Abstract
Objectives
The association between tobacco smoke exposure and critical illness is not well studied, largely because obtaining an accurate smoking history from critically ill patients is difficult. Biomarkers can provide quantitative data on active and secondhand cigarette smoke exposure. We sought to compare cigarette smoke exposure as measured by biomarkers to exposure by self-report in a cohort of critically ill patients and to determine how well biomarkers of cigarette smoke exposure correlate with each other in this population.
Design, Setting, and Patients
Serum and urine cotinine and trans-3′-hydroxycotinine, urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and hair and nail nicotine levels were measured in 60 subjects enrolled in an observational cohort of critically ill subjects at a tertiary academic medical center in Tennessee. Smoking history was obtained from patients, their surrogates, or the medical chart. Cigarette smoke exposure as measured by biomarkers was compared to exposure by history.
Measurements and Main Results
By smoking history, 29 subjects were identified as smokers, 28 were identified as nonsmokers, and 3 were identified as unknown. The combination of serum cotinine and urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol identified 27 of the 28 nonsmokers by history either as active smokers (n = 6, 21%) or as exposed to secondhand smoke (n = 21, 75%). All biomarker levels were strongly correlated with each other (r = .69–.95, p < .0001).
Conclusions
The combination of serum cotinine and urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol identified considerably more active smokers than did smoking history and detected a high prevalence of secondhand smoke exposure in a critically ill population. These markers will be important for future studies investigating the relationship between active smoking and secondhand smoke exposure and critical illness.
Keywords: biomarkers, cigarette smoking, cotinine, NNAL, critically ill
Active smoking and secondhand smoke (SHS) exposure can cause or worsen many acute and chronic cardiovascular and pulmonary diseases (1, 2). SHS is a combination of mainstream smoke (exhaled by smokers) and sidestream smoke given off by the burning end of the tobacco product. Even brief exposure to SHS has significant immediate effects on endothelial cell function, inflammation, and lung function (3). Despite public health efforts to discourage smoking, U.S. smoking prevalence has recently plateaued at 20%, and the number of smokers internationally is increasing (4, 5). In addition, even though smoking has been increasingly restricted in public places and workplaces, an estimated 40% of U.S. nonsmokers have biological evidence of significant SHS exposure (6). Thus, cigarette smoke exposure remains an important cause of preventable chronic and acute disease in both smokers and those exposed to SHS.
The association between recent tobacco smoke exposure and critical illness has been poorly studied. The lack of accurate methods to quantify exposure to tobacco smoke, and SHS in particular, in the critically ill has been a major hindrance. Historically, studies have commonly used questionnaires to measure exposure to tobacco smoke. This method is particularly difficult to use in the intensive care unit, because most critically ill patients have altered levels of consciousness or are endotracheally intubated or both. When patients are unable to provide a history, surrogate and chart reporting are often substituted; however, these sources can be subject to social desirability bias and are frequently inaccurate, out of date, or missing (7–9). Thus, to study the effects of cigarette smoke exposure on the incidence or outcomes of critical illness, a more quantitative approach to measuring cigarette smoke exposure in critically ill patients is needed.
Biomarkers of cigarette smoke exposure quantify the biologically active dose of nicotine and toxins to which patients have been exposed, overcoming the subjectivity and inaccuracy of self-reporting or surrogate reporting. Such markers have been used to distinguish active and passive smokers from nonsmokers in epidemiologic studies and to establish causal relationships between both active smoking and SHS exposure and cardiovascular and lung disease (1, 10, 11). Nicotine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (a potent lung carcinogen), and their metabolites are markers specific for tobacco exposure in smokers, smokeless tobacco users, and nonsmokers exposed to SHS.
Determining the optimal biomarker or combination of biomarkers for measuring cigarette smoke exposure in critically ill patients will be key for future studies of this population. The primary objective of our study was to compare cigarette smoke exposure, as measured by biomarkers, to exposure by self-report in a cohort of critically ill patients. A secondary objective was to determine how well biomarkers of exposure taken from different biological specimens (serum, urine, hair, and nails) correlate with each other in this population.
MATERIALS AND METHODS
Subjects
Clinical data and biological samples for this study were obtained from patients enrolled in a larger ongoing observational cohort study of critically ill adults at Vanderbilt University during 2007 and 2008. Inclusion and exclusion criteria for the parent study are described in the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/A183). Of the patients enrolled in the parent study, the first 60 enrolled subjects with all four biological samples (serum, urine, hair, toenails) collected on intensive care unit day 2 were included in the current analysis. Additional details on sample collection and storage are in the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/A183). The study protocol was approved by the Institutional Review Board at Vanderbilt University.
Smoking History
A history of active smoking was obtained from patients or their surrogates. If patients or surrogates were unavailable, then smoking history was obtained from the medical chart. Definitions of smoking history are in the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/A183). Because SHS is a complex exposure that is affected by numerous dynamic factors, including the duration of exposure and the size and ventilation of the room, subjective quantification of exposure is frequently inaccurate (1); thus, history of exposure to SHS was not elicited.
Biomarkers of Exposure
Nicotine’s metabolites cotinine and trans-3′-hydroxycotinine, and nicotine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone’s metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanol (NNAL), are well-established and highly specific markers of nicotine and nicotine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone uptake, with half-lives ranging from hours to months (serum nicotine, t1/2 = 2 hrs; serum and urine trans-3′-hydroxycotinine and cotinine, t1/2 = 16 hrs; urine NNAL, t1/2 = 2 wks; hair and nail nicotine, t1/2 = 6 months–1 yr) (12, 13). Details on measurement of cigarette smoke biomarkers are in the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/A183).
Classification of Exposure Using Biomarkers
Serum cotinine is the best-studied biomarker of cigarette smoke exposure and is considered to be the standard for measuring SHS exposure by the Surgeon General (1). A recent population-based study of >16,000 outpatients showed that a serum cotinine cut-point of 3.1 ng/mL distinguishes adult active smokers from those exposed to SHS with excellent accuracy (C statistic 0.991) (10). Additional details on the cotinine cut-point are in the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/A183).
NNAL is a metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a potent lung carcinogen that is found only in tobacco products. A recent study of 300 stable outpatients found that a urine NNAL cutoff of 64 pg/mg creatinine accurately distinguishes active smokers from passive smokers (C statistic 0.974) (14).
In the present analysis, serum cotinine levels of ≥3.1 ng/mL or urine NNAL levels of ≥64 pg/mg creatinine or both were considered consistent with active smoking. Levels less than the cutoff but greater than the limit of quantitation were considered consistent with SHS exposure.
Statistical Methods
Because biomarker levels were abnormally distributed, nonparametric analyses were used throughout. The strength of agreement between different biomarkers was assessed using Spearman’s correlation coefficient. The Mann-Whitney rank sum test was used to compare urine NNAL levels in subjects with and without renal failure. The Kruskal-Wallis test was used to determine whether hair and nail nicotine levels differed from exposure classified by serum cotinine and urine NNAL. Statistical analysis was performed with STATA/SE 9.2 (StataCorp LP, College Station, TX).
RESULTS
Baseline Characteristics of Subjects
The study population had a mean age of 52 yrs (sd ± 15); 58% were male and 93% were white (Table 1). Nearly half (48%, n = 29) of the subjects were active smokers by history, whereas 47% (n = 28) were nonsmokers by history and 5% (n = 3) had an unknown smoking history. Smoking history was most commonly obtained from the chart (87%). Smokeless tobacco use was reported by one patient, and no nicotine replacement therapy was reported. The most common primary admission diagnoses were trauma (25%), acute respiratory failure (17%), and sepsis (13%), and most subjects (88%) were enrolled within 24 hrs of hospital admission.
Table 1.
Clinical characteristics of the study participants
| Characteristic | n = 60 |
|---|---|
| Age (yrs), mean ± sd | 52 ± 15 |
| Male | 35 (58%) |
| White | 56 (93%) |
| Smoking history | |
| Never smoker | 13 (22%) |
| Former smoker | 15 (25%) |
| Active smoker | 29 (48%) |
| Unknown | 3 (5%) |
| Smoking history source | |
| Patient | 3 (5%) |
| Surrogate | 5 (8%) |
| Chart | 52 (87%) |
| Smokeless tobacco use | 1 (2%) |
| Nicotine patch or gum use | 0 (0%) |
| Enrolled within 24 hrs of hospital admission | 53 (88%) |
| Intensive care unit category | |
| Medical intensive care unit | 31 (52%) |
| Surgical intensive care unit | 9 (15%) |
| Trauma | 16 (27%) |
| Cardiac | 4 (7%) |
| Primary admission diagnosis | |
| Trauma | 15 (25%) |
| Acute respiratory failure | 10 (17%) |
| Sepsis | 8 (13%) |
| Other | 27 (45%) |
| Acute Physiology and Chronic | 26 ± 8 |
| Health Evaluation II (31), mean ± sd | |
| Insurance coverage | |
| Private | 11 (18%) |
| Federal and state sponsored insurance | 35 (58%) |
| None | 10 (17%) |
| Other/unknown | 4 (7%) |
Correlation Between Biomarker Levels
All biomarker levels were strongly correlated with each other (Table 2). The four markers with the shortest half-lives (serum and urine cotinine and trans-3′-hydroxycotinine) were most strongly correlated (r = .91–.95, p < .0001). Urine NNAL, which has an intermediate half-life of 2 wks, had the next strongest correlations with the short-lived serum and urine markers (r = .79 –.84, p < .0001). Hair nicotine and nail nicotine, which have much longer half-lives, were strongly correlated with each other (r = .80, p < .0001) and more weakly correlated with serum and urine markers (r = .69 –.79, p < .0001).
Table 2.
Correlations between biomarkers of cigarette smoke exposure
| Biomarker | Serum Cotinine |
Serum 3HC |
Urine Cotinine |
Urine 3HC |
Urine NNAL |
Nail Nicotine |
Hair Nicotine |
|---|---|---|---|---|---|---|---|
| Serum cotinine | 1 | ||||||
| Serum 3HC | .95 | 1 | |||||
| Urine cotinine | .94 | .91 | 1 | ||||
| Urine 3HC | .91 | .92 | .95 | 1 | |||
| Urine NNAL | .81 | .79 | .84 | .82 | 1 | ||
| Nail nicotine | .72 | .69 | .76 | .74 | .77 | 1 | |
| Hair nicotine | .76 | .74 | .79 | .76 | .69 | .80 | 1 |
3HC, trans-3′-hydroxycotinine; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
Values shown are Spearman correlations (n = 57–60, p < .0001 for all comparisons). Urine cotinine, 3HC, and NNAL were corrected for urine creatinine (mg).
Biochemical Evidence of Acute and Subacute Exposure With Serum Cotinine and Urine NNAL
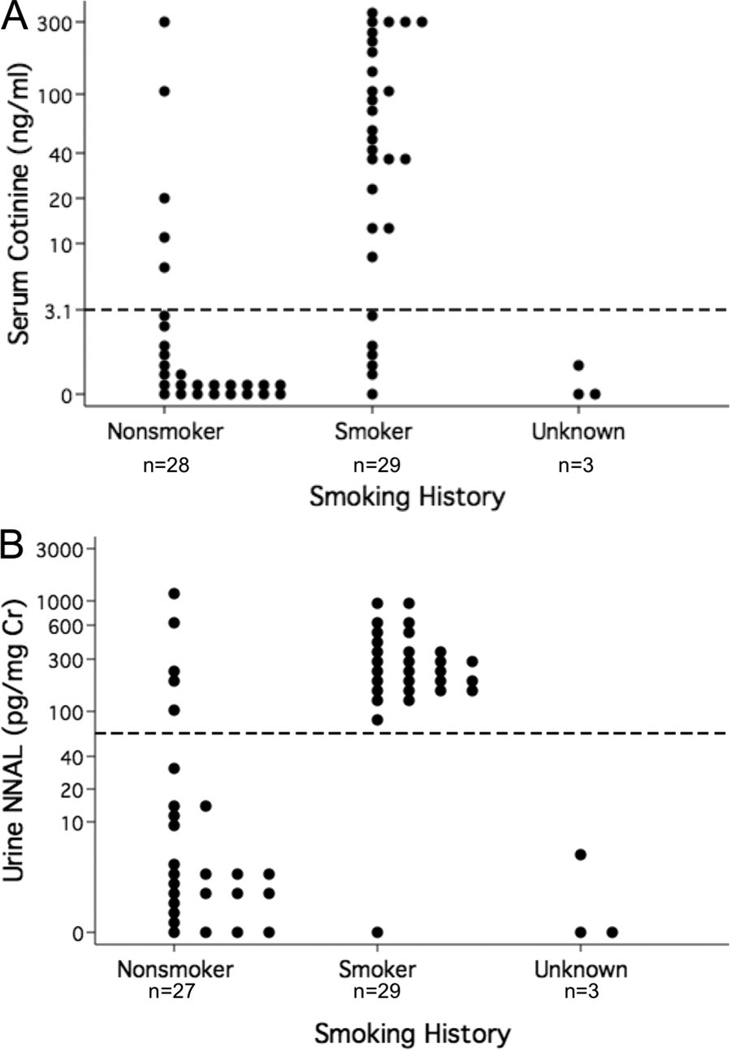
Elevated serum cotinine and urine NNAL levels identified active cigarette smoking or SHS exposure in the majority of nonsmokers by history (Fig. 1). Specifically, serum cotinine and urine NNAL each identified 5 of 28 (18%) nonsmokers by history as active smokers. Of the remaining nonsmokers by history, 15/23 (65%) and 18/23 (78%) had serum cotinine and urine NNAL levels consistent with SHS exposure, respectively. One nonsmoker’s urine NNAL sample was unable to be measured because of interference, possibly from contaminants. Nearly all of the 29 smokers by history had serum cotinine (n = 23, 79%) and urine NNAL (n = 28, 97%) levels consistent with active smoking.
Figure 1.
Most nonsmokers by history had biomarker levels consistent with active or secondhand smoke exposure. A, Serum cotinine. B, Urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL). The dots represent individual subjects. The y-axis is in log scale. The horizontal dashed line represents the cutoff between active and secondhand smoke exposure (serum cotinine = 3.1 ng/mL; urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol = 64 pg/mg creatinine). Cr, creatinine.
Level of Agreement Between Serum Cotinine and Urine NNAL
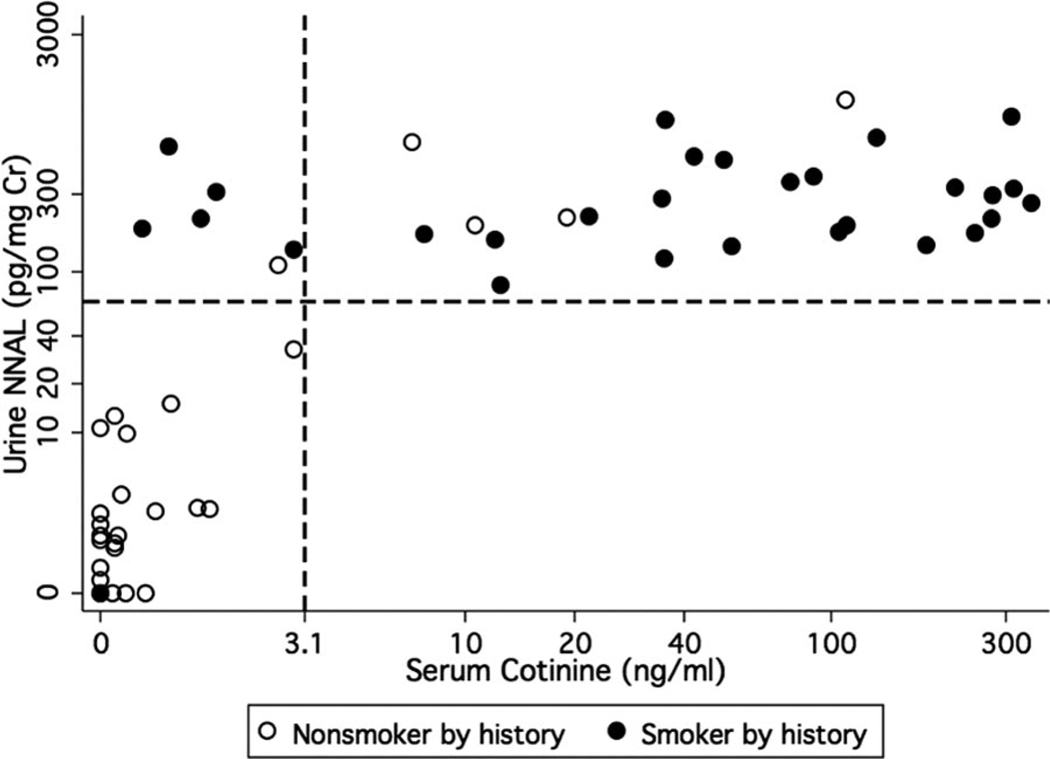
Despite the difference in half-lives between serum cotinine (16 hrs) and urine NNAL (2 wks), the two markers concurred in classifying the level of cigarette smoke exposure in the majority of cases (Fig. 2). One nonsmoker by history who was classified as an active smoker by serum cotinine (286 ng/mL) was not included because the urine NNAL measurement had interference. Concurrence in subjects enrolled within 24 hrs of hospital admission (77%) was greater than concurrence in subjects enrolled >24 hrs after admission (43%, n = 7, elapsed time 6 –36 days). Urine NNAL detected six more subjects with recent active smoke exposure and seven more subjects with passive smoke exposure than serum cotinine. Three subjects had evidence of SHS exposure by serum cotinine but no evidence of SHS exposure by urine NNAL; two of these three subjects had acute renal failure. Overall, there was no difference in urine NNAL levels between subjects with acute or chronic renal failure (n = 21) compared with subjects with normal renal function (n = 37, p = .69).
Figure 2.
Serum cotinine and urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) concurred in the classification of cigarette smoke exposure in most subjects (n = 59). The dots represent individual subjects. The dashed lines represent the cutoff between active and secondhand smoke exposure (serum cotinine = 3.1 ng/mL; urine NNAL = 64 pg/mg creatinine [Cr]). One subject was not included because of urine NNAL measurement interference (serum cotinine = 286 ng/mL).
Combination of Serum Cotinine and Urine NNAL
To assess the prevalence of recent cigarette smoke exposure, we used a combination of biomarkers that reflect acute (serum cotinine) and subacute (urine NNAL) exposure (Fig. 2, Table 3). Because biomarkers of cigarette smoke exposure decline with time, and because our subjects were enrolled on average 1 day after hospital admission, we assumed that the highest level of exposure reflected by either serum cotinine or urine NNAL was accurate. Using this approach, we found that the combination of serum cotinine and urine NNAL detected more subjects exposed to SHS than either biomarker alone. Of the 28 nonsmokers by history, all but one (96%) were biologically exposed to cigarette smoke by serum cotinine or urine NNAL levels or both. Specifically, six (21%) were classified as active smokers and 21 (75%) had SHS exposure. Twenty-eight of the 29 (97%) active smokers by history were classified as active smokers by serum cotinine and urine NNAL levels. Of the three subjects with an unknown smoking history, two were nonsmokers and one had evidence of SHS exposure. The only smokeless tobacco user in the study was classified as an active smoker by both serum cotinine and urine NNAL, but was a former smoker by chart history.
Table 3.
Concurrence between cigarette smoke exposure by history and by combined biomarker status (n = 60)
| Smoking Status by Serum Cotinine and Urine NNAL | ||||
|---|---|---|---|---|
| Smoking Status by History |
Nonsmoker (Cotinine = 0 ng/mL AND NNAL = 0 pg/mg Cr) |
Exposed to Secondhand Smoke (Cotinine >0, <3.1 ng/mL OR NNAL >0, <64 pg/mg Cr) |
Smoker (Cotinine ≥3.1 ng/mL OR NNAL ≥64 pg/mg Cr) |
Total |
| Nonsmoker | 1 | 21 | 6 | 28 |
| Smoker | 1 | 0 | 28 | 29 |
| Unknown | 2 | 1 | 0 | 3 |
| Total | 4 | 22 | 34 | 60 |
Cr, creatinine; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
Hair and Nails: Biochemical Evidence of Chronic Exposure
Data on agreement between measurements of chronic cigarette smoke exposure (hair and nail nicotine) and measurements of more recent exposure (serum cotinine and urine NNAL) are included in the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/A183).
DISCUSSION
To our knowledge, this is the first study to compare quantitatively assessed cigarette smoke exposure with smoking history in critically ill adults. The results indicate that smoking history markedly underestimates cigarette smoke exposure in the critically ill. Nearly all of the nonsmokers by history had evidence of either active smoking (21%) or SHS exposure (75%) by serum cotinine or urine NNAL levels or both. These findings have major implications for future studies on the effects of cigarette smoke exposure on critical illness, and for hospitalized patients more generally.
To date, few studies have been published on the effects of recent tobacco smoke exposure on critical illness, largely because of difficulty obtaining an accurate smoking history. In epidemiologic studies, even apparently minor degrees of misclassification of exposure can significantly bias the results (15); thus, accurate assessment of true active smoking rates and SHS exposure is essential for future research on the effects of smoking on critical illness. Because of excellent sensitivity and specificity, the measurement of cigarette smoke exposure biomarkers has been recommended to obtain a more precise estimation of active cigarette and SHS exposure in outpatient studies (1, 16). Most studies to date in the critically ill have used smoking history (in many cases obtained from the chart) and only reported active cigarette smoke exposure (17, 18). The results of our study show that biomarkers of cigarette smoke exposure substantially increase the detection of active smoking compared to smoking history in this understudied population. Furthermore, we demonstrate for the first time to our knowledge that SHS exposure in the critically ill can be quantified by cigarette smoke biomarkers and is highly prevalent.
To study the effects of recent tobacco smoke exposure on the critically ill, it will be important to assess the intensity of exposure over time relative to the course of illness, because smoking behavior may change in the setting of acute illness. Once hospitalized and no longer exposed to cigarette smoke, short-lived biomarkers (i.e., serum cotinine) will decrease quickly relative to markers with longer half-lives, and light or intermittently active smokers may be misclassified as nonsmokers with SHS exposure. Urine NNAL will be particularly useful in this setting because it has a longer half-life, and classification of exposure by urine NNAL levels should remain accurate even if specimens are collected days after admission. As evidence of this point, five (17%) smokers by history were classified as active smokers by urine NNAL levels but had serum cotinine levels in the SHS exposed range. Overall, urine NNAL detected higher levels of exposure than did serum cotinine in 13 subjects (22%).
The prevalence of active smokers in this cohort of critically ill patients (57%) was higher than the prevalence of active smokers in a cohort of urban hospitalized patients (46%) (19). It was also remarkably higher than the Tennessee average (23%) and the nationwide average (20%) (20, 21). Previous studies have measured higher rates of smoking in subjects with lower socioeconomic status and in subjects with substance abuse problems (19, 22). Compared to the national population, our cohort had fewer persons younger than age 65 yrs with private insurance (23% vs. 68%) and a higher rate of illicit drug use (17% vs. 8.3%) (23, 24). Thus, the lower socioeconomic status and higher illicit drug use of our patient population may explain, at least in part, the high prevalence of active smoking. Another possible explanation for the high prevalence of active cigarette smoke exposure in this population is that recent smoking is itself a risk factor for the development of critical illness.
The prevalence of SHS exposure in nonsmokers by history in this cohort of critically ill patients (75%) is also markedly higher than the estimated nationwide prevalence of 43% generated from National Health and Nutrition Examination Surveys data, a population-based study of nearly 30,000 subjects that used serum cotinine to identify SHS exposure (25). Despite the higher serum cotinine cut-point used in the National Health and Nutrition Examination Surveys study (10 ng/mL vs. 3.1 ng/mL), the prevalence of SHS exposure was still lower than that of our critically ill cohort. Also, the limit of quantitation for serum cotinine in the National Health and Nutrition Examination Surveys study was 0.05 ng/mL for samples analyzed from 1988 to 2000 and 0.015 ng/mL for samples analyzed from 2001 to 2002, as compared to 0.02 ng/mL in our study. For comparison, we analyzed our samples using a limit of quantitation of 0.05 ng/mL and found no difference in means and percentiles. Thus, our use of a more sensitive serum cotinine assay was unlikely to have contributed to the high prevalence of SHS exposure in this population. The prevalence of smoking among adults in Tennessee is similar to the nationwide prevalence (23% vs. 20%) (20, 21), and a public smoking ban in Tennessee was instituted for 4 of the 5 months in which our study was conducted. Therefore, it is unlikely that geographic differences contribute to the higher prevalence of SHS exposure in this study population. Another possible explanation for the high prevalence of SHS exposure is the inclusion of occasional or “social” smokers in the nonsmokers by history group. Assessing for occasional smoking requires a detailed interview with the primary subject, which is not feasible in most critically ill patients. Last, cotinine cutoff values have been shown to vary by ethnicity. Although 93% of our cohort was white, sensitivity analysis showed no difference in classification of cigarette smoke exposure with the nationally representative cutoff (3.1 ng/mL) used in this study compared to a cutoff specific for whites (4.85 ng/mL) (10). The high prevalence of SHS exposure in this cohort may be explained in part by the addition of urine NNAL, which has a longer half-life and would detect SHS exposure long after cotinine levels declined to below the limit of quantitation. Also, SHS itself could be a risk factor for the development of critical illness, an important topic that merits further study.
It should be emphasized that no gold standard exists for measuring cigarette smoke exposure, although, historically, self-report has been used as the standard for comparison. Self-reported smoking status is associated with a number of limitations, including recall bias, social desirability bias, and poor accuracy regarding quantitative aspects of exposure (8). In the critically ill, these problems are further compounded by altered mental status and respiratory failure such that most subjects are unable to provide a smoking history. Because biomarkers of cigarette smoke exposure are highly specific to tobacco products, previous epidemiologic studies have used biomarkers of cigarette smoke exposure to improve the accuracy of self-report (11, 26, 27). The results of this study confirm that quantitative biomarker data provide significantly more detailed and objective information on tobacco smoke exposure compared to smoking history in critically ill subjects.
Our study has some limitations. First, this study’s subjects were predominantly white and were enrolled from one center. Although the use of such a homogeneous sample may limit the generalizability of these results, it also eliminates the confounding effect of racial differences on nicotine metabolism (28). Second, both smokeless tobacco and nicotine replacement therapy can raise nicotine levels, potentially confounding interpretation of cigarette smoke exposure; however, nicotine replacement therapy does not affect NNAL levels (13). One subject in our study had a history of smokeless tobacco use, and interpretation of that subject’s elevated serum and urine cotinine and NNAL levels is uncertain. Third, it is unclear if renal function or medications administered in the intensive care unit interfere with the detection of urine NNAL. The method used to measure urine NNAL in this study is the most sensitive method reported to date and optimizes separation from interfering substances in the sample by converting the analyte to a relatively nonpolar derivative (29). In our 60 critically ill subjects, of whom 38% had acute or chronic renal failure, one subject with acute renal failure had interference. We adjusted all urine NNAL levels for urine creatinine concentration, and rank sum analysis showed that there was no significant difference between urine NNAL levels in patients with acute or chronic renal insufficiency compared with those with normal renal function in our study population.
CONCLUSIONS
The results of this study have important implications for research in the intensive care unit. Using a combination of serum cotinine and urine NNAL, we detected a markedly higher prevalence of both active smoking and SHS exposure in a critically ill population, compared with smoking history. The results demonstrate that smoking history markedly underestimates recent active smoking, SHS exposure can be quantified in patients in the intensive care unit, and SHS exposure is highly prevalent in critically ill patients. Biomarkers of cigarette smoke exposure will be instrumental for future studies investigating the relationship between cigarette smoke exposure and critical illness, an important topic that fits well with the U.S. government’s increasing efforts to reduce this public health threat with its recent passage of the Family Smoking Prevention and Tobacco Control Act (30).
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge the contributions of Minjiang Duan and Olivia T. Yturralde in the Division of Clinical Pharmacology and Experimental Therapeutics at the University of California, San Francisco.
Supported, in part, by HL081332 (Dr. Ware), P30 DA012393 (Drs. Jacob and Benowitz), R25CA113710–03 (Dr. Goniewicz), HL51856 (Dr. Matthay), CA78603 (Dr. Benowitz), HL090833 (Dr. Calfee), KL2RR024130 (Dr. Calfee), Flight Attendants Medical Research Institute UCSF Bland Lane Center of Excellence in Secondhand Smoke (Drs. Eisner and Benowitz), and Flight Attendants Medical Research Institute Young Clinical Scientist Award (Dr. Calfee).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the Journal’s Web site (www.ccmjournal.com).
Presented, in part, at the International Conference of the American Thoracic Society, San Diego, CA, May 20, 2009, and at the Flight Attendant Medical Research Institute Scientific Symposium, Boston, MA, May 12, 2009.
The authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.U.S. Department of Health and Human Services. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General, 2006. [Google Scholar]
- 2.U.S. Department of Health and Human Services. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. The Health Consequences of Smoking: A Report of the Surgeon General, 2004. [Google Scholar]
- 3.Flouris AD, Metsios GS, Carrillo AE, et al. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am J Respir Crit Care Med. 2009;179:1029–1033. doi: 10.1164/rccm.200812-1920OC. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. The Tobacco Atlas. [Accessed August 21, 2009];2002 Available at http://www.who.int/tobacco/resources/publications/tobacco_atlas/en/index.html.
- 5.Centers for Disease Control and Prevention. Currrent cigarette smoking among adults ≥18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1135–1140. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Vital Signs: Nonsmokers’ exposure to secondhand smoke—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2010;59:1141–1146. [PubMed] [Google Scholar]
- 7.Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. BMJ. 2009;339:b4347. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorber SC, Schofield-Hurwitz S, Hardt J, et al. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 9.Mant J, Murphy M, Rose P, et al. The accuracy of general practitioner records of smoking and alcohol use: Comparison with patient questionnaires. J Public Health Med. 2000;22:198–201. doi: 10.1093/pubmed/22.2.198. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 11.Eisner MD, Jacob P, 3rd, Benowitz NL, et al. Longer term exposure to secondhand smoke and health outcomes in COPD: Impact of urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Nicotine Tob Res. 2009;11:945–953. doi: 10.1093/ntr/ntp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goniewicz ML, Havel CM, Peng MW, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev. 2009;18:3421–3425. doi: 10.1158/1055-9965.EPI-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Hand Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goniewicz M, Peyton J, Havel C, et al. Application of urine cotinine to NNAL ratio in tobacco smoke exposure assessment. Joint Conference of Society for Research on Nicotine and Tobacco and SNRT-Europe: Poster Session Abstracts:132; Dublin, Ireland. 2009. [Accessed October 11, 2010]. Available at: http://www.srnt.org/conferences/past/2009/pdf/2009_Poster_Sessions.pdf. [Google Scholar]
- 15.Copeland KT, Checkoway H, McMichael AJ, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 16.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 17.Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA. 2004;292:2997–3005. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- 18.Iribarren C, Jacobs DR, Jr, Sidney S, et al. Cigarette smoking, alcohol consumption, and risk of ARDS: A 15-year cohort study in a managed care setting. Chest. 2000;117:163–168. doi: 10.1378/chest.117.1.163. [DOI] [PubMed] [Google Scholar]
- 19.Benowitz NL, Schultz KE, Haller CA, et al. Prevalence of smoking assessed biochemically in an urban public hospital: A rationale for routine cotinine screening. Am J Epidemiol. 2009;170:885–891. doi: 10.1093/aje/kwp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Office of Policy, Planning and Assessment. Nashville, TN: Tennessee Department of Health; 2008. Prevalence of tobacco use in Tennessee, 1997–2007. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Cigarette smoking among adults–United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1221–1226. [PubMed] [Google Scholar]
- 22.Fidler JA, Jarvis MJ, Mindell J, et al. Nicotine intake in cigarette smokers in England: Distribution and demographic correlates. Cancer Epidemiol Biomarkers Prev. 2008;17:3331–3336. doi: 10.1158/1055-9965.EPI-08-0296. [DOI] [PubMed] [Google Scholar]
- 23.Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of Applied Studies; 2008. Results from the 2007 National Survey on Drug Use and Health: National Findings. NSDUH Series H-34, DHHS Publication SMA 08-4343. [Google Scholar]
- 24.Heyman KMBP, Schiller JS. Early release of selected estimates based on data from the 2008 National Health Interview Survey National Center for Health Statistics. [Accessed June 2009]; Available at: http://www.cdc.gov/nchs/nhis.htm.
- 25.Pirkle JL, Bernert JT, Caudill SP, et al. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Delaimy WK, Willett WC. Measurement of tobacco smoke exposure: Comparison of toenail nicotine biomarkers and self-reports. Cancer Epidemiol Biomarkers Prev. 2008;17:1255–1261. doi: 10.1158/1055-9965.EPI-07-2695. [DOI] [PubMed] [Google Scholar]
- 27.Eisner MD, Klein J, Hammond SK, et al. Directly measured second hand smoke exposure and asthma health outcomes. Thorax. 2005;60:814–821. doi: 10.1136/thx.2004.037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benowitz NL, Perez-Stable EJ, Fong I, et al. Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999;291:1196–1203. [PubMed] [Google Scholar]
- 29.Jacob P, 3rd, Havel C, Lee DH, et al. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80:8115–8121. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curfman GD, Morrissey S, Drazen JM. Tobacco, public health, and the FDA. N Engl J Med. 2009;361:402–403. doi: 10.1056/NEJMe0905622. [DOI] [PubMed] [Google Scholar]
- 31.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.