Abstract
Tetracyclines (TCN) have powerful anti-inflammatory properties in addition to their anti-microbial effects. These anti-inflammatory effects are thought to play a role in inhibiting cutaneous inflammation in patients with rosacea and acne; however, the mechanism(s) of this action remains poorly understood. We have previously shown that adenosine-5′-triphosphate (ATP)γS, a hydrolysis-resistant ATP analogue, augments secretion of pro-inflammatory messengers by a human dermal microvascular endothelial cell line (HMEC-1). ATP released by the sympathetic nerves during stress may stimulate release of pro-inflammatory chemokines by dermal vessel endothelial cells, resulting in recruitment of inflammatory cells and exacerbation of inflammatory skin disease. Here we demonstrate that TCN inhibits ATPγS-induced release of pro-inflammatory mediators by HMEC-1 cells and primary human dermal microvascular endothelial cells. TCN dose-dependently inhibited ATPγS-induced augmentation of CXCL8 (interleukin-8) and CXCL1 (growth-regulated oncogene-α) production by HMEC-1 cells and primary human dermal endothelial cells in vitro. TCN and ATPγS did not affect HMEC-1 cell viability as determined by trypan-blue exclusion and cell counts. Inhibition of production of inflammatory mediators by endothelial cells may be one mechanism by which TCN improves inflammatory skin diseases. The ability to inhibit release of inflammatory mediators induced in HMEC-1 cells by purinergic agonists may be a useful way to screen for potential therapeutic agents for cutaneous inflammation.
Keywords: adenosine triphosphate, chemokines, cytokines, endothelial cells, tetracycline
Introduction
The tetracyclines (TCN) have been available for over a half-century and have proven to be a useful class of drugs because of their potent antimicrobial properties. An unexpected property of TCN was discovered in the 1980s when Golub et al. (1–4) revealed that TCN could inhibit mammalian collagenases. Later it was shown that chemically modified tetracyclines (CMT), which lack bacteriostatic qualities, also strongly inhibit mammalian collagenase (1) suggesting that the anti-inflammatory properties of TCN are independent of its antimicrobial properties. Further studies showed that TCN are potent inhibitors of several matrix metalloproteinases (MMP) associated with inflammation and connective tissue destruction and this led to the widespread use of TCN to treat chronic periodontal disease (5–8).
In addition to periodontal disease, TCN have been shown to be useful in treating many other inflammatory diseases. These include skin diseases such as acne, rosacea, bullous pemphigoid, pemphigus vulgaris, dermatitis herpetiformis, linear immunoglobulin A (IgA) bullous dermatitis as well as several other inflammatory skin disorders (9–11). TCN also have shown promise in autoimmune diseases such as rheumatoid arthritis and scleroderma (12–14) and vascular disorders such as atherosclerosis, aortic aneurism formation and in experimental models of ischemic stroke (15–17) More recently, studies have shown that TCN have neuroprotective as well as anti-inflammatory properties and show beneficial effects in animal models of chronic neuro-degenerative disease such as Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis (18–21). In addition, TCN have shown to be potentially beneficial in several other diseases involving inflammatory cascades such as endotoxic shock (22), viral encephalitis (23) and acute lung injury (24).
Rosacea is a common skin condition that is estimated to account for over 1.1 million physician visits per year (25) and may affect as many as 1 in 20 adult Americans (26). Although the cause of rosacea is unknown, the disease can be effectively treated with oral TCN (27,28). Rosacea is characterized by persistent erythema of skin, commonly on the face, which often can be worsened by triggers such as spicy foods, sunlight exposure, alcohol ingestion and stress. Inflammatory acneiform lesions are also a part of the syndrome in more advanced rosacea. Underlying bacterial infection may or may not be present in rosacea; however, it is thought that the disease is primarily inflammatory in nature (28). In an uncontrolled observational study of the use of TCN in patients with rosacea, Sneddon et al. (29) first suggested in 1966 that TCN’s ability to significantly improve pustular lesions as well as persistent erythema of the face may indicate that TCN’s beneficial action ‘may not be entirely an antibacterial [effect]’. In support of Sneddon’s findings, it was shown that after 6 months of treatment with systemic TCN (250 mg twice daily), 97% of patients had clearance of rosacea lesions (30). Interestingly, after discontinuing TCN, 24% of the patients relapsed immediately and a total of 69% relapsed over the next 4 years. Later, a double-blind, randomized, placebo-controlled trial studying 51 adults with moderate facial acne showed that subantimicrobial dose of doxycycline hyclate (20-mg tablets taken twice daily for 6 months) significantly decreased the number of inflammatory and non-inflammatory lesions (>50%) without an associated change in microbial skin flora. Moreover, it was shown that this regimen did not induce the development of resistant organisms or cross-resistance to other antimicrobial drugs suggesting its safety for clinical use (31).
It was the absence of correlation between TCN dosage and cutaneous bacterial counts that led early investigators to search for other mechanisms by which TCN exert their beneficial effects (32). An enhanced chemotactic response of leukocytes to chemotactic signals has been the subject of studies concerning the mechanisms of acne (33). Studies measuring polymorphonuclear leukocyte (PMNL) chemotaxis towards zymosan-activated serum samples showed that serum neutrophils obtained from acne patients treated with oral TCN exhibited significantly diminished chemotaxis compared with serum neutrophils obtained from acne patients receiving no medication (32). In this way, inhibition of the chemotactic response may be an important mechanism by which TCN are effective in treating inflammatory disease. We hypothesize that inhibition of chemokine release not only from neutrophils but also from endothelial cells may be a key mechanism involved in TCN’s anti-inflammatory effect.
We have chosen to study the endothelium because it is pertinent to both the pathogenesis of many inflammatory diseases such as rosacea and the actions of TCN. In particular, TCN have been shown to be very effective in inhibiting MMP-2 and MMP-9, enzymes which degrade the basement membrane of capillaries (16). Doxycycline, a second-generation TCN, has been shown to defend capillary wall and connective tissue integrity, reduce hypersensitivity to vasodilatory stimuli, prevent leakage of capillaries and inhibit cytokines involved in inflammation and erythema, all symptoms which may be associated with rosacea (33).
The anti-inflammatory activities of TCN are believed to account for the beneficial effects seen in rosacea; however, as stated before, the mechanism(s) of this action remains poorly understood. Prior studies in our laboratory showed that the adenosine-5′-triphosphate (ATP) analogue ATPγS increased the release of pro-inflammatory factors by the transformed human dermal microvascular endothelial cell line-1 (HMEC-1). Because ATP is a sympathetic nerve co-transmitter, it may mediate the exacerbation of rosacea (and perhaps other inflammatory skin disorders) reported to occur with stress (34). We hypothesized that TCN might exert its effects, at least in part, by inhibiting the endothelial release of chemokines. Thus, we examined whether TCN inhibits chemokine release by HMEC-1 cells and primary human dermal microvascular endothelial cells.
Methods
Media and cell lines
The transformed HMEC-1 line (35), immortalized by simian virus 40 transformation, was provided by T. J. Lawley (Emory University, Atlanta, GA, USA). This cell line maintains numerous properties of dermal microvascular endothelial cells including cytokine and cell adhesion molecule expression (36). Primary cultures of neonatal foreskin-derived human dermal microvascular endothelial cells were obtained commercially (Lonza, Walkersville, MD, USA). For cell culture, endothelial cell basal medium (EBM; Cambrex, Wlakersville, MD, USA) was used and supplemented with 10% fetal bovine serum (Life Technologies, Gaithersburg, MD, USA), epidermal growth factor (10 ng/ml; BD Biosciences, Bedford MA, USA), hydrocortisone (HC, 1 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) and 100 U/ml penicillin (PCN)/100 μg/ml streptomycin (Life Technologies), in a humidified atmosphere at 37°C with 5% CO2. During some experiments, such as during antibiotic treatment, the cells were kept in EBM supplemented only with 2% fetal calf serum (FCS) and without PCN/streptomycin, EGF or HC (referred to as depleted medium).
Nucleotides and cytokine
Adenosine-5′-O-(3-thiotriphosphate) (ATPγS) and ATP were purchased from Sigma-Aldrich. Tumor necrosis factor-alpha (TNFα) was purchased from R&D Systems (Minneapolis, MN, USA).
Chemokine assays
To measure CXCL8 (interleukin-8, IL-8) and CXCL1 (growth-related oncogene α) secretion by HMEC-1 cells, cells were plated at a concentration of 0.25 × 106 cells per well in 12-well plates in triplicate in medium as described before. After the cells were allowed to adhere, the medium was changed to depleted medium. Twenty-four hours later the medium was replaced with fresh medium and TCN was added at various concentrations to the appropriate wells. Thirty minutes later, 100 μm ATPγS was added to subconfluent cultures. Twenty-four hours later, the conditioned supernatants were harvested. In some experiments 0.02 μg/ml of TNFα was substituted for ATPγS.
CXCL8 secretion was quantified by sandwich enzyme-linked immunosorbent assay (ELISA), with antibodies (Ab) and standards purchased from BD Pharmingen (San Diego, CA, USA). Purified mouse anti-human CXCL8 served as the primary (capture) Ab and purified human CXCL8 as the standard. Biotinylated mouse anti-human CXCL8 mAb, the secondary (detection) Ab, was detected by steptavidin-horseradish peroxidase (HRP) in a 1:1000 dilution. The amount of bound cytokine was visualized by addition of a 1:1 mixture of colour reagent A (H2O2) and colour reagent B (tetramethylbenzidine) (R&D Systems). The colour reaction was stopped with 2N H2SO4 and the level of coloured reaction product was measured spectrophotometrically at 450 nm.
CXCL1 production was quantified by sandwich ELISA (DuoSet® ELISA Development system; R&D systems) in a similar manner as described above. These kits include mouse anti-human CXCL1 as the primary Ab and biotinylated goat anti-human CXCL1 as the secondary (detection) Ab. Streptavidin-HRP conjugate was added at a 1:1000 dilution, followed by colour development.
Statistical analysis
The Student’s t-test for independent samples was used to evaluate the difference between the mean values of chemokine production in cultures treated with varying doses of TCN compared with chemokine production in control cultures not treated with TCN. All statistical tests were two-tailed and P < 0.05 was considered statistically significant. Analyses were performed using Excel (Microsoft Office 2002; Microsoft, Redmond, VA, USA) and SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
TCN dose-dependently inhibits ATPγS-induced CXCL8 and CXCL1 release
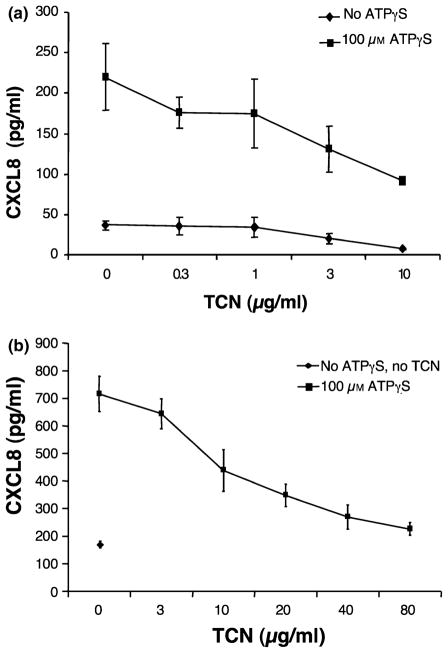
HMEC-1 cells produce CXCL8 and CXCL1 in culture and the amount produced significantly increases with exposure to ATPγS. Addition of 100 μm of ATPγS significantly increased CXCL8 production compared with basal levels (Fig. 1a). Increasing concentrations of TCN (0.3, 1, 3, 10 μg/ml) inhibited ATPγS-induced levels of CXCL8, demonstrating dose-dependence. Inhibition was statistically significant at 3 and 10 μg/ml (P = 0.04 and 0.006, respectively). This experiment is representative of five experiments performed. Four of five experiments showed a dose-dependent inhibition of CXCL8 release with increasing concentrations of TCN with two of five showing statistical significance at 0.3 μg/ml, two of five at 1 μg/ml, four of five at 3 μg/ml, and one of five at 10 μg/ml. Similar results were seen with primary human microvascular endothelial cells with statistically significant inhibition at 20, 40 and 80 μg/ml (P = 0.011, <0.001 and <0.001, respectively) (Fig. 1b). This experiment is representative of two experiments performed. Two of two experiments showed a dose-dependent inhibition of CXCL8 release with increasing concentrations of TCN with two of two showing statistical significance at 10 μg/ml, two of two at 20 μg/ml, two of two at 40 μg/ml and two of two at 80 μg/ml.
Figure 1.
Tetracycline (TCN) dose-dependently inhibits adenosine-5′-triphosphate (ATP)γS-induced CXCL8 production. (a) Human microvascular endothelial cell line-1 (HMEC-1) cells were cultured in the presence of TCN for 30 min followed by addition of ATPγS to 100 μM. Supernatants were collected 24 h later and analysed for CXCL8 content. Note that ATPγS enhances CXCL8 release. Error bars indicate ± SD (P = 0.04 and 0.006, respectively, for TCN of 3 and 10 μg/ml compared with 0 μg/ml for the ATPγS-treated groups). TCN also significantly inhibited non-stimulated production (P < 0.001 and P = 0.125, respectively, for TCN 3 μg/ml and 10 μg/ml compared with 0 μg/ml for the ATPγS groups). (b) Primary human dermal microvascular endothelial cells were cultured in the presence of TCN for 30 min followed by addition of ATPγS to 100 μm. Supernatants were collected 24 h later and analysed for CXCL8 content. Note that ATPγS enhances CXCL8 release. Error bars indicate ± SD (P = 0.008, 0.001, <0.001 and <0.001, respectively, for TCN of 10, 20, 40 and 80 μg/ml) compared with 0 μg/ml for the ATPγS-treated groups).
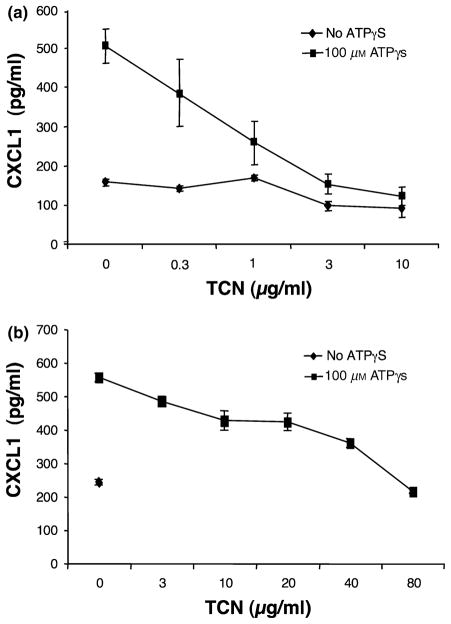
CXCL1 production was also significantly induced by ATPγS (P = 0.0002) (Fig. 2). Increasing doses of TCN (0.3, 1, 3, 10 μg/ml) inhibited ATPγS-induced levels of CXCL1, with inhibition being statistically significant at 1, 3 and 10 μg/ml (P = 0.004, 0.0002 and 0.0001, respectively). This experiment is representative of five experiments performed. Four of five showed a dose-dependent inhibition of CXCL1 release with increasing concentrations of TCN with one of five showing statistical significance at 0.3 μg/ml, three of five at 1 μg/ml, four of five at 3 μg/ml and four of five at 10 μg/ml. Similar results were seen with primary human microvascular endothelial cells with statistically significant inhibition at 3, 10, 20, 40 and 80 μg/ml (P = 0.003, 0.002, 0.001, <0.001 and <0.001, respectively) (Fig. 2b). Two experiments of this type were performed and both experiments showed a dose-dependent inhibition of CXCL1 release with increasing concentrations of TCN with one of one showing statistical significance at 3 μg/ml (only one included a 3 μg/ml group), two of two at 10 μg/ml, two of two at 20 μg/ml, two of two at 40 μg/ml and two of two at 80 μg/ml.
Figure 2.
Tetracycline (TCN) dose-dependently inhibits adenosine-5′-triphosphate (ATP)γS-induced CXCL1 production. (a) Human microvascular endothelial cell line-1 (HMEC-1) cells were cultured in the presence of TCN for 30 min followed by addition of ATPγS to 100 μM. Supernatants were collected 24 h later and analysed for CXCL1 content. Note that ATPγS enhances CXCL1 release. Error bars indicate ± SD (P = 0.004, 0.0002 and 0.0001, respectively, for TCN of 1, 3 and 10 μg/ml compared with 0 μg/ml for the ATPγS-treated groups). TCN also significantly inhibited non-stimulated production (P < 0.002 and P = 0.0125, respectively, for TCN of 3 and 10 μg/ml compared with 0 μg/ml for the ATPγS groups). (b) Primary human dermal microvascular endothelial cells were cultured in the presence of TCN for 30 min followed by addition of ATPγS to 100 μM. The supernatants were collected 24 h later and analysed for CXCL1 content. Note that ATPγS enhances CXCL1 release. Error bars indicate ± SD (P = 0.003, 0.002, 0.001, <0.001 and <0.001, respectively, for TCN 3, 10, 20, 40 and 80 μg/ml compared with 0 μg/ml for the ATPγS-treated groups).
Note that TCN also significantly decreased baseline (no ATPγS) HMEC-1 production of CXCL8 and CXCL1 at 3 and 10 μg/ml.
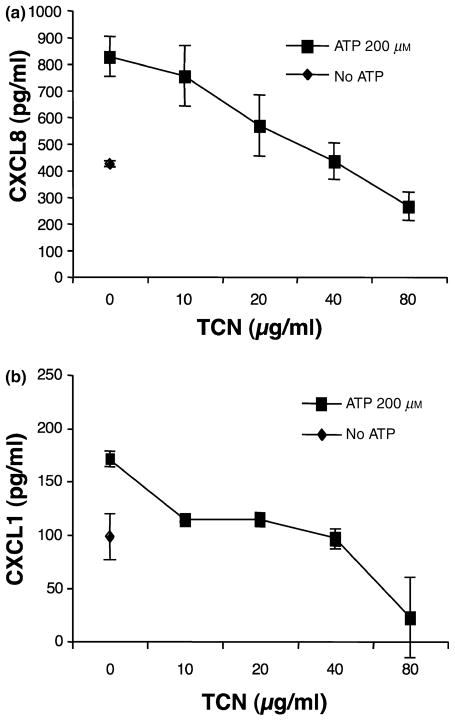
TCN dose-dependently inhibits ATP-induced CXCL8 and CXCL1 release
Experiments were also performed to examine the effect of the parent compound, ATP, on induction of CXCL8 and CXCL1 by HMEC-1 cells. As shown in Fig. 3a,b, TCN also inhibits ATP-induced production of CXCL8 and CXCL1 in a dose-dependent manner. For CXCL8 statistical significance was observed at TCN 20, 40 and 80 μg/ml (P = 0.011, <0.001 and <0.001, respectively) and for CXCL1 at TCN 10, 20, 40 and 80 μg/ml (P < 0.001 for all four groups). This experiment is representative of six experiments performed for CXCL8 and five for CXCL1. Five of the six experiments for CXCL8 showed a dose-dependent inhibition of CXCL8 release with increasing concentrations of TCN with two of six showing statistical significance at 10 μg/ml, five of six at 20 μg/ml, five of six at 40 μg/ml and five of six at 80 μg/ml. Four of the five experiments for CXCL1 showed a dose-dependent inhibition of CXCL1 release with increasing concentrations of TCN with one of five showing statistical significance at 10 μg/ml, three of five at 20 μg/ml, four of five at 40 μg/ml and three of three at 80 μg/ml (only three experiments included 80 μg/ml).
Figure 3.
Tetracycline (TCN) dose-dependently inhibits adenosine-5′-triphosphate (ATP)-induced CXCL8 and CXCL1 production of human microvascular endothelial cell line-1 (HMEC-1). (a) CXCL8: HMEC-1 cells were cultured in the presence of TCN for 30 min followed by addition of ATP to 200 μm. Supernatants were collected 24 h later and analysed for CXCL8 content. Note that ATP enhances CXCL8 release. Error bars indicate ± SD (P = 0.011, <0.001 and <0.001, respectively, for TCN 20, 40 and 80 μg/ml compared with 0 μg/ml for the ATPγS-treated groups). (b) CXCL1: HMEC-1 cells were cultured in the presence of TCN for 30 min followed by addition of ATP to 200 μm. The supernatants were collected 24 h later and analysed for CXCL1 content. Note that ATP enhances CXCL1 release. Error bars indicate ± SD (P = <0.001 for TCN 10, 20, 40 and 80 μg/ml compared with 0 μg/ml for the ATP-treated groups).
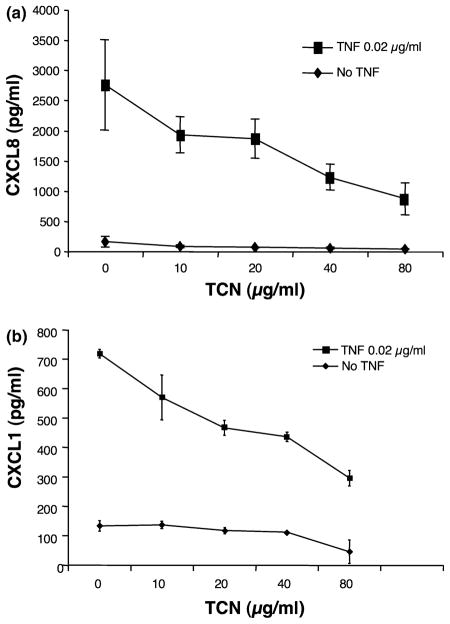
TCN dose-dependently inhibits TNFα-induced CXCL8 and CXCL1 production
TNFα is a pro-inflammatory cytokine known to induce production of CXCL8 and CXCL1 by endothelial cells (37). The ability of TCN to inhibit TNFα-induced production of CXCL8 and CXCL1 by HMEC-1 cells was also examined. Addition of 0.02 μg/ml of TNFα significantly increased CXCL8, and CXCL1 production by HMEC-1 cells. As shown in Fig. 4a,b, TCN also dose-dependently inhibited induction of these chemokines by TNFα. This experiment is representative of five experiments performed for CXCL8 and three for CXCL1. Four of the five experiments for CXCL8 showed a dose-dependent inhibition of CXCL8 release with increasing concentrations of TCN with one of five showing statistical significance at 40 μg/ml, and four of five at 80 μg/ml. Three of three experiments for CXCL1 showed a dose-dependent inhibition of CXCL1 release with increasing concentrations of TCN with one of three showing statistical significance at 10 μg/ml, one of three at 20 μg/ml, two of three at 40 μg/ml and three of three at 80 μg/ml.
Figure 4.
Tetracycline (TCN) dose-dependently inhibits tumor necrosis factor (TNF)α-induced CXCL8 and CXCL1 production by human microvascular endothelial cell line-1 (HMEC-1). (a) CXCL8: HMEC-1 cells were cultured in the presence of TCN for 30 min followed by addition of TNFα to 0.02 μg/ml. Supernatants were collected 24 h later and analysed for CXCL8 content. Note that TNFα enhances CXCL8 release. Error bars indicate ± SD (P = 0.027 and P = 0.015, respectively, for TCN of 40 and 80 μg/ml compared with 0 μg/ml for the no ATPγS groups). (b) CXCL1: HMEC-1 cells were cultured in the presence of TCN for 30 min followed by addition of TNFα to 0.02 μg/ml. The supernatants were collected 24 h later and analysed for CXCL1 content. Note that TNFα enhances CXCL1 release. Error bars indicate ± SD (P = 0.029, <0.001, <0.001 and <0.001, respectively, for TCN 10, 20, 40 and 80 μg/ml compared with 0 μg/ml for the no TNFα group).
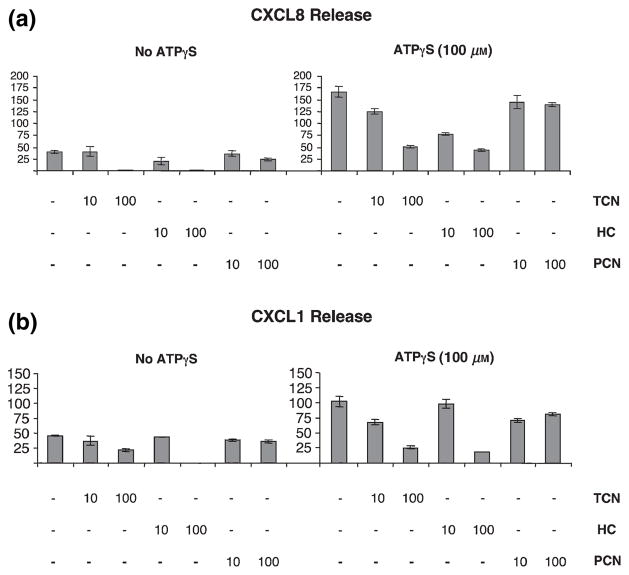
HC inhibits ATPγS-induced CXCL8 and CXCL1 production in HMEC-1 cells while PCN has no effect
As controls, ability of HC and PCN to affect ATPγS-induced chemokine production by HMEC-1 cells was examined. Ten and 100 μg/ml of HC significantly suppressed ATPγS-induced CXCL8 (P = 0.0002 and < 0.0001, respectively) and at 100 μg/ml CXCL1 (P < 0.0001) release (Fig. 5). As shown by the data in Fig. 5, PCN had a small suppressive effect on ATPγS-induced CXCL8 release at 100 μg/ml (Fig. 5a, P = 0.019); and at 10 and 100 μg/ml of PCN a small suppressive effect on CXCL1 was observed (Fig. 5b, P = 0.004, P = 0.012). In each case, the effect of PCN was not dose-dependent and was of much smaller magnitude than that seen with TCN. This experiment was performed twice and these results are representative of data obtained from both experiments. Thus, as expected, the effects observed with TCN are not a general property of any antibiotic.
Figure 5.
Hydrocortisone (HC) substantially inhibits adenosine-5′-triphosphate (ATP)γS-induced CXCL8 and CXCL1 production by human microvascular endothelial cell line-1 (HMEC-1) while penicillin (PCN) has only a small inhibitory effect on CXCL1 and CXCL8 release. HMEC-1 cell cultures were treated with tetracycline (TCN), HC or PCN and then stimulated with ATPγS (100 μm) or left unstimulated. Supernatants were collected 24 h later and analysed for CXCL8 (a) and CXCL1 (b) content. (a) CXCL8: P = 0.006 for 10 μg/ml of TCN, P < 0.0001 for 100 μg/ml of TCN, P < 0.001 for 10 μg/ml of HC, P < 0.0001 for 100 μg/ml of HC, P = 0.09 for 10 μg/ml of PCN and P = 0.020 for 100 μg/ml of PCN. (b) CXCL1: P = 0.005 for 10 μg/ml of TCN, P < 0.0001 for 100 μg/ml of TCN, P = 0.58 for 10 μg/ml of HC, P < 0.0001 for 100 μg/ml of HC, P = 0.004 for 10 μg/ml of PCN and P = 0.012 for 100 μg/ml of PCN.
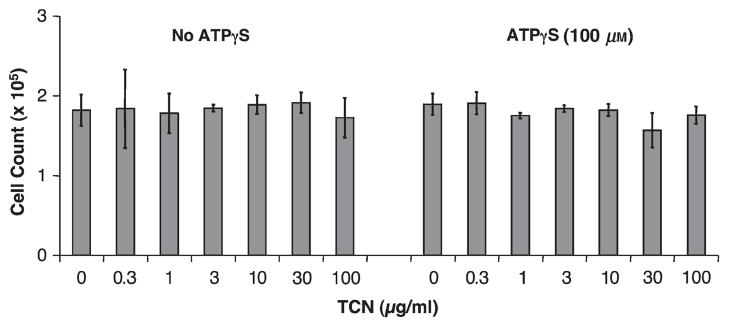
Neither ATPγS nor TCN reduce cell viability at doses tested
Neither ATPγS (100 μm) nor TCN (0.3–100 μg/ml) significantly affected HMEC-1 cell viability compared with the medium alone as measured by cell counting and trypan blue exclusion after 24 h in culture (Fig. 6). Two wells containing cells were counted for each condition treated with various amounts of TCN with or without ATPγS in each of the two experiments and the results were averaged.
Figure 6.
Neither tetracycline (TCN) nor adenosine-5′-triphosphate (ATP)γS affected human microvascular endothelial cell line-1 (HMEC-1) cell numbers after 24 h. Two hundred and fifty thousand HMEC-1 cells per well were cultured in 12-well plates in medium containing the indicated concentration of TCN with or without the addition of ATPγS to 100 μm. After 24 h, the cells were counted in two wells from each condition from each of the two experiments and averaged.
Discussion
Most studies examining the influence of TCN on cytokine release have focused on TNFα and IL-1β release and utilized human monocyte, T lymphocyte, mast cell or osteocyte cultures. Few studies have examined the role of TCN on endothelial-derived cytokines and chemokines. In a report by Shapira et al. (22), TNFα and IL-1β protein secretion in human monocytes were suppressed by TCN while cytokine mRNA accumulation remained unchanged, suggesting that the drug interferes post-transcriptionally. Another study showed that a chemically modified TCN, CMT-3, significantly inhibited TNFα in a dose-dependent manner and also inhibited CXCL8 production in mast cells (38). Later, it was shown using stimulated osteoblast cultures that TCN may act by decreasing mRNA stability of IL-6, a pro-inflammatory cytokine, thus further suggesting that TCN inhibit certain cytokine expression at a post-transcriptional level. Interestingly, these effects were found to require de novo protein synthesis as they were inhibited by cycloheximide (39,40). In addition, it has been shown that TCN suppress nitric oxide and reactive oxygen species levels and inhibits inducible nitric oxide synthase expression at the level of mRNA accumulation in lipopolysaccharide (LPS)-stimulated macrophages (41,42). It is therefore thought that some of the pleiotropic properties of TCN may be due in part to its ability to affect small molecules such as nitric oxide (43) and possibly other multi-functional signalling molecules such as nucleotides like ATP.
Endothelial cells play critical roles in the evolution of cutaneous inflammation. They can synthesize and secrete various pro-inflammatory cytokines and regulate expression of cell surface adhesion molecules. We chose to study CXCL8 and CXCL1 release from HMEC-1 cells because these chemokines were found to be upregulated upon ATPγS administration in the study by Seiffert et al. (34). CXCL8 is a chemotactic cytokine for neutrophils, CXCL1 is chemotactic for neutrophils, supports adhesion and induces activation of these cells. Hence, these chemokines enhance leukocyte recruitment to areas of inflammation. Nucleotides such as ATP have been shown to be released into the extracellular environment from damaged cells or secreted by various non-lytic means and are important signalling molecules during vascular damage or inflammation (44). It has been shown that nucleotides are released in response to a chemical, physical or microbial perturbation, and therefore may act as a ‘danger signal’ to alert other cells to the area of injury or infection. In this manner, Seiffert et al. (34) suggested that ATP may play a role as a unifying transmitter in the multifaceted system of inflammatory responses within the skin. Indeed, the cross-talk between inflammatory cells and the endothelium is integral to the development of chronic inflammation (45). Through better elucidation of the role of endothelial cells in inflammation, therapeutic drugs could be designed to target these factors in efforts to prevent the perpetuating cycle of chronic inflammatory disease.
We also performed experiments examining the effect of TCN on CCL2 (monocyte chemotactic protein 1) production by HMEC-1 cells and primary human dermal endothelial cells as Seiffert et al. (34) also found that ATPγS induced release of CCL2 from HMEC-1 cells. In most experiments, TCN suppressed induction of CCL2 production by ATPγS, ATP and TNFα. However, the degree of suppression was modest and required larger concentrations of TCN. We are still evaluating the role of TCN in regulating CCL2 expression.
In this study, we report that TCN is able to dose-dependently inhibit ATPγS-induced chemokine secretion by HMEC-1 as well as primary human dermal microvascular endothelial cells. TCN inhibited stimulated CXCL8 and CXCL1 production by HMEC-1 cells and CXCL8 and CXCL1 production by primary endothelial cells. TCN also inhibited TNFα-stimulated production of CXCXL8 and CXCL1. TCN may also inhibit CCL2 production, but the effect seems less marked. The relative importance of TNFα (and/or other cytokines) versus ATP in stimulating chemokine release from endothelial cells in vivo is difficult to assess. The concentration of ATP at the site of release by a nerve ending in situ is difficult to assess. However, release of ATP from sympathetic nerves could be relevant to stress-induced effects on inflammatory skin diseases.
The concentrations of TCN that were effective in many of our experiments are within or close to the physiologically relevant serum ranges achieved therapeutically in humans taking oral TCN. For example, a study showed mean serum concentrations of TCN in patients taking 250, 500, 750 and 1000 mg of TCN hydrochloride per day were 3.79, 5.94, 5.57 and 6.60 mg/l (μg/ml), respectively (32). Another study showed that human serum concentration of therapeutic minocycline treatment was approximately 2 μg/ml but that the gingival crevicular fluid was four to five times greater (46). Interestingly, it should be noted that TCN are lipophilic and therefore can have increased concentration in peripheral tissues such as skin compared with that of serum. Also, in our experiments, cells were exposed to TCN for 24 h. In the clinical situation, patients take TCN for weeks and long-term exposure might, hypothetically, have effects at lower concentrations. In addition, studies have shown that TCN can accumulate in leukocytes (47). There is a lack of studies evaluating whether TCN accumulate in other cell types such as endothelial cells therefore making the evaluation of concentration in an in vitro culture system difficult to assess. Nevertheless, the results of the current study support that TCN has a significant effect on endothelial chemokine release at or close to concentrations achieved therapeutically in the serum of humans.
It should be mentioned that purinergic nucleotides may also affect cutaneous inflammation through effects on keratinocytes. Pastore et al. (48) have demonstrated that ATP upregulates interferon (IFN)γ-induced expression of CCL2, CCL5 and CXCL8 while inhibiting IFNγ-induced expression of CXCR3, CXCL9, CXCL10 and CXCL11. This effect could be mimicked by adenosine diphosphate. Using pharmacologic means, these investigators demonstrated expression of most P2Y and P2X receptors on keratinocytes. They hypothesize that action on these receptors modulates skin inflammation.
Many factors have important regulatory effects on endothelial cell function. For example, the sphingolipid metabolite tetraacetyl-phytosphingosine (TAPS) was found to reduce vascular endothelial growth factor (VEGF)-induced chemotaxis and capillary-like tube formation by human umbilical vein endothelial cells (49). TAPS also inhibited VEGF-induced proteolytic enzyme production [including MMP-2, urokinase-type plasminogen activator and plasminogen activator inhibitor-1 (49)]. It was found to inhibit VEGF-induced phosphorylation of p42/44 extracellular signal-regulated kinase and c-Jun N-terminal kinase as well as VEGF-induced intracellular calcium increase (49). Through these effects, TAPS can inhibit angiogenesis. The experiments herein demonstrate that ATP and TCN can also regulate aspects of endothelial cell biology.
In summary, we hypothesize that the anti-inflammatory properties of TCN may be due in part to its ability to suppress chemokine release by endothelial cells. Future studies will be performed to investigate the potency and precise mechanism by which TCN are effective in inhibiting chemokine release. Regarding the potency of various drugs in the TCN family, it has been shown that doxycycline (IC50 = 15 μm, ~8 μg/ml) is more powerful in inhibiting collagenase than is minocycline (IC50 = 190 μm, ~94 μg/ml) or TCN (IC50 = 350 μm, ~168 μg/ml) (6). Additional research will be performed to determine the IC50 of TCN’s inhibitory actions on chemokine release in comparison with that of doxycycline and minocycline. Finally, research will further examine chemokine promoter expression, the amount of chemokine mRNA production and chemokine mRNA stability in response to TCN.
Acknowledgments
This work was funded by a grant from the National Rosacea Society and an agreement with Clinique Laboratories, LLC. Dr Granstein is the principal investigator on a research agreement with Clinique Laboratories, LLC and Clinique Laboratories, LLC has provided other support to the Department of Dermatology at the Weill Cornell Medical College.
Abbreviations
- ATP
adenosine-5′-triphosphate
- ATPγS
adenosine 5′-O-(3-thiotriphosphate)
- HC
hydrocortisone
- HMEC-1
human microvascular endothelial cell line-1
- IFNγ
interferon-gamma
- PCN
penicillin
- TAPS
tetraacetyl-phytosphingosine
- TCN
tetracycline
- VEGF
vascular endothelial growth factor
References
- 1.Golub LM, Ciancio S, Ramamamurthy NS, Leung M, McNamara TF. Low-dose doxycycline therapy: effect on gingival and crevicular fluid collagenase activity in humans. J Periodontal Res. 1990;25:321–330. doi: 10.1111/j.1600-0765.1990.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 2.Golub LM, Goodson JM, Lee HM, Vidal AM, McNamara TF, Ramamurthy NS. Tetracyclines inhibit tissue collagenases. Effects of ingested low-dose and local delivery systems. J Periodontol. 1985;56:93–97. doi: 10.1902/jop.1985.56.11s.93. [DOI] [PubMed] [Google Scholar]
- 3.Golub LM, Lee HM, Lehrer G, et al. Minocycline reduces gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. J Periodontal Res. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 4.Golub LM, Wolff M, Lee HM, et al. Further evidence that tetracyclines inhibit collagenase activity in human crevicular fluid and from other mammalian sources. J Periodontal Res. 1985;20:12–23. doi: 10.1111/j.1600-0765.1985.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 5.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 6.Burns FR, Stack MS, Gray RD, Paterson CA. Inhibition of purified collagenase from alkali-burned rabbit corneas. Invest Ophthalmol Vis Sci. 1989;30:1569–1575. [PubMed] [Google Scholar]
- 7.Choi DH, Moon IS, Choi BK, et al. Effects of subantimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J Periodontal Res. 2004;39:20–26. doi: 10.1111/j.1600-0765.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- 8.Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472–1483. doi: 10.1902/jop.2000.71.9.1472. [DOI] [PubMed] [Google Scholar]
- 9.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Tsankov N, Broshtilova V, Kazandjieva J. Tetracyclines in dermatology. Clin Dermatol. 2003;21:33–39. doi: 10.1016/s0738-081x(02)00324-3. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg JM. The anti-inflammatory effects of tetracyclines. Cutis. 2005;75:6–11. [PubMed] [Google Scholar]
- 12.Kloppenburg M, Dijkmans BA, Breedveld FC. Antimicrobial therapy for rheumatoid arthritis. Baillieres Clin Rheumatol. 1995;9:759–769. doi: 10.1016/s0950-3579(05)80312-7. [DOI] [PubMed] [Google Scholar]
- 13.Kloppenburg M, Dijkmans BA, Verweij CL, Breedveld FC. Inflammatory and immunological parameters of disease activity in rheumatoid arthritis patients treated with minocycline. Immunopharmacology. 1996;31:163–169. doi: 10.1016/0162-3109(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 14.Langevitz P, Livneh A, Bank I, Pras M. Benefits and risks of minocycline in rheumatoid arthritis. Drug Saf. 2000;22:405–414. doi: 10.2165/00002018-200022050-00007. [DOI] [PubMed] [Google Scholar]
- 15.Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 16.Berman B, Zell D. Subantimicrobial dose doxycycline: a unique treatment for rosacea. Cutis. 2005;75:19–24. [PubMed] [Google Scholar]
- 17.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu JK, Franciosi S, Sattayaprasert P, Kim SU, McLarnon JG. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia. 2004;48:85–90. doi: 10.1002/glia.20051. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Zhu S, Drozda M, et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu S, Stavrovskaya IG, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 21.Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51:215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- 22.Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996;64:825–828. doi: 10.1128/iai.64.3.825-828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson-Burns SM, Tyler KL. Minocycline delays disease onset and mortality in reovirus encephalitis. Exp Neurol. 2005;192:331–339. doi: 10.1016/j.expneurol.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Nieman GF, Zerler BR. A role for the anti-inflammatory properties of tetracyclines in the prevention of acute lung injury. Curr Med Chem. 2001;8:317–325. doi: 10.2174/0929867013373570. [DOI] [PubMed] [Google Scholar]
- 25.Feldman SR, Hollar CB, Gupta AK, Fleischer AB., Jr Women commonly seek care for rosacea: dermatologists frequently provide the care. Cutis. 2001;68:156–160. [PubMed] [Google Scholar]
- 26.Litt JZ. Rosacea: how to recognize and treat an age-related skin disease. Geriatrics. 1997;5242:39–40. 45–37. [PubMed] [Google Scholar]
- 27.Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144–150. doi: 10.1177/014107689709000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelle MT, Crawford GH, James WD. Rosacea: II. Therapy. J Am Acad Dermatol. 2004;51:499–512. doi: 10.1016/j.jaad.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Sneddon IB. A clinical trial of tetracycline in rosacea. Br J Dermatol. 1966;78:649–652. doi: 10.1111/j.1365-2133.1966.tb12168.x. [DOI] [PubMed] [Google Scholar]
- 30.Knight AG, Vickers CF. A follow-up of tetracycline-treated rosacea. With special reference to rosacea keratitis. Br J Dermatol. 1975;93:577–580. doi: 10.1111/j.1365-2133.1975.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 31.Skidmore R, Kovach R, Walker C, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Arch Dermatol. 2003;139:459–464. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- 32.Esterly NB, Koransky JS, Furey NL, Trevisan M. Neutrophil chemotaxis in patients with acne receiving oral tetracycline therapy. Arch Dermatol. 1984;120:1308–1313. [PubMed] [Google Scholar]
- 33.Golub LM, McNamara TF, D’Angelo G, Greenwald RA, Ramamurthy NS. A non-antibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J Dent Res. 1987;66:1310–1314. doi: 10.1177/00220345870660080401. [DOI] [PubMed] [Google Scholar]
- 34.Seiffert K, Ding W, Wagner JA, Granstein RD. ATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J Invest Dermatol. 2006;126:1017–1027. doi: 10.1038/sj.jid.5700135. [DOI] [PubMed] [Google Scholar]
- 35.Ades EW, Candal FJ, Swerlick RA, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 36.Swerlick RA, Lawley TJ. Role of microvascular endothelial cells in inflammation. J Invest Dermatol. 1993;100:111S–115S. doi: 10.1111/1523-1747.ep12356595. [DOI] [PubMed] [Google Scholar]
- 37.Goebeler M, Yoshimura T, Toksoy A, Ritter U, Bröcker EB, Gillitzer R. The chemokine repertoire of human dermal microvascular endothelial cells and its regulation by inflammatory cytokines. J Invest Dermatol. 1997;108:445–451. doi: 10.1111/1523-1747.ep12289711. [DOI] [PubMed] [Google Scholar]
- 38.Eklund KK, Sorsa T. Tetracycline derivative CMT-3 inhibits cytokine production, degranulation, and proliferation in cultured mouse and human mast cells. Ann N Y Acad Sci. 1999;878:689–691. doi: 10.1111/j.1749-6632.1999.tb07763.x. [DOI] [PubMed] [Google Scholar]
- 39.Kirkwood KL, Golub LM, Bradford PG. Non-antimicrobial and antimicrobial tetracyclines inhibit IL-6 expression in murine osteoblasts. Ann N Y Acad Sci. 1999;878:667–670. doi: 10.1111/j.1749-6632.1999.tb07757.x. [DOI] [PubMed] [Google Scholar]
- 40.Kirkwood K, Martin T, Andreadis ST, Kim YJ. Chemically modified tetracyclines selectively inhibit IL-6 expression in osteoblasts by decreasing mRNA stability. Biochem Pharmacol. 2003;66:1809–1819. doi: 10.1016/s0006-2952(03)00450-7. [DOI] [PubMed] [Google Scholar]
- 41.Akamatsu H, Asada M, Komura J, Asada Y, Niwa Y. Effect of doxycycline on the generation of reactive oxygen species: a possible mechanism of action of acne therapy with doxycycline. Acta Derm Venereol. 1992;72:178–179. [PubMed] [Google Scholar]
- 42.Milano S, Arcoleo F, D’Agostino P, Cillari E. Intraperitoneal injection of tetracyclines protects mice from lethal endotoxemia downregulating inducible nitric oxide synthase in various organs and cytokine and nitrate secretion in blood. Antimicrob Agents Chemother. 1997;41:117–121. doi: 10.1128/aac.41.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amin AR, Patel RN, Thakker GD, Lowenstein CJ, Attur MG, Abramson SB. Post-transcriptional regulation of inducible nitric oxide synthase mRNA in murine macrophages by doxycycline and chemically modified tetracyclines. FEBS Lett. 1997;410:259–264. doi: 10.1016/s0014-5793(97)00605-4. [DOI] [PubMed] [Google Scholar]
- 44.Di Virgilio F, Chiozzi P, Ferrari D, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 45.Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19:91–104. doi: 10.1089/107999099314234. [DOI] [PubMed] [Google Scholar]
- 46.Ciancio SG, Mather ML, McMullen JA. An evaluation of minocycline in patients with periodontal disease. J Periodontol. 1980;51:530–534. doi: 10.1902/jop.1980.51.9.530. [DOI] [PubMed] [Google Scholar]
- 47.Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15:355–366. doi: 10.2165/00003088-198815060-00001. [DOI] [PubMed] [Google Scholar]
- 48.Pastore S, Mascia F, Gulinelli S, et al. Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J Invest Dermatol. 2007;127:660–667. doi: 10.1038/sj.jid.5700591. [DOI] [PubMed] [Google Scholar]
- 49.Kwon YB, Kim CD, Kim BJ, et al. Anti-angiogenic effect of tetraacetyl-phytosphingosine. Exp Dermatol. 2007;16:311–317. doi: 10.1111/j.1600-0625.2006.00530.x. [DOI] [PubMed] [Google Scholar]