RD is indeed modulated by both task and performance (Pugh et al. 2008). Dynamic studies such as the in-scanner repetition design of Pugh and colleagues, wherein performance varied across the span of the scan but the stimuli were constant, present a promising avenue for further investigation of these issues. Thus, we suggest a more complex relationship between behavioral performance and neural activation in RD such that LH dysfunction may be lessened when stimuli are easier to process (Pugh et al. 2008) or with remediation (see Tallal, 2003; Shaywitz et al. 2004; Simos et al. 2000). Ultimately, however, RD is a neurobiological disorder with a characteristic neurobiological profile associated with relative under activation in LH cortical regions associated with both printed and spoken linguistic stimuli (and under some conditions non-linguistic stimuli, e.g., Gaab et al 2007). Thus, both poor behavioral performance on many reading related tasks and LH dysfunction are characteristics of RD, however the LH dysfunction is not necessarily a result of performance differences. Additional research is needed to examine the situations under which deficits in RD appear performance and/or task independent, and when they do not.
In terms of differential activation associated with task, although we observed some regions that were sensitive to task (significantly greater activation on the LH STG and LH AG for the semantic relative to the phonological task, consistent with previous findings), we did not find and areas with greater activation for the rhyme task, suggesting that our semantic tasks engaged an overlapping but broader cortical network. Finally, we found only a few small areas where task effects were modulated by group (see Table 2), which suggests that for our population, which included some relatively severely reading disabled adolescents, their deficit was largely task and modality independent (within the word-level linguistic domain).
One additional point of interest is our pattern of underactivation for RD relative to NI in the LH OT/fusiform region, a region that has been implicated as critical for skilled, automatic word identification (e.g, Pugh et al. 2008)4. Consistent with our data, this regions has routinely been shown to be sensitive to differences between RD and NI, with relatively increased activation for NI relative to RD. Furthermore, several studies have demonstrated that activation in this regions is positively correlated with measures of print and reading ability (Hoeft et al. 2007; Shaywitz et al. 2004; Turkeltaub et al. 2003). In addition to this regions sensitivity to reading skill, this region is often thought to be specific to visual word processing (Cohen et al. 2002), and hence has been referred to as the visual word form area or (VWFA). Our findings contradict this second finding, by showing activation differences in this region are not modulated by task – demonstrating its sensitivity to both auditory and visual processing, along with skill related differences. Some support for this finding was provided by Castro-Caldas et al. (1998), who found a marginally greater activation for literate adults in this region to spoken language relative to illiterate adults. This suggests that for skilled readers, this region might be used in both spoken and printed language processing – and that knowledge of print, or skill with printed language processing may increase the involvement of this region in language processing more generally.
One notable difference between findings in our study and findings from some previous research with RD participants is that we did not see a large amount of “compensatory” right hemisphere or frontal increases for RD relative to NI. An examination of our single subject data did reveal some areas where RD showed greater activation than NI, but these areas were quite small and limited to the phonological task (see Table 3). Potential explanations for this are that that the group of participants in the current study included some relatively more severely disabled readers than typically reported in neuroimaging studies of RD (Woodcock Johnson Word Attack Mean % = 19.2) and thus is potentially more likely to include participants with several different subtypes of RD, each with different compensation strategies (e.g., a child with a primary phonological deficit may use a different method of compensation relative to a child with a temporal processing difficulty); however our sample here is too small to attempt to divide into potential subtypes. Moreover, because little research has directly compared subtypes at the neurobiological level, it is difficult to test this possibility. Differences in treatment (uncontrolled in this sample) could also create more variablity in the sample.
Finally we note that we saw relatively large areas in the cerebellum that were significantly more active for NI than RD. This is consistent with previous findings that have found morphological differences in the cerebellum for individuals with RD (Kibby et al. 2008; Eckert et al 2005; Finch et al 2002); under-activation in the cerebellum in adults with RD relative to controls during a motor sequencing tasks (Nicolson et al. 1999); and metabolic abnormalities in the cerebellum associated with RD (Rae et al. 1998). However, the nature of the relationship between cerebellar dysfunction and RD remains an opaque one – although some have suggested a Cerebellar deficit hypothesis with primary difficulties in coordination, movement, and dexterity as causal (Nicolson & Fawcett, 2001), this hypothesis has been controversial (see Ramus et al. 2007; Nicolson & Fawcett, 2006; Zeffiro & Eden, 2001 for multiple views on this issue).
Conclusion
By demonstrating LH dysfunction in RD for both semantic and phonological processing tasks, and for both spoken and printed language tasks, our findings add to a growing body of literature that aims to understand the underlying neurobiological dysfunction associated with RD. Our finding of a significant reduction in activation for RD relative to NI, for a spoken language processing task where participants did not differ on mean in-scanner performance, underscores the utility of neuroimaging techniques for revealing dysfunction that may not be observed with behavioral measures. Critically, this finding indicates that LH dysfunction in RD is not necessarily driven by behavioral performance, suggesting a need for more sensitive behavioral measures in general, and especially in the area of spoken language processing – an area that has received relatively less attention than printed work processing in RD. We note however, that previous studies from our own lab and others have demonstrated a relationship between performance and LH dysfunction in RD and we point to the need for further investigation this relationship. Finally, consistent with our findings of similar LH dysfunction for spoken and written language processing, we observed similar patterns of LH dysfunction across both semantic and phonological processing tasks, indicating largely overlapping regions in terms of LH dysfunction in RD that is not limited to tasks that require overt phonological processing or phonological assembly.
Acknowledgments
This study is supported by NICHD Grant HD01994 to Haskins Laboratories and NICHD Grants HD40411, HD 048830 to Kenneth R. Pugh. We thank Gina DellaPorta, Kelley Delaney, Eleanor Tejada, and Priya Pugh for behavioral assessment; Hedy Serofin and Teri Hickey for help with imaging participants and Venessa DiNicola for help with initial imaging analysis. We also thank the children at the Ben Bronz Academy who generously volunteered their time to participate in our study.
Footnotes
The majority of our sample of RD adolescents came from a school for children with reading disability, and many of these children were severely impaired in reading, thus our sample includes children who are relatively more impaired than the typical RD sample with 5 of our 13 RD participants scoring below the 10th percentile on the WJ WA and 3 of our participants scoring below the 5th percentile on the WA. NI participants were recruited with flyers placed around the Yale community or by our website.
We rely on Performance IQ rather than Verbal or Full Scale IQ because of evidence that Verbal IQ scores may be artificially deflated in RD readers due to shared variance contributed by language-based abilities underlying performance on IQ and reading measures (Siegel, 1989).
Because this is not a full factorial design, statistical planned comparisons of task or stimulus effects such as modality, task and lexicality are restricted to particular task types or stimuli. That is, for modality comparisons, only the printed word and auditory words within the semantic task are compared (the words in the phonological task are not included); for Task, we compare processing of words in the semantic task to processing of words in the phonological task (auditory words and pseudowords are not included); for lexicality we compare words to pseudowords within the rhyme task (words from the semantic task are not included).
We acknowledge that differences in this region failed to reach p<.01 levels of significance for the semantic-auditory condition in the whole brain map with the FDR correction; however this was clearly region shows clear differences at a more liberal threshold of p<.05, FDR corrected – moreover, the R0I analysis suggest task independent modulation of this region by group (RD>NI).
References
- Bogliotti C, Serniclaes W, Messaoud-Galusi S, Sprenger-Charolles L. Discrimination of speech sounds by children with dyslexia: Comparisons with chronological age and reading level controls. Journal of Experimental Child Psychology. 2008;101:137–155. doi: 10.1016/j.jecp.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY. Methodological issues in pediatric neuroimaging. Mental Retardation and Developmental Disabilities. 2000;6:161–165. doi: 10.1002/1098-2779(2000)6:3<161::AID-MRDD2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Booth JR, Bebko G, Burman DD, Bitan T. Children with reading disorder show modality independent brain abnormalities during semantic tasks. Neuropsychologia. 2007;45:775–783. doi: 10.1016/j.neuropsychologia.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier JI, Simos PG, Fletcher JM, Castillo EM, Zhang W, Papanicolaou AC. Abnormal activation of tempoparietal language areas during phonetic analysis in children with dyslexia. Neuropsychology. 2003;17:610–621. doi: 10.1037/0894-4105.17.4.610. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price C, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Petersson KM, Reis A, Stone-Elander S, Ingvar M. The illiterate brain: Learning to read and write during childhood influences the functional organization of the adult brain. Brain. 1998;121:1053–1063. doi: 10.1093/brain/121.6.1053. [DOI] [PubMed] [Google Scholar]
- Chait M, Eden G, Poeppel DP, Simon JZ, Hill DF, Flowers DL. Delayed detection of tonal targets in background noise in dyslexia. Brain and Language. 2007;102:80–90. doi: 10.1016/j.bandl.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–69. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Corina DP, Richards TL, Serafini S, Richards AL, Steury K, Abbott RD, Echelard DR, Maravilla KR, Berninger VW. fMRI auditory language differences between dyslexic and able reading children. Neuroreport. 2001;12:1195–1201. doi: 10.1097/00001756-200105080-00029. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Mueller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Finch AJ, Nicolson RI, Fawcett AJ. Evidence for a neuroanatomical difference within the olivo-cerbellar pathway of adults with dyslexia. Cortex. 2002;38:529–539. doi: 10.1016/s0010-9452(08)70021-2. [DOI] [PubMed] [Google Scholar]
- Flowers DL, Wood FB, Naylor CE. Regional cerebral blood flow correlates of language processes in reading disability. Archives of Neurology. 1991;48:637–643. doi: 10.1001/archneur.1991.00530180095023. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- Frost R. Toward a strong phonological theory of visual word recognition: True issues and false trails. Psychological Bulletin. 1998;123:71–99. doi: 10.1037/0033-2909.123.1.71. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gabrieli JDE, Deutsch G, Tallal P, Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Restorative Neuroscience and Neurology. 2007;25:295–310. [PubMed] [Google Scholar]
- Hadjikhani N, Roland PE. Cross-modal transfer of information between the tactile and the visual representations in the human brain: A positron emission tomographic study. Journal of Neuroscience. 1998;18:1072–84. doi: 10.1523/JNEUROSCI.18-03-01072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalizability, random effects, and population inference. NeuroImage. 1998;7:S34. [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy Sciences. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, Whitfield-Gabrieli S, Gabrieli JDE. Neural basis of dyslexia: a comparison between dyslexic children and non-dyslexic children equated for reading ability. Journal of Neuroscience. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss A, Meyler A, Whitfield-Gabrielli S, Glover GH, Keller TA, Kobayashi N, Mazaika P, Jo B, Just A, Gabrielli JDE. Prediction of children’s reading skills using behavioral, functional and structural neuroimaging measures. Behavioral Neuroscience, 2007. 2007;12:602–613. doi: 10.1037/0735-7044.121.3.602. [DOI] [PubMed] [Google Scholar]
- Kibby MY, Fancher JB, Markanen R, Hynd GW. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. Journal of Child Neurology. 2008;23:368–380. doi: 10.1177/0883073807309235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RE. Experimental design: Procedures for the social sciences. Belmont, CA: Wadsworth; 1982. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey LS, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: The cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Nicolson RI. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;353:1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Do cerebellar deficits underlie phonological problems in dyslexia? Developmental Science. 2006;9:259–262. doi: 10.1111/j.1467-7687.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski AP, Schultz RT, Staib LH, Duncan JS. Computing 3D non-rigid brain registrations using extended robust point matching for composite multisubject fMRI analysis. In: Ellis RE, Peters TM, editors. Medical image computing and computer assisted intervention. Berlin: Springer-Verlag; 2003. pp. 788–795. [Google Scholar]
- Papanicolaou AC, Simos PG, Breier JI, Fletcher JM, Foorman BR, Francis D, Castillo EM, Davis R. Brain mechanisms for reading in children with and without dyslexia: A review of studies of normal development and plasticity. Developmental Neuropsychology. 2003;24:593–612. doi: 10.1080/87565641.2003.9651912. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Demonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. Dyslexia: Cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation & Developmental Disabilities Research Reviews. 2000a;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh K, Mencl EW, Shaywitz BA, Shaywitz SE, Fulbright RK, Skudlarski P, Constable RT, Marchione K, Jenner AR, Shankweiler DP, Katz L, Fletcher J, Lacadie C, Gore JC. The angular gyrus in developmental dyslexia: Task-specific differences in functional connectivity in posterior cortex. Psychological Science. 2000b;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pugh KP, Frost SJ, Sandak R, Landi N, Rueckl JG, Constable RT, Seidenberg MS, Fulbright RK, Katz L, Mencl WE. Effects of stimulus difficulty and repetition on printed word identification: An fMRI comparison of nonimpaired and reading-disabled adolescent cohorts. Journal of Cognitive Neuroscience. 2008;20:1146–1160. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Abdullaev YG. Neuroanatomy, circuitry and plasticity of word reading. Neuroreport. 1999;10:R12–R23. [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, More CJ, Humphreys GW, Wise RJS. Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Rae C, Lee MA, Dixon RM, Balmire AM, Thompson CH, Styles P, Talcott J, Richardson A, Stein J. Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. Lancet. 1998;351:1849–52. doi: 10.1016/S0140-6736(97)99001-2. [DOI] [PubMed] [Google Scholar]
- Ramus F. Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Current Opinion in Neurobiology. 2003;13:212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ramus F, Pidgeon E, Frith U. The relationship between motor control and phonology in dyslexic children. Journal of Child Psychology and Psychiatry. 2007;44:712–722. doi: 10.1111/1469-7610.00157. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Ruff S, Marie N, Celsis P, Cardebat D, Démonet JF. Neural substrates of impaired categorical perception of phonemes in adult dyslexics: an fMRI study. Brain and Cognition. 2003;53:331–334. doi: 10.1016/s0278-2626(03)00137-4. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, Pikus A, Rapoport JL, Cohen RM. Failure to activate the left temporoparietal cortex in dyslexia: An oxygen 15 positron emission tomographic study. Archives of Neurology. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Zametkin AJ, Andreason P, Hanahan AP, Hamburger SD, Aquino T, King AC, Pikus A, Cohen RM. Normal activation of frontotemporal language cortex in dyslexia, as measured with oxygen 15 positron emission tomography. Archives of Neurology. 1994;51:27–38. doi: 10.1001/archneur.1994.00540130037011. [DOI] [PubMed] [Google Scholar]
- Rumsey JM. Developmental dyslexia: Anatomical and functional neuroimaging. Mental Retardation and Developmental Disabilities Research Reviews. 1996;2:28–38. [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition: A PET-rCBF study. Brain: A Journal of Neurology. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Service E, Kiesila P, Uutela K, Salonen O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Annals of Neurology. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- Sarkari S, Simos PG, Fletcher JM, Castillo EM, Breier JI, Papanicolaou AC. The emergence and treatment of developmental reading disability: Contributions of functional brain imaging. Seminars in Pediatric Neurology. 2002;9:227–236. doi: 10.1053/spen.2002.35506. [DOI] [PubMed] [Google Scholar]
- Scarborough HS. Antecedents to reading disability: Preschool language development and literacy experiences of children from dyslexic families. Reading and Writing. 1991;3:219–233. [Google Scholar]
- Share DL, Jorm AF, Maclean R, Matthews R. Auditory temporal processing and specific reading disability. Reading and Writing. 2002;15:151–178. [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, Katz L, Marchione KE, Lacadie C, Gatenby C, Gore JC. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman B, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Holahan J, Marchione KE, Fletcher J, Lyon GR, Gore J. Development of left occipito-temporal systems for skilled reading following a phonologically-based intervention in children. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holohan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Breier JI, Fletcher JM, Foorman BR, Bergman E, Fishbeck K, Papanicolaou AC. Brain activation profiles in dyslexic children during nonword reading: A magnetic source imaging study. Neuroscience Letters. 2000;290:61–65. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC. Brain mechanisms for reading words and pseudowords: An integrated approach. Cerebral Cortex. 2002;12:297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Denton C, Papanicolaou AC. Altering the brain circuits for reading through intervention: A magnetic source imaging study. Neuropsychology. 2007;21:485–496. doi: 10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain & Language. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P. Language Learning Disabilities: Integrating Research Approaches. Current Directions in Psychological Science. 2003;12:206–211. [Google Scholar]
- Tallal P, Gaab N. Dynamic auditory processing, musical experience and language development. Trends in Neurosciences. 2006;29:382–390. doi: 10.1016/j.tins.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Protopapas A, Nagarajan S, Saltz T, Tallal P, Merzenich M, Gabrieli JDE. Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from functional MRI. Proceedings of the National Academy of Sciences. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JDE. Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–73. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101:192–212. [Google Scholar]
- Woods RP. Modeling for intergroup comparisons of imaging data. NeuroImage. 1996;4:S84–S94. doi: 10.1006/nimg.1996.0058. [DOI] [PubMed] [Google Scholar]
- Zeffiro T, Eden G. The cerebellum and dyslexia: Perpetrator or innocent bystander? Comment from Thomas Zeffiro and Guinevere Eden to Nicholson et al. Trends in Neurosciences. 2001;24:512–513. doi: 10.1016/s0166-2236(00)01898-1. [DOI] [PubMed] [Google Scholar]