Abstract
Background: Chronic kidney disease is a major worldwide problem. Although epidemiologic and experimental studies suggest that n–3 long-chain polyunsaturated fatty acid (n–3 LCPUFA) supplementation may prevent or slow the progression of kidney disease, evidence from clinical trials is inconsistent.
Objective: The objective was to combine evidence from controlled clinical trials to assess the effect of n–3 LCPUFA supplementation on the change in urine protein excretion (UPE) and on glomerular filtration rate (GFR).
Design: We performed a meta-analysis of clinical trials that tested the effect of n–3 LCPUFA supplementation on UPE, a marker of kidney damage, and on GFR, a marker of kidney function. A random-effects model was used to pool SD effect size (Cohen's d) across studies.
Results: Seventeen trials with 626 participants were included in the meta-analysis. Most trials focused on patients with a single underlying diagnosis: IgA nephropathy (n = 5), diabetes (n = 7), or lupus nephritis (n = 1). The dose of n–3 LCPUFAs ranged from 0.7 to 5.1 g/d, and the median follow-up was 9 mo. In the pooled analysis, there was a greater reduction in UPE in the n–3 LCPUFA group than in the control group: Cohen's d for all trials was −0.19 (95% CI: −0.34, −0.04; P = 0.01). In a patient with 1 g UPE/d , this corresponds to a reduction of 190 mg/d. Effects on GFR were reported in 12 trials. The decline in GFR was slower in the n–3 LCPUFA group than in the control group, but this effect was not significant (0.11; 95% CI: −0.07, 0.29; P = 0.24).
Conclusions: In our meta-analysis, use of n–3 LCPUFA supplements reduced UPE but not the decline in GFR. However, small numbers of participants in trials, different methods of assessing proteinuria and GFR, and inconsistent data reporting limit the strength of these conclusions. Large, high-quality trials with clinical outcomes are warranted.
INTRODUCTION
Chronic kidney disease (CKD) is increasing in prevalence in the United States. The most common causes of CKD are diabetes and hypertension. However, epidemiologic and clinical studies examining nutrient-based risk factors suggest that the dietary intake of fatty acids may prevent CKD and slow its progression (1). Because the intake of essential fatty acids is low in the US diet, many trials have been conducted to determine the effects of dietary supplementation with long-chain fatty polyunsaturated fatty acids (n–3 LCPUFAs), also known as omega-3 fatty acids, including docosahexaenoic acid (DHA, 22:6n−3) and eicosapentaenoic acid (EPA, 20:5n−3) on CKD progression.
In animal models, dietary supplementation with n–3 LCPUFAs at low doses reduces the severity of kidney disease after exposure to nephrotoxic agents (2) and slows the progression of kidney disease (3). In human studies, n–3 LCPUFA supplementation has been shown to modify intermediaries in the pathogenesis of kidney disease, ie, lowering blood pressure, oxidative stress, and inflammation and improving endothelial function (4). In cross-sectional and longitudinal observational studies in humans, n–3 LCPUFA intake was associated with a lower risk of macroalbuminuria in adults with diabetes (5) and with a lower age-related decline in kidney function (6).
Randomized trials of n–3 LCPUFA supplementation on the progression of kidney disease have been conducted, but the results have been inconsistent (7). Potential reasons for the inconsistencies include differences in the pathology of underlying kidney disease or in the prevalence of risk factors for disease progression, differences in outcome assessment, small sample sizes, and limitations in study quality (eg, noncompliance or use of concurrent medications).
The objective of this meta-analysis was to combine evidence from controlled clinical trials to assess the effect of n–3 LCPUFA supplementation on change in urine protein excretion, a well-established clinical marker of kidney damage that is a common outcome in most trials, and on glomerular filtration rate (GFR), a marker of kidney function.
METHODS
We searched the published literature for clinical trials that tested the effects of n–3 LCPUFA supplementation on urine protein excretion. We performed a Medline search using the medical subject headings fish oil, omega 3 fatty acids, kidney (renal) disease, and clinical trials. Details of the search terms are provided in Appendix A. The search period was from 1966 to October 2008. The Medline search included a search of the Cochrane database of randomized controlled trials, review of reference lists from original research, and a search of review articles.
Our prespecified inclusion criteria were as follows: 1) controlled clinical trials; 2) use of n–3 LCPUFAs, including eicosapentaenoic acid (EPA) and/or docosahexaenoic acid (DHA) or fish oils as the active treatment; 3) presence of a control or placebo group; and 4) reported effects on urine protein (or albumin) excretion. We excluded trials of patients who had undergone organ transplant or had end-stage kidney disease.
Three investigators (ERM, MM, and SPJ) independently abstracted the articles. Disagreements or uncertainties were adjudicated by consensus. From each article, we abstracted 1) characteristics of the study population, including sample size, country, age, sex, and targeted kidney disease condition; 2) characteristics of the trial design, including dose and duration of n–3 LCPUFA supplementation; 3) measurements of urine protein excretion; and 4) measured GFR or estimated GFR (eGFR) from creatinine-based estimating equations or 24-h urine creatinine clearance. The sum of the amount of EPA and DHA within intervention capsules was used to reflect the daily supplemental dose of n–3 LCPUFAs (mg/d) in each trial.
The method for evaluating urine protein excretion and GFR varied across studies. As a result, we calculated effect sizes as standardized measures of change (SD units) that are independent of the measurement unit used in individual trials. Effect sizes (Cohen's d) were computed by dividing the difference between mean change in the intervention and the mean change in the control group by their pooled SDs (8). Effect sizes are interpreted as the relative effect of n–3 LCPUFA supplementation on change in urine protein excretion compared with the change in the control group. Pooled estimates and 95% CIs were calculated by using a random-effects meta-analysis model using STATA 9.2 (STATA Corp, College Station, TX).
In each study, we used intention-to-treat analyses, if available; otherwise we used data reported on trial participants that returned for follow-up. For trials with a crossover design, change in protein excretion was determined between baseline and end of first period. In a sensitivity analysis, randomized controlled double-blind studies (high-quality trials) were analyzed separately from unblinded trials (eg, the control group did not receive capsules). Retention rates are reported as the percentage of participants within each trial who came in for end-of study measurements. We report participant retention as the percentage of participants for whom end-of study measurements were made.
RESULTS
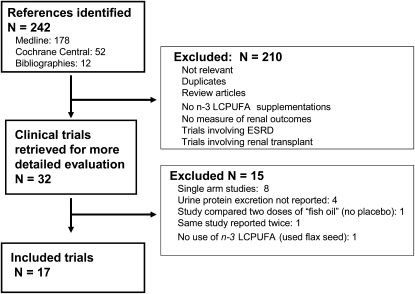
The trial selection process that resulted from our systematic literature review is shown in Figure 1. Seventeen clinical trials met our inclusion criteria. These trials, reported between 1986 and 2007, included a total of 626 participants (Table 1). The mean age in the trials ranged from 33 to 64 y. Ten trials were double-blinded randomized controlled trials, whereas the remainder did not use a placebo capsule, used concurrent controls (not randomized), or a blinded assessment of outcomes was not reported. Follow-up ranged from 6 wk to 48 mo (median: 9 mo). Most trials focused on patients with a single underlying diagnoses: IgA nephropathy (5 trials), diabetes (7 trials), and lupus nephritis (1 trial). Three trials enrolled patients with kidney disease of mixed etiologies, and one trial did not report the underlying kidney disease of the study participants. The dose of n–3 LCPUFAs (EPA and/or DHA) ranged from 0.7 to 5.1 g/d. The eGFR ranged from 24 to 116 mL · min−1 · 1.73 m−2 across studies (median: 73 mL · min−1 · 1.73 m−2).
FIGURE 1.
Flow diagram of trial selection process resulting from systematic search. ESRD, end-stage renal disease; LCPUFA, long-chain polyunsaturated fatty acids.
TABLE 1.
Characteristics of clinical trials of n−3 long-chain polyunsaturated fatty acid supplementation in patients with kidney disease, by year of publication1
| Trial | Country | Type of kidney disease | n | Mean age y | Total dose of EPA and/or DHA | eGFR2 | Month | Randomization3 | Placebo capsule | Design | Percentage of participants completing study |
| Haines et al, 1986 (15) | United Kingdom | IDDM | 41 | 42 | 4.6 | mL/min | 1.5 | Yes | Olive oil | Parallel | 93 |
| Bennett et al, 1989 (16) | United States | IgA nephropathy | 37 | 39 | 3.0 | 78 | 24 | Yes | None | Parallel | 97 |
| Jensen et al, 1989 (17) | Denmark | IDDM | 18 | 37 | 4.6 | 81 | 2 | Yes | Olive oil | Crossover | 94 |
| Hamazaki et al, 1990 (18) | Japan | IDDM or NIDDM | 26 | 64 | 1.8 | — | 6 | No | None | Parallel | 100 |
| Clark et al, 1993 (11) | Canada | Lupus | 26 | 39 | 4.4 | 75 | 27 | Yes | Olive oil | Crossover | 81 |
| Gentile et al, 1993 (19) | Italy | CKD, protein > 2.5 g/d | 20 | 45 | 1.5 | 64 | 9 | Yes | None | Crossover | 100 |
| Pettersson et al, 1994 (20) | Sweden | IgA nephropathy | 32 | 41 | 5.1 | 66 | 6 | Yes | Corn oil | Parallel | 94 |
| Shimizu et al, 1995 (21) | Japan | NIDDM | 45 | 62 | 0.9 | — | 12 | No | None | Parallel | 100 |
| Rossing et al, 1996 (22) | Denmark | IDDM | 36 | 33 | 4.6 | 112 | 12 | Yes | Olive oil | Parallel | 83 |
| Cappelli et al, 1997 (23) | Italy | Mixed etiolgy | 20 | 52 | 3.4 | 29 | 12 | Yes | None | Parallel | 100 |
| Lungershausen et al, 1997 (24) | Australia | IDDM or NIDDM | 32 | 55 | 3.4 | 116 | 3 | Yes | Corn oil | Parallel | 100 |
| Donadio et al, 1999 (25) | United States | IgA nephropathy | 106 | 37 | 3.2 | 82 | 24 | Yes | Olive oil | Parallel | 71 |
| Branten et al, 2002 (26) | Netherlands | IgA nephropathy | 25 | 38 | 3.0 | 45 | 12 | No | None | Parallel | 100 |
| Alexopoulos et al, 2004 (27) | Greece | IgA nephropathy | 34 | 40 | 1.4 | 46 | 48 | Yes | None | Parallel | 82 |
| Svensson et al, 2004 (28) | Denmark | Mixed etiolgy | 64 | 59 | 2.4 | 24 | 2 | Yes | Olive oil | Parallel | 91 |
| Zeman et al, 2006 (12) | Europe | NIDDM | 24 | 49 | 3.1 | — | 6 | No | Olive oil | Crossover (sequential) | 100 |
| Theobald et al, 2007 (29) | United Kingdom | None reported | 38 | 49 | 0.7 | — | 3 | Yes | Olive oil | Crossover | 98 |
| Median (all trials) | 34 | 42 | 3.1 | 73 | 9 | 97 | |||||
| Range (minimum, maximum) | (18, 106) | (33, 64) | (0.7, 5.1) | (24, 116) | (1.5, 48) | (71, 100) |
IDDM, insulin-dependent (type 1) diabetes; NIDDM, non-insulin-dependent (type 2) diabetes; eGFR, estimated glomerular filtration rate; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; CKD, chronic kidney disease.
Dashes represent GFR that was not reported or unable to be estimated.
Trials that were not randomized made comparisons with concurrent control patients.
Retention rates within trials were high (median: 97% of participants had end-of-study measurements). Five of 17 trials reported side effects from supplementation. Three of these trials did not quantify the proportion of patients experiencing these symptoms [eg, “fishy aftertaste” in some patients (25), “occasional smell of fish on belching” (20), “eructation with fish taste” (27)]. One trial reported gastrointestinal side effects in 11% of the intervention group (28), and one trial reported that 17% of those assigned to fish-oil supplements had nausea at some point in the trial (22). There was no evidence of a differential dropout rate between the n–3 LCPUFA and control groups within trials.
The outcome variable of urine protein excretion was reported differently in trials (Table 2): protein in g/d (5 studies), albumin in μg/min (3 studies), albumin as a percentage of excretion per hour, albumin as g/d, albumin as mg/g creatinine, albumin as mg/d, protein as μg/creatinine clearance, protein as g/L, albumin as mg/L, and albumin as μg/creatinine mmol (one study each). The method for assessing GFR varied across trials (Table 3). Several studies measured GFR using one of several “gold standard” radioisotope determinations of kidney function (10, 19, 21, 26). Other studies used either 24-h creatinine clearance rates (15, 16, 18, 23, 25) or creatinine-based estimating equations (22, 24, 27). This variation in trial outcomes provided the rational for calculating individual trial effect size in SD units and overall effects using the Cohen's d method. The Cohen's d value is the effect size in SD units. For example, in a trial in which baseline urine protein excretion is 1000 mg/d, an effect size (Cohen's d) of −0.20 means that n–3 LCPUFA supplementation reduced urine protein excretion compared with the control by 200 mg/d (20%).
TABLE 2.
Urine protein excretion at baseline by group assignment and difference in mean change in urine protein excretion in the n−3 long-chain polyunsaturated fatty acid (LCPUFA) treatment (T) minus control (C) group (Δm) ± pooled SD
| Baseline urine protein excretion |
Difference in mean change in urine protein excretion (n−3 LCPUFA minus control) |
||||||||
| n−3 LCPUFA |
Control |
||||||||
| Trial | Urine protein | nT | Baseline mean ± SDT | End mean ± SDT | nC | Baseline mean ± SDC | End mean ± SDC | Δm1 | Pooled SD2 |
| Haines et al, 1986 (15) | Albumin (μg/min) | 19 | 6.09 | 7.45 | 22 | 6.70 | 6.71 | 0.223 | 1.523 |
| Bennett et al, 1989 (16) | Protein (g/d) | 17 | 1.86 ± 2.00 | 1.35 ± 1.68 | 20 | 2.05 ± 2.49 | 1.51 ± 1.60 | 0.03 | 2.81 |
| Jensen et al, 1989 (17) | Albumin (%/h) | 18 | 8.7 ± 2.1 | 6.9 ± 2.5 | 18 | 8.7 ± 2.1 | 8.5 ± 2.5 | −1.64 | 3.3 |
| Hamazaki et al, 1990 (18) | Albumin (mg/g creatinine) | 16 | 65 ± 2 | 36 ± 2 | 10 | 55 ± 3 | 54 ± 3 | −0.574,5 | 1.325 |
| Clark et al, 1993 (11) | Protein (mg/d) | 13 | 1424 ± 2029 | 897 ± 1614 | 13 | 1271 ± 2049 | 1548 ± 2139 | −804 | 2783 |
| Gentile et al, 1993 (19) | Albumin (g/d) | 20 | 5.43 ± 1.74 | 3.96 ± 1.43 | 20 | 5.43 ± 1.74 | 4.20 ± 2.19 | −0.24 | 2.54 |
| Pettersson et al, 1994 (20) | Protein (g/d) | 15 | 1.8 ± 1.2 | 1.7 ± 0.9 | 17 | 2.0 ± 1.4 | 1.8 ± 1.2 | 0.1 | 1.7 |
| Shimizu et al, 1995 (21) | Albumin (mg/g creatinine) | 29 | 447 ± 680 | 59 ± 67 | 16 | 200 ± 240 | 114 ± 50 | −3024 | 570 |
| Rossing et al, 1996 (22) | Albumin (g/d) | 14 | 0.77 ± 1.21 | 0.94 ± 1.20 | 15 | 0.71 ± 1.30 | 0.82 ± 1.30 | 0.055 | 1.235 |
| Cappelli et al, 1997 (23) | Protein (μg/mL creatinine clearance) | 10 | 43.2 ± 18.7 | 56.3 ± 24.5 | 10 | 41.3 ± 23.9 | 68.0 ± 29.4 | −13.6 | 34.5 |
| Lungershausen et al, 1997 (24) | Albumin (μg/min) | 16 | 60 ± 44 | 70 ± 64 | 16 | 83 ± 48 | 95 ± 64 | −2 | 79 |
| Donadio et al, 1999 (25) | Protein (g/d) | 55 | 2.55 ± 1.71 | 2.32 ± 1.716 | 51 | 3.22 ± 3.21 | 3.12 ± 3.216 | −0.13 | 3.606 |
| Branten et al, 2002 (26) | Albumin (μg/min) | 12 | 1594 ± 984 | 951 ± 693 | 13 | 1509 ± 782 | 947 ± 977 | −81 | 1229 |
| Alexopoulos et al, 2004 (27) | Protein (g/d) | 14 | 2.0 ± 1.9 | 0.8 ± 0.4 | 14 | 1.6 ± 1.4 | 0.9 ± 0.6 | −0.54 | 1.7 |
| Svensson et al, 2004 (28) | Protein (g/L) | 28 | 0.7 ± 1.0 | 0.6 ± 1.0 | 30 | 0.6 ± 0.8 | 0.8 ± 1.0 | −0.3 | 1.3 |
| Zeman et al, 2006 (12) | Albumin (mg/L) | 24 | 27.0 ± 41.2 | 20.5 ± 15.7 | 24 | 27.0 ± 41.2 | 29.5 ± 29.9 | −9.04 | 47.6 |
| Theobald et al, 2007 (29) | Albumin (μg/mmol creatinine) | 38 | 0.59 ± 1.48 | 0.51 ± 1.66 | 38 | 0.53 ± 0.97 | 0.60 ± 1.36 | −0.275 | 0.505 |
Δm is equal to the end-study mean of the treatment (eicosapentaenoic acid and/or docosahexaenoic acid) group minus the end-study mean of the control group; the trial by Haines et al (15) reported the percentage difference only.
Pooled SD is equal to the square root of {[(nT − 1)SDT2 + (nC – 1)SDC2]/(nT + nC – 2)}.
Based on reported difference, as a percentage of change.
Individual trial reports significant reduction in urine protein excretion with n–3 LCPUFA supplementation.
Δm and pooled SD are based on logarithmic conversion of trial-reported geometric means.
Reported baseline SD used for pooled estimate.
TABLE 3.
Measured glomerular filtration rate (GFR), estimated GFR (eGFR), or creatinine clearance at baseline by group assignment and difference in mean change in measured GFR, eGFR, or creatinine clearance in the n−3 long-chain polyunsaturated fatty acid (LCPUFA) treatment (T) minus control (C) group (Δm) ± pooled SD
| Baseline eGFR |
Difference in mean change in eGFR (n–3 LCPUFA minus control) |
||||||||
| n–3 LCPUFA |
Control |
||||||||
| Trial | Method of estimating GFR1 | nT | Baseline mean ± SDT | End mean (SDT) | nC | Baseline mean ± SDC | End mean ± SDT | Δm2 | Pooled SD3 |
| Haines et al, 1986 (15) | NA | 19 | — | — | 22 | — | — | — | — |
| Bennett et al, 1989 (16) | 24-h creatinine clearance (mL/min) | 17 | 80 ± 16 | 57 ± 17 | 20 | 76 ± 18 | 55 ± 14 | −2 | 23 |
| Jensen et al, 1989 (17) | 24-h creatinine clearance (mL · min−1 · 1.73 m−2) | 18 | 81 ± 21 | 81 ± 21 | 18 | 81 ± 21 | 80 ± 21 | 1 | 30 |
| Hamazaki et al, 1990 (18) | NA | 16 | — | — | 10 | — | — | — | — |
| Clark et al, 1993 (11) | Tc-DPTA GFR (mL · min−1 · 1.73 m−2) | 13 | 76 ± 31 | 75 ± 30 | 13 | 73 ± 38 | 69 ± 35 | 3 | 48 |
| Gentile et al, 1993 (19) | 24-h creatinine clearance (mL · min−1 · 1.73 m−2) | 20 | 64 ± 38 | 59 ± 34 | 20 | 64 ± 38 | 56 ± 31 | 3 | 50 |
| Pettersson et al, 1994 (20) | 51 cr-EDTA GFR (mL · min−1 · 1.73 m−2) | 15 | 63 ± 22 | 59 ± 21 | 17 | 69 ± 25 | 68 ± 27 | −3 | 34 |
| Shimizu et al, 1995 (21) | NA | 29 | — | — | 16 | — | — | — | — |
| Rossing et al, 1996 (22) | 51 cr-EDTA GFR (mL · min−1 · 1.73 m−2) | 14 | 116 ± 30 | 105 ± 30 | 15 | 108 ± 25 | 103 ± 30 | −6 | 41 |
| Cappelli et al, 1997 (23) | CCr (Cockcroft-Gault) (mL/min) | 10 | 28 ± 15 | 25 ± 15 | 10 | 29 ± 13 | 16 ± 14 | 10 | 20 |
| Lungershausen et al, 1997 (24) | 24-h creatinine clearance (mL · min−1 · 1.73 m−2) | 16 | 120 ± 32 | 118 ± 36 | 16 | 112 ± 52 | 108 ± 48 | 2 | 61 |
| Donadio et al, 1999 (25)4 | eGFR5 (mL · min−1 · 1.73 m−2) | 55 | 83 ± 30 | 82 ± 306 | 51 | 81 ± 27 | 66 ± 276 | 14 | 40 |
| Branten et al, 2002 (26) | 24-h creatinine clearance (mL · min−1 · 1.73 m−2) | 12 | 44 ± 12 | 38 ± 15 | 13 | 46 ± 14 | 40 ± 14 | 0 | 20 |
| Alexopoulos et al, 2004 (27)4 | 51 cr-EDTA GFR (mL · min−1 · 1.73 m−2) | 14 | 46 ± 12 | 41 ± 13 | 14 | 45 ± 23 | 34 ± 30 | 6 | 30 |
| Svensson et al, 2004 (28) | eGFR5 (mL · min−1 · 1.73 m−2) | 28 | 24 ± 13 | 24 ± 14 | 30 | 24 ± 10 | 23 ± 10 | 1 | 17 |
| Zeman et al, 2006 (12) | NA | 24 | — | — | 24 | — | — | — | — |
| Theobald et al, 2007 (29) | NA | 38 | — | — | 38 | — | — | — | — |
NA, not available; Tc-DPTA, technetium-labeled diethylenetriamine pentaacetate; cr-EDTA; creatinine-EDTA; CCr, creatinine clearance rate.
Δm is equal to the end-study mean of the treatment (eicosapentaenoic acid and/or docosahexaenoic acid) group minus the end-study mean of the control group; the trial by Haines et al (15) reported the percentage difference only.
Pooled SD is equal to the square root of {[(nT − 1)SDT2 + (nC – 1)SDC2]/(nT + nC – 2)}.
Individual trial reports a significant reduction in GFR in the n−3 LCPUFA supplement group.
eGFR estimated by modified MDRD (Modification of Diet in Renal Disease) equation based on race, sex, and serum creatinine data reported in the trials.
End means estimated from reported rate of decline in GFR at 2 y.
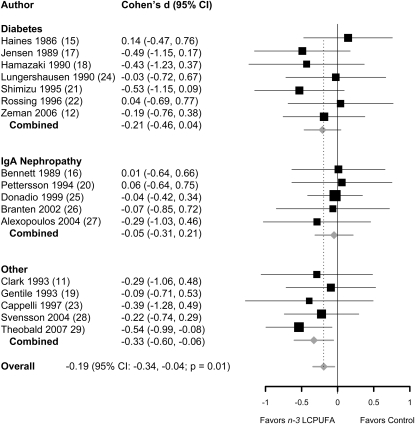
Effects of n–3 LCPUFA supplementation on urine protein excretion was greater in the n–3 LCPUFA supplementation group than in the control group in 13 of 17 trials. Only 5 trials (17, 18, 21, 27, 28), however, reported significant reductions in urine protein excretion. In the pooled analysis, n–3 LCPUFA supplementation significantly reduced urine protein excretion compared with the control. The pooled effect size (Cohen's d) for all trials was −0.19 (95% CI: −0.34, −0.04; P = 0.01). The test for heterogeneity was nonsignificant (P = 0.84). The effect sizes (Cohen's d) in studies of patients with IgA nephropathy (n = 5), diabetes (n = 7), and other conditions (n = 5) were −0.05 (95% CI: −0.31, 0.21; P = 0.71), −0.21 (95% CI: −0.46, 0.04; P = 0.10), and −0.33 (95% CI: −0.60, −0.06; P = 0.02), respectively (Figure 2). In meta-regression analyses, trial characteristics including trial duration, n–3 LCPUFA dose, use of olive oil as placebo, randomized controlled clinical trial compared with other trial designs, and type of underlying kidney disease were not associated with the efficacy of n–3 LCPUFA supplements (data not shown).
FIGURE 2.
Effect size (Cohen's d and 95% CI) for differences in urine protein excretion between the n−3 long-chain polyunsaturated fatty acid (LCPUFA) supplementation and control groups, presented by preexisting condition: IgA nephropathy, diabetes, or mixed etiology (other). Pooled effect size was derived from a random-effects model. The area of each square is proportional to the inverse of study variance in the analysis. Horizontal lines represent 95% CIs.
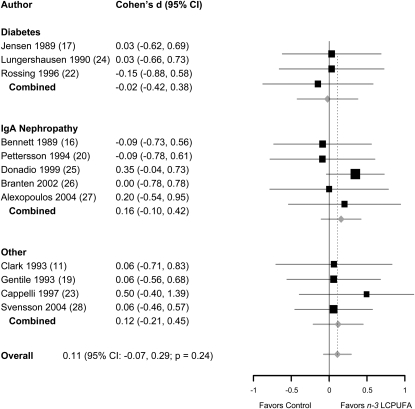
Effects of n–3 LCPUFA supplements on GFR or eGFR were reported in 12 of 17 trials (Table 3). The GFR decline was slower in the n–3 LCPUFA supplementation group than in the control group in 9 of 12 trials. Only 2 trials (24, 26), however, reported significant protective effects of n–3 LCPUFA supplementation in preventing GFR decline. Nonetheless, the pooled effect size (Cohen's d) was not significant (0.11; 95% CI: −0.07, 0.29; P = 0.24) (Figure 3). The test for heterogeneity was nonsignificant (P = 0.98). In a meta-regression analysis, the change in urine protein excretion was not associated with change in GFR (−0.03; 95% CI: −0.98, 0.28; P = 0.24).
FIGURE 3.
Effect size (Cohen's d and 95% CI) for difference in glomerular filtration rate between the n−3 long-chain polyunsaturated fatty acid (LCPUFA) supplementation and control groups, presented by preexisting condition: IgA nephropathy, diabetes, or mixed etiology (other). Pooled effect sizes were derived from a random-effects model. The area of each square is proportional to the inverse of study variance in the analysis. Horizontal lines represent 95% CIs.
DISCUSSION
In pooled analyses of clinical trials of patients with kidney disease, n–3 LCPUFA supplementation significantly reduced urine protein excretion but had no significant effect on decline in GFR. Whereas most individual trials (13 of 17) showed greater reductions in urine protein excretion in the n–3 LCPUFA supplementation groups than in the control groups, only 5 of 17 trials reported significant reductions. This likely reflects the fact that most trials in this meta-analysis were underpowered to examine the effects of n–3 LCPUFA supplementation on urine protein excretion. Whereas pooling results from these small trials increased our ability to estimate an overall effect, precise estimates of the effect of n–3 LCPUFA supplementation on urine protein excretion require adequately powered high-quality trials. Future studies should determine whether n–3 LCPUFAs reduce kidney damage by studying effects on other biomarkers of kidney injury as well as effects on clinically relevant endpoints.
n–3 LCPUFAs may reduce urine protein excretion through effects on both renal disease activity and glomerular hemodynamics. Previous meta-analyses examining the effects of n–3 LCPUFA supplements on blood pressure reported that supplementation with a relatively high dose of n–3 LCPUFAs, generally >3 g/d, results in clinically relevant, albeit modest, reductions in blood pressure (8, 9). Lower blood pressure may reduce renal perfusion and reduce GFR; lower urine protein excretion may simply parallel these renal hemodynamic effects. The trial by Clark et al (11) provides indirect evidence of a transient, potentially hemodynamic effect of n–3 LCPUFA supplements that may account for the observed reduced protein excretion in patients with lupus nephritis. In this trial, n–3 LCPUFA supplementation (4.4 g/d) reduced urine protein excretion by 37% at 1 y, but the effect disappeared after a 10-wk washout period. However, because the effect of n–3 LCPUFA supplementation on blood pressure is modest even at high doses (9), it is unlikely that this effect will completely explain the effects of n–3 LCPUFAs on urine protein excretion.
Changes in urine protein excretion do not appear to simply reflect the effects of n–3 LCPUFAs on GFR. In all studies, GFR declined in both the intervention and control arms of each study. The rate of decline was slower in the n–3 LCPUFA supplementation group, but this effect was not statistically significant. Hence, the observed significant effects of n–3 LCPUFA supplementation on urine protein excretion are not likely the result of renal hemodynamic effects (blood pressure or renal perfusion effects), because we would have expected parallel changes in GFR. Alternatively, n–3 LCPUFAs may have beneficial effects on intermediaries in the pathophysiology of kidney disease, such as inflammation or reduced oxidative stress (4). Zeman et al (12) reported that n–3 LCPUFA supplementation lowered urine protein excretion while lowering circulating conjugated dienes—an in vivo marker of oxidative stress. In a mouse model of type 2 diabetes and obesity (KKA/T), n–3 LCPUFA supplementation reduced urine albumin excretion and inhibited the up-regulation of the inflammatory marker monocyte chemoattractant protein (MCP-1) expression without having measurable effects on blood pressure or GFR, results that suggest benefits beyond hemodynamic mechanisms (3).
Variability in the effects of n–3 LCPUFA supplementation on urine protein excretion rates may be due to differences in underlying etiologies of kidney disease in the patient populations. Five trials were conducted in patients with IgA nephropathy, which is often a very slowly progressive disease with alternating periods of activity and quiescence. In all 5 of the trials in patients with IgA nephropathy and in the lupus nephritis trial, the placebo groups had significant reductions in urine protein excretion from baseline, which suggests that participants were randomly assigned during a period of high disease activity. In the other trials that enrolled participants with diabetic kidney or hypertensive nephrosclerosis, a reversal of the underlying pathology responsible for progression is less likely. Whereas this may be an argument to pool results of trials that include participants with only a single underlying disease diagnosis, our results showed a similar magnitude of effect of n–3 LCPUFA supplementation on urine protein excretion across the trials pooled by diagnoses (IgA nephropathy, diabetic nephropathy, and mixed etiologies). Future trials will need to clearly define the patient population because findings in kidney diseases with a specific pathology may not be generalizable to other causes of urine protein excretion.
Whereas our meta-analysis offers insight into the possibility of a beneficial effect of n–3 LCPUFA supplements on urine protein excretion, a well-accepted marker of kidney damage and strong predictor of kidney disease progression, the included studies were small and many had methodologic limitations. In addition, these trials typically used very high doses (up to 5.1 g/d) and did not consistently collect measurements of physiologic explanatory variables, including GFR, blood pressure, or measurements of inflammation or oxidation.
As an additional limitation, our results were based on urine protein (albumin) excretion, a surrogate endpoint. There is interest and controversy associated with the use of urine protein excretion as a surrogate marker in kidney disease (13). There are many examples in which therapies that lower urine protein excretion are thought to be renoprotective (eg, angiotensin-converting enzyme inhibitors) and provide evidence of concordance between reduced proteinuria and a lower risk of kidney disease progression. However, there are counterexamples with discordant results. For instance, nonsteroidal antiinflammatory drugs acutely decrease proteinuria but increase the risk of kidney injury, whereas dietary protein restriction or lower therapeutic blood pressures lower urine protein excretion but have not been linked to a slower progression of kidney disease (14). Many trials included in this meta-analysis did not report effects on renal glomerular function, and the correlation between changes in urine protein excretion and eGFR in those trials that did report or in which GFR could be estimated did not show a consistent pattern.
In summary, our meta-analysis suggests that n–3 LCPUFA supplementation lowers urine protein excretion. However, because many of the trials included in this meta-analysis were small, future studies designed to examine the potential benefits of n–3 LCPUFA therapy must improve on the quality of prior trials in several ways. First, trials should be adequately powered and use high-quality measures to estimate changes in urine protein excretion. Second, the trials should include measurements of explanatory variables in the pathophysiology of the underlying disease, including effects on GFR, blood pressure, markers of oxidation and inflammation, and urine protein fingerprinting, which may provide insight into whether glomerular or tubulointerstitial injury has been modified. Finally, the efficacy of n–3 LCPUFA supplements in population groups with different underlying causes of kidney disease needs to be determined.
Acknowledgments
The authors' responsibilities were as follows— ERM and EG: concept and design of the study, critical revision of the article for important intellectual content, and draft of the manuscript; ERM, SPJ, JB, CAMA, LJA, and EG: analysis and interpretation of the data; EG and JB: statistical expertise; and ERM, MM, and SPJ: collection and assembly of the data. All authors were responsible for final approval of the manuscript. None of the authors declared a conflict of interest.
APPENDIX A
MESH terms used to search for trials within MEDLINE (PUBMED):
((“Docosahexaenoic Acids”[MH] OR “Eicosapentaenoic Acids”[MH] OR “Fatty acids, unsaturated”[MH] OR “Fatty acids, omega-3”[MH] OR (fish [TIAB] AND oils [TIAB]) OR (eicosapentaenoic [TIAB] AND acids [TIAB]) OR (Docosahexaenoic [TIAB] AND acids [TIAB]) OR (polyunsaturated [TIAB] AND fatty [TIAB] acids [TIAB]) OR PUFA [TIAB] OR (OMEGA-3[TIAB] AND fatty [TIAB] AND Acids[TIAB]) OR (N3 [TIAB] AND FATTY [TIAB] AND ACIDS [TIAB]) OR “fish oils”[MH])) AND ((“kidney diseases” [MH] OR “Diabetic nephropathies” [MH] OR “Glomerulonephritis” [MH] OR “Glomerulonephritis, IGA”[MH OR “renal insufficiency” [MH] OR “Kidney neoplasms” [MH] OR “Nephrosclerosis” [MH] OR “pyelonephritis”[MH] OR (kidney [TIAB] AND diseases [TIAB]) OR (Diabetic [TIAB] AND nephropathy [TIAB]) OR proteinuria [TIAB] OR (renal[TIAB] AND insufficiency [TIAB]) OR (glomerulonephropathy [TIAB] AND membranous [TIAB]) OR (Kidney [TIAB] AND neoplasms [TIAB]) OR Nephrosclerosis [TIAB] OR pyelonephritis [TIAB] OR (glomerulonephropathy [TIAB] AND IgA [TIAB]) OR FSGS [TIAB])) AND ((“Randomized Controlled Trials” [MH] OR “Humans” [MH] OR “Double blind method”[MH] OR “single-blind method”[mh]))
REFERENCES
- 1.De Caterina R, Bertolotto A, Madonna R, Schmidt EB. n−3 Fatty acids in the treatment of diabetic patients. Diabetes Care 2007;30:1012–26 [DOI] [PubMed] [Google Scholar]
- 2.Brown SA, Brown CA, Crowell WA, et al. Effects of dietary polyunsaturated fatty acid supplementation in early renal insufficiency in dogs. J Lab Clin Med 2000;135:275–86 [DOI] [PubMed] [Google Scholar]
- 3.Hagiwara S, Makita Y, Gu L, et al. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant 2006;21:605–15 [DOI] [PubMed] [Google Scholar]
- 4.Abeywardena MY, Head RJ. Longchain n−3 polyunsaturated fatty acids and blood vessel function. Cardiovasc Res 2001;52:361–71 [DOI] [PubMed] [Google Scholar]
- 5.Lee CT, Adler AI, Forouhi NG, et al. Cross-sectional association between fish consumption and albuminuria: the European Prospective Investigation Of Cancer-Norfolk Study. Am J Kidney Dis 2008;52:876–86 [DOI] [PubMed] [Google Scholar]
- 6.Lauretani F, Semba RD, Bandinelli S, et al. Plasma polyunsaturated fatty acids and the decline of renal function. Clin Chem 2008;54:475–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLean CH, Mojica WA, Morton SC, et al. Effects of omega-3 fatty acids on lipids and glycemic control in type II diabetes and the metabolic syndrome and on inflammatory bowel disease, rheumatoid arthritis, renal disease, systemic lupus erythematosus, and osteoporosis. Evid Rep Technol Assess (Summ) 2004;89:1–4 [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal R. Parametric measures of effect size. : Handbook of research synthesis. New York, NY: Russell Sage Foundation, 1994 [Google Scholar]
- 9.Appel LJ, Miller ER, III, Seidler AJ, Whelton PK. Does supplementation of diet with ‘fish oil’ reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med 1993;153:1429–38 [PubMed] [Google Scholar]
- 10.Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens 2006;24:215–33 [DOI] [PubMed] [Google Scholar]
- 11.Clark WF, Parbtani A, Naylor CD, et al. Fish oil in lupus nephritis: clinical findings and methodological implications. Kidney Int 1993;44:75–86 [DOI] [PubMed] [Google Scholar]
- 12.Zeman M, Zak A, Vecka M, Tvrzicka E, Pisarikova A, Stankova B. n–3 fatty acid supplementation decreases plasma homocysteine in diabetic dyslipidemia treated with statin-fibrate combination. J Nutr Biochem 2006;17:379–84 [DOI] [PubMed] [Google Scholar]
- 13.de Zeeuw D. Targeting proteinuria as a valid surrogate for individualized kidney protective therapy. Am J Kidney Dis 2008;51:713–6 [DOI] [PubMed] [Google Scholar]
- 14.Agodoa LY, Appel L, Bakris GL, et al. African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001;285:2719–28 [DOI] [PubMed] [Google Scholar]
- 15.Haines AP, Sanders TA, Imeson JD, et al. Effects of a fish oil supplement on platelet function, haemostatic variables and albuminuria in insulin-dependent diabetics. Thromb Res 1986;43:643–55 [DOI] [PubMed] [Google Scholar]
- 16.Bennett WM, Walker RG, Kincaid-Smith P. Treatment of IgA nephropathy with eicosapentanoic acid (EPA): a two-year prospective trial. Clin Nephrol 1989;31:128–31 [PubMed] [Google Scholar]
- 17.Jensen T, Stender S, Goldstein K, Holmer G, Deckert T. Partial normalization by dietary cod-liver oil of increased microvascular albumin leakage in patients with insulin-dependent diabetes and albuminuria. N Engl J Med 1989;321:1572–7 [DOI] [PubMed] [Google Scholar]
- 18.Hamazaki T, Takazakura E, Osawa K, Urakaze M, Yano S. Reduction in microalbuminuria in diabetics by eicosapentaenoic acid ethyl ester. Lipids 1990;25:541–5 [DOI] [PubMed] [Google Scholar]
- 19.Gentile MG, Fellin G, Cofano F, et al. Treatment of proteinuric patients with a vegetarian soy diet and fish oil. Clin Nephrol 1993;40:315–20 [PubMed] [Google Scholar]
- 20.Pettersson EE, Rekola S, Berglund L, et al. Treatment of IgA nephropathy with omega-3-polyunsaturated fatty acids: a prospective, double-blind, randomized study. Clin Nephrol 1994;41:183–90 [PubMed] [Google Scholar]
- 21.Shimizu H, Ohtani K, Tanaka Y, Sato N, Mori M, Shimomura Y. Long-term effect of eicosapentaenoic acid ethyl (EPA-E) on albuminuria of non-insulin dependent diabetic patients. Diabetes Res Clin Pract 1995;28:35–40 [DOI] [PubMed] [Google Scholar]
- 22.Rossing P, Hansen BV, Nielsen FS, Myrup B, Holmer G, Parving HH. Fish oil in diabetic nephropathy. Diabetes Care 1996;19:1214–9 [DOI] [PubMed] [Google Scholar]
- 23.Cappelli P, Di Liberato L, Stuard S, Ballone E, Albertazzi A. n–3 Polyunsaturated fatty acid supplementation in chronic progressive renal disease. J Nephrol 1997;10:157–62 [PubMed] [Google Scholar]
- 24.Lungershausen YK, Howe PR, Clifton PM, et al. Evaluation of an omega-3 fatty acid supplement in diabetics with microalbuminuria. Ann N Y Acad Sci 1997;827:369–81 [DOI] [PubMed] [Google Scholar]
- 25.Donadio JV, Jr, Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J Am Soc Nephrol 1999;10:1772–7 [DOI] [PubMed] [Google Scholar]
- 26.Branten AJ, Klasen IS, Wetzels JF. Short-term effects of fish oil treatment on urinary excretion of high- and low-molecular weight proteins in patients with IgA nephropathy. Clin Nephrol 2002;58:267–74 [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos E, Stangou M, Pantzaki A, Kirmizis D, Memmos D. Treatment of severe IgA nephropathy with omega-3 fatty acids: The effect of a “very low dose” regimen. Ren Fail 2004;26:453–9 [DOI] [PubMed] [Google Scholar]
- 28.Svensson M, Christensen JH, Solling J, Schmidt EB. The effect of n–3 fatty acids on plasma lipids and lipoproteins and blood pressure in patients with CRF. Am J Kidney Dis 2004;44:77–83 [DOI] [PubMed] [Google Scholar]
- 29.Theobald HE, Goodall AH, Sattar N, Talbot DC, Chowienczyk PJ, Sanders TA. Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J Nutr 2007;137:973–8 [DOI] [PubMed] [Google Scholar]