Abstract
Clinical investigation of levodopa-induced dyskinesia (LID) in Parkinson Disease (PD) is limited because of lack of objective measurements, and no consensus on use of a standard measuring tool. Currently, clinical trials use subject-completed diaries of dyskinesia throughout the day or investigator-administered clinical rating scales. An objective and valid method of measuring LID would reduce bias, variability, and decrease the time and number needed in trials of potential anti-dyskinetic agents. We have investigated using a force plate on which the subject stands which records movement of the center of pressure (CoP) to quantify LID over the course of a levodopa (LD) cycle. 24 PD subjects (17 with LID, 7 without LID) admitted to an inpatient research facility had their anti-parkinsonian meds withheld overnight, and the following morning a 2-hour intravenous LD infusion was administered. The root mean squared of the velocity in the anterior-posterior direction (RMSV) derived from an analysis of the CoP, and a common clinical dyskinesia rating scale (CDRS) were performed repeatedly for 6 hours, initially as subjects were OFF before the LD infusion, through infusion and until OFF again. There was a high correlation between the area under the curve (AUC) of the clinical rating score and the RMSV within and between subjects. As a measure of LID, the RMSV of the CoP had excellent feasibility, validity and reliability.between subjects. Thus, CoP recordings during stance on a force plate can objectively quantify LID in PD and may be very useful in clinical trials or other investigations of dyskinesia.
Levodopa induced dyskinesia (LID) is a major problem associated with chronic use of levodopa (LD) for symptomatic treatment of Parkinson's disease (PD). LD remains our most potent therapy and nearly all PD patients will use it. A substantial portion of them will experience LID, with the varying impact on functional ability and quality of life.1-3 There are few options to reduce the severity of LID once established. Patients may need to reduce their symptomatic therapies, leading to possible intolerable worsening of parkinsonism. Deep brain stimulation can reduce LID, but is not a viable option for many4,5. There are very few drugs that have established anti-dyskinetic properties. Research into LID prevention and treatment has been limited by the subjective nature of clinical rating methods, including diaries kept by subjects, or clinical rating scales administered by observers6-11. A variety of more objective measures have been investigated, however none have entered clinical methodology12-15. We present here the results of using a force plate to objectively quantify LID.
Subjects and Methods
Subjects
We enrolled 24 subjects with idiopathic PD with a spectrum of LID severity, including 7 who had no dyskinesia. All PD subjects had previously been taking LD as part of their therapeutic regimen. Subjects were recruited from the Oregon Health & Science University (OHSU) Movement Disorders Outpatient Program. The selection criteria included clinically diagnosed idiopathic PD, established LD use, ability to consent, and no medical or psychiatric contraindications. Subjects were excluded if deficits were detected in proprioception. We also recruited 6 healthy controls. The study was completed with OHSU Institutional Review Board and the Oregon Clinical and Translational Research Institute (OCTRI) approval, and all subjects gave informed consent.
Protocol
Screening and inpatient procedures took place in the OCTRI. Subjects were admitted the evening before the study procedures, and all anti-PD medications were withheld after 10 pm. A LD infusion at either 1 or 1.5 mg/kg /h was administered the following morning from 9am-11am. Those who normally took <50 mg/hour of oral LD or LD equivalents were given 1 mg/kg/h IV LD. Those who took >50 mg/h of oral LD received 1.5 mg/kg/h LD. Carbidopa was given every 2 hours from 8am until noon. Beginning at 8am when subjects were off, measurements of parkinsonism and dyskinesia were performed, which continued throughout the 2 hour LD infusion and afterwards until the subject turned off as determined by tapping speed and Unified Parkinson Disease Rating Scale (UPDRS) scores. At the conclusion of testing, subjects were administered their usual antiparkinsonian medications and discharged to home.
Clinical dyskinesia ratings were performed repeatedly throughout the testing day at regular intervals of 30-60 minutes. Additionally, dyskinesia measurements were performed 3 times at 8am and 11am for test-retest reliability. Non-PD control subjects were measured repeatedly beginning at 8am following the same protocol as PD subjects, though no drug treatment was administered.
Measurements
Clinical Dyskinesia Rating Scale
Subjects were visually inspected for hyperkinetic movements in each of 7 body parts, including the face, neck, trunk and each limb. A summation score was generated by adding single scores of 0-4 in each body part with 0 representing no LID and 4 representing disabling dyskinesia 6. These clinical ratings occurred at the same time that the subject was standing on the force plate.
Force Plate
In this study, we utilized the force plate function of an RSS Footscan 2m gait mat (serial number 11/5/8). Subjects used their natural upright stance on the plate looking straight ahead, with arms at their sides for 50 seconds, once while standing quietly, then again while verbally performing a moderately difficult cognitive task such as a categorical fluency test or mathematical computation. Subjects were also instructed to avoid automatic repetitive gestures that may accompany counting-off tasks like tapping their fingers in turn, or nodding with each answer. Between test-retest periods, subjects briefly rested off the plate.
The Footscan software acquired the data from the plate and computed total body Center of Pressure (CoP), and the location of the ground reaction force vectors in the Anterior-Posterior (A/P) and Medial-Lateral (M/L) directions. The data was filtered using a zero phase, low-pass Butterworth filter with a cutoff frequency of 4 Hz to eliminate tremor. The body's CoP is used to keep the center of gravity within the base of support. This parameter is often used in studies of postural movement.
Tests of parkinsonism
The UPDRS III was administered at 8am, 10:30 am and when subjects appeared to turn off, or 2:30pm, whichever came first. Finger tapping scores were collected every 30-60 minutes, and acquired by having a subject repetitively press two counter keys 20 cm apart for 1 minute using the index finger on the most affected side.
Heart rate, rhythm, and blood pressure were measured every 30-60 minutes for safety monitoring throughout the study.
Data Analysis
A. Validity
Numerous variables comprising the CoP plot were calculated16 including total sway area (95% confidence ellipse), total excursion (total length of the CoP path), root mean square (RMS) distance, which describes the variability around the mean CoP trajectory, RMS of the velolcity, f95—which is the frequency below which 95% of the total power is found (spectral analysis), the rectified peak-to-peak distance, the mean velocity and root mean square of the velocity. When appropriate, these calculations were performed for directions in both the anterior-posterior (AP) and medial-lateral (ML) planes.
By inspection of the correlations for each CoP variable, we found that the area under the curve (AUC) of RMSV in the anterior-posterior direction correlated highly with the CDRS and it was chosen as our main outcome variable. The AUC for each subject was calculated using the trapezoidal method. Each subject's unique baseline was used by computing the mean of the test-retest period at 8am.
B. Reliability
The test-retest properties of each of the outcome measurements of the force plate were explored by calculating the intra- and inter- subject variation. The test-retest properties of the forceplate were explored using the 8 am test-retest and 11 am test-retest periods, corresponding to times of zero and probable maximum LID. The intraclass correlation coefficient (ICC) was computed for each of the outcome measurements of the forceplate at these two time points. The ICC would approach 1.0 as the between-groups effect (the effect of different subjects) is large relative to the within-groups effects (the measurement over time).
C. Responsiveness
Performing a mental task (MT) is known to provoke dyskinesia17. We took advantage of this to determine how the force plate and the CDRS responded to changes in severity of LID at a group level. We calculated a standardized change from baseline of the AUCs of the RMSV and the CDRS in subjects performing and also not performing a MT. From this, we could calculate what new proportion of subjects would be required to adequately power a study of LID.
Results
Twenty-three PD subjects and 6 non-PD controls completed the protocol. Three PD subjects were eliminated from the analysis, one subject could not complete the study procedures because of severe postural instability and inability to stand while OFF and two dropped out before ratings were collected. No adverse events occurred during the study. The baseline characteristics of our subjects are presented in Table 1. The mean age of our predominantly male subjects was 62.4 (SD = 8.5). Those who had more severe dyskinesia tended to have longer duration of disease and higher medication usage.
Table 1. Demographics and Baseline Clinical Features.
| Number of Subjects | Sex (male:female) | Mean Age | Duration of disease in years | UPDRS (part III) | CDRS at screening | |
|---|---|---|---|---|---|---|
| No LID | 7 | 6:1 | 64.7 (6.9) | 4.7 (3.1) | 21.3 (7.9) | 0 |
| Mild to moderate LID (CDRS, ≤7) | 11 | 10:1 | 62.5 (10.4) | 10.2 (5.0) | 23.5 (12.7) | 1.6 (2.4) |
| Moderate to Severe LID CDRS ≥8) | 4 | 3:1 | 59.7 (7.4) | 10.5 (4.8) | 12.0 (8.0) | 11.8 (3.5) |
| Controls | 6 | 5:1 | 67.8 (8.4) | n/a | n/a | 0 |
Clinical Dyskinesia Rating Scale: (0-4 in 7 body parts, including face, neck, trunk, and each limb, the maximum score possible is 28)
Data for continuous variables are presented as mean (SD).
A. Validity
Convergent Validity
In order to examine the validity of the force plate as a measurement of LID, spearman rank order correlations of the AUC of the 14 variables and the CDRS scores were computed. Table 2 shows that all but the frequency of movement measures were highly correlated with the CDRS. More specifically, the RMSV in the anterior-posterior direction demonstrated the strongest relationship with a spearman's rho of 0.81
Table 2. Anterior-Posterior RMSV and CDRS AUC s are Highly Correlated.
| CDRS | P | |
|---|---|---|
| RMSV_AP | .814** | <.0001 |
| MV_AP | .811** | <.0001 |
| TOTEX | .804** | <.0001 |
| SWAYAREA | .757** | .001 |
| MV_ML | .746** | .001 |
| RMSV_ML | .718** | .003 |
| P2P_ML | .675** | .006 |
| P2P_AP | .675** | .006 |
| RMS | .604* | .017 |
| RMS_ML | .604* | .017 |
| RMS_AP | .568* | .027 |
| F95 | .336 | .221 |
| F95_ML | .129 | .648 |
| F95_AP | .004 | .990 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
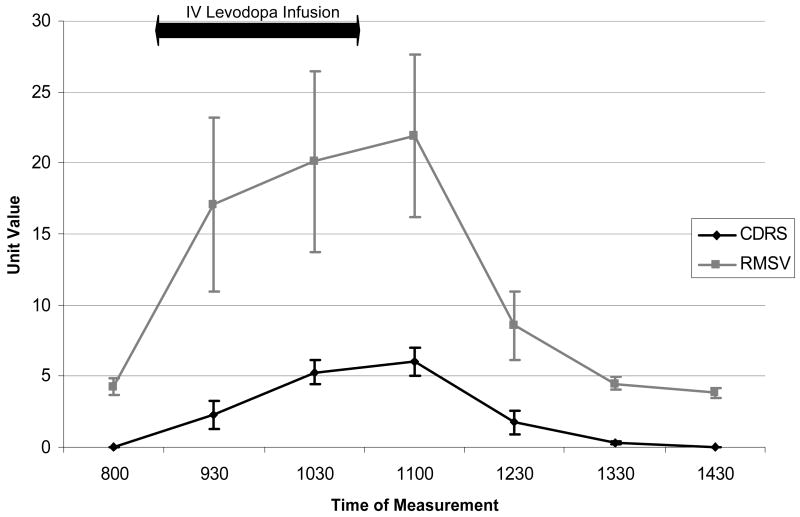
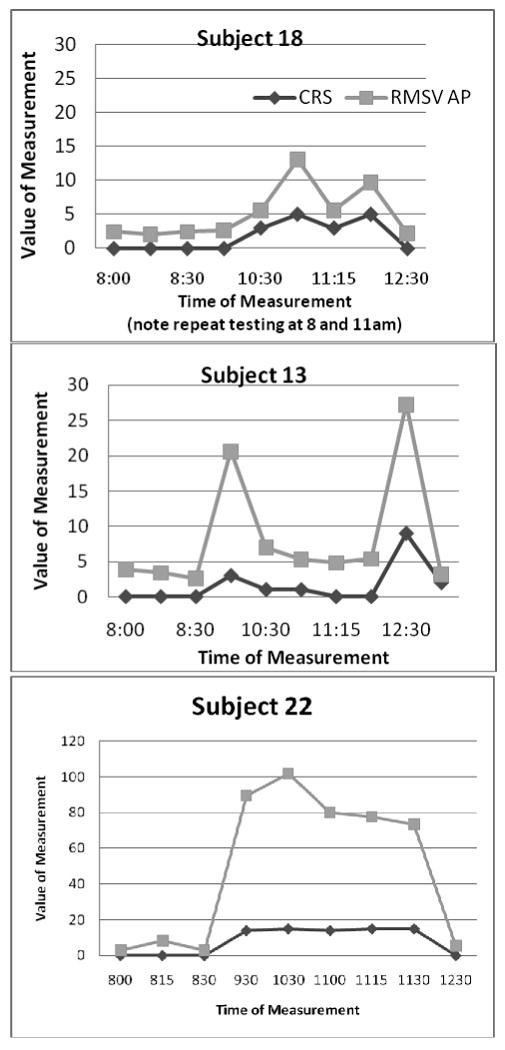
Examination of the RMSV and the CDRS curves across the testing period in subjects with LID show a parallel shape (Figure 1). This parallel shape may indicate that the two variables are measuring the same underlying construct, dyskinesia. This is supported by the persistence of the parallel shapes during complex patterns of LID (Figures 2 a-c).
Figure 1. Group Means (+/- SEM) of the CDRS and RMSV during an ON-OFF cycle.
Figure 2. RMSV parallels CDRS in simple or complex LID patterns.
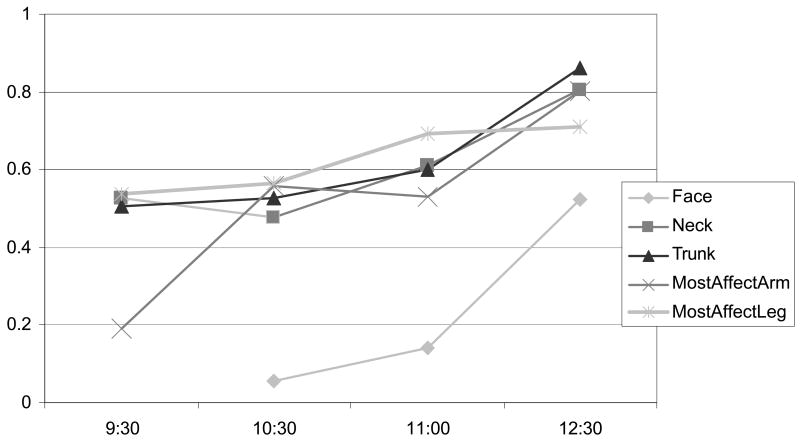
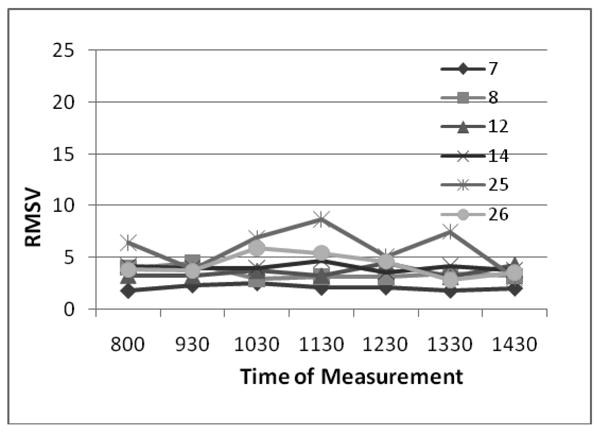
A breakdown of individual body part subscores across subjects shows similar performance, with the exception of the face (Figure 3).
Figure 3. Non-PD Control values do not vary (CDRS=0).
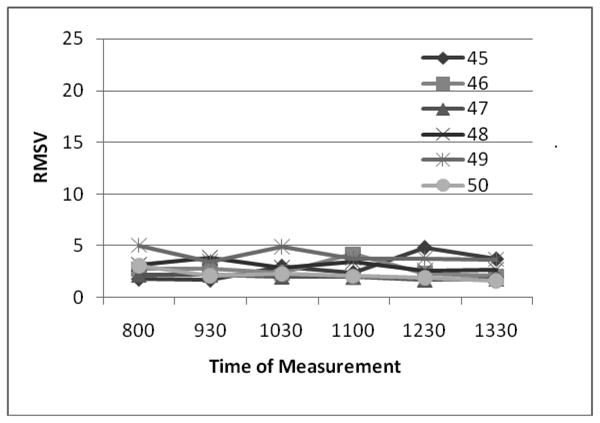
Discriminant Validity
To examine how well the RMSV classifies LID, we observed the results of measuring subjects with and without clinically detectable LID. Detectable LID is defined as a CDRS score of >0. There is little variability in RMSV in control subjects without PD (mean= 2.8 +/- 0.95 SD) and in those with PD but no clinically apparent dyskinesia (4.4 +/- 2.1) The low, consistent values of RMSV in control subjects without PD and in PD subjects without dyskinesia demonstrates that RMSV can distinguish those without LID. (Figures 3 and 4).
Figure 4. PD subjects without LID have low RMSV values (CDRS=0).
Compared to control subjects, the mean RMSV in subjects with dyskinesia was larger; 10.8 +/-12.1 in those with mild to moderate LID, and 20.9 in those with moderate to severe LID.
B. Reliability
Test-Retest Reliability
At 11am, the intraclass correlation was 0.96 for the RSMV compared with 0.92 for the CDRS. At 8am, an ICC could not be calculated for the clinical ratings because of insufficient range of values. An overall standard deviation in CDRS values was 0.09, and 2.22 for RMSV values at 8am.
C. Responsiveness
We compared the results measured by the CDRS and the RMSV when a mental task was applied, a technique known to increase LID. Both the CDRS and RMSV increased with the effort of a mentally focusing task. During the highest LID rating of the day, the mean RMSV during quiet stance was 12.5, SD =18.9 compared with 28.4, SD=27.3 during a mental task (p<0.0001). During the same testing periods, the mean CDRS increased from 2.9 (SD=3.8) to 7.7 (SD=4.5).
We computed standardized scores for the RMSV and CDRS. The difference in AUCs were calculated for mental task and no-mental task conditions. The change from baseline scores in the RSMV was 0.11, versus 0.07 for the CDRS.
While an exploratory analysis only, a comparison of the average of 9:30 RSMV values in those who had known LID but scored zero on the CDRS and in those with no LID ever (ie CDRS scores are always zero) tended to be higher (mean of 6.2 +/- SD 4.5 compared with 3.7 +/- 0.79 p<0.18). This time was examined because it represented a higher likelihood of early motor state transition. This higher average RSMV in a group with the capability of expressing LID but during a time the examiner recorded a LID-free state may represent evidence that the RSMV is more sensitive to an early manifestation of LID compared to the CDRS.
Discussion
To study the effectiveness of treatments for dyskinesia, an objective device that can discriminate between normal and abnormal, involuntary movements is needed. We have demonstrated that a force plate, measuring changes in CoP under the feet of standing subjects, can closely replicate a clinical rating scale, the current gold standard measurement of LID. In addition to showing that the device has convergent and disciminant validity, we have demonstrated that it produces reliable results during test-retest conditions as well as sensitivity to change in the dyskinetic state.
It is not possible to determine whether subjects with dyskinesia had normal postural control, superimposed with involuntary movements from dyskinesia or they had abnormally large and fast postural sway associated with dyskinesia. Since RMSV was normal in subjects with PD but without dyskinesia and because sway frequency was within the normal range in the dyskinetic PD subjects, we favor the interpretation that the increased variability of postural sway velocity represents ground reaction forces reflecting every involuntary action of a limb or trunk18. Facial dyskinesia did not produce forces large enough to be related to reactions on the ground. If having PD or dyskinesia impaired neural control of posture, we would expect to see large forceplate measures in all subjects, with out without dyskinesia. Previous studies reporting larger postural sway velocity in patients with severe PD, eligible for DBS surgery, may have been detecting dyskinesia.19 The strong convergent and discriminate validity of forceplate measures with clinical rating of dyskinesia are consistent with the forceplate detecting involuntary movements transmitted down to the surface reaction forces directly, superimposed upon normal postural sway.
We considered the influence of postural sway or some other unanticipated effect of merely having a therapeutic level of LD on our findings. Given the parallel nature of the AUC curves in complicated patterns of LID, we believe that it is LID that is being measured. If a completely different measurement was developed that also correlated well with the CDRS and then was demonstrated to correlate with the RMSV, we would be more confident that we are measuring LID.
A new instrument to measure LID must be responsive or sensitive to changes, and we have utilized the observation that mentally focusing tasks can provoke dyskinesia to demonstrate that the effect size reported is greater with the force plate than with the CDRS. In fact, if a study used change in AUC of RSMV over baseline through an ON-OFF cycle, it would require (0.07/0.11)2 or only 41% of the subjects as with a study using a change in CDRS.
The widespread availability, ease of use and portability of a force plate is attractive, as previous monitoring attempts have involved rather cumbersome wearable sensors with intrusive wiring, or mounted optical systems that were practically useful only in a laboratory setting. Measurements are completed in short periods of time with relative ease as subjects are free of attachments, and collection of data does not require the presence of an expert in PD, dyskinesia or devices. These features suggest good feasibility.
There are limitations to using this device for LID evaluation. It may not be sensitive to those who have only facial dyskinesia. We did not differentiate between dystonic and the more classic hyperkinetic or choreiform form of dyskinesia as some investigators have, though some of our subjects did have mixed presentations which did not appear to cause loosening of the correlations. Subjects must be able to stand for one minute at a time, and therefore some PD subjects with presumably very advanced disease and inability to balance safely will not be testable. Because a cognitive task is an important part of the methodology, those with advanced cognitive problems may not be able to participate. We did not test the utility of an alternate motor activity (beyond speaking), as we were concerned it would alter the movement of CoP, and would make it difficult to score the working limb.
We selected RMSV after inspection of the performance of all the CoP metrics, which could have introduced systematic bias due to the issues surrounding testing hypotheses suggested by data. Further validation of the RMSV on a separate group of subjects is needed to confirm that the RMSV is a precise measurement of dyskinesia.
We have demonstrated evidence of greater sensitivity of this device compared with the often used clinical rating scale. Further exploration of the utility of this device should concentrate on responsiveness of the force plate by showing a reduction of RMSV values in those who undergo a treatment with known anti-dyskinetic properties such as amantadine.
In conclusion, the limitations of semi-objective measurements in monitoring dyskinesia in PD patients have contributed to uncertainty about the incidence, prevalence, prevention strategies and even treatment of LID. Previously, dyskinesia investigators relied upon diary reporting, the UPDRS items 32 and 33, and various clinical rating scales. Diaries are quite likely to underreport dyskinesia, the UPDRS dyskinesia items rely entirely upon subject reporting and similarly are subject to poor sensitivity, perhaps only providing a gross estimate of dyskinesia. Clinical rating scales are more accurate, but are subject to the problems of bias, ceiling effect, and poor resolution. While we have demonstrated that the plate is valid, reliable, and feasible, it is also advantageous in that it does not rely on clinical ratings by an observer who may be subject to bias or inexperience. Additionally, improved resolution that electronic data can provide compared with the interval groupings of only 0-4, as well as the demonstrated higher sensitivity of the RSMV will likely lead to significant findings in clinical trials with fewer numbers of subjects needed, and shorter periods of observation.
Figure 5.
The CDRS is a composite score of ratings in 7 body parts, therefore we examined each individual subscore for its performance. The trunk, neck, and most affected arm and leg correlated well, whereas the face produced the worst correlations. This is especially true in the subject with only facial dyskinesia.
Acknowledgments
Supported by a Veteran's Administration Career Development Award, US Public Health Service Grant ULIRR024140-02, NIH R01-NS21062, National Institutes on Aging AG006457, and a grant from Intel Corporation.
References
- 1.Pechevis M, Clarke CE, Vieregge P, Khoshnood B, schaseaux-Voinet C, Berdeaux G, Ziegler M. Effects of dyskinesias in Parkinson's disease on quality of life and health-related costs: a prospective European stud. Eur J Neurol. 2005 Dec;12(12):956–63. doi: 10.1111/j.1468-1331.2005.01096.x. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007 Jul;22(10):1379–89. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- 3.Grandas F, Galiano ML, Tabernero C. Risk factors for levodopa-induced dyskinesias in Parkinson's disease. J Neurol. 1999 Dec;246(12):1127–33. doi: 10.1007/s004150050530. [DOI] [PubMed] [Google Scholar]
- 4.Limousin P, Martinez-Torres I. Deep brain stimulation for Parkinson's disease. Neurotherapeutics. 2008 Apr;5(2):309–19. doi: 10.1016/j.nurt.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkmann J. Deep brain stimulation for Parkinson's diseas. Parkinsonism Relat Disord. 2007;13 3:S462–S465. doi: 10.1016/S1353-8020(08)70050-6. [DOI] [PubMed] [Google Scholar]
- 6.Hagell P, Widner H. Clinical rating of dyskinesias in Parkinson's disease: use and reliability of a new rating scale. Mov Disord. 1999 May;14(3):448–55. doi: 10.1002/1531-8257(199905)14:3<448::aid-mds1010>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Goetz CG, Stebbins GT, Shale HM, Lang AE, Chernik DA, Chmura TA, Ahlskog JE, Dorflinger EE. Utility of an objective dyskinesia rating scale for Parkinson's disease: inter- and intrarater reliability assessment. Mov Disord. 1994 Jul;9(4):390–4. doi: 10.1002/mds.870090403. [DOI] [PubMed] [Google Scholar]
- 8.Hauser RA, Friedlander J, Zesiewicz TA, Adler CH, Seeberger LC, O'Brien CF, Molho ES, Factor SA. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clin Neuropharmacol. 2000 Mar;23(2):75–81. doi: 10.1097/00002826-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Katzenschlager R, Schrag A, Evans A, Manson A, Carroll CB, Ottaviani D, Lees AJ, Hobart J. Quantifying the impact of dyskinesias in PD: the PDYS-26: a patient-based outcome measure. Neurology. 2007 Aug;69(6):555–63. doi: 10.1212/01.wnl.0000266669.18308.af. [DOI] [PubMed] [Google Scholar]
- 10.Marsden CD, Schachter M. Assessment of extrapyramidal disorders. Br J Clin Pharmacol. 1981 Feb;11(2):129–51. doi: 10.1111/j.1365-2125.1981.tb01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uitti RJ, Wharen RE, Jr, Turk MF, Lucas JA, Finton MJ, Graff-Radford NR, Boylan KB, Goerss SJ, Kall BA, Adler CH, Caviness JN, Atkinson EJ. Unilateral pallidotomy for Parkinson's disease: comparison of outcome in younger versus elderly patients. Neurology. 1997 Oct;49(4):1072–7. doi: 10.1212/wnl.49.4.1072. [DOI] [PubMed] [Google Scholar]
- 12.Keijsers NL, Horstink MW, Gielen SC. Automatic assessment of levodopa-induced dyskinesias in daily life by neural network. Mov Disord. 2003 Jan;18(1):70–80. doi: 10.1002/mds.10310. [DOI] [PubMed] [Google Scholar]
- 13.Keijsers NL, Horstink MW, Gielen SC. Ambulatory motor assessment in Parkinson's disease. Mov Disord. 2006 Jan;21(1):34–44. doi: 10.1002/mds.20633. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Carroll CB, Wang SY, Zajicek J, Bain PG. Quantifying drug-induced dyskinesias in the arms using digitised spiral-drawing tasks. J Neurosci Methods. 2005 May;144(1):47–52. doi: 10.1016/j.jneumeth.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Manson AJ, Brown P, O'Sullivan JD, Asselman P, Buckwell D, Lees AJ. An ambulatory dyskinesia monitor. J Neurol Neurosurg Psychiatry. 2000 Feb;68(2):196–201. doi: 10.1136/jnnp.68.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adult 18. IEEE Trans Biomed Eng. 1996 Sep;43(9):956–66. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 17.Durif F, Vidailhet M, Debilly B, Agid Y. Worsening of levodopa-induced dyskinesias by motor and mental tasks. Mov Disord. 1999 Mar;14(2):242–5. doi: 10.1002/1531-8257(199903)14:2<242::aid-mds1007>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Horak FB, Manson A. Handbook of Physiology. New York: Oxford University Press; 1996. p. 255. [Google Scholar]
- 19.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002 Sep;73(3):267–74. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]