Abstract
Background
Maternal minority status is a risk factor for iron deficiency in infancy and pregnancy. Because language and cultural differences may limit research participation, a prospective study examining iron deficiency included maternal minority status as an inclusionary criterion. Cognizant of potential barriers to recruitment, goals were to quantify eligible Latina enrollees and refusals, examine participation barriers, and devise possible solutions.
Methods
Mothers and their full-term newborns were eligible if the women were anemic, diabetic during pregnancy, of minority and/or lower socioeconomic status, and/or delivered an infant outside the average weight range for gestational age. Self-reported ethnicity and reasons for participation refusal were documented.
Results
During the first 18 months, 255 mothers and their infants were enrolled. Based on inclusionary criteria and the percentage of minority women admitted to the birthing center in a year, we anticipated 25% minority enrollees, with 16.3% Latina. Although 27% minority enrollment was obtained, only 8% were Latina (P < 0.01). System barriers, researcher perception barriers, and participant perception barriers were encountered. Over the next 8 months, addressing these recruitment barriers improved Latina enrollment.
Conclusion
Enrollment barriers are significant hurdles to overcome, but with increased understanding and effort, more successful inclusion of Latina families can be achieved.
INTRODUCTION
Adequate representation of ethnic and racial minorities in clinical health research is challenging yet necessary to evaluate and reduce health disparities among minority groups. Progress is often hindered by low overall rates of minority participation in studies and the paucity of information regarding the influence of these demographic variables in many clinical situations.1,2 Increased awareness of minority under-enrollment among health care professionals and researchers may motivate increased representation of minority groups in clinical studies, allowing for the discovery of scientific knowledge that could benefit diverse populations.
In 1993, the National Institutes of Health (NIH) implemented the Revitalization Act to mandate the inclusion of women and people of minority status in all NIH-funded clinical trials.2,3 The NIH also required that clinical research grant proposals include recruitment strategies that enable addressing the impact of racial and ethnic minority status on clinical outcomes.4
The rapidly growing nature of the Latino population in the United States further highlights the need for adequate minority representation in clinical research.5,6 In Wisconsin, the site of this study, the Latino population has increased by 48.2% since 2000 and now constitutes 5.1% of the state's total population.7
The results being reported are a small portion of a larger prospective study in Madison, Wisconsin, that is investigating whether iron deficiency anemia (IDA) in infancy can be predicted by screening at-risk newborns for iron status at birth. Among other factors, maternal minority status is an important inclusionary criterion because iron deficiency is more prevalent among minorities.8–10 Moreover, up to one-fifth of Latinas may not see a physician before the mid-trimester, likely impacting iron nutrition and the use of prenatal vitamins.11 Inclusion of this at-risk and fast-growing population in clinical research is vital.
The research team anticipated the potential of recruitment barriers, although not to the extent that they were encountered. In the first 18 months of the study, half of the anticipated Latina enrollment was achieved, but enrollment improved as study adjustments were made. The goal of this paper is to increase awareness of several recruitment challenges by describing the barriers to Latina participation encountered in a prospective study of infantile IDA. We also describe adjustments to recruitment strategies as the study progressed and provide recommendations for optimizing minority representation in clinical research.
STUDY OBJECTIVES/METHODS
Subjects
Joint approval from the Meriter Hospital and University of Wisconsin Institutional Review Boards (IRBs) was received and recruitment of English-speaking patients began in June 2008. After a 3-month delay for a formal Spanish-language translation process, joint approval to recruit Spanish-speaking patients was obtained. Following delivery, but before hospital discharge, mothers and newborns meeting inclusionary criteria were recruited.
Inclusion Criteria
Risk for IDA in infancy includes a number of pregnancy and demographic factors, including at least 1 of the following: maternal minority status, low socioeconomic status, maternal anemia, maternal diabetes during pregnancy, and/or infants showing evidence of fetal overgrowth or undergrowth.9,10 In this report, the term “Latina” refers to women who self-reported to be of Hispanic background. Mothers ≥ 18 or ≤ 40 years old with healthy term newborns born ≥ 35 weeks gestation were enrolled. From June 2008 to August 2010, research personnel screened electronic medical records for births with 1 or more of the 5 listed risk factors. According to IRB guidance, approval from the bedside nurse was required before approaching the mother for informed consent. When bedside nurses disapproved of approaching a screened candidate, the patient's ethnic background was recorded. For potential enrollees whose primary language was Spanish, a hospital-approved interpreter was required to interpret the consent process. For those who refused participation, ethnic background and reasons for refusal were recorded.
Study Procedures
Study requirements included the authorization to release maternal and child computerized medical records, including labor and delivery data, and follow-up data. Umbilical cord blood from delivery was obtained. To measure iron status, the study requested follow-up blood draws on the infant at an outpatient laboratory, and subsequently, in the second year of the study, in the participant's home upon request. The study was low-risk, non-interventional, and minimally invasive. Participants received a grocery store gift certificate worth $25 upon completion of each follow-up blood draw.
Data Management
The Meriter Hospital Birthing Center delivers approximately 3800 newborns per year. Using the hospital's electronic data-base, the ethnic and racial minority demographics of deliveries were collected. Chi-square and Fisher exact testing were used to examine observed and predicted enrollment rates on the basis of enrollee ethnicity.
RESULTS
Enrollment
In the first 18 months of the study, 255 mothers and their newborns were enrolled. Based on demographic information about ethnicity of deliveries from the prior year, 25% minority enrollment was predicted. Minority recruitment of 27% was observed, consistent with prior predictions. Using the African American enrollee percentage (13.5%) as the criterion to gauge the participation of other minorities, 16.3% Latina enrollment was anticipated. Although the expected values for other minority enrollees (ie, Asian) were observed, the number of Latina enrollees was half of the anticipated amount (P < 0.005). Recruitment strategies were adjusted by increasing identification of Latina enrollees, increasing recruiter work hours, involving family members earlier in the study consent process, and working more closely with hospital interpreters. Over the next 8 months of the study, 20% of the additional enrollees were Latina, increasing Latina enrollment to 10% of the overall study population.
Refusals
For the study's duration, there were 167 refusals, 65 (39%) of which were from women of minority status. Of the minority refusals, 18 were Latinas. Rates of refusal, based on potential enrollees screened are portrayed by race/ethnicity in Figure 1.
Figure 1.
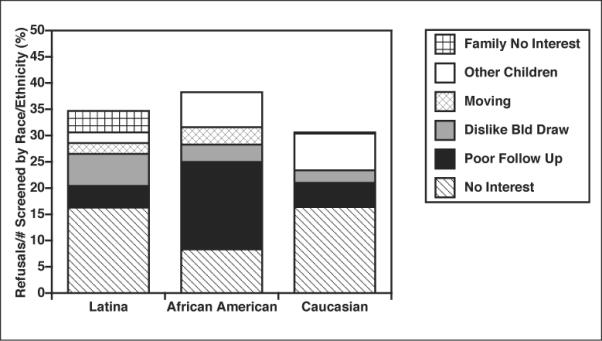
Refusals by race/ethnicity. Potential participants who refused or denied are divided by total number screened. The percentages are displayed by race/ethnicity, and are also separated by reasons for refusal. “No Interest” meant refusal by the woman (diagonal lines), “Poor Follow Up” was an assumption of poor follow-up by either denial to approach by nursing caregiver or similar assessment by researcher (black), “Dislike Blood Draw” reflects a maternal concern about the infant follow-up blood draws (gray), “Moving” connotes moving out of the country (diamonds), “Other Children” indicates the family was too busy with other children for follow-up (white), and “Family No Interest” conveys a refusal by another family member (checks). The overall rate of refusals, based on those screened, was similar across the race/ethnic groups.
DISCUSSION
The goal of this paper is to increase awareness of barriers to enrolling Latino subjects in clinical studies by describing our experience and to provide recommendations from the literature for optimizing minority representation in clinical research. Although similar overall refusal rates were seen for each ethnic group, lower than anticipated rates of Latina enrollment were observed initially. One unique reason for Latina refusal was a disinterest in the study from other family members. However, the percent refusing did not fully account for the 50% lower initial Latina enrollment. It is probable that a culmination of factors initially prevented a proportionate number of Latina women from being approached by recruiters. With recognition of the hurdles discussed below and adjustments made to recruitment strategies, improved Latina participation was observed.
Problems with recruiting minority populations are often attributed to 3 barrier types.3,6,12 System barriers are caused by issues in study design and implementation, participant perception barriers are due to their understanding of research based on their personal history and prior experiences,12 and researcher perception barriers are attributed to research staff avoidance of hard-to-reach populations due to limited time and resources.1 All 3 barrier types were experienced during our study of IDA. The system barriers included difficulties with the IRB approval process, challenges in obtaining interpreters within the required window of time, and a shortage of bilingual study staff. Researcher barriers included recruiter and bedside nursing bias. Participant perception barriers included language and cultural barriers, and family members discouraging enrollment.
System Barriers—IRB Process and Expense
Better anticipation of expenses and delays is important for ensuring recruitment success. Although our hospital has a teaching mission as a component of the university's obstetrical service, it supports fewer active research protocols than a typical university hospital. Consequently, our research protocol was the first in this clinical setting to require and offer Spanish-translated study consent forms and materials, making the translation and approval processes challenging. In an effort to protect the rights of the study subjects, an independent, for-profit translation service was required to convert study materials to Spanish. At study conception, it was not anticipated that the translation process would cost $800 and delay our ability to enroll Spanish-speaking participants by 3 months. Based on data from computerized medical logs, we estimate that at least 17 potential Spanish-speaking participants were excluded during this delay.
System Barriers—Interpreters
Understanding IRB requirements and/or the process by which an interpreter becomes approved was also a critical issue, as a hospital-approved interpreter was required to translate the consent process. This requirement was not evident initially and proved to be challenging. In our study, women delivering vaginally were hospitalized for only 36 – 48 hours, offering a small window of time to allow for recovery, screening of potential participants, and obtaining interpreters. Because of their other important responsibilities, the interpreters' schedules were often unpredictable, and part-time research recruiters or potential subjects commonly could not wait for the interpreter. Recruiters estimated that at least 11 Spanish-speaking potential participants were missed because the interpreters were unavailable.
Although interpreters are present to ensure linguistic proficiency, other researchers have noted that interpreters not directly involved with a study may also inadvertently impersonalize communication, making it more difficult to engender and build trust with the research team.13 While well-trained for clinical duties, interpreters may not necessarily be trained or as invested in clinical research. This experiential background may be critical, because precise wording during recruitment is essential, ie, the use of the Spanish word for “study” rather than the more threatening “experiment.”3
System Barriers—Resources
In our study, the lack of full-time, approved bilingual research staff may have impaired recruitment of Latina subjects. Sources explain that a lack of research staff diversity may be detrimental, as potential participants prefer study personnel who “look (and speak) like them.”14,15 Financial support for bilingual perinatal research nurses was not available through our Clinical Research and Translational Core grant. E-mail correspondence with staff from the Wisconsin Nurses Association revealed no mechanism to quantify the number of bilingual nurses currently practicing in the state. However, the Wisconsin Nurse Faculty Task Force has acknowledged, “the current workforce and the nurse educator workforce does not reflect the diversity of the state.”16
This type of staffing problem seems to be less salient in the geographic West and Southwest, which have larger bilingual health care workforces, as compared to a region such as the Midwest. Perinatal studies in the Southwest with similar sample sizes report more Latina enrollees, supporting fewer barriers to enrollment.17,18
System Barriers—Study Design
Recognizing potential barriers in study design is important to ensure a more diverse representation. Previous reports recommended using community outreach and direct access to clinics serving hard-to-reach targeted populations.6,14,19 Both approaches were investigated but proved unhelpful, because Latino health fairs were held infrequently, and the prenatal clinics predominantly serving Latinos were not interested in collaborating.5 Our project was somewhat constrained by limited budgetary resources to support research nurses, deeming inpatient screening to be more time efficient than clinic screening.
Perception Barriers—Researcher Bias
While other published reports have noted some mistrust and misperception of Latinos toward research, a reverse bias may be as important.1 Because refusal rates were similar between all ethnic groups, it is probable that in the first 18 months of the study, recruiters did not approach qualified Latina candidates at a rate proportional to other groups. In the next 8 months, after adjustment of recruitment strategies, improved Latina recruitment was seen. Although candidates were identified by the use of electronic census logs, additional required steps were necessary to determine whether a Spanish interpreter was necessary. If so, time invested for recruitment of Latinas was commonly doubled, likely causing the busy recruiters to preferentially seek easier-to-identify subjects. The literature discusses the theory of “The Good Study Patient,” which proposes that with a short timeline, unpredictability of interpreter arrival, limited resources, and need for follow-up, recruiters may be pressured to seek out participants implicitly perceived to be most compliant.1,14 Additionally, bedside nurses may inadvertently introduce some bias because they determine whether researchers are allowed to approach potential enrollees. Six of the 18 Latina refusals were because bedside nurses suggested avoiding potential subjects due to their perceived likelihood of poor follow-up, social issues, or because the patient “appeared” overwhelmed.
Participant Perception Barriers—Communication
Translating legal terminology and the sometimes subtle intent of an English consent form into a written Spanish document can be challenging. Additionally, the enrollee's spoken and written Spanish proficiency may not be equivalent.15,20 Many Spanish dialects are spoken, and assuming “one Spanish translation of a consent form fits all” is unrealistic.5
The support and involvement of “la familia” is crucial.6,15,21 Ineffective communication with the potential participants may cause a reliance on family members for the information,3,6,13,22 potentially contributing to miscommunication and lower enrollment. We observed a reliance on family and hesitation to independently make participation decisions by Latinas more often than in African American or white mothers. In at least 1 situation, English proficiency of a mother, but not of other family members, negatively influenced enrollment, suggesting that using interpreters involved with the study may be helpful, even with a mother who comfortably speaks English.
Participation Perception Barriers—Culture
Despite the use of interpreters and translation services, it is important to recognize that a study is not guaranteed to be culturally appropriate.5 Minority mistrust and fear of medical research is widely recognized,23 with the expectation of poor service, lack of culturally competent providers, and long waiting times for interpreters.24 In research involving genetic testing, where the donation of a blood or tissue sample was required, minority status was linked to lower rates of participation, with concerns about data misuse, racial discrimination, and unequal access to the potential research benefits.25 History of inappropriate use of minorities in medical research is also of concern, specifically as a result of the infamous Tuskegee Study on syphilis for African Americans.2,23,25
Undocumented immigrant status among the Latino population may contribute to under-enrollment because of a fear that their status may be discovered.5,6,15 Because of this concern, grocery cards or check rewards may not be culturally appropriate participation incentives as identifying documents are required for redemption.26 Cash as a research incentive may be optimal, but university policies may require social security numbers and contact information for tax and accounting purposes.
Changes of contact address and phone number were often encountered, illustrating the more transient nature of the Latino population. To compensate, the study design allowed tracking of the updated address and/or 12-month hemoglobin value, the primary study endpoint, in the electronic medical record. Literature demonstrates that mobile residency patterns have also been an issue in other clinical studies.19,21 Other researchers have speculated that potential Latina participants are unwilling to commit to long-term research when their living situations are temporary, the study is a low priority for them, and medical concerns of potential participants and researchers are mismatched.6,12,14 For example, when surveyed, neonatal and immediate pediatric care were reported as being of utmost importance to health care professionals, but not for the Latinos.24
CONCLUSIONS AND SUGGESTIONS
The difficulty in recruitment and retention of Latinos in clinical health research is an ongoing challenge. The list of barriers to recruitment is substantial, but with careful study design and practice, can be minimized or largely eliminated.15 The goal of sharing this experience is to offer suggestions for improving recruitment of Latino, as well as other minority and hard-to-reach populations.
More collaboration between researchers, providers, and the surrounding community is key. An initial step could be gaining better support from community gatekeepers, ie, church leaders, civic leaders, and community health care professionals to help garner trust within a community.6,13–15 Researchers and community leaders can then help to refine a greater awareness of the health priorities of a given community,14,27 and when possible, frame study goals within these priorities. Although most studies, like this one, set forth a broad goal to include minorities as a critical subset of the participants, the study name and materials could be modified to be more appropriate for all subgroups. For example, in our case, some Latinos were more familiar with the term “low hemoglobin” than “iron deficiency,” so the study name could ideally accommodate this cultural preference.
Some of the steps toward a balanced representation in clinical research likely will be costly, and funding agencies will need to recognize the added cost. The availability of some financial support from the university or hospital to cover required professional translation expenses would be helpful. Institutions should focus on training more bilingual clinical and research personnel or increasing training of clinical hospital interpreter teams in research methodology.16 Ideally, bilingual research team members could make recruitment and follow-up phone calls to help build more empathetic relationships between the subject and the researcher,15 and generally, to serve as health care advocates for the participant.23,26
Sensitive approaches to recruitment begin with recognizing cultural values.6,14,15 Because of the importance of family in the Latino community,6,15,21 research methods should encourage family involvement. The study budget could also include the cost of taxicabs to transport participants without vehicles to and from follow-up appointments, as well as to provide a child-friendly research site for the other children.11,15,21,26 Flexibility with the times of phone calls and appointments and Spanish signage in clinics where follow-up appointments take place would increase ease of participation. Incentives should be culturally appropriate, including cash rewards if possible. To give more back to participants, researchers should provide study updates and employ results in a way that would benefit the community,14,27 including the distribution of educational materials to promote health and well-being.6,26
Despite lower than anticipated initial enrollment in our study, Latina representation was improved after awareness of several of the discussed barriers to participation. By increasing the availability of the recruiters, employing a native Spanish speaker to make study-related phone calls, recognizing some of the culturally sensitive issues for the participants, and accommodating family involvement in the consent process, a more balanced study population was achieved.
As maternal minority is a risk factor for iron deficiency and other health disparities, it is imperative that the mothers and infants of diverse populations be adequately represented. Although it may require an extra investment of time and resources, communities, health care professionals, and researchers must continue to prioritize the attainment of greater diversity in studies to achieve the goal of improving health among all populations.
Acknowledgments
We thank the participating families, Meriter Hospital Birthing Center, Deb Krumpos, RN, Patricia Green-Sotos, RN, Sharon E. Blohowiak, BS, Melinda E. Chen, BS, Sheila C. Roy, BS, Vidya Sridhar, MBBS, Lauren K. Dahlin, BS, Brian R. Pisula, BS, Mary Bacsik, BS, Karen Flores, UW COAST Research Team, Murray L. Katcher MD, PhD, Daphne Pham, PhD, and Anthony Auger, PhD.
Support/Funding: Meriter Foundation, NIH 1 ULRR026011 UW CTSA Program, NIH T32HD048302 Health Disparities Research Scholar, UW Medical Education & Research Committee—Wisconsin Partnership Program, Thrasher Research Fund. UW Medical Student Shapiro Research Fund and UW Medical Student Cardiovascular Research Center Grant
Footnotes
Financial Disclosures: None declared.
REFERENCES
- 1.Joseph G, Dohan D. Diversity of participants in clinical trials in an academic medical center: the role of the `Good Study Patient?'. Cancer. 2009;115(3):608–615. doi: 10.1002/cncr.24028. [DOI] [PubMed] [Google Scholar]
- 2.Bustillos D. Limited English proficiency and disparities in clinical research. J Law Med Ethics. 2009;37(1):28–37. doi: 10.1111/j.1748-720X.2009.00348.x. [DOI] [PubMed] [Google Scholar]
- 3.Joseph G, Dohan D. Recruiting minorities where they receive care: institutional barriers to cancer clinical trials recruitment in a safety-net hospital. Contemp Clin Trials. 2009;30(6):552–559. doi: 10.1016/j.cct.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Ness RB, Nelson DB, Kumanyika SK, Grisso JA. Evaluating minority recruitment into clinical studies: how good are the data? Ann Epidemiol. 1997;7(7):472–478. doi: 10.1016/s1047-2797(97)00080-x. [DOI] [PubMed] [Google Scholar]
- 5.Suarez-Morales L, Matthews J, Martino S, et al. Issues in designing and implementing a Spanish-language multi-site clinical trial. Am J Addict. 2007;16(3):206–215. doi: 10.1080/10550490701375707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelman CR. Learning from recruitment challenges: barriers to diagnosis, treatment, and research participation for Latinos with symptoms of Alzheimer's disease. J Gerontol Soc Work. 2010;53(1):94–113. doi: 10.1080/01634370903361847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poston B. Hispanic residents increasing in state; population rises 48% in 8 years, furthering integration trends. Milwaukee Journal Sentinel. 2009 May 14; [Google Scholar]
- 8.Baumann-Blackmore NL, Goetz E, Blohowiak SE, Zaka O, Kling PJ. Cord blood zinc protoporphyrin/heme ratio in minority neonates at risk for iron deficiency. J Pediatr. 2008;153(1):133–136. doi: 10.1016/j.jpeds.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: risk factors and racial/ethnic disparities. Pediatrics. 2008;121(3):651–652. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- 10.Lozoff BJE, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. 2007;161(5):523. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes-Bautista DE, Baezconde-Garbanati L, Hayes-Bautista M. Latino health in Los Angeles: family medicine in a changing minority context. Fam Pract. 1994;11(3):318–324. doi: 10.1093/fampra/11.3.318. [DOI] [PubMed] [Google Scholar]
- 12.Robinson JM, Trochim WM. An examination of community members', researchers' and health professionals' perceptions of barriers to minority participation in medical research: an application of concept mapping. Ethn Health. 2007;12(5):521–539. doi: 10.1080/13557850701616987. [DOI] [PubMed] [Google Scholar]
- 13.Cristancho S, Garces DM, Peters KE, Mueller BC. Listening to rural Hispanic immigrants in the Midwest: a community-based participatory assessment of major barriers to health care access and use. Qual Health Res. 2008;18(5):633–646. doi: 10.1177/1049732308316669. [DOI] [PubMed] [Google Scholar]
- 14.Stark N, Paskett E, Bell R, et al. Increasing participation of minorities in cancer clinical trials: summary of the “Moving Beyond the Barriers” Conference in North Carolina. J Natl Med Assoc. 2002;94(1):31–39. [PMC free article] [PubMed] [Google Scholar]
- 15.Daunt DJ. Ethnicity and recruitment rates in clinical research studies. Appl Nurs Res. 2003;16(3):189–195. doi: 10.1016/s0897-1897(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 16.Force WNFST. Educating the Nursing Workforce: The Nurse Faculty Shortage in Wisconsin. 2007 June; [Google Scholar]
- 17.Wadhwa PD, Garite TJ, Porto M, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz RJ, Stowe RP, Goluszko E, Clark MC, Tan A. The relationships among acculturation, body mass index, depression, and interleukin 1-receptor antagonist in Hispanic pregnant women. Ethn Dis. 2007;17(2):338–343. [PubMed] [Google Scholar]
- 19.Eakin EG, Bull SS, Riley K, Reeves MM, Gutierrez S, McLaughlin P. Recruitment and retention of Latinos in a primary care-based physical activity and diet trial: The Resources for Health study. Health Educ Res. 2007;22(3):361–371. doi: 10.1093/her/cyl095. [DOI] [PubMed] [Google Scholar]
- 20.Resnik DB, Jones CW. Research subjects with limited English proficiency: ethical and legal issues. Account Res. 2006;13(2):157–177. doi: 10.1080/08989620600654043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman A, Richardson IM, Gesell S, Barkin SL. Recruitment and retention of Latino children in a lifestyle intervention. Am J Health Behav. 2009;33(5):581–586. [PMC free article] [PubMed] [Google Scholar]
- 22.Carter-Pokras O, Zambrana RE, Mora SE, Aaby KA. Emergency preparedness: knowledge and perceptions of Latin American immigrants. J Health Care Poor Underserved. 2007;18(2):465–481. doi: 10.1353/hpu.2007.0026. [DOI] [PubMed] [Google Scholar]
- 23.Broome B. Research and under represented groups. J Cult Divers. 2007;14(2):55. [PubMed] [Google Scholar]
- 24.Martinez IL, Carter-Pokras O. Assessing health concerns and barriers in a heterogeneous Latino community. J Health Care Poor Underserved. 2006;17(4):899–909. doi: 10.1353/hpu.2006.0129. [DOI] [PubMed] [Google Scholar]
- 25.Bussey-Jones J, Garrett J, Henderson G, Moloney M, Blumenthal C, Corbie-Smith G. The role of race and trust in tissue/blood donation for genetic research. Genet Med. 2010;12(2):116–121. doi: 10.1097/GIM.0b013e3181cd6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberg KC-DK, Anderson B. The key to successful recruitment and retention in clinical research programs. Monitor. 2006:13–16. [Google Scholar]
- 27.Martinez IL, Carter-Pokras O, Brown PB. Addressing the challenges of Latino health research: participatory approaches in an emergent urban community. J Natl Med Assoc. 2009;101(9):908–914. doi: 10.1016/s0027-9684(15)31038-5. [DOI] [PubMed] [Google Scholar]


