Abstract
The embryonic zebrafish model offers the power of whole-animal investigations (e.g. intact organism, functional homeostatic feedback mechanisms and intercellular signaling) with the convenience of cell culture (e.g. cost- and time-efficient, minimal infrastructure, small quantities of nanomaterial solutions required). The model system overcomes many of the current limitations in rapid to high-throughput screening of drugs/compounds and casts a broad net to rapidly evaluate integrate system effects. Additionally, it is an ideal platform to follow up with targeted studies aimed at the mechanisms of toxic action. Exposures are carried out in 96-well plates so minimal solution volumes are required for the assessments. Numerous morphological, developmental and behavioral endpoints can be evaluated non-invasively due to the transparent nature of the embryos.
Keywords: Zebrafish, development, embryos, in vivo, vertebrate, rapid screening
1. Introduction
Numerous biological models can be employed for toxicity evaluations. In vitro techniques, such as cell culture systems, are often preferred because of they are both cost- and time-efficient. While these studies are useful, direct translation to whole organisms and human health is often difficult to infer. In vivo studies can provide improved prediction of biological response in intact systems but often require extensive facilities and infrastructure (1). Zebrafish (Danio rerio) offer a number of practical advantages as a model organism that overcome these limitations, making these vertebrates highly amenable for toxicologically relevant research. Zebrafish can be employed as a powerful in vivo model system to assess biological interactions and are an outstanding platform to detail the mechanisms by which substances elicit specific biological responses. A remarkable similarity in cellular structure, signaling processes, anatomy and physiology exist among zebrafish and other high-order vertebrates, particularly early in development (2–6). Current estimates indicate that over 90% of the human open reading frames are homologous to genes in fish (7). Thus, investigations using this model system can reveal subtle interactions that are likely to be conserved across species.
Features of the zebrafish’s biology are favorable for adapting this model system to high-throughput assays. Female zebrafish are able to produce hundreds of eggs weekly, so large sample sizes are easily achieved, allowing for statistically powerful dose-response studies. This abundant supply of embryos also makes it possible to simultaneously assess the toxicity of a large number of substances in a short period. The vertebrate’s rapid developmental progression compared to other mammals makes it an ideal model for high-throughput screening (8). For example, neuronal plate formation occurs at 10 hours post fertilization (hpf), followed by organogenesis at 24 hpf, which compared to a rat occurs at 9.5 days and 5–6 days respectively. The first heartbeat occurs at 30 hpf for the zebrafish and 10.2 days for rats (9).
Zebrafish embryos can be individually exposed in wells of a multi-well plate so the required volume needed for the model is small; thus, only limited amounts of materials are needed to assess an entire suite of biological interactions and responses. Early developmental life stages are often uniquely sensitive to environmental insult, due in part to the enormous changes in cellular differentiation, proliferation and migration required to form multiple cell types, tissues and organs (2, 5, 6, 10). Since development is highly coordinated requiring specific cell-to-cell communications, if exposure to a substance during that critical period perturbed these interactions, development would be expected to be disrupted. Embryos are waterborne–exposed to a chemical using a continuous method in which 24 embryos are exposed per concentration in individual wells of a multi-well plate from 8 to 120 hpf. Exposure until 120 hpf is the ideal duration for a developmental toxicity testing; primarily due to the vertebrate model’s ability to obtain its nutrients from its yolk sac until five days, which will not introduce new confounding factors. Perturbed development can manifest as morphological malformations, behavioral abnormalities or death of the embryos. Zebrafish embryos develop externally and are optically transparent so it is possible to resolve individual cells in vivo throughout the duration of an exposure using simple microscopic techniques and numerous effects can be assessed non-invasively over the course of development.
2. Materials
2.1. Zebrafish Husbandry
Fish water: 0.3 g/L Instant Ocean salts (Aquatic Ecosystems, Apopka, FL) in reverse osmosis (RO) water.
Incubator set at 28 ± 0.1 °C.
2.2. Dechorination
Compound stereo microscope for viewing embryos.
60 mm glass petri dish.
50 mg/mL pronase (Sigma-Aldrich, cat # 81750) in RO water. Measure 50 mg of pronase into a 1.5 mL microcentrifuge tube and fill it with 1 mL of RO water. Aliquot 50 μl into 1.5 mL microcentrifuge tube and place them into a freezer box, then immediately place into the box into the freezer. This will make 20 1.5mL microcentrifuge tubes that can be stored for up to 4 months. Aliquots can be thawed just prior to use.
Timer.
2.3. Exposure
Multi-well plates.
8 or 12 multichannel pipette.
50 mL reagent reservoir.
Wide-bore Pasteur pipette.
2.4. Assessment
Anesthesia: 4mg/mL of 3-aminobenzoate ethyl ester methanesulfonate salt (tricaine, Sigma-Aldrich, cat # A-5040) in RO water, pH adjusted to 7.0 with Tris-HCl, pH 9.0.
Methyl cellulose: 10 mg/mL of methyl cellulose (Sigma-Aldrich, cat # 274429, see Note 1).
3. Methods
3.1. Zebrafish Husbandry
Rear adult zebrafish Danio rerio in standard laboratory conditions of 28°C with a pH of 7 ± 0.2 on a 14 h light/10 h dark photoperiod (11).
House zebrafish in 2.0-liter polycarbonate tanks with recirculating water system. Keep adult zebrafish in groups to allow for large quantities of embryos to be collected. Group spawning also helps to increase genetic diversity.
Feed the fish twice daily with either crushed TetraMinR Tropical Flake or live Artemia from INVE (Salt Lake City, UT).
Spawning: place male and female zebrafish into spawning baskets in polycarbonate tanks the afternoon before the embryos are needed. Zebrafish will typically spawn when the lights come on after the 10 h dark period.
The following morning, newly fertilized eggs are collected, rinsed several times in system water and placed into fresh fish water in a 150 mm plastic petri dish.
Remove embryos that are unfertilized or necrotic prior to placing the petri dish into the incubator to keep warm until the embryos reach six hours post fertilization (hpf) (Fig. 1) [8].
Remove embryos that are not the same stage as the majority prior to experimental use (see Note 2).
Fig. 1. Six hours post fertilization embryos.
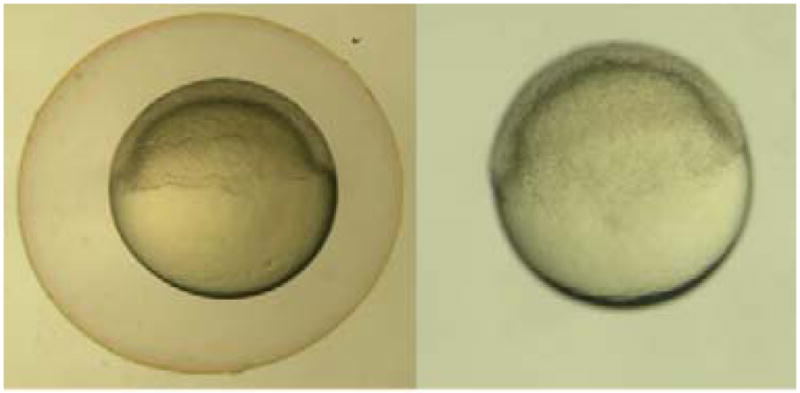
a) Six hpf embryo with its chorion. b) Six hpf embryo after using pronase to enzymatically remove its chorion.
3.2. Dechorination
To avoid barrier effects potentially posed by the chorion, all embryos should be dechorionated at six hours post fertilization (hpf) using a modified version of Westerfields (2000) (11) protocol for pronase enzyme degradation.
Place six hpf embryos into a 60 mm glass petri dish with 25 mL fish water (see Note 3). Up to 1200 embryos can be processed in a single dish using this method.
Add 50 μl of 50 mg/mL pronase to the center of the dish and continuously swirl gently to mix the solution.
Set a timer for seven minutes, and continuously swirl the embryos while occasionally observing the petri dish under the microscope to check for embryos without chorions, chorion pieces in the solution and ‘deflated’ chorions.
When seven minutes have passed, or when the above are observed, remove the pronase solution by diluting the solution with fresh fish water, slowly decanting over the edge of the petri dish continuously for one minute, then repeat this procedure for a total of 10 minutes (see Note 4).
After the rinse, allow the embryos to recover in the petri dish in an incubator (or a room at 28°C) until eight hpf (see Note 5).
3.3. Exposure
3.3.1. Waterborne exposure
Chemicals should be dissolved in fish water if possible (see Note 6). In the case that this is not possible, the solvent of choice for exposure utilizing the embryonic zebrafish is dimethyl sulfoxide (DMSO) (see Note 7).
Pour each test solution into a 50 mL reagent reservoir, which will fit a multichannel pipette.
For each exposure concentration tested, use a multichannel pipette to fill 24 individual wells in a multi-well plate with 100 μl of chemical solution. Seven concentrations and one control group can be tested using two 96-well plates.
At eight hpf, transfer viable, appropriately developing embryos into individual wells of a multi-well plate using a wide-bore glass pipette (see Note 8).
Incubate at 28°C until 24 hpf, then perform assessments.
3.3.2. Microinjection exposure
If direct delivery of a chemical is necessary to ensure accurate dose delivery, embryos should be microinjected at eight hpf (see Note 9).
Align eight hpf embryos in troughs embedded in a 1% agarose plate filled with fish water as described by The Zebrafish Book (9, 11).
Inject each embryo with 2.3 nL of the desired chemical concentration or the appropriate vehicle control directly into the yolk.
Place each embryo into individual wells of a 96-well plate, each filled with 100 μl of fish water. When directly delivering a chemical into the yolk sac, any concentration above 0.1% DMSO caused developmental defects not attributed to the chemical. If a chemical requires a solvent, two sets of serial dilutions should be made. The first serial dilution should be 100 times higher than the final concentration desired made with 100% DMSO. (see Note 10) For the second set of serial dilutions, from the 100% DMSO serial dilution, make a 1:10 dilution from the first serial dilution. Make sure to have an appropriate control for each chemical, which includes the correct percentage of solvent used in each solution.
Incubate at 28°C until first assessments at 24 hpf.
3.4. Assessment
At 24 hpf, embryos are assessed for viability, developmental progression and spontaneous movements (earliest behavior in zebrafish). Developmental progression is considered perturbed if zebrafish are more than 12 hours delayed compared to control animals. Spontaneous movements are assessed over a 2 minute period and is considered perturbed if there is a lack of embryonic contractions and/or movement.
At 120 hpf, larval morphology (body axis, eye, snout, jaw, otic vesicle, notochord, heart, brain, somite, fin, yolk sac, trunk, circulation, pigment, swim bladder; Fig. 2) is evaluated and recorded and behavioral endpoints (motility, tactile response) are thoroughly evaluated in vivo. Test for behavioral endpoints and then anesthetize animals for thorough morphological analysis. At the end of the assessments, zebrafish are euthanized with tricaine.
Evaluations are completed in a binary notation (present or not present) (see Note 11). Control and chemical-exposed groups are statistically compared using Fisher’s Exact test at p<0.05 (Sigma Stat, SPSS Inc., Chicago, IL) for each endpoint evaluated (see Note 12).
Fig. 2. Visual assessment of zebrafish morphology.
Images are given as examples of typical chemical-induced malformations observed in the zebrafish.
Acknowledgments
The authors would like to thank the Sinhubber Aquatic Research Laboratory and the Environmental Health Sciences Center at Oregon State University where much of the protocols were developed. This work was supported by EPA STAR grant RD-833320 and NIEHS grants ES03850 and ES07060.
Footnotes
Methyl cellulose is unique in that it ‘melts’ when cold and solidifies when hot. It dissolves best in cold water; however, it is best to disperse the powder form in warm water and then continue to mix while chilling. An alternate to the methyl cellulose is Protoslo® (Carolina Biological Supply Company, Burlington, NC).
Eggs can sometimes be laid and fertilized at different times in a group spawns, therefore always remove embryos that are developing more rapidly or significantly slower prior to using them for an experiment. As an alternate, male and female pairs can be set up in several divided tanks, and the dividers can be removed at the same time. The resulting stage matched embryos can then be pooled, prior to random embryo selection.
Do not bleach embryos is their chorions are to be removed by pronase digestion. Bleaching modifies the chorion and pronase treatment is completely ineffective. In addition, when dechorinating embryos it is essential to use glass petri dishes. Dechorinated embryos will stick to the bottom of plastic dishes and will be severely damaged during the procedure.
The newly dechorionated embryos are very delicate. Water should be administered with a gentle flow and not directly onto the embryos. Some of the embryos will not be out of their chorion even once the ten minute rinsing period is done. More will emerge during the recovery period.
Once an embryo is dechorinated, do not bleach the embryos.
Chemicals or drugs that are thought to be inactive until metabolized to an active form, may be pre-exposed to induce and active conformation prior to waterborne exposures.
The Sinnhuber Aquatic Research Laboratory at Oregon State University has demonstrated that an embryo elicited no developmental deformities at 1% DMSO when waterborne-exposed (1, 12, 13).
Be sure to allow the embryo to fall to the bottom of the wide-bore Pasteur pipette prior to touching the solution in the wells. If an embryo disintegrates when it reaches the solution, make sure to replace the solution and place a new embryo in the well.
All methods discussed are continuous waterborne exposure, but if no analytical method is available to determine biological uptake, an alternative is to directly deliver the chemical into the animal through microinjection. Because embryos are transparent, tissue dose and distribution can also be determined using fluorescently labeled materials and laser scanning confocal microscopy.
Make sure to vortex each microcentrifuge tube prior the next dilution to ensure it is a homogenous solution.
If more than 2 animals in the control group die, then the experiment is not valid and will need to be repeated. Test chemicals may have specific targets in humans, but this target may not be completely conserved-structurally in other vertebrate models. The structural differences between vertebrates and humans can result in either false negatives or false positives. For example, if a drug is designed to target a human specific structure that is not well-conserved in zebrafish, upon exposure, the drug would not influence the zebrafish target. The effects observed when this occurs are considered false negatives. Vice versa, a false positive can also occur when effects observed due to a drug impacting a specific target expressed only in zebrafish, but this target is not structurally conserved in humans. Another consideration is that chemical toxicity may be dependent on metabolic activity. False negatives and false positives may also occur if the metabolic activity in the zebrafish embryo is distinct from human metabolic activity. It is possible to use exogenous mammalian metabolic activation system to reduce false positive and false negatives (14).
References
- 1.Akimenko MA, Johnson SL, et al. Differential induction of four msx homeobox genes during fin development and regeneration in zebrafish. Development. 1995;121(2):347–57. doi: 10.1242/dev.121.2.347. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio S, Chapman J, et al. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 2002;297(5585):1301–10. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- 3.Blechinger SR, Warren JT, Jr, et al. Developmental toxicology of cadmium in living embryos of a stable transgenic zebrafish line. Environ Health Perspect. 2002;110(10):1041–6. doi: 10.1289/ehp.021101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busquet F, Nagel R, et al. Development of a new screening assay to identify proteratogenic substances using zebrafish danio rerio embryo combined with an exogenous mammalian metabolic activation system (mDarT) Toxicol Sci. 2008;104(1):177–88. doi: 10.1093/toxsci/kfn065. [DOI] [PubMed] [Google Scholar]
- 5.Harper SL, Dahl JL, et al. Proactively designing nanomaterials to enhance performance and minimize hazard. International Journal of Nanotechnology. 2008;5(1):124–142. [Google Scholar]
- 6.Henken DB, Rasooly RS, et al. Recent Papers on Zebrafish and Other Aquarium Fish Models. Zebrafish. 2003;1:305–311. doi: 10.1089/zeb.2004.1.305. [DOI] [PubMed] [Google Scholar]
- 7.Kimmel CB, Ballard WW, et al. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 8.Levin ED, Swain HA, et al. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26(6):719–23. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Rasooly RS, Henken D, et al. Genetic and genomic tools for zebrafish research: the NIH zebrafish initiative. Dev Dyn. 2003;228(3):490–6. doi: 10.1002/dvdy.10366. [DOI] [PubMed] [Google Scholar]
- 10.Rubinstein AL. Zebrafish: from disease modeling to drug discovery. Curr Opin Drug Discov Devel. 2003;6(2):218–23. [PubMed] [Google Scholar]
- 11.Spitsbergen J, Kent M. The state of the art of the zebrafish model for toxicology and toxicologic pathology research - advantages and current limitations. Toxicological Pathology. 2003;31:62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usenko CY, Harper SL, et al. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usenko CY, Harper SL, et al. Exposure to fullerene C60 elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol. 2008;(229):44–55. doi: 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westerfield M. The Zebrafish Book. Eugene, OR: University of Oregon Press; 1995. [Google Scholar]



