Abstract
Optical imaging possesses similar sensitivity to nuclear imaging and has led to the emergence of multimodal approaches with dual-labeled nuclear/near-infrared (NIR) agents. The growing impact of 68Ga (t1/2 = 68 min) labeled peptides on preclinical and clinical research offers a promising opportunity to merge the high spatial resolution of NIR imaging with the clinically-accepted positron emission tomography (PET). Previously, dual-labeled agents have been prepared with longer-lived radiometals and showed no detrimental effects on optical properties as a result of radiolabeling. In this study, we selected a peptide (M2) that targets MMP-2/9 and is dual-labeled with IRDye 800CW and 68Ga. Since 68Ga chelation typically requires low pH (3.5–4) and elevated heating temperatures (95°C), we sought to evaluate the impact of 68Ga labeling on the optical properties of M2. An efficient method for preparation of 68Ga-M2 was developed and reaction conditions were optimized. Stability studies in PBS, DTPA, and serum were performed and high levels of intact agent were evident under each condition. The addition of multiple reporters to a targeting agent adds further complexity to the characterization and validation and thus requires not only testing to ensure the agent is stable chemically and radiochemically, but also optically. Therefore, fluorescence properties were evaluated using a spectrofluorometer as well as by fluorescence detection via HPLC. It was determined that 68Ga-labeling conditions did not impair the fluorescent properties of the agent. The agent was then used for in vivo imaging in a mouse model of heterotopic ossification (HO) with activated MMP-9 expression as an early biomarker which precedes mineralization. Although 68Ga-complexation greatly reduced binding affinity of the peptide and negated tracer uptake on PET, NIR imaging showed consistent fluorescent signal that correlated to MMP-9 expression. This attests to the feasibility of using 68Ga/NIR for dual-labeling of other peptides or small molecules for multimodality molecular imaging.
Keywords: Dual-labeling, Near-infrared fluorescence, Gallium-68, MMP
1. Introduction
Multimodality imaging provides complementary functional and anatomical information for diagnosis, treatment planning, and therapeutic monitoring. Clinical hybrid systems that combine functional imaging modalities such as Positron Emission Tomography (PET) and Single-Photon Emission Computed Tomography (SPECT) with Computed Tomography (CT) offer the ability to obtain molecular imaging data that can be co-registered with anatomical imaging, playing substantial roles in patient care. The development of a single agent capable of carrying dual-contrast for both nuclear and CT or MRI imaging modalities proves challenging due to the inherent differences in measurement sensitivities between nuclear and all other conventional imaging modalities. While the pico- to femto- molar sensitivity of nuclear imaging permits the use of microdosing and minimizes potential pharmacologic effects and toxicity of a radiotracer, MR and CT contrast agents currently require millimolar tissue concentrations for acquisition.1
Near-infrared fluorescence (NIRF) optical imaging is an emerging imaging modality that promises comparable sensitivity to nuclear imaging.2–3 Because of the comparable sensitivity, dual optical/nuclear labeling of molecularly-targeted imaging agents can provide specific advantages. Foremost, if an imaging agent can be dual-labeled with a radionuclide and a NIR excitable fluorophore, then a single imaging agent can be used for non-invasive imaging of diseased tissues (via PET and possibly NIRF imaging) as well as intraoperative guidance for accurate surgical removal of corresponding tissues and tissue margins (via NIR fluorescence imaging). Second, because the NIR signal does not have a physical half-live, it can facilitate the validation of agent targeting capabilities long after physical decay of radiotracer. Lastly, if NIR fluorescent agents are to be used in molecular imaging, dual-labeling provides a strategy for comparative assessment against the conventional nuclear imaging modalities.
To date, the only NIR fluorophore that has been employed in human NIRF imaging studies is indocyanine green (ICG).4 However, dyes that possess better optical properties (i.e. increased fluorescent yield, preferable hydrophilicity, and enhanced stability) and can be subjected to reaction in organic, aqueous and solid phase chemistries with reduced risk of physical degradation or loss of fluorescence following radiolabeling are of great interest. IRDye 800CW is a NIR dye that is functionalized with either an N-hydroxysuccinimideor maleimide reactive group, allowing it to be attached to a number of biomolecules. Owing to its NIR excitation which abrogates tissue autofluorescence as a complicating background signal5 as well as to its unprecedented stability, IRDye 800CW has been used in a number of preclinical studies.6–12 While the imaging sensitivity to IRDye 800CW and other NIR fluorophores ultimately depends upon instrumentation design3, efficiency of dual-labeled agents also depends upon the stability and efficient fluorescent yield of the NIR fluorophore following conjugation and radiolabeling.
Different nuclear/optical dual-labeling strategies have been reported for antibody6–7, 13–14 and peptide-based agents.15–19 Our previous experiences with dual-labeled agents were with antibodies and required longer-lived radionuclides, such as 64Cu (t1/2 = 12.7 h) and 111In (t1/2 = 2.8 d), to allow for sufficient clearance from background tissues and permit imaging at delayed time points (i.e. 24 h). We and others are also developing peptide agents as they are ideal molecules for dual-labeling since they can be synthesized by solid-phase peptide synthesis and have a clearly defined structure to which site-specific conjugations can be performed. Also, peptides clear rapidly from circulation and provide high target-to-background ratios at early time points. Our initial studies with dual-labeled peptides used 64Cu to allow for longitudinal PET imaging at early and delayed time points to monitor distribution, clearance routes, agent stability, and determine the optimal time point for imaging. Due to limitations in the production of 64Cu, we explored the use of more readily-available PET radionuclides, such as 68Ga, which possesses a shorter half-life (68 min) and is widely-used for peptide imaging. 68Ga is a positron-emitting radionuclide that is produced from commercially-available generators. Since the generator is housed locally and can be eluted multiple times per day, it allows for rapid method development and clinical deployment of new radiotracers. 68Ga is formed as the decay product of the long-lived parent radionuclide Germanium-68 (68Ge, t1/2= 270 d), thus allowing routine use of the 68Ga-generator for nearly one year.
In this study, we evaluated the feasibility of using a 68Ga/IRDye 800CW dual-labeling strategy for a peptide that targets the gelatinases, matrix metalloproteinases-2 and -9 (MMP-2/-9). MMPs are a family of enzymes that participate in extracellular matrix (ECM) degradation. Altered MMP expression has been reported in physiological conditions including rheumatoid arthritis, atherosclerosis, heart failure, pulmonary emphysema, and tumor growth and metastasis, and bone formation.20–25 A model of heterotopic ossification (HO) has been described using bone morphogenetic protein (BMP) signaling which activates MMP-9 and contributes to the new bone formation.26 While there are several strategies for targeting the entire family of MMPs, Koivunen et al. originally described several peptide sequences containing the HWGF motif that showed excellent inhibition of the gelatinases, MMP-2 and MMP-9.27 The CTTHWGFTLC (CTT) peptide showed the best inhibitory properties and led to its functionalization by others to generate different imaging probes with Iodine-125 (125I), Indium-111 (111In), and Copper-64 (64Cu) labels.28–30 In an attempt to generate a variant of the CTT peptide for fluorescence imaging, Wang et al. modified the N-terminus in order to attach a red-excitable fluorophore, Cy5.5, and to improve the in vivo stability of the peptide.31 This agent showed specific tumor uptake and served as the basis for the NIR/PET dual-labeled compound shown in Figure 1.
Figure 1.
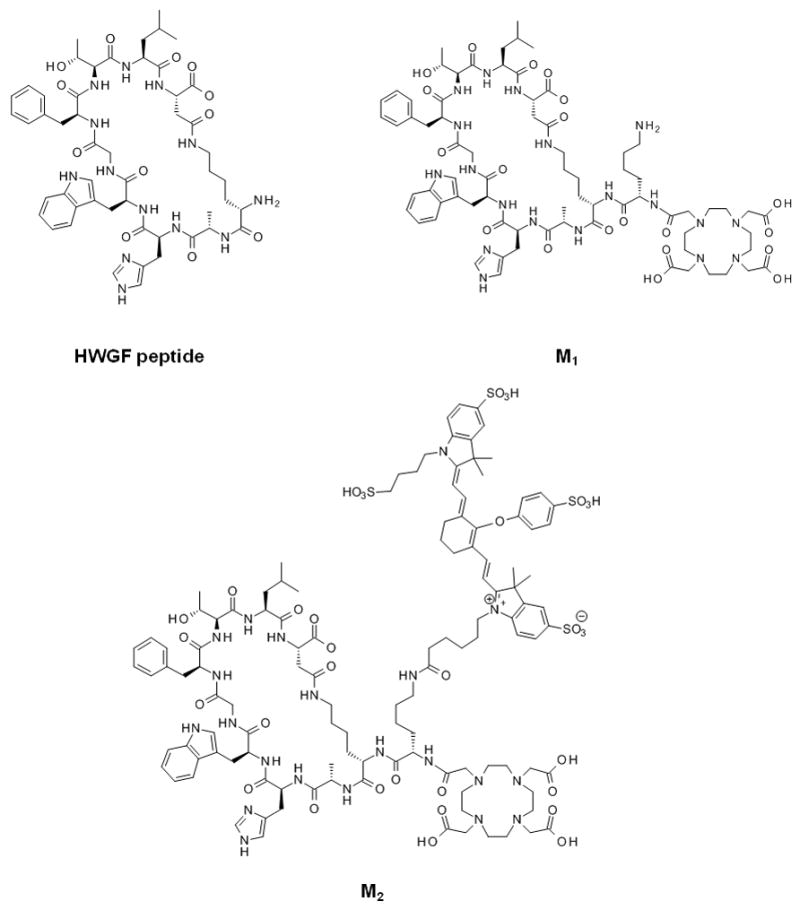
Chemical structures of HWGF peptide, DOTA-derivatized peptide (M1) and dual-conjugate (M2).
2. Results
2.1. Conjugation of IRDye 800CW
The dual-conjugate M2 was formed through attachment of IRDye 800CW to M1. Analysis of the spin column-purified sample by HPLC showed >90 % purity with a small amount of unreacted M1 present in the final product and was used for all subsequent studies. Figure 2 shows HPLC chromatograms with UV detection at 280 nm and fluorescence detection. Retention times of 5.9 min and 6.1 min were observed for M1 and M2 (at 280 nm), respectively, while fluorescence detection of IRDye 800CW had a retention time of 5.6 min. The HPLC data shows a single fluorescent peak for M2, confirming formation and purity of the dual-conjugate. Analysis by ESI-MS showed that the observed molecular weight (2555.9) was in excellent agreement with the calculated value (2555.92).
Figure 2.
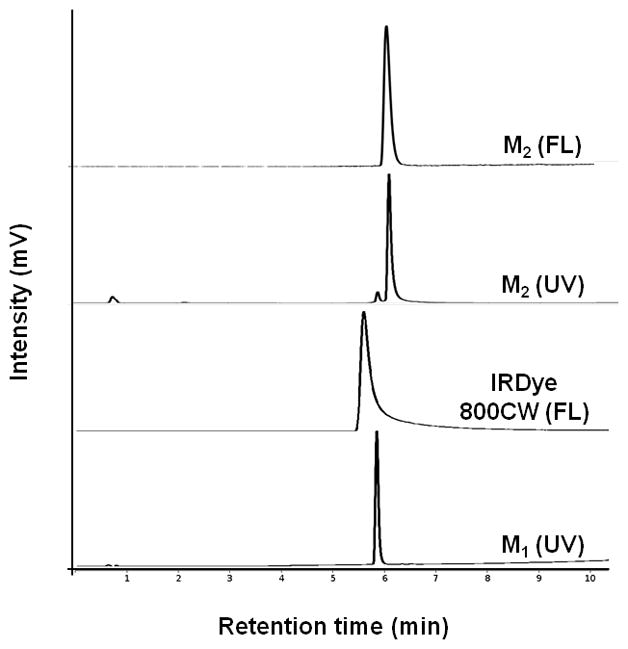
HPLC traces for M1, IRDye 800CW and M2. Traces were acquired at 280 nm or with a fluorescence detector.
2.2. MMP-9 binding assays
HWGF-peptides described by Wang et al. were further modified by DOTA and IRDye 800CW conjugation and enzyme binding was evaluated. Binding of M2 with MMP-9 could be visualized with 400 nM peptide, whereas a 10-fold increase in concentration of processed M2 was required for detection (Figure 3A,B). This indicates a detrimental effect from the labeling conditions which may be mainly due to the elevated heat required for Ga complexation by DOTA. natGa-M2 binding occurred at concentrations >400 μM (data not shown) showing aneven further reduction in binding affinity. In blocking studies, a 200 μM concentration of M1 was needed to produce a modest decrease in M2 signal and substantial inhibition was only observed at concentrations >1 mM (Figure 3C), thus no further steps were taken to remove the small amounts of 68Ga-M1 from the radiolabeled product.
Figure 3.
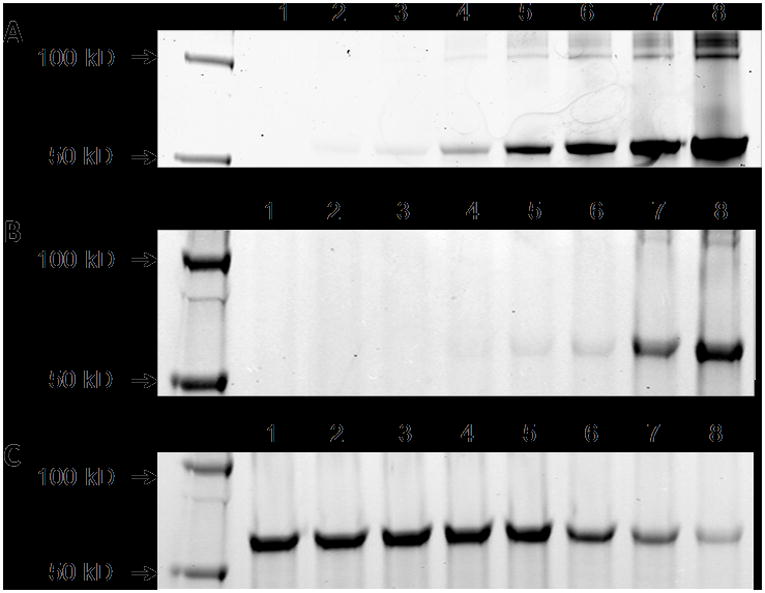
MMP-9 enzyme binding assays. Shown are the fluorescent scans acquired on the LICOR infrared imaging system. In all cases, 10 ng of purified MMP-9 was used and binding was resolved by SDS gel electrophoresis. The binding experiments shown are as follows: MMP-9 was incubated with increasing amounts of M2 (A) and processed M2 (B) at concentrations of 0, 0.1, 0.2, 0.4, 1, 2, 4 and 10 μM (lanes 1–8). For the blocking study (C), MMP-9 was incubated with M1 atconcentrations of 0.2, 0.5, 1, 2, 20, 200, 500 and 1000 μM (lanes 1–8). M2 (2 μM) was then added and incubated for 1 h. Bands resolved at 62 kDa correspond to M2 binding with MMP-9.
2.3. Radiochemistry
M2 was radiolabeled with 68Ga using the fractionation method and radiochemical purity (RCP) of >95% were achieved within 10 min, with 13 min selected as the optimal heating time. 68Ga-M2 was formed with high labeling efficiency over the range of peptide amounts investigated. Similar RCP were observed between the peptide amounts tested, therefore, 6 nmol (15 μg) was selected for radiolabeling experiments to achieve the highest specific activity and reaction conditions were optimized using 0.1 N NaOAc buffer. A specific activity of 42 Ci/mmol was used for imaging studies to ensure sufficient NIR signal. Co-injection of natGa-M2 and 68Ga-M2 on HPLC showed excellent correlation between the UV, fluorescent, and radiometric peaks (Figure 4). The log P value for 68Ga-M2 was calculated to be −2.09 ± 0.02.
Figure 4.
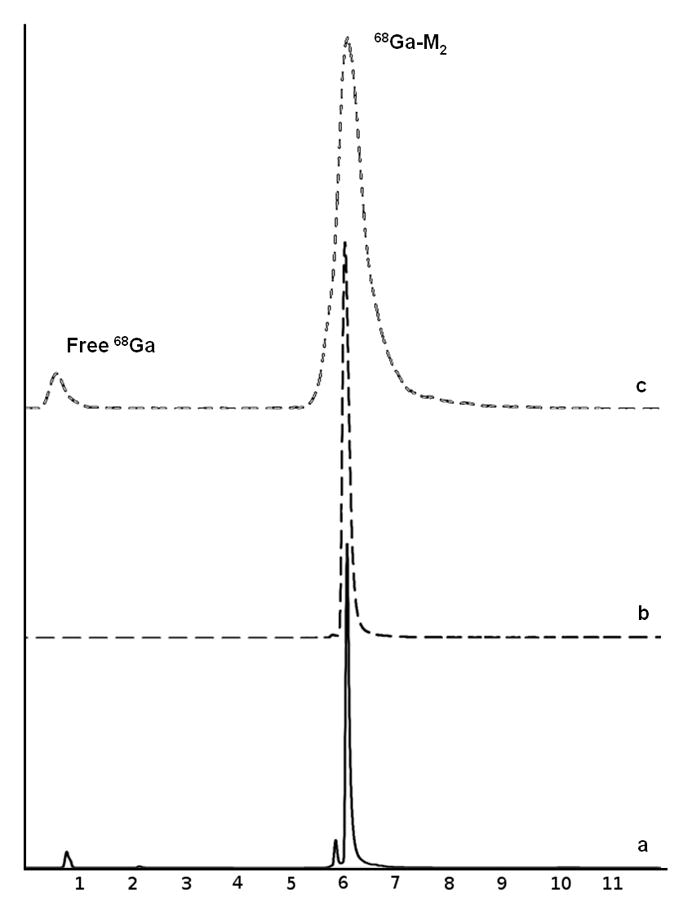
HPLC chromatograms for 68Ga-M2: UV at 280 nm (A), fluorescent (B), and radiometric (C).
2.4. Stability studies
Peptide and optical stability were evaluated in water and PBS at 4°C and room temperature. Over the 14 day incubation period, HPLC analysis revealed no significant degradation of peptide or fluorescent signal. Retention times were consistent and peak values did not significantly change based on quantification from the HPLC fluorescence detector. The effects of Ga-labeling conditions on the fluorescent properties of M2 were evaluated using radioactive and natGa and showed no deterioration in fluorescence signal of the dual-labeled agent. 68Ga-M2was stable in PBS, DTPA and mouse serum as indicated by >95% radiochemical purity at 3 h incubation (Figure 5).
Figure 5.
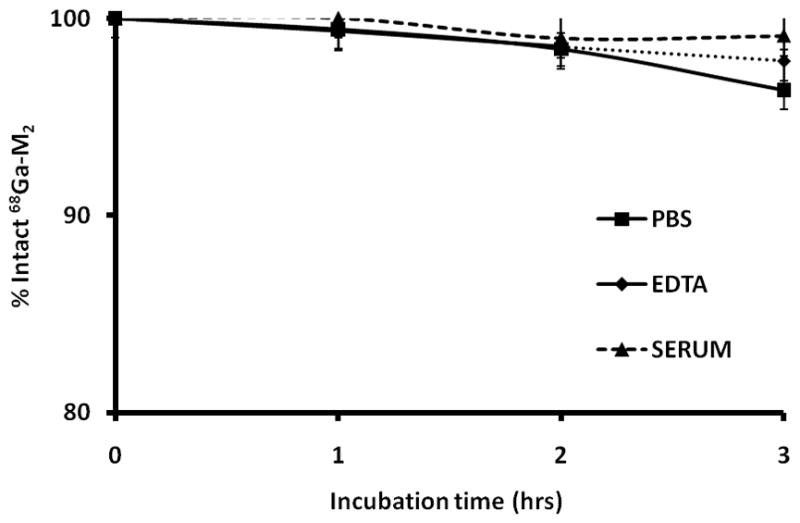
Stability studies for 68Ga-M2 in PBS, DTPA challenge and serum.
2.5. Fluorescent properties
Table 1 shows a summary of the spectral properties of IRDye 800CW, M2 and 68Ga-M2. The optical spectrum of M2 exhibited an absorbance maximum at 785 nm with an average extinction coefficient of 160,530 ± 3802 M−1cm−1 which is similar to that calculated for IRDye 800CW. Similarly, 68Ga-M2 showed maximum absorbance at 785 nm but reported a lower extinction coefficient value of 108,069 ± 7,918M−1cm−1. Using an excitation wavelength of 785 nm, M2 and 68Ga-M2 demonstrated fluorescence quantum yields (Φ) of 0.034 and 0.031, respectively, relative to ICG. These values were in reasonable agreement with the calculated quantum yield of IRDye 800CW (Φ =0.034) and attests to the efficiency of these IRDye 800-based peptide conjugates.
2.6. Quantification of BMP2 protein and activity
We have characterized BMP-2 expression from the adenovirus transduced cells in vitro and shown expression level as compared to control cells (Figure 6). W20-17 cells, a mouse bone marrow stem cell line, have increased alkaline phosphatase (AP) activity in response to BMP-2 treatment which corresponds to osteogenic differentiation of cells and BMP-2 functional activity in vitro. Using the known concentrations of BMP-2 with their corresponding AP activity readings, the transduced MRC5 cells had significantly more BMP-2 protein (0.74 ng) in comparison to the control cells (0.19 ng) (p < 0.0005).
Figure 6.
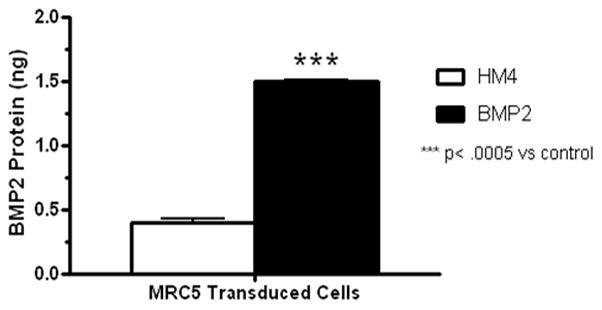
In vitro characterization of BMP-2 expression from adenovirus transduced cells.
2.7. In vivo imaging
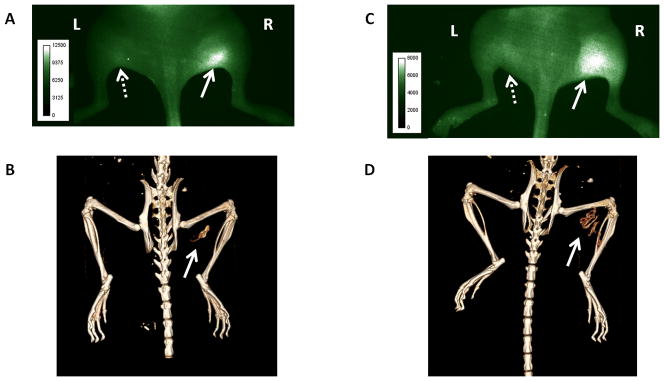
Figures 7A and 7C present typical NIR fluorescence images of mice at 4 days after implantation. The images were taken 18 h post-injection of 68Ga-M2 and show the dorsal view of mice with right hind limbs injected with AdBMP2 cells (solid arrows) and the left hind limbs injected with Adempty cells (dashed arrows). Localization of 68Ga-M2 was observed in the tissue region with BMP-2 producing cells with minimal fluorescence detected in the contralateral region. Figures 7B and 7D show CTs acquired for each mouse on day 11 post-implantation and provide evidence of new bone formation at the site corresponding to agent uptake on the NIR images. The findings demonstrate the feasibility of NIR imaging following 68Ga-labeling with consistent fluorescent signal obtained in all cases, and also indicate the mechanism of tracer accumulation may be related to MMP-9 expression during HO. In contrast with previous results using 64Cu-M226, PET imaging of 68Ga-M2 was confounded by the overwhelming signal from the nearby bladder and showed only negligible uptake at the target site (data not shown). Nonetheless, mouse imaging shows that in vivo NIR signal and targeting of MMP-9 was not perturbed by 68Ga labeling.
Figure 7.
Multimodality imaging of mice with fracture putty implants. Human fibroblast cells transduced with AdBMP2 were injected into the right hind limb of NOD/SCID mice. Mice were injected with 68Ga-M2 and imaged at 18 h. NIR images (A,C) were acquired on day 4 post-implantation and follow-up CTs (B,D) were taken on day 11. Solid arrows indicate site of new bone formation and agent accumulation. Dashed arrows designate control (empty cassette) injection sites in the contralateral limb.
3. Discussion
The use of hybrid imaging has gained acceptance clinically led by the advent and utility of combining the functional imaging of nuclear imaging with anatomical correlation by CT, and more recently with the introduction of PET/MRI. In the case of cancer, multimodal imaging platforms have improved diagnosis through co-registration of images, providing physicians with methods to identify lesions with better certainty and accuracy and tailor treatment strategies.32–33
As a result, tumors can be detected earlier and therapeutic intervention can be initiated prior to reaching advanced stages of the disease. An even further extension of multimodal imaging applications is intraoperative use for image-guided surgery by combining PET/NIR into a single agent. For example, an agent can be dual-labeled with a radionuclide and a NIR fluorophore and injected a day prior to surgery for PET/CT imaging for lesion detection and surgical planning. On the following day, the radioactivity will have decayed and the surgeon can remove the tumor using conventional methods, but could now incorporate intraoperative optical imaging to detect any existing NIR signal from residual tumor tissue. This directly permits visualization of any positive margins that still remain via molecular imaging and guides the surgeon on the potential need for further surgical intervention in real-time.
Since the sensitivities of CT and MRI are far lower than nuclear modalities, the design of agents bearing beacons for both nuclear and anatomical imaging is challenging. Conversely, optical imaging possesses similar sensitivity to nuclear imaging and the feasibility of a multimodality imaging approach with dual-labeled nuclear/NIR peptides has been described in a recent review.34 We have previously shown that dual-labeling of antibodies with a radionuclide and a NIR fluorophore can be achieved using various combinations of IRDye 800CW and metal chelates with either 111In or 64Cu.6–7, 14 In the case of antibodies, it is known that extended plasma circulation times are critical for therapy as they reduce the need for frequent dosing. However, for imaging this creates high levels of radioactivity present in the blood and liver and mandates later imaging time points to achieve lower background levels and sufficient contrast. Longer-lived radionuclides such as 111In and 64Cu allow for delayed imaging and clearing of the agent from circulation. Thus, these radiometals are widely-used for antibody imaging and have been adopted in many dual-labeling strategies.
Peptides are of particular importance in molecular imaging due to favorable pharmacokinetic properties, specific interaction with cell surface receptors, preparation in high specific activities, and robust manufacturing schemes by solid phase synthesis. The tendency of peptides to rapidly associate with receptors and clear from non-target sites permits imaging at early time points through the use of shorter-lived radionuclides such as 68Ga. As such, the use of 68Ga-peptides has experienced tremendous growth over the past decade.35–37 Since previous reports with dual-labeled nuclear/NIR agents predominantly used 64Cu or 111In, we examined the potential role of 68Ga in dual-labeling primarily because of the availability of the generator systems and the possibility of imaging at earlier time points due to the rapid clearance of 68Ga-tracers. Also, since 64Cu and 111In have milder radiolabeling conditions (pH > 5, heat at RT-80°C) than 68Ga (pH 3.5–4, heat at 80–98°C for DOTA chelates), we sought to examine the effects of 68Ga labeling conditions on the optical properties of IRDye 800CW. Multiple 68Ga labeling schemes have been reported which minimize the effect of 68Ge breakthrough and concentrate the generator eluate through fractionation or ion exchange methods, but a common thread shared by most DOTA-based schemes is the need to label in acidic conditions with elevated heating.38–41 Thus, we explored these effects on M2 to evaluate chemical and optical stability in response to 68Ga labeling using a modified version of the method described by Breeman et al.39
The two week stability study of M2 revealed no degradation of peptide or fluorescent signal at 4°C or room temperature. Initially, care was taken to shield samples from light, but the data showed no added benefit as samples exposed to ambient light showed identical fluorescent intensity by HPLC analysis. The stability of the dye was further confirmed when M2 was labeled with 68Ga/natGa and showed no alteration in fluorescence profile. Optical properties were assessed for IRDye 800CW, M2 and 68Ga-M2 and showed minor changes in extinction coefficient and quantum yield in response to conjugation and radiolabeling, indicating the ability of the dye to withstand synthesis conditions. A somewhat lower extinction coefficient was observed for 68Ga-M2 and may be attributable to the presence of acetate buffer in the reaction mixture post-radiolabeling, in contrast to the other samples which were analyzed in water. Nevertheless, the fluorescent signal from 68Ga-M2 was retained and confirmed by in vivo imaging. Stability studies looking at the radiochemical stability also served as opportunities to confirm fluorescence signal in response to the effects of 68Ga labeling over time.
The conserved fluorescent yield of the NIR fluorophore indicated no significant changes under any of the test conditions and allowed us to proceed to a model of HO where targeting specificity and fluorescent properties of 68Ga-M2 were assessed in vivo. Qualitatively, a high degree of similarity between pattern and location of new bone formation was observed on both NIR and CT images. Due to the inherent variability of the transduced cell implantations, various degrees of new bone formation were present, but in each case the anatomic rendering of new bone by CT corresponded to the location and relative shape of the functional image from NIR fluorescence. The mouse in Figures 7A,B had a smaller bone mass as shown by the day 11 CT, whereas the mouse in Figures 7C,D had much larger bone formation. Variation in size was evident as early as day 4 by NIRF imaging and preceded the anatomical appearance of bone by CT.
The rationale for acquiring PET images at 1 h post-injection was based on the rapid clearance of the tracer from circulation via the kidneys and absence of non-specific binding. However, the PET images did not support the NIR findings as poor accumulation of 68Ga-M2 was observed at sites of HO. Our prior experiments with 64Cu-M2 required delayed imaging (18 h post-injection) due to high levels of background, liver, and gut uptake at early time points, but could clearly delineate new bone formation by PET.26 The discrepancies between the PET findings in both studies may be attributed to changing of the radiometal from 64Cu to 68Ga. A study evaluating various somatostatin octapeptides radiolabeled with 67/68Ga, 111In, and Yttrium-90 (90Y) found that changing the radiometal does indeed cause variation in receptor-binding affinities, cellular internalization rates, hepatic clearance, and in vivo pharmacology.42 This can result from different conformations of metal-chelator complex, or differences in labeling schemes (i.e. pH or heat) that may impact the peptide adversely. We initially suspected that our imaging time point was premature for detecting tracer localization, however, upon examining the effect of nat/68Ga on M2 in enzyme binding assays, it was determined that the presence of metal played a key role in outcome of the PET studies. The enzyme binding assays showed a dramatic reduction in affinity of natGa-M2 compared with unlabeled M2. Further examination using M2 that was processed under labeling conditions in the absence of natGa/68Ga (i.e. acetate buffer and heat) revealed a 10-fold decrease in enzyme binding affinity compared with unprocessed agent. This may explain the differences between the NIR and PET images in the context of peptide amount used for labeling. In our studies, we selected 6 nmol of peptide 1) for sufficient labeling yield and 2) to provide fluorescent signal for delayed NIR imaging. Under these conditions there is a mixture of 68Ga-M2, which poorly binds MMP-9, along with a large excess of non-radioactive (processed) M2 that still retains binding potential. Therefore, the drastic differences in binding affinity and selection of imaging time points may have contributed to the positive signal observed in NIR imaging and the absence of signal on PET.
Our plan to substitute 68Ga for 64Cu did not yield comparable imaging findings. A change in composition to replace DOTA with 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) as the chelator may offer an effective alternative since NOTA possesses a different coordination structure and can label 68Ga at ambient temperature. The NOTA-bioconjugate may have a different affinity profile observed with the metal-bound complex and eliminate any negative effects that resulted from heating. Increasing the radiospecific activity may also minimize differential effects from radioactive and non-radioactive components. Nonetheless, the in vivo findings demonstrate the feasibility of generating and applying a dual-labeled probe with 68Ga and NIRF for multimodality PET/NIRF imaging.
4. Conclusions
We prepared a dual-labeled MMP-9 targeting peptide using 68Ga and IRDye 800CW. The addition of multiple reporters to a targeting agent has the ability to enhance each modality, but also requires additional testing and validation to ensure the agent is stable across biologic, chemical, radiochemical and optical criteria. The dual conjugate in this study showed excellent stability and retention of optical properties. 68Ga labeling methods were developed and optimized to yield a dual-labeled peptide for PET/NIRF imaging which had excellent radiochemical stability. This study showed that 68Ga labeling conditions did not adversely affect the optical properties of IRDye 800CW and that this strategy can be applied to other dual-labeled peptides and other NIR fluorophores. Modification with a different bifunctional chelator may be useful for further evaluation of the peptide by PET/CT/NIRF imaging in models of HO.
5. Experimental procedures
5.1. Reagents
All reagents were purchased from commercial sources and used without further purification. Chelex-100 resin was purchased from Bio-Rad Laboratories (Richmond, CA) and used with all aqueous buffers to ensure metal-free conditions. A commercially-available 68Ge/68Ga generator was purchased from Eckert & Ziegler (Berlin, Germany). Analytical high-performance liquid chromatography (HPLC) was performed on a Hitachi LaChrom system equipped with a 2.6 μm Kinetex C-18 column (Phenomenex, Torrance, CA) with a mobile phase of A = 0.1% TFA in H2O, B = 0.1% TFA in CH3CN; gradient, 0 min = 10% B, 10 min = 90% B; flow rate, 1 mL/min. Radio-thin-layer chromatography (radio-TLC) was carried out on a AR-2000 scanner (Bioscan, Washington, D.C.) using instant thin-layer chromatography (ITLC) strips and 1:1 methanol/0.1 M ammonium acetate. Molecular weight measurement was carried out by ESI on a Waters UPLC system equipped with a Waters PDA detector and a Waters TQD mass spectrometer.
5.2. Peptide synthesis
The DOTA (1,4,7,10-tetraazacyclotetradecane-N′,N″,N‴,N″″-tetraacetic acid)-modified cyclic MMP-targeting peptide (lactam 2,10) DOTA-KKAHWGFTLD (M1) was synthesized by New England Peptide (Gardner, MA) according to standard Fmoc-protocols. The peptide was made on a low load Rink amide resin (~0.3 mmol/g, NovaBiochem) using HCTU as the coupling agent. A three-dimensional orthogonal strategy developed by Kates et al.43 for making cyclic lactam peptides on resin with allyl-based side-chain protection was utilized. The C-terminal Asp was added as the Fmoc-Asp(OAll)-OH derivative, the Lys2 residue was added as Fmoc-Lys(Alloc)-OH, and the N-terminal Lys was added as Fmoc-Lys(Boc)-OH and the N-terminal Fmoc group was left on the peptide. After chain assembly, the allyl-based protection was remove from Lys2 and Asp10 using catalytic Pd(PPh3)4 and excess phenylsilane (~5 equiv relative to allyl-based protection). Cyclization was accomplished with PyClock (2 equiv) with DIPEA (4 equiv) in DMF. The N-terminal Fmoc was removed and DOTA-tris (t-Bu ester) (Macrocyclics, Dallas, TX) was added to the N-term. The peptide was cleaved with 90% TFA and purified by reversed-phase chromatography to >95% purity.
5.3. Conjugation of IRDye 800CW
In order to generate a peptide with two reactive amine groups for conjugation to DOTA and IRDye 800CW, we modified the HWGF peptide sequence described by Wang et al. by adding an additional lysine to the N-terminus of the peptide followed by DOTA-conjugation by solid-phase synthesis to yield the cyclic decapeptide M1. Conjugation of IRDye 800CW (LICOR Biosciences, Lincoln, NE) was performed by adding 1.52 mg (1.3 μmol) of dye to a stirred solution of M1 (2 mg, 1.3 μmol) in 0.1 M sodium phosphate buffer (pH 8.33). The reaction was carried out at 4oC overnight and the crude mixture was purified with a 2000 MWCO Sartorius Vivaspin spin column (VWR International) or semi-preparative-HPLC to yield M2.
5.4. MMP-9 binding assays
To examine the binding of the modified peptides, 10 ng of purified MMP-9 (AnaSpec, San Jose, CA) was preincubated with a) M2, b) M2 processed in non-radioactive labeling conditions (i.e. acetate buffer and heat), or c)natGa-M2 for 1 h at concentrations ranging from 100 nM-500 μM. Excess peptide was separated from enzyme-peptide complex by non-reducing SDS gel electrophoresis and the fluorescent signal of the bound complex was detected by scanning with the Odyssey Infrared Imaging System (LICOR Biosciences, Lincoln, NE). The binding of M1 and M2 were compared in a blocking study. MMP-9 (10 ng) was incubated with increasing concentrations of M1 (200 nM-1 mM) for 1 h. M2 (2 μM) was then added and incubated for 1 h followed by analysis using non-reducing SDS gel electrophoresis as described above.
5.5. Radiolabeling
68Ga-M2 was prepared by eluting a 10 mCi 68Ge/68Ga generator with 0.1 N HCl and collecting the 2 mL peak fraction. The eluted 68GaCl3 was buffered to pH 4 with solid NaOAc. The effects of peptide concentration, buffer concentration, and heating time were tested to determine optimal labeling conditions. Different peptide amounts were added to 0.1 M or 1.25 M NaOAc buffer (pH 4) with reaction volumes ranging from 150–710 μL. Samples were heated at 95°C for 5–20 min. For pharmacological studies, 1 N NaOH (30 μL) was used to adjust to pH 7. Radiochemical purity was assessed by radio-TLC and confirmed by radio-HPLC.
5.6. Synthesis of natGa-M2
natGa-M2 was synthesized based on conditions developed with 68Ga. M2 (150 μg, 60 nmol) was mixed with an excess of non-radioactive Ga and the reaction was heated at 95°C for 13 min. The crude mixture was HPLC purified and characterized by mass spectrometry.
5.7. Stability studies
The chemical, radiochemical and optical stability of radioactive and non-radioactive M2 were examined using a series of in vitro stability studies. M2 was incubated in PBS and water at room temperature and 4°C for 14 days, followed by HPLC analysis of peptide and fluorescent stability. To assess radiochemical stability, 68Ga-M2 was added to a solution of PBS or DTPA (500-fold excess), kept at room temperature for 1, 2 and 3 h, and analyzed by radio-HPLC. To evaluate serum stability, 150 μL of 68Ga-M2 was added to 50% mouse serum and incubated at 37°C. An 80 μL aliquot was taken at each of the above-mentioned time points and added to 160 μL ice-cold acetonitrile. The samples were centrifuged at 14,000 rpm for 5 min and the supernatant was collected, filtered and analyzed by radio-HPLC.
5.8. Determination of log P value
The lipophilicity of 68Ga-M2 was assessed by determination of the water-octanol partition coefficient. 1-Octanol (1 mL) was added to a solution of approximately 25 μCi of 68Ga-M2in water (1 mL) and the layers were vigorously mixed for 5 min at room temperature. The tubes were centrifuged at 14,000 rpm for 2–3 min. Three samples of 100 μL of each layer were taken in pre-weighed vials, re-weighed, and counted in a γ-counter (Wizard-2, Perkin Elmer). The partition coefficient was determined by calculating the ratio of counts per minute (cpm) in weight (g) of octanol/cpm in weight (g) of water and expressed as log P. At least three independent experiments were performed in triplicate to give the log P as the mean value ± standard deviation (SD).
5.9. Characterization of fluorescent properties
Fluorescence intensity of the fluorophores was determined using the Fluorolog Tau-3 Spectrofluorometer (Horiba JobinYvon, Edison, NJ) with excitation from a xenon arc lamp and absorbance was recorded using the DU-800 Spectrophotometer (Beckman Coulter, Brea CA). Fluorescence excitation and emission spectra were obtained at wavelengths of 785 and 830 nm respectively, with an integration time of 0.3 seconds for 1 μM solutions of IRDye 800CW, M2 and 68Ga-M2 (n=4). Fluorescence measurements for IRDye 800CW and M2 were performed in aqueous solution under ambient conditions, whereas 68Ga-M2 was prepared as previously described in NaOAc (pH 4) to provide an accurate representation of the 68Ga-labeling scheme. Extinction coefficients were determined from the slope of absorbance at 785 nm as a function of the concentration of serial dilutions of each agent. Fluorescence quantum yield was determined by the comparative method of Williams et al.44 using the quantum yield of ICG (ξ= 0.016) at 785/830 nm as a standard.
5.10. Quantification of BMP2 protein and activity from adenovirus-transduced MRC cells
The culture supernatant was collected after transduction with AdHM4 (control) or AdBMP2 in MRC5 cells at 2,500 vp/cell and BMP-2 protein levels assessed using an AP assay.45 W20-17 cells were assayed for AP activity 72 h after the addition of conditioned culture supernatant or various known concentrations of recombinant BMP-2. A Student’s t-test was applied to demonstrate significance.
5.11. Animal model
All animal studies were performed in accordance with the standards of Baylor College of Medicine (Houston, TX), Department of Comparative Medicine and The University of Texas Health Science Center (Houston TX), Center for Molecular Imaging after review and approval of the protocol by their respective Institutional Animal Care and Use Committee (IACUC) or Animal Welfare Committee (AWC). A murine model of BMP-2 induced HO was used by transducing human fibroblast (MRC5) cells with either Adempty (control) or AdBMP2, followed by intramuscular injection into each hind limb quadriceps muscle of non-obese diabetic/severely compromised immunodeficient (NOD/SCID) mice. Control transduced cells were injected into the contralateral limb. The data showed that elevated RNA protein expression and active MMP-9 content in tissues injected with AdBMP2 transduced cells were maximal 4 days after implantation and were significantly elevated when compared to the contralateral tissues receiving Adempty transduced cells.
5.12. In vivo imaging
Based on the findings from the ex vivo analysis, mice (n=3) were imaged on day 4 post-implantation with multimodality imaging to assess localization and in vivo fluorescence of M2 following 68Ga-labeling. For all imaging procedures, mice were anesthetized with 1% isoflurane. μPET/CT imaging was performed on day 4 post-implantation using a Siemens Inveon μPET/CT scanner (Siemens Medical, Knoxville, TN) with instrument parameters as previously described.14 The anesthetized mice were injected intravenously with 68Ga-M2 (200 μCi, 6 nmol) and μPET/CT images were acquired 1 h post-injection. To visualize the formation of ectopic bone, CT imaging was performed on days 4 and 11 post-implantation
NIR fluorescence images were acquired 18 h after intravenous administration of 68Ga-M2 using a custom-built fluorescence imaging systems previously described.2 Briefly, a field of view was illuminated with 785 nm of light from a laser diode, outfitted with a convex lens and diffuser to create a uniform excitation field. The fluorescence was collected through holographic and interference filters placed before a Nikon camera lens. The images were finally captured by an electron-multiplying charge-coupled device camera (PhotonMax 512; Princeton Instruments, Princeton, NJ) with 200 to 400 milliseconds of integration time. For acquisition of white-light images, the optical filters were removed, and a low-power lamp illuminated the subject. Image acquisition was accomplished by V++ software (Auckland, New Zealand).
Acknowledgments
The authors would like to acknowledge Robert P. Hammer (New England Peptide) for his technical assistance with design and synthesis of the peptide conjugate. This work was supported by Defense Advanced Research Projects Agency W911NF-09-1-0040 and Department of Defense W81XWH-07-0281 (EO-D) and W81XWH-07-1-025 (EO-D).
References
- 1.Culver J, Akers W, Achilefu S. J Nucl Med. 2008;49:169. doi: 10.2967/jnumed.107.043331. [DOI] [PubMed] [Google Scholar]
- 2.Houston JP, Ke S, Wang W, Li C, Sevick-Muraca EM. J Biomed Opt. 2005;10:054010. doi: 10.1117/1.2114748. [DOI] [PubMed] [Google Scholar]
- 3.Sevick-Muraca EM, Rasmussen JC. J Biomed Opt. 2008;13:041303. doi: 10.1117/1.2953185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall MV, Rasmussen JC, Tan I-CAMB, Adams KE, Wang X, Fife CE, Maus EA, Smith LA, Sevick-Muraca EM. The Open Surgical Oncology Journal. 2010;2:12. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams KE, Ke S, Kwon S, Liang F, Fan Z, Lu Y, Hirschi K, Mawad ME, Barry MA, Sevick-Muraca EM. J Biomed Opt. 2007;12:024017. doi: 10.1117/1.2717137. [DOI] [PubMed] [Google Scholar]
- 6.Sampath L, Wang W, Sevick-Muraca EM. J Biomed Opt. 2008;13:041312. doi: 10.1117/1.2953498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampath L, Kwon S, Ke S, Wang W, Schiff R, Mawad ME, Sevick-Muraca EM. J Nucl Med. 2007;48:1501. doi: 10.2967/jnumed.107.042234. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Dhara S, Banerjee SR, Byun Y, Pullambhatla M, Mease RC, Pomper MG. Biochem Biophys Res Commun. 2009;390:624. doi: 10.1016/j.bbrc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Liu S, Niu G, Wang F, Chen X. Mol Imaging. 2010;9:21. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Ke S, Wu Q, Charnsangavej C, Gurfinkel M, Gelovani JG, Abbruzzese JL, Sevick-Muraca EM, Li C. Mol Imaging. 2004;3:343. doi: 10.1162/15353500200404148. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka E, Choi HS, Humblet V, Ohnishi S, Laurence RG, Frangioni JV. Surgery. 2008;144:39. doi: 10.1016/j.surg.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Q, Liu S, Niu G, Chen K, Yan Y, Liu Z, Chen X. Amino Acids. 2010 doi: 10.1007/s00726-010-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa M, Regino CA, Seidel J, Green MV, Xi W, Williams M, Kosaka N, Choyke PL, Kobayashi H. Bioconjug Chem. 2009;20:2177. doi: 10.1021/bc900362k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampath L, Kwon S, Hall MA, Price RE, Sevick-Muraca EM. Transl Oncol. 2010;3:307. doi: 10.1593/tlo.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grotzinger C. Nat Biotechnol. 2001;19:327. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 16.Achilefu S, Jimenez HN, Dorshow RB, Bugaj JE, Webb EG, Wilhelm RR, Rajagopalan R, Johler J, Erion JL. J Med Chem. 2002;45:2003. doi: 10.1021/jm010519l. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Wang W, Wu Q, Ke S, Houston J, Sevick-Muraca E, Dong L, Chow D, Charnsangavej C, Gelovani JG. Nucl Med Biol. 2006;33:349. doi: 10.1016/j.nucmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Edwards WB, Xu B, Akers W, Cheney PP, Liang K, Rogers BE, Anderson CJ, Achilefu S. Bioconjug Chem. 2008;19:192. doi: 10.1021/bc700291m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura RH, Miao Z, Cheng Z, Gambhir SS, Cochran JR. Bioconjug Chem. 2010 doi: 10.1021/bc9003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YH, Lin IL, Tsay GJ, Yang SC, Yang TP, Ho KT, Hsu TC, Shiau MY. Clin Biochem. 2008;41:955. doi: 10.1016/j.clinbiochem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Lancelot E, Amirbekian V, Brigger I, Raynaud JS, Ballet S, David C, Rousseaux O, Le Greneur S, Port M, Lijnen HR, Bruneval P, Michel JB, Ouimet T, Roques B, Amirbekian S, Hyafil F, Vucic E, Aguinaldo JG, Corot C, Fayad ZA. Arterioscler Thromb Vasc Biol. 2008;28:425. doi: 10.1161/ATVBAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 22.Muroski ME, Roycik MD, Newcomer RG, Van den Steen PE, Opdenakker G, Monroe HR, Sahab ZJ, Sang QX. Curr Pharm Biotechnol. 2008;9:34. doi: 10.2174/138920108783497631. [DOI] [PubMed] [Google Scholar]
- 23.Boschetto P, Quintavalle S, Zeni E, Leprotti S, Potena A, Ballerin L, Papi A, Palladini G, Luisetti M, Annovazzi L, Iadarola P, De Rosa E, Fabbri LM, Mapp CE. Thorax. 2006;61:1037. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deryugina EI, Quigley JP. Cancer Metastasis Rev. 2006;25:9. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 25.Manduca P, Castagnino A, Lombardini D, Marchisio S, Soldano S, Ulivi V, Zanotti S, Garbi C, Ferrari N, Palmieri D. Bone. 2009;44:251. doi: 10.1016/j.bone.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Rodenberg E, Azhdarinia A, Lazard Z, Hall M, Kwon S, Wilganowski N, Merched-Sauvage M, Salisbury EA, Davis AR, Sevick-Muraca EM, Olmsted-Davis E. 2010 Manuscript submitted for publication (copy on file with author) [Google Scholar]
- 27.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R. Nat Biotechnol. 1999;17:768. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 28.Kuhnast B, Bodenstein C, Haubner R, Wester HJ, Senekowitsch-Schmidtke R, Schwaiger M, Weber WA. Nucl Med Biol. 2004;31:337. doi: 10.1016/j.nucmedbio.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Sprague JE, Li WP, Liang K, Achilefu S, Anderson CJ. Nucl Med Biol. 2006;33:227. doi: 10.1016/j.nucmedbio.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Hanaoka H, Mukai T, Habashita S, Asano D, Ogawa K, Kuroda Y, Akizawa H, Iida Y, Endo K, Saga T, Saji H. Nucl Med Biol. 2007;34:503. doi: 10.1016/j.nucmedbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Shao R, Wu Q, Ke S, McMurray J, Lang FF, Jr, Charnsangavej C, Gelovani JG, Li C. Mol Imaging Biol. 2009;11:424. doi: 10.1007/s11307-009-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poeppel TD, Krause BJ, Heusner TA, Boy C, Bockisch A, Antoch G. Eur J Radiol. 2009;70:382. doi: 10.1016/j.ejrad.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 33.Cronin CG, Swords R, Truong MT, Viswanathan C, Rohren E, Giles FJ, O’Dwyer M, Bruzzi JF. AJR Am J Roentgenol. 2010;194:W91. doi: 10.2214/AJR.09.2637. [DOI] [PubMed] [Google Scholar]
- 34.Kuil J, Velders AH, van Leeuwen FW. Bioconjug Chem. 2010;21:1709. doi: 10.1021/bc100276j. [DOI] [PubMed] [Google Scholar]
- 35.Hofmann M, Maecke H, Borner R, Weckesser E, Schoffski P, Oei L, Schumacher J, Henze M, Heppeler A, Meyer J, Knapp H. Eur J Nucl Med. 2001;28:1751. doi: 10.1007/s002590100639. [DOI] [PubMed] [Google Scholar]
- 36.Maecke HR, Hofmann M, Haberkorn U. J Nucl Med. 2005;46(Suppl 1):172S. [PubMed] [Google Scholar]
- 37.Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, Papathanasiou ND, Pepe G, Oyen W, De Cristoforo C, Chiti A. Eur J Nucl Med Mol Imaging. 2010;37:2004. doi: 10.1007/s00259-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 38.Velikyan I, Beyer GJ, Langstrom B. Bioconjug Chem. 2004;15:554. doi: 10.1021/bc030078f. [DOI] [PubMed] [Google Scholar]
- 39.Breeman WA, de Jong M, de Blois E, Bernard BF, Konijnenberg M, Krenning EP. Eur J Nucl Med Mol Imaging. 2005;32:478. doi: 10.1007/s00259-004-1702-y. [DOI] [PubMed] [Google Scholar]
- 40.Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, Jahn M, Jennewein M, Rosch F. J Nucl Med. 2007;48:1741. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 41.Meyer GJ, Macke H, Schuhmacher J, Knapp WH, Hofmann M. Eur J Nucl Med Mol Imaging. 2004;31:1097. doi: 10.1007/s00259-004-1486-0. [DOI] [PubMed] [Google Scholar]
- 42.Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, Maecke H. Eur J Nucl Med Mol Imaging. 2007;34:982. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 43.Kates SA, Sole NA, Johnson CR, Hudson D, Barany G, Albericio F. Tetrahedron Letters. 1993;34:1549. [Google Scholar]
- 44.Williams ATR, Winfield SA, JNM Analyst. 1983;108:1067. [Google Scholar]
- 45.Olmsted EA, Blum JS, Rill D, Yotnda P, Gugala Z, Lindsey RW, Davis AR. Journal of Cellular Biochemistry. 2001;82:11. doi: 10.1002/jcb.1106. [DOI] [PubMed] [Google Scholar]