Abstract
Some have characterized patients living with intractable pain as a vulnerable population in both clinical and research settings. Labeling the population as vulnerable, however, does not provide clarity regarding the potential risks that they face when they participate in research. Instead, research vulnerability for patients in pain is a function of an interaction between their pain conditions and elements of the research enterprise. Therefore, the identification of potential risks requires consideration not only of characteristics of patients with chronic pain, but also consideration of features of researchers, the quality of institutional oversight, and the medical/social environment within which the research is conducted. This paper provides an analysis of those risks and provides some suggestions as to how the risks might be better managed.
Introduction
The concept of vulnerability has been the topic of considerable discussion in research bioethics, largely because of dissatisfaction with early constructions of the concept that were based on subpopulations of research subjects. These subpopulations have attributes likely to undermine their capacity to provide autonomous informed consent:1 “persons who are relatively or absolutely incapable of protecting their own interests through negotiations for informed consent.” Several subpopulations were seen as requiring special protections, including children, pregnant women, prisoners, racial minorities, the economically disadvantaged, the very sick, and the institutionalized. Recent years have witnessed the identification of other subpopulations with attributes that could render them vulnerable, as well. For example, the Council for the International Organization of Medical Societies has named such potentially vulnerable groups as the homeless, nursing home residents, patients with incurable diseases, patients in the emergency department, employees, students, and members of communities who are unfamiliar with modern medicine.2
The proliferation of vulnerable subgroups has raised questions about the utility of population-based categories. Some have noted that the diversity within a given subpopulation is sufficiently great, making some members vulnerable, while others not.3 Similarly, others have noted that the increase in subpopulations has diluted the concept of vulnerability so much that any research subject could be considered vulnerable according to some criterion, rendering safeguards for vulnerable subpopulations nebulous.4 While the above considerations suggest that a subpopulation approach is too broad, other considerations suggest that sub-population-based definitions are not broad enough: a range of situational factors in a research enterprise can render any subject vulnerable, regardless of the subpopulation to which he might belong.5
Similar considerations certainly apply to any discussion of research vulnerability for patients in pain. After all, pain has been termed “a more terrible lord of mankind than even death itself,”6 and unrelieved pain is recognized as potentially impacting a person’s autonomy to make free choices.7 Moreover, people who are affliicted with unremitting pain are subject to physical, emotional, cognitive, and socioeconomic sequelae.8 Both in its potential impact on autonomy and in its sequelae, persistent pain demonstrates characteristics that echo features of the vulnerable subpopulations described above. On the other hand, pain is a universal affliiction that affects everyone in one form or another at some point in their lives: acute (e.g., post-surgical pain), chronic and unremitting (e.g., neuropathic pain), chronic and remitting (e.g., migraine pain), or malignant and progressive (e.g., cancer pain). Indeed, most of us will experience pain in several of its forms over the course of a lifetime. Hence, like other subpopulations of research subjects, patients in pain are so diverse that a population-based perspective on vulnerability provides little or no direction regarding ethical safeguards.
Alternative Approaches to Vulnerability
Given that little ethical direction derives from viewing vulnerability solely from the perspective of the subpopulation to which the research subject might belong, alternative perspectives must be considered. One such alternative describes vulnerability relative to the investigator.9 According to this view, research subjects are significantly more vulnerable to unethical research practices when factors exist that may impact a researcher’s priorities. When those conflict with priorities relevant to the protection of human subjects, subject vulnerability is enhanced.
Another model of vulnerability considers elements relevant to both the subject and the investigator. According to this view, vulnerability is relational, involving factors that apply to both.10 For example, research subjects that also are patients of an investigator may be susceptible to decision making that is unduly influenced by that relationship (whether or not the investigator intentionally exerts such influence). Similarly, investigators who also serve as consultants to study sponsors may have their judgment compromised by the conflict of interest entailed by that relationship.
Yet another perspective locates vulnerability at various places in the research context.11 For example, local issues such as deficient quality control standards12 and/or inadequate IRB oversight13 can occasion increased vulnerability. Further, the larger sociopolitical environment also can render subjects more or less vulnerable to unethical research practices.14 The increased regulatory attention now being accorded to conflicts of interest,15 recruitment incentives,16 ghost-writing,17 and other sponsor-investigator relationships bears witness to the impact that the sociopolitical environment can have on research activities that previously were countenanced with little question.
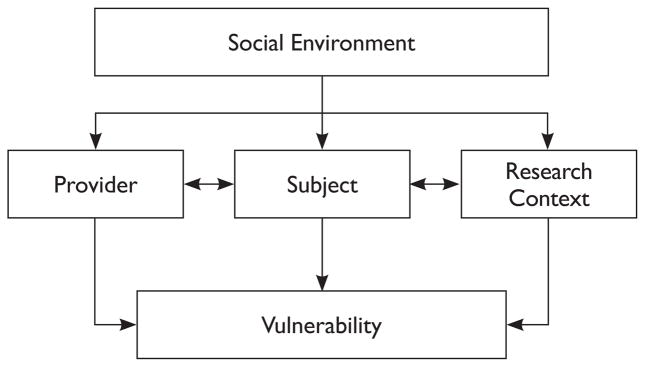
Clearly, each of the above perspectives on vulnerability has merit, as well as the potential to provide ethical direction. For the same reasons, it is difficult to select one perspective, when it is clear that vulnerability can be impacted by factors associated with the subject, the investigator, and the research environment. Therefore, this paper adopts an analytic model to guide the discussion.18 This model considers the interface of the clinical condition under study (in this case, pain) in conjunction with features of the research enterprise, including each of the perspectives described above: the subject, the investigator, and the research environment. See Figure 1 below for representation of the sources of vulnerability to be discussed with this model.
Figure 1.
Sources of Vulnerability for the Research Subject
In our analysis of vulnerability, we will focus particularly on judgments made by either the investigator or the subject. Because the judgments may be unduly influenced by the three features of the research enterprise described above, they may guide decisions that place subjects at “special risk of unethical treatment,”19 i.e., unacceptable levels of pain, distress, or the likelihood of negative clinical outcomes associated with research participation. Some discussion of the experience of pain is needed before moving to a consideration of research-related risks for patients in clinical pain studies. This discussion will underscore the important role that judgments play in pain assessment and treatment, independent of research matters.
The Experience of Pain
Pain has been defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”20 It is highly prevalent, as it constitutes the most common complaint that brings patients to physicians.21 Of course, the experience of acute pain is universal; the prevalence of chronic pain, either remitting, unremitting, or progressive, also is high. For example, recent estimates indicate that 16% of people worldwide experience migraine headaches at some point in their lives22 and approximately 6% live with chronic low back pain.23 While accurate diagnosis and effective treatment for acute pain is generally expected, chronic pain conditions are characterized by diagnostic uncertainty, a low likelihood of cure, a high risk for psychiatric co-morbidities and significant pain-related dysfunction.24 A high risk of undertreatment further complicates the picture.25
The primary focus of this paper is on patients/subjects with severe, chronic pain, rather than those with acute pain. This is because the multiple sequelae described above expose chronic pain patients to risks greater than those faced by persons experiencing pain of an acute nature, making this population particularly vulnerable. While a similar argument could be made for patients with pain secondary to malignancy, a discussion of research ethics for the latter group would have to include issues related to cancer and palliative care, a topic that already has received considerable attention.26
Whether acute or chronic, however, several features characterize the experience of pain that render it challenging from both research and clinical perspectives: (1) it is highly subjective; (2) it is comprised of sensory, affective, and cognitive elements; and (3) its relationship to indicators of tissue damage is uncertain and variable. Indeed, in chronic pain conditions it is common for pain to be reported with little (if any) objective evidence of tissue damage.27 Because of these features, patient self-reports are central to pain assessment and provide guidance for medical judgments, such as those involved in planning treatment and in evaluating treatment effectiveness. These judgments, clearly, are common to both medical practice and to clinical research.
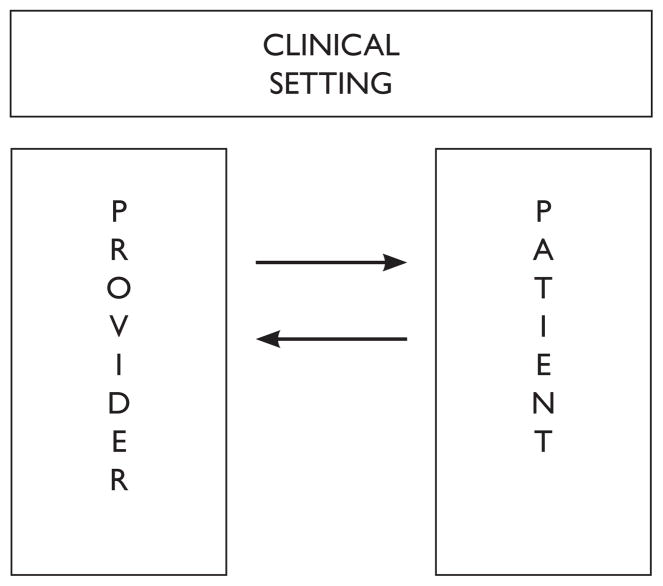
Although self-reported pain is the backbone of assessment, the validity of those reports is often questioned when pain is reported to be severe. When questioned, of course, pain severity typically is underestimated.28 A recent review of the pain literature suggests that the uncertainty inherent in any self-report opens pain assessment to a range of social psychological factors that can drive underestimation, especially in the absence of other, confirmatory information.29 Those social psychological factors derive from attributes of the actor/patient/subject, the observer/provider/investigator, and the situation within which the assessment occurs30 and, through their impact on judgments of pain, likely contribute to the persistent pattern of pain under-treatment that has been reported in the medical and bioethical literature.31 The following figure provides a model of the social context that can influence judgments of pain.
The parallels between the factors that may influence judgments made in the context of pain treatment and those made in the context of research are obvious. Moreover, they reflect a form of double jeopardy to which patients with chronic pain are exposed when they participate as subjects in a clinical research enterprise. First, they are exposed to the risks of symptom underestimation and undertreatment that are well documented in the pain literature and which may characterize their pre-study (and/or post-study) status. Second, they are exposed to the relatively unexplored risks related to judgments that are made in the course of research participation. The manner in which the risks of the first type interact with risks of the second constitutes the remainder of this paper. Those risks will be examined relative to the three general dimensions described above: the subject, the investigator, and the research environment.
Sources of Vulnerability: The Research Subject
When considering risks potentially faced by patients in pain as a consequence of research participation, the temporal phases of a research trial provide a useful framework. The first phase, of course, involves the consent process: understanding both the rationale for the research and what research participation might entail, appreciating the potential risks and benefits that an individual who participates might incur, and, finally, deciding whether to participate. The second phase involves actual study participation. Such participation includes not only the mechanics of the research (e.g., taking the study article according to the protocol, attending study visits), but also weighing the apparent benefits and risks experienced in the course of participation relative to the alternatives that are available outside the research context. Based on these considerations, judgments are made (by both subjects and investigators) as to whether the subject should continue to the end. The third and final phase involves study termination, particularly the steps taken to move a subject from protocol-driven treatment to standard of care in a manner that minimizes exposure to risk.
Informed Consent
As noted previously, the experience of pain has sensory, cognitive, and affective elements that can influence decisions relevant to research participation. To the degree that pain is poorly controlled, the sensory component can involve pain that is both severe and immediate (i.e., it is present as subjects make decisions). These qualities have led some to observe that poorly controlled pain may reduce the autonomy that a prospective subject might have relative to research participation decisions.33
There are two likely mechanisms that may impinge on autonomy. One is directly related to the aversive nature of severe pain: absent a viable alternative, prospective research subjects are likely to have a general bias toward options perceived as holding promise for pain relief and/or reduction. The other mechanism that may impact autonomy may be mediated through a therapeutic misconception, characterized by a tendency “to overestimate the likely benefits of entry into research studies, to underestimate risks, to be confused about the nature of randomized assignment, and generally to conflate research with ordinary treatment.”34 Patients with chronic pain have several characteristics that they share with research subjects most at risk for holding therapeutic misconceptions:35 they may have lower levels of education, be limited in physical functioning, and exhibit decrements in the performance of customary roles. Further, many have failed to benefit substantially from FDA-approved medicines used in standard medical care. Finally, there is evidence that the disappointing results of standard care predispose patients with severe, intractable pain toward nonstandard (i.e., complementary and alternative) forms of care.36 While the latter forms of treatment differ in a number of ways from the investigational treatments discussed in this paper, both reflect a willingness to look beyond standard care. Patients in severe pain, for whom standard care has failed to provide pain relief, may be predisposed to participate in such activities.
Aside from the sensory dynamics described above, pain duration also may play a role in the decision-making process. Chronic pain has many of the elements of an incurable disease: the prospect of a cure is remote, management of pain and dysfunction can be uncertain, and severe exacerbations may be common. Hence, patients with intractable pain depend on ongoing medical care to a high degree. The dependent relationship that can develop between patients and their long-term providers, while understandable, can make these patients susceptible to undue influence on research-related decisions. If providers are perceived as invested in a study, then patients may be inclined toward research participation in order to be seen as “good” patients.37
In addition to the potential impact of the sensory elements of pain, pain also has cognitive sequelae that may impact decision-making processes. There is evidence that pain reduces attention, concentration, and short-term memory to a degree that interferes with complex tasks38 and may interfere with the performance of common, everyday activities.39 The deficits can affect information retention when patients are confronted with complex information (such as a consent form), so that patients with severe pain may be at risk of failing to understand a research project and, more importantly, failing to grasp the implications of research participation for their specific condition. On the other hand, the resulting deficits are generally not at levels that would render a subject incapable of providing meaningful informed consent, as long as the consent process is carefully conducted.40 Available evidence, however, suggests that researchers may fail to recognize such deficits.41 If unrecognized, the failure to manage the consent process for patients with cognitive deficits associated with severe pain could undermine the patient’s autonomy and potentially expose the patient to avoidable risk if he entered the trial without fully understanding and appreciating it.
Finally, depression and anxiety are common co-morbidities for persons experiencing pain of a chronic nature.42 Previous bioethical analyses have recognized patients with affective disorders as a vulnerable group relative to research participation, secondary to the potential impact of affective disorders on cognition and on judgments of benefit and risk.43 The weight of prevailing opinion indicates that most patients with such disorders are capable of executing an autonomous informed consent, whether the disorders are of moderate44 or high severity.45 On the other hand, in order to assure autonomous and informed consent, it is crucial that the informed consent is managed effectively.46 Unfortunately, psychiatric co-morbidities often are not addressed in patients presenting with a primary complaint of pain,47 in part because chronic pain patients often are reluctant to admit to such disorders, secondary to concerns that pain symptoms may be attributed to psychiatric causes.48 Whether because of provider oversight or patient under-reporting, the failure to recognize psychiatric co-morbidities in this patient group reduces the likelihood that the consent process will be administered in a manner that accommodates such co-morbidities.
To summarize, multiple elements can undermine a subject’s capacity to provide an autonomous, informed consent. The patient with severe, poorly controlled pain may experience limitations in autonomy secondary to the aversive nature of pain and a susceptibility to misperceiving benefits and risks. The patient with a longstanding relationship with a provider may be unduly influenced to participate in a research project, especially when the patient anticipates continued dependence on a provider for pain management. Additional risks faced by a patient involve pain’s cognitive and affective sequelae, factors that may influence a subject’s understanding of a protocol, appreciation for its personal implications, and judgments of benefit and risk. Although each of these risks can be managed by an effectively managed consent process, the likelihood of such a consent process is reduced if these risks are unrecognized.
STUDY PARTICIPATION
Of course, the risk of unethical treatment does not end at the time of study enrollment. Once involved in a trial, both the research subject and the investigator make ongoing judgments regarding continued participation. These judgments often are informed by substantive factors such as the experience of benefits and/or adverse effects, as well as convenience and the availability of alternative treatments. Not all of the factors that color these judgments are substantive because of the highly subjective nature of pain and the degree to which assessment relies on a social exchange. This section examines social psychological factors that may color judgments in a pain trial more than in trials where more objective indicators can drive decisions regarding efficacy and/or the safety of continued participation.
Consider a subject that is involved in a clinical study of an analgesic compound with unproven efficacy and a moderate side effect profile. Such trials commonly have guidelines regarding both titration and “stop rules” to protect subjects who demonstrate a negative response to study treatment of a significant magnitude. Stop rules in pain trials typically target the frequency and severity of pain exacerbations, information derived from patient self-reports and investigator judgments regarding those reports. Subjects who underreport levels of pain may bear risks that exceed levels expected in the design of a trial. While such risks may be acceptable if they are uncommon, they may be more frequent for subjects in pain trials. There is evidence that patients systematically report lower levels of pain to physicians than to health care providers with other levels of training.49 Because physicians typically serve as the person who is charged with managing stop rules in a trial, patients may fail to accurately report pain at levels of severity otherwise sufficient to terminate study participation. As already noted, subjects also may be motivated to behave as “good” patients, often viewed as patients who do not complain.50 Ironically, the risk of underreporting may be greater for subjects who have a positively valenced relationship with an investigator than for those with a negatively valenced relationship; subjects in the former group may be more motivated to be viewed as a “good patient.” Of course, subjects having a negatively valenced relationship may be more likely to incur other risks that may impact study participation, such as the risk of having their symptoms discounted.51
STUDY TERMINATION
Because of the need for ongoing management of chronic symptoms, subjects in pain trials continue to incur risks after study termination. Those risks fall into two primary categories that relate to the transition from investigational treatment to standard care: (1) managing the aftermath of the study drug (e.g., withdrawal effects), and (2) establishing effective pain care with standard treatments. Although any analgesic trial carries some level of transitional risk, opioid trials may carry greater risks than do trials of other pharmacologic agents.
Most trials provide a follow-up period after active drug is no longer administered, during which time withdrawal problems can be managed such that care can shift back to the primary provider. Neuroleptic compounds typically engender a predictable withdrawal course that can be managed by steady down-titration of the drug with relatively minimal complications, so long as the time period for down-titration is of reasonable length. Although some patients exhibit difficulties with neuroleptic down-titration, strategies for dealing with these complications are well accepted, so that these transitional risks can be managed for most patients in a reasonably straightforward manner. Further, a number of neuroleptic compounds currently are marketed with analgesic indications, making the crossover from a study drug to a comparable neuroleptic drug usually (but not always) straightforward. Finally, the use of neuroleptics for their analgesic value is a well-established standard of care.52 Because this use is widely accepted, continuity of care usually is not problematic for patients transitioning from an investigational neuroleptic compound to standard care.
The transitional risks for patients in opioid trials differ from those described above and, ironically, may be greater for study subjects who realize the greatest analgesic benefit from the study drug. For trials of a reasonable duration (e.g., six weeks or more), study subjects may develop drug tolerance, and tolerance can become more pronounced as the duration of a trial is extended. While the parameters that inform the management of opioids, including their discontinuation, also are reasonably well established, complications from opioid therapy, such as aberrant medication-taking, are not uncommon among patients with chronic pain conditions.53 These complications may extend well beyond the usual length of time allocated in a pharmaceutical trial for follow-up after the termination of study drug.
Aside from the management of complications directly associated with the termination of opioid therapy, subjects who received effective pain management in opioid trials also are faced with establishing effective pain management in standard care. While a number of opioid compounds are marketed for pain control, the use of opioids on a chronic basis is much more controversial than is the long-term use of neuroleptics.54 Hence, unlike subjects in neuroleptic trials, those in opioid trials may not have a straightforward crossover strategy available to them. Because of the controversial nature of long-term opioid therapy for non-malignant pain, it can be difficult to establish an alternative approach to effective pain management, especially if pain was not effectively managed prior to trial participation. Hence, both continuity and effectiveness of care can be problematic for this patient population. This is exemplified in a recent study that examined the effects of escalating doses of morphine administered intrathecally to patients with inadequately controlled neuropathic pain receiving intrathecal ziconitide.55 Patients received gradually increasing doses of morphine over the course of approximately one year and reported both a decrease in pain severity and a reduction in the use of oral opiates. These results supported the effectiveness of this analgesic regimen, as did the retention rate (17 of the 22 surviving subjects completed the extension study). While the management of these patients after study completion was not discussed, it is likely that they returned to their (inadequate) pre-study levels of pain control.
Of course, neuroleptic compounds and opioids do not exhaust the compounds studied in analgesic trials. For example, medicinal marijuana has demonstrated significant analgesic benefit in patients with chronic pain.56 Obviously, if societal attitudes toward the long-term use of opioids can be described as pejorative, attitudes toward and the legal implications for long-term marijuana use may be worse. Clearly, patients who participate in clinical trials using such substances could face significant issues related to continuity of care, as well as the problems with effective pain management described previously.
Sources of Vulnerability: The Investigator
As noted previously, sources of vulnerability referable to investigators generally involve relationships that may influence an investigator’s judgments so that those judgments may conflict with the priority of protecting human subjects.57 Several relationships are of interest to this paper, including relationships with the research subject and those with study sponsors. Of course, these relationships are not automatically inappropriate. Indeed, research could not be conducted without them. Instead, the relationships are problematic primarily to the degree that they affect investigator judgments of benefit and risk.
Relationships with Subjects
Two risks associated with investigator relationships involving subjects merit discussion: (1) those that are heightened when an investigator also serves as a patient’s health care provider, and (2) those that are influenced by the valence (positive/negative) of an investigator’s relationship with a study subject. Each of these relationships may influence the level of risk that subjects incur with study participation.
Of course, it is common for research subjects to be drawn from a physician’s practice. Indeed, a variety of benefits can ensue from a pre-existing clinical relationship. The physician/investigator is more likely to be familiar with not only a patient’s primary problem (neuropathic or nociceptive pain), but also with co-morbidities and with elements of a patient’s history likely to predict compliance with a research protocol. Similarly, when the patient-physician relationship is positive, the physician/investigator can reasonably expect good communication with the patient/subject in the course of the research project.
Disadvantages also can result to research subjects accrued from a physician’s clinical practice. Foremost among these is the possibility that patients may not appreciate the role changes inherent in shifting from clinical to research status, i.e., the patients may be at greater risk for therapeutic misconception. While this issue exists for any patients that face participation in research, it is likely to be exacerbated for patients who have a pre-existing clinical relationship. Relative to standard care, the patient who becomes a research subject will encounter new responsibilities (e.g., increased visits secondary to the requirements of a research protocol), new risks (e.g., related to the study drug/placebo), and less flexibility in treatment (changes in treatment will be protocol driven). Similarly, the provider will have new responsibilities (e.g., to assess study parameters that reflect response to treatment), new risks (e.g., to manage study-related side effects), and less flexibility (with limited options available for managing side effects and/or titrating study drug).
With patients in pain, the complications may be heightened by other factors, including a prospective subject’s dependence on the provider for long-term care. As noted above, this can place the investigator in a “one-up” position in the research relationship, potentially amplifying the effect of investigator attitudes and beliefs such that a prospective research subject may experience subtle pressure to enroll. Another complication is associated with the patient’s clinical status at the time of enrollment. If pain is inadequately controlled (a common inclusion criterion for analgesic trials), the pressure to avoid that aversive state also may foster enrollment, especially if the physician offers no alternative treatments outside study enrollment.
Within the context of a pre-existing patient-physician relationship, inadequately controlled pain may heighten the likelihood of therapeutic misconception. Patients who are approached by their treating physicians about study participation may assume that the invitation implies continuity with clinical care, driven primarily by their individual needs, rather than the inflexibility of protocol-driven treatment. Further, investigators also may entertain unrealistic expectations of clinical benefit for patients who participate in research.58 If misconceptions are held by the patient, the provider, or both, then the patient-provider dynamic can presage difficulties for an incipient subject/investigator relationship.
In addition to issues posed by a pre-existing patient-provider relationship, several that are related to the valence of the subject/investigator relationship are relevant to studies of pain. As previously noted, judgments of pain at high levels of severity are influenced by a range of factors; many of these factors can lead to symptom discounting. Patient attributes that can occasion discounted judgments of pain severity include demographic variables such as gender, age, and race/ethnicity. Similarly, the absence of objective medical evidence and the presence of psychiatric symptoms can negatively impact ratings of pain.59 Several physician attributes also can influence ratings of pain, including the previously noted valence of the physician-patient relationship.60 In addition, physicians with higher acuity practices may be predisposed to discount levels of pain severity, and surgeons may rate pain at levels lower than internists.
Such systematic variation in pain assessment can impact several phases of a study. For example, the determination of inclusion/exclusion criteria typically relies on investigator judgment (e.g., of pain severity). As noted previously, those judgments can be colored by a range of factors. For example, patients who report low-to-moderate levels of pain are not only likely to have their symptoms validated, but may have the severity of their symptoms amplified by the investigator.61 This could eventuate in patients being enrolled inappropriately into a trial (and exposed to study risks). Of course, the process also could be reversed, with patients inappropriately excluded from a trial with symptoms that are discounted. Minorities, older patients, and women are demographic sets that have been shown particularly susceptible to pain discounting, thus raising concerns about possible violations of justice in the conduct of a study.
Of course, investigator judgments of pain severity could affect other decisions related to research participation, including the “stop rules.” In studies of pain treatment, stop rules often are soft, based upon the severity, duration, and controllability of pain exacerbations. As discussed previously, these determinations are subject to the influences described above, potentially exposing subjects to risks in a trial beyond the point where they might reasonably be withdrawn.
Relationships with Sponsors
Recent years have witnessed considerable attention to conflicts of interest between physicians and sponsors that have the potential to influence physician judgments and behavior. Conflicts of interest, such as membership on an advisory board or a speaker’s bureau, have been shown to influence medical education62 and practice.63 They also have been identified in the research realm through recruitment incentives64 and through the use of unacknowledged ghostwriters on manuscripts for which authorship credit is given to others.65 Whatever their form, conflicts of interests are similar across the various forms of clinical research (including pain). Because they have been addressed at length elsewhere, this paper will touch only briefly on those of relevance to potential risks that research subjects might encounter in pain studies.
The previous section already has outlined several choice points where investigators must make judgments regarding research subjects. These include judgments made regarding inclusion/exclusion criteria and those made at various points during the time of study participation where subjects either experience a potentially inadequate response to treatment or significant side effects. Conflicts of interest in either case have the potential to blur an investigator’s decisions regarding study management. For example, enrollment incentives may sway judgments regarding a subject’s suitability for a study. In the case of a pain study, conflicts of interest may lead an investigator to augment symptom severity for prospective subjects who are on the margin; as noted previously, physicians may be prone to such augmentation, anyway, for symptoms in the low-to-moderate range.66 Similarly, financial conflicts may influence an investigator’s estimation of benefits relative to risks, thus inflating any pre-existing therapeutic misconceptions that the investigator might have. Of course, this then may be communicated to prospective subjects during the consent process. As noted previously, patients requiring long-term pain management may be particularly sensitive to such biases.
Just as conflict-of-interest biases might affect subject recruitment, so might they impact judgments made during the actual conduct of a trial. Again, the potential biases are particularly problematic in pain research because of the subjective nature of pain assessment and its susceptibility to social psychological influences. As noted earlier, the potential risk of primary concern involves the underassessment of pain. This can arise from either of two sources, some that may be directly influenced by an investigator’s conflict of interest and some that may be indirectly affected.
The direct effects are mediated by assessments of pain severity. One potential effect applies to unblinded treatment studies: a conflicted investigator may be influenced to overestimate the effect of study drug (e.g., through ratings of clinical global impressions, a widely utilized but notoriously unreliable measure67) and/or to underestimate the level of reported pain over the course of a trial (reinforcing a documented predisposition to discount severe levels of pain).68 This effect, of course, would not apply to research involving blinded, placebo-controlled designs. A second potential effect, involving the application of stop rules, could apply. Subject retention obviously is important to a study’s sponsor — attrition decreases study power and the likelihood of finding a statistically significant effect. A conflict of interest could enhance the pre-existing bias to discount judgments that an investigator makes about the severity of a subject’s pain. Such a bias may militate against withdrawing a subject from a study and expose the subject to further risk of poorly controlled pain.
Conflicts of interest also may have an indirect effect on subject reporting. As noted previously, subjects tend to report lower levels of pain to physicians than to other health care professionals, a tendency that may be reinforced by a desire to be viewed as a good patient when in a dependent relationship. There is potential for these effects to be amplified when an investigator manifests enthusiasm for a study, something that may be more likely when an investigator has a conflict of interest.
Sources of Vulnerability: The Environment
The research subject operates in a research environment of multiple layers. The first layer involves the immediate research context: after deciding to participate in a study, the subject lives in relationship to the study as designed by the study sponsor and as executed by the research team. A second layer involves the institutional regulatory structure that oversees research activities, generally through a local or central IRB/ethics committee. At a greater remove is the broad sociopolitical environment within which the research enterprise occurs. As noted earlier in this paper, bio-ethicists have identified influences at each of these levels that may contribute to risks associated with research participation. This section reviews potential influences, starting at the level of the immediate research context (including aspects of study design) that may contribute to subject risks in pain trials. It then addresses influences associated with deficiencies in institutional oversight that might impact pain trials and, finally, reviews factors at the sociopolitical level that also may influence study risks.
The Research Context
The research context involves both local aspects (i.e., the research team) and more remote aspects (i.e., the study design). The risks associated with the research team relate primarily to the investigator and have been addressed previously. Therefore, this section focuses on potential risks that a subject with chronic pain might face secondary to study design.
Of the design-related risks, possibly the most important involves potential exposure to a placebo condition. The Food and Drug Administration (FDA) often requires placebo-controlled trials, particularly for psychoactive and analgesic compounds, in order to evaluate efficacy preparatory to licensure. The ethics of placebo-controlled research have been the subject of numerous articles, including articles critical of the design69 and those more supportive of it.70 Several articles have explicitly addressed the ethics of placebo-controlled research in pain, generally supporting the need for such research.71
While a review of that literature is beyond the scope of this paper, the articles generally cite the subjectivity of outcome measures and the power of placebo effects as justification for such designs in conditions having strong subjective components such as the experience of pain. The use of a placebo condition as the sensitivity index controls for error in outcome measures and allows an investigator to determine whether there is an effect of active treatment. These issues are confounded when an active treatment is compared against a positive control. Further, non-inferiority trials that use positive controls require larger samples. In turn, larger samples can lead to more logistical and methodological problems. Even with large samples that are successfully managed, however, such trials are vulnerable to findings of negative results (i.e., comparable effects for both conditions that suggest equivalent efficacy), even when compounds are not equivalent.72 Without placebo-controlled trials to demonstrate the efficacy of an investigational analgesic compound, ineffective compounds would be more likely to find their way to the marketplace, representing a source of jeopardy to the common good.73 Of course, these advantages of placebo-controlled studies would be moot if several other conditions also were not met: (1) that a compelling case cannot be made for the use of an approved compound (i.e., clinical equipoise exists); (2) that the use of placebo does not place the subject population at risk of severe or irreversible harm; and (3) that the informed consent process must clearly describe the risks involved in study participation, including the risks of placebo.
Although the arguments favoring the use of placebo controls in analgesic research are persuasive, risks of those studies specific to patients with chronic pain should be recognized. Several have been raised previously: (1) pain can impact affect and cognition, potentially compromising a patient’s understanding of a study and its implications for his specific condition, which increases the risk of therapeutic misconception; (2) the patient who finds a physician willing to work with him to manage pain effectively over the long term may be loathe to decline study participation in order to be viewed as a “good patient”; and (3) secondary to the long-term nature of the medical relationship, the patient may fail to perceive the changes in that relationship that study participation occasions (secondary to the constraints associated with protocol-driven decisions).
Finally, some consideration should be given to the assumption that any symptom exacerbation that a subject might experience will be of a relatively brief duration (i.e., not severe) and can be resolved by resuming active treatment (i.e., not irreversible). Placebo-controlled, analgesic studies would appear to meet these criteria: the patient who is randomized to placebo may experience a relatively brief increase in pain that should be controlled once analgesic treatment is reinitiated. The latter assumptions, however, may not hold true for analgesic trials in patients with chronic pain: (1) secondary to the social psychological mechanisms discussed earlier, study subjects may be exposed to higher levels of pain for longer periods of time than intended in a study protocol; (2) if inclusion criteria include pain that was inadequately controlled with standard care, the pain exacerbation may not be readily controlled upon termination of study participation; and (3) psychiatric co-morbidities often associated with pain also complicate the situation. Relative to the latter issue, there is evidence that depression among patients in pain is related not only to high levels of pain severity, but also to the number of exacerbations that a patient experiences.74 Thus, the patient who is randomized to placebo may be at increased risk of a depressive episode, a potentially severe consequence requiring additional care. These complications raise questions as to whether decision rules for placebo-controlled trials with patients in chronic pain should consider increased risks associated with common, co-morbid psychiatric disorders.
The Institutional Regulatory Environment
Considerable responsibility for the ethical and regulatory oversight of clinical research is vested in central and local Institutional Review Boards (IRBs). Indeed, IRBs are charged with affording particular protections to research subjects who are identified as vulnerable. Secondary to these statutory requirements, IRBs generally are vigilant in regard to the protection of identified vulnerable populations (e.g., children, patients with neurocognitive/neuropsychiatric disorders). IRB vigilance may be diminished, however, for research with potentially vulnerable populations that have not been granted regulatory safeguards. A recent study examined IRB member perceptions of decisional capacity and risk for hypothetical patients participating in either medical or psychiatric research.75 IRB members clearly saw subjects in the psychiatric studies as at high levels of risk relative to those in medical studies. Of more concern, this risk assessment was obtained even when the medical conditions under study were of such severity as to make psychiatric co-morbidities very likely (i.e., neuropathic pain > 7/10, cancer with less than three months to live). Thus, the pattern suggested ample protection (and possible over-protection) of psychiatric patients, but relative indifference to vulnerabilities in patients with medical diagnoses with likely psychiatric co-morbidities.
The above findings suggest that the vulnerabilities of patients with chronic pain, for whom psychiatric co-morbidities are common, may be underestimated by IRBs, the primary institutional structure charged with overseeing human subject research protections. Hence, it appears unrealistic to expect IRBs to be sensitive to research designs having the potential to engender special risks in this subject group. The lack of such oversight, in turn, potentially increases the risks that these subjects may face.
The Sociopolitical Environment
It has been suggested that any patient with chronic pain is vulnerable, secondary to the under-treatment that often characterizes that population.76 We already have identified a fundamental contributor to under-treatment, the subjectivity of the experience of pain. The subjectivity of pain, coupled with the frequent lack of supporting medical evidence, also contributes to negative stereotypes currently affecting public perceptions. Such perceptions are reflected in the numerous unflattering labels attached to people in pain: low back loser, compensation neurosis, railway spine. Negative stereotypes also are evident within the medical profession, where patients with chronic pain routinely rank near the top of physician surveys regarding the most frustrating types of patients.77 Aside from negative stereotypes, bona fide obstacles to the effective treatment of patients with chronic pain are evident in public policies, including an emphasis in government policy on limiting access to classes of analgesics (e.g., opioids), rather than assuring their availability to people in need.78 Such stances have contributed to fear among physicians regarding the use of opioid analgesics, especially in the treatment of chronic pain conditions.79 In turn, this has led many physicians to limit the number of such patients in their practices and/or to limit the availability of opioids to them.80
This negative backdrop has several implications for research because of its effects on the availability of treatment outside the research context. First, because of limitations in access to adequate analgesia, patients with chronic pain commonly have pain that is ineffectively managed.81 This unfortunate state of affairs, of course, underscores the need for clinical research aimed at identifying more effective treatments for pain, although it also is implicated in having a pool of patients that are likely to be motivated to participate in studies that hold promise (or are perceived as holding promise) of effective pain management. On the other hand, as noted previously, the inadequately controlled pain that patients may experience may compromise their capacity to provide a truly autonomous informed consent. Further, the negative backdrop has implications for subjects during the course of study participation, particularly if they experienced inadequate pain control prior to study participation. If they also experience inadequate pain control during study participation, then they face a dilemma: do they remain in the study (and continue to experience inadequately controlled pain), or do they terminate study participation (knowing that they will have inadequate pain control outside the study)? This dilemma would be less problematic if investigators shared some responsibility for effective pain management after study participation, either by assuming pain management care or identifying other providers for that care. Outside study parameters, however, study investigators generally avoid either role. Hence, the research subject who qualified for trial participation secondary to inadequately controlled pain faces a Hobson’s choice that is not likely to be resolved so long the current socio-political environment continues to exist relative to the treatment of pain.
Concluding Comments
Although patients living with intractable pain may indeed constitute a vulnerable population, labeling the population as vulnerable does not adequately specify the risks that they may face when participating in research. Instead, research vulnerability for patients in pain is a function of risks incurred because of the interaction of their pain conditions with elements of the research enterprise in which they participate. Therefore, the identification of potential risks requires consideration not only of factors endemic to chronic pain, but also features of researchers, local oversight, and the medical/social environment within which the research is conducted.
This paper has identified a number of risks that patients in pain might incur secondary to research participation. Unfortunately, those that are inherent in the subjective experience of pain, especially those related to the self-report mechanisms (levels of pain severity) by which that experience is assessed, are not remediable as pain can be misreported for various reasons. Similarly, levels of pain severity, if reported accurately, may be misperceived (augmented/discounted) by investigators for various reasons. Because no clear remedy exists by which to ensure accurate and reliable assessment information to guide decisions, the only corrective action may be recognizing those pitfalls, applying caution in the conduct of pain assessment, and, perhaps, having pain assessed by several members of the investigative team in order to ensure that discrepancies in ratings are identified and their causes addressed.
If there is no ready solution for the former problem, several others have potentially actionable implications with the potential to minimize research risks for patients in pain. One such problem involves the cognitive and affective co-morbidities often associated with chronic pain conditions; these can contribute to participant risk in several ways. Clearly, such co-morbidities deserve greater attention, both at the level of the investigative team and at the regulatory level. At the investigator level, greater attention should be directed at assessing and managing these co-morbidities in the consent process. It is reasonable to formally evaluate not only a subject’s understanding of the proposed research, but also his appreciation of how the research applies specifically to him (e.g., through the use of such checklists as the McArthur Competence Assessment Tool).82 The use of a systematic approach to assessing the effects of mood and cognition in this patient group would serve to identify patients whose levels of depression and/or cognitive dysfunction are clinically significant. That subgroup may require further management to ascertain that they provide a truly autonomous and informed decision regarding research participation.
Similarly, ongoing attention to subject cognitive and affective status is needed throughout a trial. Subjects who demonstrate inadequate pain control are at particular need for such attention as they are likely to demonstrate an increased risk for a depressive episode. Early identification and treatment of a developing depressive disorder can prevent it from becoming a severe problem that challenges the ethics of a research project.
At the regulatory (IRB) level, research indicates that psychiatric co-morbidities may not be appreciated at the time that protocols undergo review.83 These results suggest that mechanisms intended to ensure attention to these matters (e.g., the composition of a board) may be ineffective, perhaps, because the information available to the board is insufficiently specific regarding such matters. The decision-making literature has demonstrated that, in the absence of specific information, decisions tend to underestimate the likelihood of an event.84 Thus, in the case of psychiatric co-morbidities, the absence of information regarding their prevalence in a given condition (e.g., chronic pain) is likely to eventuate in decisions that discount such co-morbidities. Given the above, IRBs should consider requiring information that speaks specifically to psychiatric co-morbidities in research protocols.
Another problem that calls for solution involves the availability of effective pain management for patients considering research, both before and after study participation. Currently, patient selection criteria for many analgesic trials include the presence of poorly controlled pain (often defined as levels of pain exceeding 4/10). While lower boundaries are well described, upper boundaries for admissible pain (e.g., 8/10) are seldom specified. These criteria capitalize on the current state of pain management in this and other countries, where the undertreatment of pain is widespread secondary to a range of barriers: societal attitudes, political and legal concerns (e.g., over drug diversion), fear of opioids, and fear of prosecution for providing such treatment.85
Clearly, an inclusion criterion that restricts enrollment to patients for whom pain can be severe is understandable in an analgesic trial (there must be room to demonstrate analgesic efficacy in ratings of pain severity). While the potential for pain to be severe is understandable, it is not clear that current pain must be poorly controlled. Indeed, patients with severe, poorly controlled pain may be unduly influenced by their discomfort to accede to study participation. Moreover, to the degree that their discomfort reflects ineffective provider practices, the presence of poorly controlled pain may represent undue provider influence (i.e., compromised autonomy) in the consent process. Such compromise would be particularly problematic in patients with very severe pain (e.g., 8/10), who may be driven by desperation to participate in a research trial in the absence of reasonable relief through other, clinical channels. Perhaps consideration should be given to a different inclusion criterion, such that patients would be eligible for study participation if their clinical record reflected previously high levels of pain severity that are reasonably controlled with standard care.
Although the above analyses apply to the consent process, similar arguments could be made in regard to the management of study subjects participating in a study or facing study termination. For active subjects whose pain was poorly controlled prior to the study, the promise of ineffective pain control upon return to standard care may represent a significant barrier to study withdrawal, even when pain is poorly controlled by the study regimen. Similarly, subjects facing study termination with the prospect of poor pain control are at risk of significant emotional distress, both in anticipation of this development and, of course, when faced again with coping with severe pain. Each of these issues would be minimized if adequate pain control was available to subjects at study termination.
In summary, this manuscript argues for the consideration of research vulnerability as a construct that reflects risks arising from the intersection of the medical condition under study (i.e., pain) with the context within which research participation occurs. This approach recognizes both the clinical vagaries of a research subpopulation and situational factors that might present specific risks to that population. Not only does this approach provide a model within which to consider the concept of research vulnerability, but, when applied to a particular subpopulation, it also suggests corrective actions that might be taken to minimize specific risks that study subjects from that subpopulation might encounter.
Figure 2.
The Social Context of Pain Judgments32 (used with permission)
Acknowledgments
The author would like to acknowledge support for the prepration of this article from a grant, R01 MH075958, from the National Institutes of Mental Health.
Biography
Raymond C. Tait, Ph.D., is a Professor of Neurology and Psychiatry at Saint Louis University in St. Louis, Missouri. He received his B.A. from Amherst College in Amherst, Massachusetts, his M.A. and Ph.D. from the University of Illinois at Chicago in Chicago, Illinois, and completed a post-doctoral fellowship in pain management at the University of Virginia School of Medicine in Charlottesville, Virginia. He has longstanding interests in chronic pain and more recent interests in research ethics, having chaired the Institutional Review Board at Saint Louis University for approximately five years.
References
- 1.U.S. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Guidelines for the Protection of Human Subjects of Research. Washington, D.C: U.S. Government Printing Office; 1979. p. 8. [Google Scholar]
- 2.Council for International Organization of Medical Societies. International Ethical Guidelines for Biomedical Research involving Human Subjects, Commentary on Guideline. 2002;13 available at < www.cioms.ch> (last visited July 15, 2008) [PubMed]
- 3.DeBruin D. Reflections on “Vulnerability”. Bioethics Examiner. 2001;5(3):1–7. [Google Scholar]; Levine C, Faden R, Grady C, Hammerschmidt D, Eckenwiler L, Sugarman J. The Limitations of ‘Vulnerability’ as a Protection for Human Research Subjects. American Journal of Bioethics. 2004;4(3):44–49. doi: 10.1080/15265160490497083. [DOI] [PubMed] [Google Scholar]
- 4.Grinnell F. Subject Vulnerability: The Precautionary Principle of Human Research. American Journal of Bioethics. 2004;4(3):72–74. doi: 10.1080/15265160490497416. see Levine et al., supra note 3. [DOI] [PubMed] [Google Scholar]
- 5.Kottow MH. The Vulnerable and the Susceptible. Bioethics. 2003;17(5–6):460–471. doi: 10.1111/1467-8519.00361. see also Levine, supra note 3. [DOI] [PubMed] [Google Scholar]
- 6.Schweitzer A. On the Edge of the Primeval Forest. New York: Macmillan; 1931. p. 2. [Google Scholar]
- 7.Blacksher E. Hearing from Pain: Using Ethics to Reframe, Prevent, and Resolve the Problem of Unrelieved Pain. Pain Medicine. 2001;2(2):169–175. doi: 10.1046/j.1526-4637.2001.002002169.x. [DOI] [PubMed] [Google Scholar]
- 8.Brennan F, Carr DB, Cousins M. Pain Management: A Fundamental Right. Anesthesia & Analgesia. 2007;105(1):205–221. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- 9.VanderWalde AM. Vulnerability as the Inability of Researchers to Act in the Best Interest of a Subject. American Journal of Bioethics. 2004;4(3):65–66. doi: 10.1080/15265160490497092. [DOI] [PubMed] [Google Scholar]
- 10.Henderson GE, Davis AM, King NMP. Vulnerability to Influence: A Two-Way Street. American Journal of Bioethics. 2004;4(3):50–52. doi: 10.1080/15265160490497371. [DOI] [PubMed] [Google Scholar]
- 11.Shivas T. Contextualizing the Vulnerability Standard. American Journal of Bioethics. 2004;4(3):84–86. doi: 10.1080/15265160490497137. [DOI] [PubMed] [Google Scholar]
- 12.Marshall MF. Vulnerable Subjects and Civic Professionalism: Would Six-Sigma Research and Research Ethics Consultation Solve the Vulnerability Problem? American Journal of Bioethics. 2004;4(3):54–55. doi: 10.1080/15265160490497533. [DOI] [PubMed] [Google Scholar]
- 13.Vawter DE, Gervais KG, Freeman TB. Strategies for Achieving High-Quality IRB Review. American Journal of Bioethics. 2004;4(3):74–76. doi: 10.1080/15265160490497407. [DOI] [PubMed] [Google Scholar]
- 14.Campbell AT. ‘Vulnerability’ in Context: Recognizing the Sociopolitical Influences. American Journal of Bioethics. 2004;4(3):58–59. doi: 10.1080/15265160490497100. [DOI] [PubMed] [Google Scholar]
- 15.Healy D, Cattel D. Interface between Authorship, Industry and Science in the Domain of Therapeutics. British Journal of Psychiatry. 2003;183(3–4):22–27. [PubMed] [Google Scholar]; Psaty BM, Kronmal RA. Reporting Mortality Findings in Trials of Rofecoxib for Alzheimer Disease or Cognitive Impairment. JAMA. 2008;299(15):1813–1817. doi: 10.1001/jama.299.15.1813. [DOI] [PubMed] [Google Scholar]
- 16.Lemmens T, Miller PB. The Human Subjects Trade: Ethical and Legal Issues Surrounding Recruitment Incentives. Journal of Law, Medicine & Ethics. 2003;31(3):398–418. doi: 10.1111/j.1748-720x.2003.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 17.DeAngelis CD, Fontanarosa PB. Impugning the Integrity of Medical Science: The Adverse Effects of Industry Influence. JAMA. 2008;299(15):1833–1835. doi: 10.1001/jama.299.15.1833. [DOI] [PubMed] [Google Scholar]; Ross JS, Hill KP, Egilman DS, Krumholz HM. Guest Authorship and Ghostwriting in Publications Related to Rofecoxib: A Case Study of Industry Documents from Rofecoxib Litigation. JAMA. 2008;299(15):1800–1812. doi: 10.1001/jama.299.15.1800. [DOI] [PubMed] [Google Scholar]
- 18.DeBruin DA. Looking Beyond the Limitations of ‘Vulnerability’: Reforming Safeguards in Research. American Journal of Bioethics. 2004;4(3):76–78. doi: 10.1080/15265160490497579. [DOI] [PubMed] [Google Scholar]
- 19.See DeBruin, supra note 3, at 7.
- 20.Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2. Seattle: IASP Press; 1994. p. 210. [Google Scholar]
- 21.Turk DC, Melzack R. The Measurement of Pain and the Assessment of People Experiencing Pain. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. 2. New York: The Guilford Press; 2001. p. 3. [Google Scholar]
- 22.Jensen R, Tfelt-Hansen P. Headache. In: Jensen TS, Wilson PR, Rice ASC, editors. Chronic Pain: Clinical Pain Management. London: Arnold Publishers; 2003. p. 467. [Google Scholar]
- 23.Campbell FA, Atcheson R. Chronic Back Pain. In: Jensen TS, Wilson PR, Rice ASC, editors. Chronic Pain: Clinical Pain Management. London: Arnold Publishers; 2003. p. 524. [Google Scholar]
- 24.Turner JA, LeResche L, Von Korff M, Ehrlich K. Back Pain in Primary Care: Patient Characteristics, Content of Initial Visit, and Short-Term Outcomes. Spine. 1998;23(4):463–469. doi: 10.1097/00007632-199802150-00011. [DOI] [PubMed] [Google Scholar]; Turner JA, Franklin G, Turk DC. Predictors of Chronic Disability in Injured Workers: A Systematic Literature Synthesis. American Journal of Industrial Medicine. 2000;38(6):707–722. doi: 10.1002/1097-0274(200012)38:6<707::aid-ajim10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]; Gatchel RJ, Dersh J. Psychological Disorders and Chronic Pain: Are There Cause-and-Effect Relationships? In: Turk DC, Gatchel RJ, editors. Psychological Approaches to Pain Management: A Practitioner’s Handbook. 2. London: The Guilford Press; 2002. p. 33. [Google Scholar]
- 25.Merskey H, Teasell RW. The Disparagement of Pain: Social Influences on Medical Thinking. Pain Research and Management. 2000;5(4):259–270. [Google Scholar]
- 26.Agrawal M, Emanuel EJ. Ethics of Phase 1 Oncology Studies: Reexamining the Arguments and Data. JAMA. 2003;290(8):1075–1082. doi: 10.1001/jama.290.8.1075. [DOI] [PubMed] [Google Scholar]; Cantor NL, Thomas GC. Pain Relief, Acceleration of Death, and Criminal Law. Kennedy Institute of Ethics Journal. 1996;6(2):107–128. doi: 10.1353/ken.1996.0017. [DOI] [PubMed] [Google Scholar]; Emanuel EJ. Pain and Symptom Control: Patient Rights and Physician Responsibilities. Hematology/Oncology Clinics of North America. 1996;10(1):41–56. doi: 10.1016/s0889-8588(05)70326-9. [DOI] [PubMed] [Google Scholar]; Emanuel EJ. Attending to Psychologic Symptoms and Palliative Care. Journal of Clinical Oncology. 2002;20:624–626. doi: 10.1200/JCO.2002.20.3.624. [DOI] [PubMed] [Google Scholar]; Pellegrino ED. Emerging Ethical Issues in Palliative Care. JAMA. 1998;279(19):1521–1522. doi: 10.1001/jama.279.19.1521. [DOI] [PubMed] [Google Scholar]
- 27.Hadler NM. MRI for Regional Back Pain: Need for Less Imaging, Better Understanding. JAMA. 2003;289(21):2863–2865. doi: 10.1001/jama.289.21.2863. [DOI] [PubMed] [Google Scholar]; Rudy TE, Turk DC, Brena SF. Differential Utility of Medical Procedures in the Assessment of Chronic Pain Patients. Pain. 1988;34(1):53–60. doi: 10.1016/0304-3959(88)90181-9. [DOI] [PubMed] [Google Scholar]
- 28.Chibnall JT, Tait RC, Ross LR. The Effects of Medical Evidence and Pain Intensity on Medical Student Judgments of Chronic Pain Patients. Journal of Behavioral Medicine. 1997;20(3):257–271. doi: 10.1023/a:1025504827787. [DOI] [PubMed] [Google Scholar]; Grossman SA, Sheidler VR, Swedeen K, Mucenski J, Piantadosi S. Correlation of Patient and Caregiver Ratings of Cancer Pain. Journal of Pain and Symptom Management. 1991;6(2):53–57. doi: 10.1016/0885-3924(91)90518-9. [DOI] [PubMed] [Google Scholar]
- 29.Tait RC, Chibnall JT, Kalauokalani DA. Provider Judgments of Patients in Pain: Seeking Symptom Certainty. Pain Medicine. doi: 10.1111/j.1526-4637.2008.00527.x. (in press) [DOI] [PubMed] [Google Scholar]
- 30.Fiske ST, Taylor SE. Social Cognition. 2. New York: McGraw-Hill; 1991. p. 14. [Google Scholar]
- 31.See Brennan et al., supra note 8 Cassell EJ. The Nature of Suffering and the Goals of Medicine. New England Journal of Medicine. 1982;306(11):639–645. doi: 10.1056/NEJM198203183061104.Rich BA. A Legacy of Silence: Bioethics and the Culture of Pain. Journal of Medical Humanities. 1997;18(4):233–259. doi: 10.1023/a:1025697920944.Johnson SH. Legal and Ethical Perspectives on Pain Management. Anesthesia & Analgesia. 2007;105(1):5–7. doi: 10.1213/01.ane.0000268148.38688.e7.Merskey and Teasell, supra note 25.
- 32.Tait RC. The Social Context of Pain Management. Pain Medicine. 2007;8(1):1–2. doi: 10.1111/j.1526-4637.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 33.Blacksher E. Hearing from Pain: Using Ethics to Reframe, Prevent, and Resolve the Problem of Unrelieved Pain. Pain Medicine. 2001;2(2):169–175. 170. doi: 10.1046/j.1526-4637.2001.002002169.x. [DOI] [PubMed] [Google Scholar]
- 34.Appelbaum PS, Lidz CW, Grisso T. Therapeutic Misconception in Clinical Research: Frequency and Risk Factors. IRB: Ethics & Human Research. 2004;26(2):1–8. 1. [PubMed] [Google Scholar]
- 35.Id
- 36.Fleming S, Rabago DP, Mundt MP, Fleming MF. CAM Therapies among Primary Care Patients Using Opioid Therapy for Chronic Pain. BMC Complementary and Alternative Medicine. 2007;7(15):15–22. doi: 10.1186/1472-6882-7-15. available at < http://www.biomedcentral.com/1472-6882> (last visited July 15, 2008) [DOI] [PMC free article] [PubMed]; Upchurch DM, Chyu L, Greendale GA, Utts J, Bair YA, Zhang G, Gold EB. Complementary and Alternative Medicine Use among American Women: Findings from the National Health Interview Survey, 2002. Journal of Women’s Health. 2007;16(1):102–113. doi: 10.1089/jwh.2006.M074. [DOI] [PubMed] [Google Scholar]
- 37.Werner A, Malterud K. It Is Hard Work Behaving as a Credible Patient: Encounters between Women with Chronic Pain and Their Doctors. Social Science & Medicine. 2003;57(8):1409–1419. doi: 10.1016/s0277-9536(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 38.Camarda C, Monastero R, Pipia C, Reca D, Camarda R. Interictal Executive Dysfunction in Migraineurs without Aura: Relationship with Duration and Intensity of Attacks. Cephalalgia. 2007;27(10):1094–1100. doi: 10.1111/j.1468-2982.2007.01394.x. [DOI] [PubMed] [Google Scholar]; Dick BD, Rashiq S. Disruption of Attention and Working Memory Traces in Individuals with Chronic Pain. Anesthesia & Analgesia. 2007;104(5):1223–1239. doi: 10.1213/01.ane.0000263280.49786.f5. [DOI] [PubMed] [Google Scholar]
- 39.Dick BD, Eccleston C, Crombez G. Attentional Functioning in Fibromyalgia, Rheumatoid Arthritis, and Musculoskeletal Pain Patients. Arthritis & Rheumatism. 2002;47(6):639–644. doi: 10.1002/art.10800. [DOI] [PubMed] [Google Scholar]
- 40.Bayer A, Fish M. The Doctor’s Duty to the Elderly Patient in Clinical Trials. Drugs & Aging. 2003;20(15):1087–1097. doi: 10.2165/00002512-200320150-00002. [DOI] [PubMed] [Google Scholar]; Dick B. Fitness of Patients in Pain to Make Optimal Decisions. Anesthesia & Analgesia. 2008;106(2):669–670. doi: 10.1213/ane.0b013e31816197b1. [DOI] [PubMed] [Google Scholar]
- 41.Kim SYH, Caine ED, Currier GW, Leibovici A, Ryan JM. Assessing the Competence of Persons with Alzheimer’s Disease in Providing Informed Consent for Participation in Research. American Journal of Psychiatry. 2001;158(5):712–717. doi: 10.1176/appi.ajp.158.5.712. [DOI] [PubMed] [Google Scholar]; Raymont V, Bingley W, Buchanan A, David AS, Hayward P, Wessely S, Hotopf M. Prevalence of Mental Incapacity in Medical Inpatients and Associated Risk Factors: Cross-sectional Study. The Lancet. 2004;364(9443):1421–1427. doi: 10.1016/S0140-6736(04)17224-3. [DOI] [PubMed] [Google Scholar]
- 42.See Gatchel and Dersh, supra note 24 Sullivan MD. Assessment of Psychiatric Disorders. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. 2. New York: The Guilford Press; 2001. pp. 275–291.pp. 275
- 43.Dresser JD. Research Involving Persons with Mental Disabilities: A Review of Policy Issues and Proposals. National Bioethics Advisory Commission, Research Involving Persons with Mental Disorders That May Affect Decisionmaking Capacity: Commissioned Papers. 1999:5–28. [Google Scholar]
- 44.Appelbaum PS, Grisso T, Frank E, O’Donnell S, Kupfer DJ. Competence of Depressed Patients for Consent to Research. American Journal of Psychiatry. 1999;156(9):1380–1384. doi: 10.1176/ajp.156.9.1380. [DOI] [PubMed] [Google Scholar]
- 45.Roberts LW. Ethics and Mental Illness Research. Psychiatric Clinics of North America. 2002;25(3):525–545. doi: 10.1016/s0193-953x(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 46.Chen DT, Miller FG, Rosenstein DL. Enrolling Decisionally Impaired Adults in Clinical Research. Medical Care. 2002;40(Supplement 9):V20–V29. doi: 10.1097/01.MLR.0000023952.15394.88. [DOI] [PubMed] [Google Scholar]; Wirshing DA, Wirshing WC, Marder SR, Liberman RP, Mintz J. Informed Consent: Assessment of Comprehension. American Journal of Psychiatry. 1998;155(9, Supplement):1508–1511. doi: 10.1176/ajp.155.11.1508. [DOI] [PubMed] [Google Scholar]
- 47.See Turner et al. (1998), supra note 24.
- 48.Deshields TL, Tait RC, Gfeller J, Chibnall JT. The Influence of Social Desirability on Self-Report in Chronic Pain Patients. Clinical Journal of Pain. 1995;11(3):189–193. doi: 10.1097/00002508-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Williams DA, Park KM, Ambrose KR, Clauw DJ. Assessor Status Influences Pain Recall. Journal of Pain. 2007;8(4):343–348. doi: 10.1016/j.jpain.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Jones KR, Fink RM, Clark L, Hutt E, Vojir CP, Mellis BK. Nursing Home Resident Barriers to Effective Pain Management: Why Nursing Home Residents May Not Seek Pain Medication. Journal of the American Medical Directors Association. 2005;6(1):10–17. doi: 10.1016/j.jamda.2004.12.010. [DOI] [PubMed] [Google Scholar]; Ward S, Goldberg N, Miller-McCauley V, Mueller C, Nolan A, Pawlik-Plank D, Robbins A, Stormoen D, Weissman DE. Patient-Related Barriers to Management of Cancer Pain. Pain. 1993;52(3):319–324. doi: 10.1016/0304-3959(93)90165-L. [DOI] [PubMed] [Google Scholar]
- 51.Tait RC, Chibnall JT. Observer Perceptions of Chronic Low Back Pain. Journal of Applied Social Psychology. 1994;24(5):415–431. [Google Scholar]
- 52.Nurmikko TJ. Anticonvulsants and Antiarrhythmics. In: Jensen TS, Wilson PR, Rice ASC, editors. Chronic Pain: Clinical Pain Management. London: Arnold Publishers; 2003. pp. 251–260.pp. 252 [Google Scholar]
- 53.Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic Review: Opioid Treatment for Chronic Back Pain: Prevalence, Efficacy, and Association with Addiction. Annals of Internal Medicine. 2007;146(2):116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 54.Fanciullo GJ, Ball PA, Girault G, Rose RJ, Hanscom B, Weinstein JN. An Observational Study on the Prevalence and Pattern of Opioid Use in 25,479 Patients with Spine and Radicular Pain. Spine. 2002;27(2):201–205. 201. doi: 10.1097/00007632-200201150-00016. [DOI] [PubMed] [Google Scholar]; Rich BA. Ethics of Opioid Analgesia for Chronic Noncancer Pain. Pain: Clinical Updates. 2007;15(9):1–4. [Google Scholar]
- 55.Webster LR, Fakata KL, Charapata S, Fisher R, MineHart M. Open-Label, Multicenter Study of Combined Intrathecal Morphine and Ziconitide: Addition of Morphine in Patients Receiving Ziconitide for Severe Chronic Pain. Pain Medicine. 2008;9(3):282–290. doi: 10.1111/j.1526-4637.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 56.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, Controlled Trial of Cannabis-based Medicine in Central Pain in Multiple Sclerosis. Neurology. 2005;65(6):812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]; Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis Use for Chronic Non-cancer Pain: Results of a Prospective Survey. Pain. 2003;102(1–2):211–216. doi: 10.1016/s0304-3959(02)00400-1. [DOI] [PubMed] [Google Scholar]; Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, Fishman S. A Randomized, Placebo-Controlled, Crossover Trial of Cannabis Cigarettes in Neuropathic Pain. Journal of Pain. 2008;9(6):506–521. doi: 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.See Henderson et al., supra note 10.
- 58.Dresser R. The Ubiquity and Utility of the Therapeutic Misconception. Social Philosophy and Policy. 2002;19(2):271–294. doi: 10.1017/s0265052502192119. [DOI] [PubMed] [Google Scholar]
- 59.See Tait et al., supra note 29.
- 60.See Tait and Chibnall, supra note 51.
- 61.See Chibnall et al., supra note 28.
- 62.Wazana A. Physicians and the Pharmaceutical Industry: Is a Gift Ever Just a Gift? JAMA. 2000;283(3):373–380. doi: 10.1001/jama.283.3.373. [DOI] [PubMed] [Google Scholar]; Steinbrook R. Financial Support of Continuing Medical Education. JAMA. 2008;299(9):1060–1062. doi: 10.1001/jama.299.9.1060. [DOI] [PubMed] [Google Scholar]
- 63.Choudhry NK, Stelfox HT, Detsky AS. Relationships between Authors of Clinical Practice Guidelines and the Pharmaceutical Industry. JAMA. 2002;287(5):612–617. doi: 10.1001/jama.287.5.612. [DOI] [PubMed] [Google Scholar]
- 64.Lemmons T, Miller PB. The Human Subjects Trade: Ethical and Legal Issues Surrounding Recruitment Incentives. Journal of Law, Medicine & Ethics. 2003;31(3):398–418. doi: 10.1111/j.1748-720x.2003.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 65.See Ross et al., supra note 17.
- 66.See Chibnall et al., supra note 28.
- 67.Beneke M, Rasmus W. Clinical Global Impressions (ECDEU): Some Critical Comments. Pharmacopsychiatry. 1992;25(4):171–176. doi: 10.1055/s-2007-1014401. [DOI] [PubMed] [Google Scholar]
- 68.See Chibnall et al., supra note 28.
- 69.Rothman KJ, Michels KB. The Continuing Unethical Use of Placebo Controls. New England Journal of Medicine. 1994;331(6):394–398. doi: 10.1056/NEJM199408113310611. [DOI] [PubMed] [Google Scholar]; Khan A, Warner HA, Brown WA. Symptom Reduction and Suicide Risk in Patients Treated with Placebo in Antidepressant Clinical Trials: An Analysis of the Food and Drug Administration Database. Archives of General Psychiatry. 2000;57(4):311–317. doi: 10.1001/archpsyc.57.4.311. [DOI] [PubMed] [Google Scholar]
- 70.McQuay HJ, Moore RA. Placebo. Postgraduate Medical Journal. 2005;81(953):155–160. doi: 10.1136/pgmj.2004.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]; Temple R, Ellenberg SS. Placebo-Controlled Trials and Active-Control Trials in the Evaluation of New Treatments. Annals of Internal Medicine. 2000;133(6):455–463. doi: 10.7326/0003-4819-133-6-200009190-00014. [DOI] [PubMed] [Google Scholar]
- 71.Dworkin RH, Katz J, Gitlin MJ. Placebo Response in Clinical Trials of Depression and Its Implications for Research on Chronic Neuropathic Pain. Neurology. 2005;65(12, Supplement 4):S7–S19. doi: 10.1212/wnl.65.12_suppl_4.s7. [DOI] [PubMed] [Google Scholar]; Nagasako EM, Kalauokalani DA. Ethical Aspects of Placebo Groups in Pain Trials. Neurology. 2005;65(12, Supplement 4):S59–S65. doi: 10.1212/wnl.65.12_suppl_4.s59. [DOI] [PubMed] [Google Scholar]
- 72.See McQuay and Moore, supra note 70; Temple and Ellenberg, supra note 70; Nagasako and Kalauokalani, supra note 71.
- 73.See Dworkin et al., supra note 71.
- 74.Erdal KJ, Zautra AJ. Psychological Impact of Illness Downturns: A Comparison of New and Chronic Conditions. Psychology and Aging. 1995;10(4):570–575. doi: 10.1037//0882-7974.10.4.570. [DOI] [PubMed] [Google Scholar]
- 75.Luebbert R, Tait RC, Chibnall JT, Deshields TL. IRB member Judgments of Decisional Capacity, Coercion, and Risk in Medical and Psychiatric Studies. Journal of Empirical Research in Human Research Ethics. 2008;3(1):15–24. doi: 10.1525/jer.2008.3.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.See Rich, supra notes 31 and 54 Tait RC, Miller L. The Multidisciplinary Treatment of Pain in Vulnerable Populations. In: Schatman ME, Campbell A, editors. Chronic Pain Management: A Guidebook for Multidisciplinary Program Development. New York: Informa Healthcare; 2008. pp. 129–150.pp. 129
- 77.Sharpe M, Mayou R, Seagroatt V, Surawy C, Warwick H. Why Do Doctors Find Some Patients Difficult to Help? Quarterly Journal of Medicine. 1994;87(3):187–193. [PubMed] [Google Scholar]
- 78.Gevirtz C. Imbalance and Inconsistency Continue in DEA Policy on Medical Use of Opioids. Topics in Pain Management: Current Concepts and Treatment Strategies. 2006;21(10):6–7.see Rich, supra note 54.
- 79.See Brennan et al., supra note 8; Fishman SM. Risk of the View through the Keyhole: There Is Much More to Physician Reactions to the DEA Than the Number of Formal Actions. Pain Medicine. 2006;7(4):360–362. doi: 10.1111/j.1526-4637.2006.00194.x.Jung B, Reidenberg MM. The Risk of Action by the Drug Enforcement Administration against Physicians Prescribing Opioids for Pain. Pain Medicine. 2006;7(4):353–357. doi: 10.1111/j.1526-4637.2006.00164.x.
- 80.Lin JJ, Alfandre D, Moore C. Physician Attitudes toward Opioid Prescribing for Patients with Persistent Non-cancer Pain. Clinical Journal of Pain. 2007;23(9):799–803. 802. doi: 10.1097/AJP.0b013e3181565cf1. [DOI] [PubMed] [Google Scholar]
- 81.See Brennan et al., supra note 8; see Rich, supra note 31.
- 82.Grisso T, Appelbaum PS, Mulvey EP, Fletcher K. The MacArthur Treatment Competence Study, II: Measures of Abilities Related to Competence to Consent to Treatment. Law and Human Behavior. 1995;19(2):127–148. doi: 10.1007/BF01499322. [DOI] [PubMed] [Google Scholar]
- 83.See Luebbert et al., supra note 75.
- 84.Redelmeier DA, Koehler DJ, Liberman V, Tversky A. Probability Judgment in Medicine: Discounting Unspecified Possibilities. Medical Decision Making. 1995;15(3):227–230. doi: 10.1177/0272989X9501500305. [DOI] [PubMed] [Google Scholar]
- 85.See Brennan et al., supra note 8.