Abstract
Background
Incidental papillary thyroid microcarcinoma (PTMC), a frequent clinical problem, is usually associated with a favorable outcome. During long-term follow-up, only a minority of cases show aggressive behavior with either lymph node or distant metastases. Recently, we had an opportunity to evaluate the efficacy of nonsurgical, ultrasound (US)-guided percutaneous laser ablation (PLA) for local treatment of PTMC in an otherwise inoperable patient.
Patient and Methods
Neck US examination revealed an incidental, solitary, 8 × 7 × 7 mm hypoechoic nodule with microcalcifications of the right thyroid lobe. The patient suffered from decompensated liver cirrhosis, renal failure, and recent surgery followed by external beam radiation therapy for breast cancer. Cytologic diagnosis showed papillary thyroid carcinoma, but the patient declined surgery because of high risk of thyroid surgery. After local anesthesia with 2% xylocaine, PLA was performed according to the previously reported procedure with an Nd:YAG laser.
Summary
The procedure was well tolerated, without side effects, and the patient required no analgesics. US-guided fine-needle aspiration biopsy and core-needle biopsy were performed at 1 and 12 months after PLA, which demonstrated necrotic material and inflammatory cells with no viable neoplastic cell. At the 24 months US follow-up examination, the area of necrosis further decreased, demonstrating a 4 × 4 mm hypoechoic zone and a small hyperechoic area due to fibrotic changes. A fine-needle aspiration biopsy confirmed the absence of malignant cells.
Conclusions
Laser-induced thermal ablation was a safe and effective ablative treatment for a patient with PTMC confined to the thyroid gland who was at high surgical risk. This approach should be considered only in elderly patients and/or in those with comorbidities that might expose the patients to an undue high surgical risk and only after the evaluation by neck US, computed tomography, magnetic resonance imaging, or positron emission tomography/computed tomography rules out lymph-node involvement or metastatic disease.
Introduction
Incidental papillary thyroid microcarcinoma (PTMC), a frequent clinical problem, is usually associated with a favorable outcome (1–4). During long-term follow-up, only a minority of cases show aggressive behavior with either lymph node or distant metastases (3). Although most patients can effectively be treated with lobectomy or thyroidectomy, management occasionally becomes a dilemma because of either patient refusal to undergo surgery or high surgical risk in an elderly patient (3). Recently, we had an opportunity to evaluate the efficacy of nonsurgical, ultrasound (US)-guided percutaneous laser ablation (PLA) for local treatment of PTMC in an otherwise inoperable patient.
Patient and Methods
An 81-year-old woman with decompensated liver cirrhosis, renal failure, and recent surgery followed by external beam radiation therapy for breast cancer was found to have, at US examination, an incidental, solitary, 8 × 7 × 7 mm hypoechoic nodule with microcalcifications in the right thyroid lobe (Fig. 1A). Cytologic diagnosis showed papillary thyroid carcinoma (Fig. 1B), but the patient declined surgery because of high risk of thyroid surgery. Neck US examination and a chest computed tomography (CT) scan revealed no cervical or lung metastasis. Serum thyroid-stimulating hormone, thyroglobulin, antithyroglobulin antibodies, and thyroid hormones were within normal limits. On the basis of previous experience on the use of PLA for benign lesions and distant metastases (5–7), and after the local bioethics committee approval, a thermal ablation of PTMC was proposed and the patient gave her informed consent.
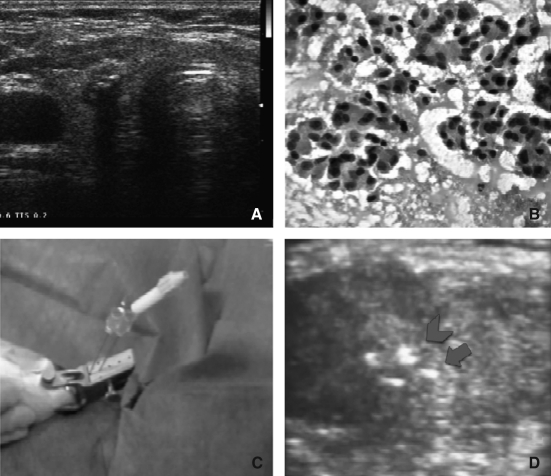
FIG. 1.
Baseline imaging and laser treatment. (A) US scan of the thyroid gland. An 8 × 8 mm hypoechoic nodule with intranodular microcalcification is shown in the right lobe. No further lesions were present in the contralateral lobe. (B) US-guided fine-needle aspiration biopsy of the nodule shows a cytologic pattern diagnostic for papillary carcinoma, oxyphilic variant (Papanicolaou stain). (C) US-guided percutaneous laser ablation procedure. Under US guidance, two 300 μm fiberoptics are inserted through the sheath of spinal needles into the thyroid gland; (D) US image showing the hyperechoic spots due to fiberoptics tips (arrows) near the spots due to microcalcifications (arrowheads). US, ultrasound.
Under sterile conditions and after local anesthesia with 2% xylocaine (3 mL subcutaneous and intracapsular), PLA was performed according to a previously reported procedure (8) with an Nd:YAG laser (Ecolaser-Elesta, Firenze, Italy). Under continuous US guidance, two 21-gauge spinal needles were positioned in correspondence of the lesion. After removal of the stylet, two fibers were inserted 1 cm apart in correspondence of the thyroid lesion and 3600 J of energy was delivered with an output power of 3 W for 10 minutes (Fig. 1C, D). After PLA treatment, an intravenous injection of 4 mg betametasone and an intramuscular injection of 160 mg ketoprofene were given. US and color-Doppler control of the neck were performed at the end of the procedure and after 4 hours. PLA was performed as a day-hospital procedure and the patient returned home at 6 hours after treatment.
Results
The procedure was well tolerated, without side effects, and the patient required no analgesics during the following days. An ENT evaluation confirmed the absence of any laryngeal nerve damage. A rapid increase of Tg value (from 40 to 200 ng/mL), observed after the first 24 hours, returned to baseline values after 1 month. No significant change was observed in serum thyroid hormone levels. Renal and liver functions were not affected by the treatment. Posttreatment US examination showed a 12 × 15 mm hypoechoic area in the right lobe devoid of vascular signals at color-Doppler evaluation. Contrast-enhanced US (9) performed after 12 months (SonoVue, Bracco, Italy) showed a broad avascular area completely encompassing the lesion (Fig. 2A). US-guided fine-needle aspiration biopsy (FNA) and US-guided core-needle biopsy with a 20-gauge cutting needle were performed at 1 and 12 months after PLA and demonstrated necrotic material and inflammatory cells with no viable neoplastic cells (Fig. 2B). At 24 months US follow-up examination, the area of necrosis had further decreased; there was a 4 × 4 mm hypoechoic zone and a small hyperechoic area due to fibrotic changes (Fig. 2C). No suspicious adenopathy was noted by either clinical and US examination or distant metastases by chest and upper abdomen CT scans. A US-guided FNA confirmed the absence of malignant cells, showing inflammatory cells, charred debris, and fibrous tissue (Fig. 2D).
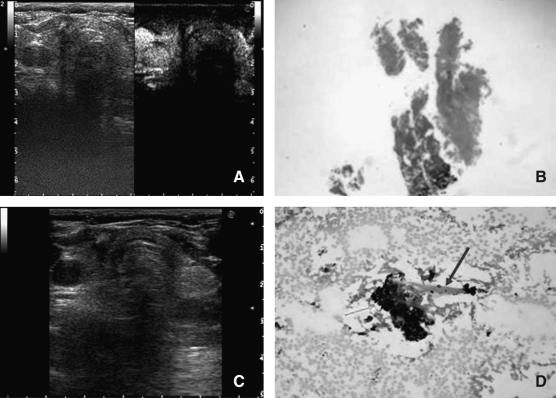
FIG. 2.
Follow-up after treatment. (A) A contrast-enhanced US scan of the right thyroid lobe performed at 12 months after PLA shows a broad hypoechoic and completely avascular area fully encompassing the treated lesion. (B) Core-needle biopsy of the treated area performed at 12 months after PLA shows the presence of inhomogeneous necrotic material with absence of neoplastic tissue (haematoxylin–eosin stain). (C) US scan performed at 24 months after PLA. Right thyroid lobe presents a small avascular hypoechoic area corresponding to the treated lesion (5 × 4 mm). (D) Fine-needle aspiration biopsy of the treated area performed at 24 months after PLA. Necrotic material and inflammatory cells are present, with no viable neoplastic cell (Papanicolaou stain). Few charred debris (green arrow) and fibrous tissue (blue arrow) are depicted. PLA, percutaneous laser ablation.
Discussion
The widespread use of neck imaging procedures and US-guided FNA has resulted in an increasing number of incidentally discovered PTMCs even in elderly patients who are at increased surgical risk because of relevant comorbidities (10). In many of these cases, PMTC is followed up without treatment (3). Although disease progression is infrequent, a few cases of local spread or nodal metastases are reported in the long term (11). Moreover, most patients are understandably concerned about their cancer and ask for repeated, regular follow-up if PTMC is left untreated.
In the present case, PLA treatment of an incidentally discovered PTMC in an otherwise severely ill patient was performed rapidly and without any side effects or complications. The procedure was safe and inexpensive and did not require hospitalization or posttreatment monitoring. The neoplastic lesion, which was unifocal at US examination, appeared completely ablated on the basis of posttreatment cytological, microhistological, and imaging findings. No evidence of disease recurrence was apparent during a 2-year follow-up.
Mini-invasive ablation of incidental PTMCs that appear unifocal and confined to the thyroid gland at US examination may be used to decrease the risk of local growth or extraglandular spread in elderly patients or in patients with relevant comorbidities who are not candidates for surgical resection. After PLA treatment, patients can be followed up with clinical and US evaluations at yearly intervals and with a chest CT scan every 2 years.
Percutaneous ethanol injection has been proposed for the treatment of metastatic cervical lymph nodes (12). The procedure is reported as effective and safe (13,14), but ethanol is randomly distributed in tissue and the size and shape of the area of coagulative necrosis cannot be predicted (15). Ethanol injection may be followed by alcohol seeping into cervical tissues, which may cause neck pain, laryngeal nerve damage, and posttreatment fibrosis (15). Because of these limitations, thermal ablation seems to be more suited for inducing a predictable and well-defined area of necrosis in small thyroid lesions close to vital structures in the neck (9,16). The role of thermal ablation procedures as part of a multimodality therapeutic approach for the cytoreduction of persistent/recurrent thyroid cancer in 131-I resistant patients who have had repeat neck surgery and external beam radiation therapy is promising but should still be considered experimental (17).
Conclusions
PLA was a rapid, safe, and inexpensive procedure for the ablation of a small-sized papillary thyroid tumor without evidence of extraglandular spread or nodal metastases. In experienced centers, PLA can be performed as either an outpatient or a day-hospital procedure, even for patients with significant coexistent comorbidities (16). This novel approach should not be considered as an alternative to surgery because of the frequent multifocality of PTMC and the limitations of US evaluation in assessing the presence of extrathyroid extension (18,19). Laser-induced thermal ablation should be considered only in elderly patients and/or in patients with comorbidities that might expose them to a high surgical risk and only after the morphological evaluation rules out potential lymph-node involvement or metastatic disease by neck US, CT, magnetic resonance imaging, or positron emission tomography/CT.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Bernet V. Approach to the patient with incidental papillary microcarcinoma. J Clin Endocrinol Metab. 2010;95:3586–3592. doi: 10.1210/jc.2010-0698. [DOI] [PubMed] [Google Scholar]
- 2.Roti E. degli Uberti EC. Bondanelli M. Braverman LE. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 2008;159:659–673. doi: 10.1530/EJE-07-0896. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y. Masuoka H. Fukushima M. Inoue H. Kihara M. Tomoda C. Higashiyama T. Takamura Y. Kobayashi K. Miya A. Miyauchi A. Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg. 2010;34:1285–1290. doi: 10.1007/s00268-009-0356-0. [DOI] [PubMed] [Google Scholar]
- 4.Sugitani I. Toda K. Yamamoto N. Ikenaga M. Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. Worl J Surg. 2010;34:1222–1231. doi: 10.1007/s00268-009-0359-x. [DOI] [PubMed] [Google Scholar]
- 5.Papini E. Guglielmi R. Bizzarri G. Graziano F. Bianchini A. Brufani C. Pacella S. Valle D. Pacella CM. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy. Thyroid Mar. 2007;17:229–235. doi: 10.1089/thy.2006.0204. [DOI] [PubMed] [Google Scholar]
- 6.Guglielmi R. Pacella CM. Dottorini ME. Bizzarri G. Todino V. Crescenzi A. Rinaldi R. Panunzi C. Rossi Z. Colombo L. Papini E. Severe thyrotoxicosis due to hyperfunctioning liver metastasis from follicular carcinoma: treatment with (131)I and interstitial laser ablation. Thyroid. 1999;9:173–177. doi: 10.1089/thy.1999.9.173. [DOI] [PubMed] [Google Scholar]
- 7.Hegedüs L. Therapy: a new nonsurgical therapy option for benign thyroid nodules? Nat Rev Endocrinol. 2009;5:476–478. doi: 10.1038/nrendo.2009.152. [DOI] [PubMed] [Google Scholar]
- 8.Cakir B. Ugras NS. Gul K. Ersoy R. Korukluoglu B. Initial report of the results of percutaneous laser ablation of benign cold thyroid nodules: evaluation of histopathological changes after 2 years. Endocr Pathol. 2009;20:170–176. doi: 10.1007/s12022-009-9081-3. [DOI] [PubMed] [Google Scholar]
- 9.Papini E. Bizzarri G. Bianchini A. Guglielmi R. Graziano F. Lonero F. Pacella S. Pacella C. Contrast-enhanced ultrasound in the management of thyroid nodules. In: Baskin HJ, editor; Duick D, editor; Levine RA, editor. Thyroid Utrasound and Ultrasound-Guided FNA. 2nd. Springer; NJ: 2008. pp. 151–172. [Google Scholar]
- 10.Zhai G. Zhang M. Xu H. Zhu C. Li B. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography whole body imaging in the evaluation of focal thyroid incidentaloma. J Endocrinol Invest. 2010;33:151–155. doi: 10.1007/BF03346574. [DOI] [PubMed] [Google Scholar]
- 11.Pellegriti G. Scollo C. Lumera G. Regalbuto C. Vigneri R. Belfiore A. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metab. 2004;89:3713–3720. doi: 10.1210/jc.2003-031982. [DOI] [PubMed] [Google Scholar]
- 12.Lewis BD. Hay ID. Charboneau JW. McIver B. Reading CC. Goellner JR. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. AJR Am J Roentgenol. 2002;178:699–704. doi: 10.2214/ajr.178.3.1780699. [DOI] [PubMed] [Google Scholar]
- 13.Kim BM. Kim MJ. Kim EK. Park SI. Park CS. Chung WY. Controlling recurrent papillary thyroid carcinoma in the neck by ultrasonography-guided percutaneous ethanol injection. Eur Radiol. 2008;18:835–842. doi: 10.1007/s00330-007-0809-5. [DOI] [PubMed] [Google Scholar]
- 14.Sohn YM. Hong SW. Kim EK. Kim MJ. Moon HJ. Kim SJ. Son EJ. Kwak JY. Complete eradication of metastatic lymph node after percutaneous ethanol injection therapy: pathologic correlation. Thyroid. 2009;19:317–319. doi: 10.1089/thy.2008.0370. [DOI] [PubMed] [Google Scholar]
- 15.Guglielmi R. Pacella CM. Bianchini A. Bizzarri G. Rinaldi R. Graziano FM. Petrucci L. Toscano V. Palma E. Poggi M. Papini E. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid. 2004;14:125–131. doi: 10.1089/105072504322880364. [DOI] [PubMed] [Google Scholar]
- 16.Pacella CM. Bizzarri G. Guglielmi R. Anelli V. Bianchini A. Crescenzi A. Pacella S. Papini E. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology. 2000;217:673–677. doi: 10.1148/radiology.217.3.r00dc09673. [DOI] [PubMed] [Google Scholar]
- 17.Papini E. Bizzarri G. Pacella CM. Percutaneous laser ablation of benign and malignant thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2008;15:434–439. doi: 10.1097/MED.0b013e32830eb89a. [DOI] [PubMed] [Google Scholar]
- 18.So YK. Son YI. Hong SD. Seo MY. Baek CH. Jeong HS. Chung MK. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. 2010;148:526–531. doi: 10.1016/j.surg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Koo BS. Lim HS. Lim YC. Yoon YH. Kim YM. Park YH. Rha KS. Occult contralateral carcinoma in patients with unilateral papillary thyroid microcarcinoma. Ann Surg Oncol. 2010;17:1101–1105. doi: 10.1245/s10434-009-0906-6. [DOI] [PubMed] [Google Scholar]