Abstract
The success in screening for drug candidates is highly dependent on the power of the strategy implemented. In this work, we report and characterize a novel fluorescent benzodiazepine antagonist of the type 1 cholecystokinin receptor (3-(3-(7-fluoro-1-(2-isopropyl(4-methoxyphenyl)amino)-2-oxoethyl)-2,4-dioxo-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[b][1,4]-diazepin-3-yl)ureido)benzoic acid) that can be used as a receptor ligand in a fluorescence polarization assay, which is ideally suited for the identification of small molecule allosteric modulators of this physiologically important receptor. By binding directly to the small molecule-docking region within the helical bundle of this receptor, this indicator can be displaced by many small molecule candidate drugs, even those that might not affect the binding of an orthosteric cholecystokinin-like peptide ligand. The biological, pharmacological, and fluorescence properties of this reagent are described, and proof-of-concept is provided in a fluorescence polarization assay utilizing this fluorescent benzodiazepine ligand.
Introduction
Adetailed understanding of receptor structure and the molecular basis of ligand binding and receptor activation can facilitate the development of receptor-active drugs that have a broad spectrum of activities.1 Ligands can be chemically diverse and can theoretically interact with a variety of potential binding sites within a given target receptor. Bioavailable small molecule drugs can possess a number of advantages over peptide or protein ligands, such as increased stability in the protease-rich milieu of the upper gastrointestinal tract. In addition, they often possess the major advantage of being absorbable through the gastrointestinal tract mucosa, thus allowing them to be effectively and efficiently administered orally. Therefore, the goal of most drug development programs is to strategically target this type of ligand.
The type 1 cholecystokinin (CCK) receptor (CCK1R) is a family A guanine nucleotide-binding protein (G protein)-coupled receptor that naturally binds CCK peptide hormone ligands.1,2 When acting through this receptor, CCK stimulates gallbladder contraction, pancreatic exocrine secretion, gastrointestinal motility, and postcibal satiety.3,4 Naturally occurring CCK peptides vary in length from 8 to 58 amino acids, with all peptides sharing their common carboxyl-terminal region that has been shown to be essential for full efficacy and potency at the CCK1R.5 The full-agonist, natural CCK can be changed into a partial agonist by replacing the carboxyl-terminal phenylalanine-amide with a phenylethyl ester.6 This peptide can be further modified by replacement of the L-tryptophan in position 30 with a D-tryptophan to yield an antagonist.7 The molecular basis for natural CCK peptide binding to the CCK receptor has been explored using structure-activity relationships, receptor mutagenesis, photoaffinity labeling, and fluorescent ligand analysis.8–13 Based on these studies, CCK has been shown to interact with extracellular loop and amino-terminal tail regions of this receptor.9,13
The characteristics for the binding of a number of nonpeptidyl ligands of the CCK1R have also been described.14 One such class of nonpeptidyl ligands is the 1,5-benzodiazepine derivatives, which have been shown to dock within the intramembranous helical bundle domain.15,16 This domain has been demonstrated to represent an allosteric site of action, which has been shown to be fully distinct from the orthosteric site of action of the natural peptide ligand of this receptor.17
By definition, allosteric ligands bind to receptor sites that are distinct from the site of binding of the natural orthosteric ligand. It is now clear that allosteric ligands can alter the binding affinity and/or efficacy of ligands that bind to the orthosteric site within the same receptor.1,18 Allosteric modulators can exert either positive (positive allosteric modulators or enhancers) or negative (negative allosteric modulators) influence on orthosteric ligands by facilitating or interfering with the conformational changes stimulated by those agents. Additionally, allosteric ligands can possess intrinsic efficacy as full or partial agonists, presumably by inducing analogous conformational changes in the receptor to those stimulated by full or partial agonists binding to the distinct orthosteric ligand-binding site. It is also possible for allosteric ligands to be virtually neutral, having no effect on the binding or action of the natural orthosteric agonist.19 These effects of allosteric ligands on orthosteric ligands can be quantified by the cooperativity index, ranging from negative to positive, with a value of zero reflecting the possibility of having no observable effect. It is for this reason that screening for allosteric drug candidates using a competition assay for the binding of an orthosteric radioligand might not be optimal. In such a situation, the use of a radiolabeled or fluorescent tracer that binds directly to the allosteric domain of interest within the receptor may identify a larger spectrum of candidate ligands than that which could otherwise have been identified in such a screen.
In the current article, we describe and characterize a new fluorescent benzodiazepine ligand of the CCK1R. Its fluorescence properties suggest its utility for the screening of small-molecule drug candidates that could be useful as new novel therapeutics.
Materials and Methods
Materials
The fluorescent benzodiazepine compound, GI224329 (3-(3-(7-fluoro-1-(2-isopropyl(4-methoxyphenyl)amino)-2-oxoethyl)-2,4-dioxo-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[b][1,4]-diazepin-3-yl)ureido)benzoic acid), was kindly provided by Drs. E.E. Sugg and R. Sherrill of GlaxoSmithKline Research Laboratories (Research Triangle Park, NC) (Fig. 1). Synthetic CCK octapeptide (CCK-8, CCK-26-33) was from Peninsula Laboratories (Belmont, CA). The fluorescent CCK peptide analog, Alexa488-CCK, was synthesized, purified to homogeneity on reversed-phase high performance liquid chromatography, and characterized in our laboratory.20 The CCK1R antagonist, L-364,718 (devazepide), was provided by Merck Research Laboratories (West Pointe, PA). The radioiodinated benzodiazepine previously identified as compound 9,21 was prepared by oxidative radioiodination using Na125I (Perkin-Elmer Life Sciences, Waltham, MA) and Iodobeads (Pierce Chemical Company, Rockford, IL). The CCK-like peptide radioligand, 125I-D-Tyr-Gly-[(Nle28,31)CCK-26-33], was synthesized, purified to homogeneity on reversed-phase high performance liquid chromatography, and radioiodinated in our laboratory, as was previously described.22 Black 96-well Optiplates and 96-well white microplates with bonded GF/B filters were from Perkin-Elmer Life Sciences. Costar 96-well black assay plates with clear bottoms and V-bottoms were from Corning (Corning, NY). Probenecid, 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) was from Sigma (St. Louis, MO). Fura-2-acetoxymethyl ester, Ham's F-12 medium, and other tissue culture supplements were obtained from Invitrogen (Carlsbad, CA). Fetal clone II cell culture medium supplement was from Hyclone Laboratories (Logan, UT). All other reagents were analytical grade.
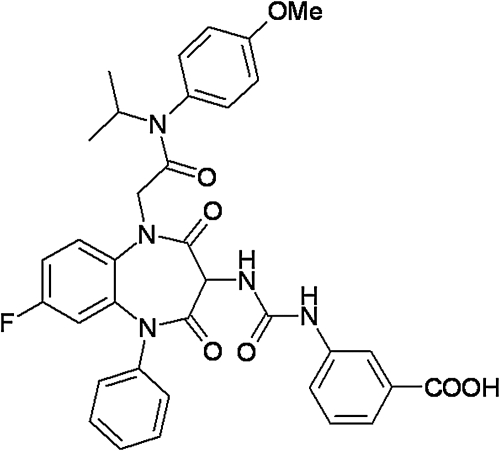
Fig. 1.
Structure of GI224329. Shown is the chemical structure of the fluorescent benzodiazepine, GI224329, used in the current study.
Cell Culture
A Chinese hamster ovary cell line stably expressing the human CCK1R (CHO-CCK1R cells) was used as a source of receptor for this study. We previously characterized and demonstrated that these cells have the ability to bind and signal normally in response to CCK.23 CHO-CCK1R cells were grown in Ham's F-12 medium supplemented with 5% fetal clone II in tissue culture plasticware in a 37°C incubator in an environment including 5% CO2. Cells were passaged approximately twice per week.
Radioligand Binding Studies
Receptor-enriched membranes were isolated from confluent CHO-CCK1R cells using a previously described protocol that involves sucrose density centrifugation.20 Membranes were suspended in Krebs-Ringers-HEPES (KRH) medium containing 25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 2 mM CaCl2,1 mM KH2PO4, 1.2 mM MgSO4, 0.01% soybean trypsin inhibitor, and 1 mM phenylmethylsulfonyl fluoride and were stored at −80°C until use.
Radioligand binding assays were carried out as was previously described.24 Receptor-enriched membrane suspensions were mixed with 1–2 pM (∼20,000 counts per minute) of the CCK-like radioligand, 125I-D-Tyr-Gly-[(Nle28,31)CCK-26-33], in the absence or presence of unlabeled CCK or benzodiazepine GI224329 (1 pM–1 μM) for 1 h at room temperature in KRH medium, pH 7.4, containing 0.2% bovine serum albumin and 0.01% soybean trypsin inhibitor. Nonsaturable binding was determined in the presence of 1 μM CCK. The receptor-bound fraction was separated from free radioligand by centrifugation and washing, with bound radioactivity quantified using a gamma spectrometer.
Binding of the radioiodinated benzodiazepine, compound 9,21 to the CCK1R was evaluated using a filtration assay. Receptor-bearing membranes (5 μg protein) were mixed with 0.5–1.0 pM (∼10,000 counts per minute/well) of compound 921 in a low-retention polypropylene plate for 1 h at room temperature in the presence or absence of GI224329 or compound 5 (the 127I-labeled analog of compound 921). The receptor-bound fraction was separated from the free ligand by filtration using Unifilter-96 GF/B filtermats in a FilterMate Harvester (PerkinElmer, Shelton, CT). The plate was then washed six times with wash buffer (0.9% NaCl and 0.2% bovine serum albumin), air-dried overnight, and subsequently counted on a TopCount® NXT™ (Packard, Meriden, CT) after addition of 30 μL of MicroScint™-O (PerkinElmer). Data were analyzed and plotted using the nonlinear least-squares curve-fitting routine in the Prism program (GraphPad version 4.0, San Diego, CA).
Biological Activity Studies
Ligand-stimulated intracellular calcium responses were measured in CHO-CCK1R cells. Cells were seeded in a clear-bottom sterilized tissue culture 96-well plate at a density of ∼20,000 cells/well 24 h before the assay. At the beginning of the assay, cells were washed with buffer 1 (25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1.0 mM KH2PO4, 1.2 mM MgSO4, 1.2 mM MgCl2, 0.2% bovine serum albumin, and 2.5 mM probenecid) and then were incubated with cell-permeant Fura-2-acetoxymethyl ester (1.5 μM in buffer 1) for 1 h at 37°C. After incubation, the fluorescence indicator was aspirated from the wells and washed with buffer 1 before being assayed in a Molecular Devices Flexstation 3.0 (Molecular Devices, Sunnyvale, CA) with robotic addition of ligands (1 pM–1 μM) using Softmax Pro 5.4 software. Receptor-mediated intracellular calcium responses were measured at 37°C by setting up excitations at 340 and 380 nm, and emission at 510 nm, with data collection for 120 s at intervals of 4 s. Data were analyzed and plotted using the nonlinear least-squares curve-fitting routine in the Prism program (GraphPad version 4.0).
Fluorescence Spectrometry
Fluorescence properties of GI224329 while free in solution and while bound to the receptor were measured in a Fluorolog spectrofluorometer (SPEX Industries, Edison, NJ). The receptor-bound samples were prepared by incubating GI224329 (100 nM) with the CCK1R-bearing membranes (50 μg of membrane protein) in KRH medium for 30 min at room temperature,25 before being isolated by centrifugation at 20,000 g for 10 min at 4°C. The membranes were then washed and resuspended in cold KRH medium to avoid ligand dissociation. Steady-state fluorescence measurements were acquired in a spectrofluorometer with a 4 nm band pass filters and an integration rate of 1 nm/s. Emission spectra were collected from 300 to 500 nm with excitation wavelength fixed at 290 nm. Corrected spectra were prepared by subtracting Raman spectra as well as the spectra obtained from membrane samples incubated with buffer alone. Fluorescence quantum yield was calculated by using tryptophan in water as reference standard (0.14). Quantum yield (Qx) was calculated based on the equation, Qx = Qs(Fx/Fs)(As/Ax), where s and x refer to standard and sample, respectively. F refers to corrected emission spectra with constant slit opening, and A represents the absorbance at the excitation wavelength to avoid inner filter effect.
Fluorescence Quenching Analysis
Fluorescence collisional quenching experiments were performed using a hydrophilic quencher, potassium iodide (KI), and a hydrophobic quencher, TEMPO, as previously described.26 Here, the fluorescence of free and receptor-bound probe was monitored in the spectrofluorometer using excitation wavelength of 290 nm and fixed emission wavelength of 333 nm. Fluorescence emission intensities were recorded 10 s after sequential additions of increasing concentrations of KI (1 M aqueous stock solution incorporating 10 mM sodium thiosulfate) or TEMPO (100 mM stock solution in 10% dimethylsulfoxide). For correcting the effects of dilution and ionic strength, measurements were carried out using control samples containing the same concentrations of potassium chloride and dimethylsulfoxide. The corrected fluorescence data were determined by subtracting both membrane and buffer blank values and were plotted according to the Stern-Volmer equation, Fo/F = 1 + KSV [Q], where Fo/F is the ratio of fluorescence intensities in the absence and presence of quencher. The Stern-Volmer quenching constant, KSV, was determined from the slope of Fo/F as a function of the quencher concentration.
Fluorescence Anisotropy Analysis
Anisotropy measurements were acquired using an Edinburgh spectrofluorometer equipped with automatic polarizer and thermostatically adjusted cuvette holder, as previously described.20 Fluorescence polarization (FP) measurements were performed using 10 s integration times. Emission measurements were performed with the excitation side polarizer in a vertical position and the emission side polarizer in horizontal and vertical positions. Excitation wavelength was fixed at 290 nm, and the emission wavelength was fixed at 333 nm.
Fluorescence Lifetime Analysis
The fluorescence lifetimes of GI224329 were determined using time-correlated single-photon counting, as previously described.20 Receptor-bound probe was analyzed in a cuvette with path length of 1 cm. Samples were excited at 290 nm using a frequency-doubled dye laser (Antares A76 mode-locked pump laser and model 700 cavity-dumped dye laser with R6G; Coherent, Palo Alto, CA). The excitation wavelength was tunable and had a pulse width of ∼2 picoseconds full-width half-maximum. Fluorescence emission was collected at 25°C through a monochrometer centered at 333 nm having a 6.8 nm bandwidth. Data were collected in 1,080 channels, with a width of 10.05 picosecond/channel. Fluorescence decay data were collected using NIM modules for signal processing and a time and amplitude converter for A/D conversion to the Ortec Maestro-32 pulse height analyzer and software package. Fluorescence intensity decay analysis was performed using version 1.2 of the GLOBALS Unlimited program. Models of single exponential and dual, discrete exponential lifetime components were utilized, and the average lifetimes were calculated as previously described,20 with the quality of fit determined using Chi-squared (χ2) statistics.
FP Assays
FP assays were carried out in a Spectramax M5E fluorescence plate reader (Molecular Devices) using either GI224329 or Alexa488-CCK bound to the CCK1R. Receptor-enriched membrane suspensions (5 μg) were mixed with either 5 nM Alexa488-CCK or 100 nM GI224329 in KRH medium, pH 7.4, in the absence or presence of increasing concentrations of nonfluorescent ligand (CCK or L-364,718). The membrane suspensions were incubated in final volumes of 200 μL at room temperature in a 96-well black Optiplate (Perkin-Elmer Life Sciences) for 1 h. FP was determined by the FP detection mode using tunable excitation and emission wavelengths (Ex. 482 nm, Em. 521 nm for Alexa488-CCK; Ex. 300 nm, Em. 333 nm for GI224329). Nonsaturable binding was measured in the presence of 1 μM concentration of the appropriate nonfluorescent ligand. The influence of solvent carrier on this assay was determined by performing the assay in the presence of dimethylsulfoxide concentrations ranging from 0.5% to 5% in KRH medium, pH 7.4. Polarization was calculated using the equation P = (V–H)/(V+H), where P is polarization, V is the vertical component of the emitted light, and H is the horizontal component of the emitted light when the fluorophore is excited using polarized light. Polarization values were expressed in millipoise (mP) units. The data were collected by the automated FP mode and analyzed using nonlinear regression analysis with Prism (GraphPad version 3.0). The value of the Z-factor for this assay was determined using the equation Z = 1–(3σb + 3σf)/(mPb–mPf), where σb is the standard deviation of the bound signal in the absence of competitor, σf is the standard deviation of the free signal in the presence of a saturating concentration of competitor, mPb is the mean bound signal, and mPf is the mean-free signal.
Statistical Analysis
Statistical analysis was performed using the two-tailed t-test in the Instat program (GraphPad), unless specifically noted otherwise. P values <0.05 were considered statistically significant.
Results
Receptor Binding and Biological Activity of the Fluorescent Tracer
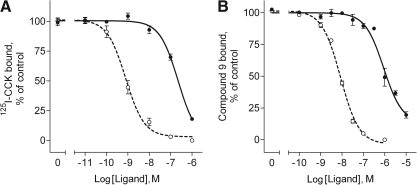
The new benzodiazepine ligand being reported (GI224329, structure shown in Fig. 1) represents a specific antagonist of the CCK1R. This compound competed for binding of the CCK-like peptide radioligand in a concentration-dependent manner, although this required much higher concentrations of GI224329 than CCK (IC50 values for binding of the CCK-like peptide radioligand were the following: GI224329, 230 ± 40 nM; CCK 0.5 ± 0.2 nM) (Fig. 2A). Previously, we developed a radioiodinated benzodiazepine antagonist ligand (compound 9) that binds with high affinity and specificity to the CCK1R.21 Competition-binding assays were also performed with this radioligand, using GI224329 and the 127I-labeled analog of compound 9, previously identified as compound 5.21 Both these compounds were able to compete for the binding of compound 9 in a concentration-dependent manner (Fig. 2B). The IC50 values for binding of radioiodinated compound 9 were the following: GI224329, 890 ± 230 nM; compound 5, 9 ± 1 nM.
Fig. 2.
Receptor binding characteristics for GI224329. Shown are the binding curves reflecting GI224329 competition for binding of 125I-CCK radioligand (A) and radioiodinated benzodiazepine compound 9 (B) to membranes bearing CCK1Rs. Unlabeled CCK (○ in A) and the 127I-labeled analog of compound 9, compound 5 (○ in B), were used as control ligands for the respective radioligand binding assays. Benzodiazepine GI224329 (●) was able to compete for the binding of each radioligand in a concentration-dependent manner. Data are expressed as means ± SEM of values from three to five independent experiments performed in duplicate. CCK1R, type 1 cholecystokinin receptor; SEM, standard error of the mean.
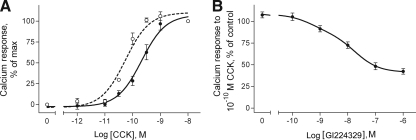
The ability of GI224329 to modify intracellular calcium release was also studied (Fig. 3). GI224329 did not stimulate any significant increase in intracellular calcium. Figure 3A, shows that 100 nM GI224329 shifted the ability of CCK to stimulate intracellular calcium to the right. The ratio of EC50 values of agonist in the presence and absence of antagonist was 4 ± 1, supporting a Kb value for GI224329 of 40 ± 10 nM. Additionally, preincubation of cells with GI224329 inhibited the subsequent intracellular calcium response to 0.1 nM CCK in a concentration-dependent manner (Fig. 3B). The IC50 value for this inhibition of intracellular calcium response to CCK was 50 ± 20 nM.
Fig. 3.
Biological activity characteristics of GI224329. Shown are biological response curves reflecting the ability of 0.1 μM GI224329 (●) to shift CCK-stimulated intracellular calcium responses (○) to the right (A) and the ability of various concentrations of GI224329 (●) to inhibit intracellular calcium responses stimulated by 0.1 nM CCK in CHO-CCK1R cells (B). GI224329 acted as a competitive antagonist that inhibited the calcium response elicited by CCK. Kb (40 ± 10 nM) and IC50 (50 ± 20 nM) values were in a similar range. Data are expressed as means ± SEM of values from three independent experiments performed in duplicate.
Fluorescence Characteristics of the Tracer
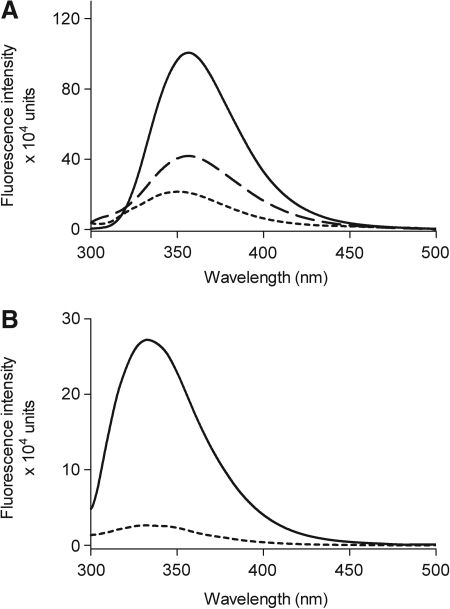
The fluorescence emission spectra of 100 nM GI224329 in various solvents are shown in Figure 4A. When excited with light at 290 nm, the emission maximum was at 355 nm. These spectra were affected by the polarity of the solvent, with reduced emission intensities observed as the polarity of the solvent was increased. The quantum yield of fluorescence in aqueous medium was 0.053. Specificity and saturability of binding of GI224329 to the CCK1R were demonstrated by demonstrating the reduction in its fluorescence emission in the presence of a saturating concentration of nonfluorescent CCK peptide (Fig. 4B). The maximal emission in the receptor-bound state was observed to be shifted to 333 nm.
Fig. 4.
Fluorescence spectral characteristics of GI224329. Shown are the spectral emission profiles for the benzodiazepine compound of interest in solvents having different polarities and dielectric constants (A). Shown are spectra in aqueous buffer (-----), acetonitrile (––), and dimethylsulfoxide (–––). Emission intensities were observed to increase as the dielectric constants of the solvent decreased. Also shown are spectra for this compound when it was allowed to bind to the CCK1R-bearing membranes in the absence (–––) and presence (-----) of 1 μM competing nonfluorescent ligand performed in aqueous Krebs-Ringers-HEPES medium (B). Spectral profiles showed maximal emission at 355 nm when free in solution and 333 nm when bound to the receptor.
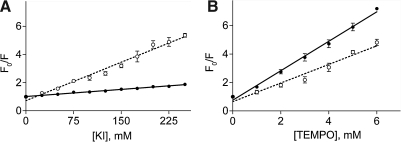
Fluorescence quenching of the probe was determined by quantifying the intensity of its fluorescence in the presence of ether hydrophilic or hydrophobic quenchers. Figure 5 shows the concentration dependence of the quenching of the fluorescence of GI224329, when free in solution and when bound to the CCK receptor, using hydrophilic KI (Fig. 5A) and hydrophobic TEMPO (Fig. 5B). The Stern-Volmer fluorescence quenching constants for these data are shown in Table 1. The fluorescence of GI224329 in solution was more easily quenched by the hydrophilic reagent than by the hydrophobic reagent, whereas the opposite was true when this compound was bound to its receptor. The fluorescence quenching pattern of the receptor-bound probe reflects its accessibility in its respective environments.
Fig. 5.
Quenching of GI224329 fluorescence. Shown are Stern-Volmer plots of collisional quenching of the fluorescence of the benzodiazepine GI224329 with KI (A) and TEMPO (B) when free in solution (○) and when bound to CCK1Rs (●). Steady-state fluorescence was measured after sequential addition of quenchers into the samples and measured the fluorescence intensity (Ex. 290 nm, Em. 333 nm). Data are expressed as means ± SEM of values from four to five independent experiments performed in duplicate. KI, potassium iodide; TEMPO, 2,2,6,6-tetramethylpiperidine 1-oxyl.
Table 1.
Fluorescence Quenching Constants for GI224329
Values are expressed as means ± SEM from a minimum of four experiments.
P < 0.01 relative to data from the compound free in solution.
KI, potassium iodide; SEM, standard error of the mean; TEMPO, 2,2,6,6-tetramethylpiperidine 1-oxyl.
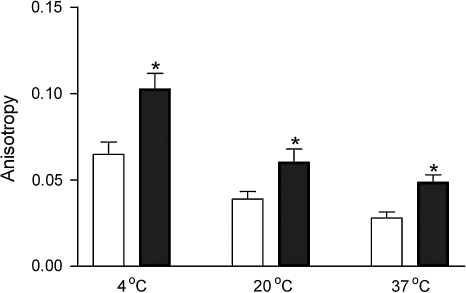
Figure 6 shows the fluorescence anisotropy measurements for GI224329 at different temperatures when free in solution and when bound to the CCK receptor. The anisotropy values varied with temperature and were significantly higher when bound to the receptor than when free in solution at all temperatures tested. Table 2 lists the fluorescence lifetimes of GI224329 when free in solution and when bound to the CCK receptor. The lifetimes of receptor-bound GI224329 were significantly longer than under the same temperatures when this compound was free in solution.
Fig. 6.
Fluorescence anisotropy of GI224329. Shown are fluorescence anisotropy values for the benzodiazepine, GI224329, when free in solution (open bars) and when bound to the CCK1R (filled bars), with experiments performed at three different temperatures (noted). Fluorescence anisotropy was calculated by measuring the polarization values of the compound. Studies utilized a fixed excitation wavelength of 290 nm and an emission wavelength chosen to coincide with emission maximum of 333 nm. Data are expressed as means ± SEM of values from four to five independent experiments performed in duplicate. *Data that are significantly different from free in solution at three different temperatures (P < 0.01).
Table 2.
Fluorescence Lifetimes of GI224329
| τ1 (ns) | f1 | τ2 (ns) | f2 | χ2 | Average lifetime (τ) (ns) | |
|---|---|---|---|---|---|---|
| Free in solution | 1.7 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.1 | 0.4 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.1 |
| Receptor-bound | 4.0 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.2 ± 0.1 | 1.1 ± 0.1 | 3.2 ± 0.1a |
The lifetimes of receptor-bound GI224329 were significantly longer than when it was free in solution. Data are expressed as means ± SEM of duplicate values from four experiments.
P < 0.05 relative to data obtained with GI224329 free in solution.
FP Assays
FP reflects the degree of rotational freedom of motion of a fluorescent molecule and it is utilized to differentiate the status of the fluorescent ligand when free in solution and when bound to the receptor. A major advantage of an FP assay relative to traditional radioligand-binding assays is the absence of requirement for separation of bound and free ligand. Here, we performed FP assays with both Alexa488-CCK, a fluorescent peptide ligand that has previously been fully characterized,20 and the fluorescent benzodiazepine compound that is the focus of the current report (Fig. 7). In the FP assay with the fluorescent benzodiazepine GI224329 (Fig. 7A), nonfluorescent benzodiazepine (L-364,718) inhibited the signal more effectively than the peptide ligand, CCK. The opposite was true in the FP assay with the fluorescent CCK analog (Fig. 7B). Table 3 illustrates the standard conditions for the application of this reagent in an FP assay. Assay values were stable under conditions of dimethylsulfoxide concentrations ranging from 0.5% to 5%, supporting a broad range of compound solubilization conditions. Under the assay conditions, there was no detectable fluorescent signal from either CCK peptides or the L-364,718 used to generate the standard curve. The value of the Z-factor for the assay utilizing fluorescent GI224329 was 0.6. The interassay variation was 9.9%.
Fig. 7.
FP assays. Shown are data from FP assays utilizing GI224329 (A) and Alexa488-CCK (B). Studies were carried out with fixed excitation and emission wavelengths (GI224329; Ex. 300 nm, Em. 333 nm) and (Alexa488-CCK; Ex. 482 nm, Em. 521 nm), using both L-364,718 (●) and CCK (○) ligands. Data are expressed as means ± SEM from four independent experiments performed in duplicate. FP, fluorescence polarization.
Table 3.
Protocol for Fluorescence Polarization Assay Using GI224329
| Step | Parameter | Reagents | Total volume added | Final concentration or incubation time |
|---|---|---|---|---|
| 1 | Source of CCK receptor | CHO-CCK1R cell membranes | 4 μL | 5 μg |
| 2 | Fluorescent ligand | GI224329 | 20 μL | 100 nM |
| 3 | Nonfluorescent ligands | Library compounds and standards | 20 μL | 10−5 M |
| 4 | Buffer | KRH medium, pH 7.4 | 156 μL | N/A |
| 5 | Incubation time | N/A | N/A | 1 h |
| 6 | Assay readout | Spectramax M5E, FP mode | N/A | Ex. 300 nm, Em. 333 nm |
1. Black Optiplate, 96-well format, 8-tip dispensing to all wells.
2. KRH medium used to bring final assay volume to 200 μL per well.
3. Library compounds and standard (L-364,718) diluted to a stock concentration of 1 mM in 100% DMSO. For standard curve, L-364,718 diluted serially in KRH medium from 10−5 to 10−10 M (highest final DMSO concentration being 1%). Column 1-2, row 1, buffer only control, duplicate; row 2, L-364,718 10−11 M; row 3, L-364,718 10−10 M; row 4, L-364,718 10−9 M; row 5, L-364,718 10−8 M; row 6, L-364,718 10−7 M; row 7, L-364,718 10−6 M. The use of the L-364,718 dilution series not only provides a positive control but also allows for the measurement of interassay reproducibility at each of the serial dilutions. L-364,718 being diluted in DMSO to the same stock concentration as library compounds provides a control for the effect of DMSO on the assay. Column 3-12, 6 wells per compound (20 μL of 10−4 M/well) added using an 8-tip dispensing multichannel pipette.
4. GI224329 fluorescent ligand stock concentration of 1 μM in 100% DMSO (0.01% final DMSO concentration per well) added to all rows in column 1 and 2 using an 8-tip dispensing multichannel pipette. GI224329 added to 4 of 6 wells per library compound. Potential fluorescence emitted by each library compound assessed in duplicate in wells in which GI224329 is absent.
5. Plates covered with aluminum foil during incubation period to avoid photobleaching.
6. FP = (V−H)/(V+H), V is the vertical component of the emitted light; H is the horizontal component of the emitted light when the fluorophore is excited using polarized light. FP signal expressed in mP units. Specific binding (%) = {(total binding [FP signal]–nonspecific binding [FP signal])/(total binding [FP signal])} × 100Total binding = polarization value in row 1 (without the competitor); Nonspecific binding = polarization value in row 7 (in the presence of 10−6 M competitor).
CCK1R, type 1 cholecystokinin receptor; CHO, Chinese hamster ovary cells; DMSO, dimethylsulfoxide; Em, emission; Ex, excitation; FP, fluorescence polarization; KRH, Krebs-Ringers-HEPES; N/A, not applicable; mP, millipoise.
Discussion
In the process of drug development, it is important to align screening strategies with the ultimate goals of the project. There is a compelling case for the development of small-molecule drugs active at the CCK1R, given the physiological importance of this receptor in the stimulation of satiety, as well as multiple other actions related to nutritional homeostasis.3 In this work, we report a new reagent that has the potential to be useful in the screening for small-molecule ligands of this receptor that could serve as candidates for new types of CCK1R-active drugs. These could include positive or negative allosteric modulators of the action of CCK as well as allosteric ligands with biological effects that might represent a subset of those of natural hormones or that could even be distinct from those stimulated by natural hormones. Since allosteric ligands bind by definition to receptor regions that are distinct from the orthosteric ligand-binding site, they can even exhibit an additional level of specificity for a subset of receptors within distinct tissues or cells.
For a peptide hormone-binding G protein-coupled receptor such as the CCK receptor, there is strong precedent for the existence of small-molecule ligands that can bind to an allosteric site in the helical bundle within the lipid bilayer.15,21,27 Although most of the small-molecule drug candidates characterized to date have been antagonists, there is also precedent for small-molecule agonists to bind to an allosteric site of the CCK receptor.1 Some of these small-molecule candidate ligands have had little or no demonstrable effect on CCK peptide radioligand binding, which likely reflects differences in the cooperativity index of such allosteric compounds.28 Due to this, a high throughput screen for competitive peptide binding to the orthosteric site of this receptor might miss compounds that could represent leads for the development of important new types of drugs. Therefore, there are clear advantages for a radiolabeled or fluorescent tracer, such as is being currently described, which binds directly to the region of interest in the allosteric binding site within the helical bundle.
In the current article, we introduce a new, fluorescent small-molecule benzodiazepine ligand that represents a specific small-molecule antagonist acting at the CCK1R. By analogy to other structurally related benzodiazepine ligands, it likely binds in the helical bundle within the lipid bilayer.15,16 Its fluorescence characteristics are described in the current work. These data reveal an emission maximum of the receptor-bound state of this ligand at 333 nm, when excited by light at 290 nm. The anisotropy and lifetime data for the receptor-bound ligand reflect the docking of this ligand in a protected intrahelical, intramembranous site. This is further supported by the fluorescence quenching data, showing that the docked ligand is most effectively quenched by the hydrophobic TEMPO reagent, in contrast to GI224329 free in solution that is most effectively quenched by the hydrophilic KI reagent. Similar results were reported with a fluorescent nonpeptidyl antagonist of the neurokinin 1 receptor29 and with the β2-adrenergic receptor ligand, carazolol,30 both of which bind to sites within the lipid bilayer.
These characteristics suggest the utility of this type of fluorescent tracer for an FP assay. Such an assay also has the advantage of not being dependent on a step to separate bound ligand from free ligand. In the initial application of this fluorescent benzodiazepine compound in this type of assay, a known small-molecule ligand for this receptor, L-364,718, is shown to have a clear signal, reducing the FP of this ligand in a concentration-dependent manner, whereas a natural peptide ligand of this receptor that is known to bind to extracellular loops and tail regions is less potent in its effect. Despite having similar binding affinities for the CCK receptor, L-364,718 exhibited an IC50 value for inhibiting half of the FP signal with a concentration >10-fold less than that of CCK (IC50 values: L-364,718, 16 ± 4 nM; CCK, 340 ± 180 nM). This reflects the nature of this assay, being directly competitive for the benzodiazepine, while having indirect effects through negative cooperativity for CCK. The converse of this was true for a fluorescent peptide analog of CCK that can also be utilized in an FP assay. As might be expected, the peptide-based assay was shown to be more useful for examining the receptor binding of orthosteric peptide ligands than for that of allosteric small-molecule ligands. Unfortunately, this FP assay was less sensitive than a direct competition-binding assay using a high-affinity radioligand.21 Identification of a higher-affinity fluorescent benzodiazepine ligand with higher fluorescence quantum yield would likely be even more useful than GI224329 in the screening for novel small-molecule ligands for this receptor.
In this work, we have identified and characterized a new fluorescent benzodiazepine ligand that could be useful in the screening for allosteric small-molecule ligands of the CCK receptor. The ability to use this type of screen for such ligands with a simple, one-step FP assay may have great utility.
Abbreviations
- CCK
cholecystokinin
- CCK1R
type 1 cholecystokinin receptor
- CHO
Chinese hamster ovary cells
- DMSO
dimethylsulfoxide
- Em
emission
- Ex
excitation
- FP
fluorescence polarization
- KI
potassium iodide
- KRH
Krebs-Ringers-HEPES
- mP
millipoise
- TEMPO
2,2,6,6-tetramethylpiperidine 1-oxyl
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK32878) and the Fiterman Foundation. The authors thank E.E. Sugg and R. Sherrill of GlaxoSmithKline Research Laboratories for providing GI224329. We also thank F.G. Prendergast for sharing his instrumentation and P.J. Calahan, W.S. Wessels, E.L. Holicky, and R. Kini for their assistance in these studies.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kenakin T. Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LJ. Dong M. Harikumar KG. Lisenbee CS. Biochemical and cell biological mechanisms of cholecystokinin receptor regulation. Curr Top Med Chem. 2007;7:1166–1172. doi: 10.2174/156802607780960474. [DOI] [PubMed] [Google Scholar]
- 3.Dufresne M. Seva C. Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 4.Liddle RA. Cholecystokinin cells. Annu Rev Physiol. 1997;59:221–242. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- 5.Rehfeld JF. Cholecystokinin. Clin Gastroenterol. 1980;9:593–607. [PubMed] [Google Scholar]
- 6.Gaisano HY. Klueppelberg UG. Pinon DI. Pfenning MA. Powers SP. Miller LJ. Novel tool for the study of cholecystokinin-stimulated pancreatic enzyme secretion. J Clin Invest. 1989;83:321–325. doi: 10.1172/JCI113877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulcrand P. Rodriguez M. Galas MC. Lignon MF. Laur J. Aumelas A. Martinez J. 2-Phenylethyl ester and 2-phenylethyl amide derivative analogues of the C-terminal hepta- and octapeptide of cholecystokinin. Int J Pept Protein Res. 1988;32:384–395. doi: 10.1111/j.1399-3011.1988.tb01273.x. [DOI] [PubMed] [Google Scholar]
- 8.Ding XQ. Pinon DI. Furse KE. Lybrand TP. Miller LJ. Refinement of the conformation of a critical region of charge-charge interaction between cholecystokinin and its receptor. Mol Pharmacol. 2002;61:1041–1052. doi: 10.1124/mol.61.5.1041. [DOI] [PubMed] [Google Scholar]
- 9.Dong M. Lam PC. Pinon DI. Abagyan R. Miller LJ. Elucidation of the molecular basis of cholecystokinin Peptide docking to its receptor using site-specific intrinsic photoaffinity labeling and molecular modeling. Biochemistry. 2009;48:5303–5312. doi: 10.1021/bi9004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadac EM. Pinon DI. Ji Z. Holicky EL. Henne RM. Lybrand TP. Miller LJ. Direct identification of a second distinct site of contact between cholecystokinin and its receptor. J Biol Chem. 1998;273:12988–12993. doi: 10.1074/jbc.273.21.12988. [DOI] [PubMed] [Google Scholar]
- 11.Harikumar KG. Miller LJ. Fluorescence resonance energy transfer analysis of the antagonist- and partial agonist-occupied states of the cholecystokinin receptor. J Biol Chem. 2005;280:18631–18635. doi: 10.1074/jbc.M410834200. [DOI] [PubMed] [Google Scholar]
- 12.Harikumar KG. Pinon DI. Wessels WS. Dawson ES. Lybrand TP. Prendergast FG. Miller LJ. Measurement of intermolecular distances for the natural agonist Peptide docked at the cholecystokinin receptor expressed in situ using fluorescence resonance energy transfer. Mol Pharmacol. 2004;65:28–35. doi: 10.1124/mol.65.1.28. [DOI] [PubMed] [Google Scholar]
- 13.Ji Z. Hadac EM. Henne RM. Patel SA. Lybrand TP. Miller LJ. Direct identification of a distinct site of interaction between the carboxyl-terminal residue of cholecystokinin and the type A cholecystokinin receptor using photoaffinity labeling. J Biol Chem. 1997;272:24393–24401. doi: 10.1074/jbc.272.39.24393. [DOI] [PubMed] [Google Scholar]
- 14.Cawston EE. Miller LJ. Therapeutic potential for novel drugs targeting the type 1 cholecystokinin receptor. Br J Pharmacol. 2010;159:1009–1021. doi: 10.1111/j.1476-5381.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadac EM. Dawson ES. Darrow JW. Sugg EE. Lybrand TP. Miller LJ. Novel benzodiazepine photoaffinity probe stereoselectively labels a site deep within the membrane-spanning domain of the cholecystokinin receptor. J Med Chem. 2006;49:850–863. doi: 10.1021/jm049072h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smeets RL. Ap IJ. Hermsen HP. Ophorst OJ. Van Emst-De Vries SE. De Pont JJ. Willems PH. Mutational analysis of the putative devazepide binding site of the CCK(A) receptor. Eur J Pharmacol. 1997;325:93–99. doi: 10.1016/s0014-2999(97)00106-4. [DOI] [PubMed] [Google Scholar]
- 17.Gao F. Sexton PM. Christopoulos A. Miller LJ. Benzodiazepine ligands can act as allosteric modulators of the Type 1 cholecystokinin receptor. Bioorg Med Chem Lett. 2008;18:4401–4404. doi: 10.1016/j.bmcl.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenakin TP. ′7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol Sci. 2009;30:460–469. doi: 10.1016/j.tips.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 19.May LT. Leach K. Sexton PM. Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 20.Harikumar KG. Pinon DI. Wessels WS. Prendergast FG. Miller LJ. Environment and mobility of a series of fluorescent reporters at the amino terminus of structurally related peptide agonists and antagonists bound to the cholecystokinin receptor. J Biol Chem. 2002;277:18552–18560. doi: 10.1074/jbc.M201164200. [DOI] [PubMed] [Google Scholar]
- 21.Akgun E. Korner M. Gao F. Harikumar KG. Waser B. Reubi JC. Portoghese PS. Miller LJ. Synthesis and in vitro characterization of radioiodinatable benzodiazepines selective for type 1 and type 2 cholecystokinin receptors. J Med Chem. 2009;52:2138–2147. doi: 10.1021/jm801439x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powers SP. Pinon DI. Miller LJ. Use of N,O-bis-Fmoc-D-Tyr-ONSu for introduction of an oxidative iodination site into cholecystokinin family peptides. Int J Pept Protein Res. 1988;31:429–434. doi: 10.1111/j.1399-3011.1988.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 23.Hadac EM. Ghanekar DV. Holicky EL. Pinon DI. Dougherty RW. Miller LJ. Relationship between native and recombinant cholecystokinin receptors: role of differential glycosylation. Pancreas. 1996;13:130–139. doi: 10.1097/00006676-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Harikumar KG. Hosohata K. Pinon DI. Miller LJ. Use of probes with fluorescence indicator distributed throughout the pharmacophore to examine the peptide agonist-binding environment of the family B G protein-coupled secretin receptor. J Biol Chem. 2006;281:2543–2550. doi: 10.1074/jbc.M509197200. [DOI] [PubMed] [Google Scholar]
- 25.Harikumar KG. Miller LJ. Use of fluorescence indicators in receptor ligands. Methods Mol Biol. 2009;552:279–291. doi: 10.1007/978-1-60327-317-6_20. [DOI] [PubMed] [Google Scholar]
- 26.Harikumar KG. Pinon DI. Miller LJ. Fluorescence characteristics of hydrophobic partial agonist probes of the cholecystokinin receptor. Biosci Rep. 2006;26:89–100. doi: 10.1007/s10540-006-9008-x. [DOI] [PubMed] [Google Scholar]
- 27.Aquino CJ. Armour DR. Berman JM. Birkemo LS. Carr RA. Croom DK. Dezube M. Dougherty RW., Jr. Ervin GN. Grizzle MK. Head JE. Hirst GC. James MK. Johnson MF. Miller LJ. Queen KL. Rimele TJ. Smith DN. Sugg EE. Discovery of 1,5-benzodiazepines with peripheral cholecystokinin (CCK-A) receptor agonist activity. 1. Optimization of the agonist “trigger”. J Med Chem. 1996;39:562–569. doi: 10.1021/jm950626d. [DOI] [PubMed] [Google Scholar]
- 28.Christopoulos A. Mitchelson F. Application of an allosteric ternary complex model to the technique of pharmacological resultant analysis. J Pharm Pharmacol. 1997;49:781–786. doi: 10.1111/j.2042-7158.1997.tb06112.x. [DOI] [PubMed] [Google Scholar]
- 29.Turcatti G. Zoffmann S. Lowe JA., 3rd Drozda SE. Chassaing G. Schwartz TW. Chollet A. Characterization of non-peptide antagonist and peptide agonist binding sites of the NK1 receptor with fluorescent ligands. J Biol Chem. 1997;272:21167–21175. doi: 10.1074/jbc.272.34.21167. [DOI] [PubMed] [Google Scholar]
- 30.Tota MR. Strader CD. Characterization of the binding domain of the beta-adrenergic receptor with the fluorescent antagonist carazolol. Evidence for a buried ligand binding site. J Biol Chem. 1990;265:16891–16897. [PubMed] [Google Scholar]