Abstract
Introduction
Profound hypoglycemia occurs rarely as a late complication after Roux-en-Y gastric bypass (RYGB). We investigated the role of glucagon-like-peptide-1 (GLP-1) in four subjects) who developed recurrent neuro-glycopenia 2-3 years after RYGB.
METHODS
A standardized test meal (STM) was administered to all four subjects. A 2 hr hyperglycemic clamp with GLP-1 infusion during the second hr was performed in one subject, before, during a 4 wk trial of octreotide (Oc) and after 85% distal pancreatectomy. After cessation of both glucose and GLP-1 infusion at the end of the 2 hr clamp, blood glucose levels were monitored for 30 minutes. Responses were compared to a control group (5 subjects 12 mo status post RYGB without hypoglycemic symptoms).
RESULTS
During STM, both GLP-1 and insulin levels were elevated 3-4 fold in all subjects, and plasma glucose-dependent insulinotropic peptide (GIP) levels were elevated 2-fold. Insulin responses to hyperglycemia ± GLP-1 infusion in one subject were comparable to controls, but after cessation of glucose infusion, glucose levels fell to 40 mg/dl. During Oc, the GLP-1 and insulin responses to STM were reduced (>50%). During the clamp, insulin response to hyperglycemia alone was reduced, but remained unchanged during GLP-1. Glucagon levels during hyperglycemia alone were suppressed and further suppressed after the addition of GLP-1. With the substantial drop in glucose during the 30 minute follow-up, glucagon levels failed to rise. Due to persistent symptoms, one subject underwent 85% distal pancreatectomy; post-operatively, the subject remained asymptomatic (blood glucose: 119-220 mg/dl), but a repeat STM showed persistence of elevated levels of GLP-1. Histologically enlarged islets, and beta cell clusters scattered throughout the acinar parenchyma was seen, as well as beta-cells present within pancreatic duct epithelium. An increase in pancreatic and duodenal homeobox-1 protein (PDX-1) expression was observed in the subject compared to control pancreatic tissue.
CONCLUSIONS
A persistent exaggerated hypersecretion of GLP-1, which has been shown to be insulinotropic, insulinomimetic and glucagonostatic, is the likely cause of post-RYGB hypoglycemia. The hypertrophy and ectopic location of beta-cells is likely due to over-expression of the islet cell transcription factor, PDX-1, caused by prolonged hypersecretion of GLP-1.
Introduction
Hypoglycemia is a rare complication occurring after Roux-en-Y gastric bypass (RYGB) surgery. Reports have described a handful of patients in whom frank hypoglycemic episodes, some resulting in motor vehicle accidents, have occurred one to three years after RYGB(1-5). Most of the reports implicate inappropriate insulin secretion as the cause, due to diffuse islet hyperplasia and expansion of beta cell mass. It is unclear whether the increased beta cell mass develops prior to surgery due to extreme obesity or whether it is activated as a consequence of the surgical procedure. Some reports attribute the hyperinsulinemia to the development of nesidioblastosis(2, 3) while others report that an increase in beta cell mass was not present in the pancreas of patients undergoing pancreatic resection (4).
Hypoglycemia in patients who have undergone RYGB has been attributed to several possible mechanisms. These mechanisms include a) lack of reduction of beta cell mass which was constitutively increased during the obese state prior to surgery, b) gut hormonal activation of new beta cell formation due to surgically-induced changes in the secretion of insulinotropic enteric hormones, or incretins, or other regulatory peptides, c) increased insulin sensitivity following weight loss, d) inappropriate beta cell secretion following early entry of ingested nutrients into the small intestine (dumping syndrome), and/or e) abnormal counter-regulatory hormonal (glucagon) responses(6-9). However, none of the proposed mechanisms has been clearly established, and it is possible that the abnormal glucose homeostasis is mediated by more than one of these mechanisms. Glucagon-like-peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), have been clearly established as potent incretins, and GLP-1 is more potent than GIP on a molar basis(10). We hypothesized that a change in gut hormonal milieu is responsible for the hyperinsulinemic hypoglycemia following RYGB.
We report four cases of post-RYBG hypoglycemia in which a standardized test meal (STM) was used to measure hormonal responses. In one subject, the hyperglycemic clamp technique was used to evaluate beta cell response to fixed hyperglycemia as well as to a fixed infusion of GLP-1. In this subject, the response to the STM and the clamp study were performed initially, after one month of treatment with octreotide, and after 85% distal pancreatectomy. Histologic evaluation of the resected pancreas was performed to evaluate islet cell mass and the expression of the beta cell growth-promoting transcription factor, pancreatic duodenal homeobox-1 protein (PDX-1).
Methods
A 47 year old caucasian female with severe fasting, post-prandial, and exercise-induced hypoglycemia three years after RYGB was referred to this institution for further evaluation. The patient underwent RYGB for morbid obesity (BMI = 44.5 kg/m2) associated with type 2 diabetes mellitus in January 2006. Her diabetes resolved promptly after surgery and she lost over 54.5 kg over a 15 month period. Approximately 30 months post-operatively, she developed symptoms of confusion, dizziness and unconsciousness which were accompanied by documented post prandial hypoglycemia in the range of 30-40 mg/dL. She was treated with dietary alteration (low carbohydrate, high protein intake), followed by a two week trial of diazoxide. She stopped using diazoxide due to edema and weight gain, but did not experience any symptoms of hypoglycemia during the two week period. At this time, she was referred for other treatment options.
To evaluate the underlying cause of her symptoms, we performed a standardized test meal (STM) consisting of oral ingestion of 475 cc of Ensure Plus(r) followed three days later by a two hour hyperglycemic clamp (98 mg/dl above basal) at which evaluation, after treatment with octerotide and after pancreatectomy. During the second hour of the clamp we infused GLP-1 (7-36) amide in a primed continuous manner (5 pmol·kg−1·min-1) as previously described(10). At 120 minutes both glucose and GLP-1 infusion were terminated and the fall of blood glucose was followed for 30 minutes. She was then treated with octreotide (400 mcg/day) for four weeks, during which the frequency of her symptoms decreased, until she abruptly suffered a profound episode of fasting hypoglycemia (25 mg/dL) associated with seizure development and unconsciousness, that required resuscitation and hospitalization. Both the STM and the hyperglycemic clamp were repeated during the fourth week of treatment. After recovery and re-evaluation, she underwent an 85% distal pancreatectomy, which resulted in resolution of her hypoglycemic episodes. She was evaluated again with the STM and the hyperglycemic clamp eight weeks after pancreatectomy. Paraffin-embedded tissue samples from the tail, mid portion and head of the pancreas were evaluated by routine histology. The specimen was fixed in 10% formalin and processed according to routine practice in the Johns Hopkins Hospital Surgical Pathology Laboratory. Routine hematoxylin and eosin staining was done and immunostains for insulin, chromogranin, glucagon and somatostatin were performed as per routine using the Ventana Benchmark XT automated system. Islet diameter was determined using a calibrated reticle in an Olympus BX40 microscope. Immuno-histologic staining for beta cells, alpha cells, pancreatic polypeptide (PP) cells and PDX-1 were also performed.
We also studied three other female patients with severe hypoglycemia who were referred to us for evaluation. All three had undergone a RYGB procedure 2-3 years prior to the development of hypoglycemic symptoms. Two of them were treated with dietary alteration with no success and one was taking acarbose at the time of evaluation. We performed a STM and measured their active GLP-1 and total GIP responses following the ingestion of the test meal for 180 minutes. Plasma glucose, active GLP-1, total GIP and insulin were measured as previously described(10).
Results
Glucose and Hormonal Responses During STM
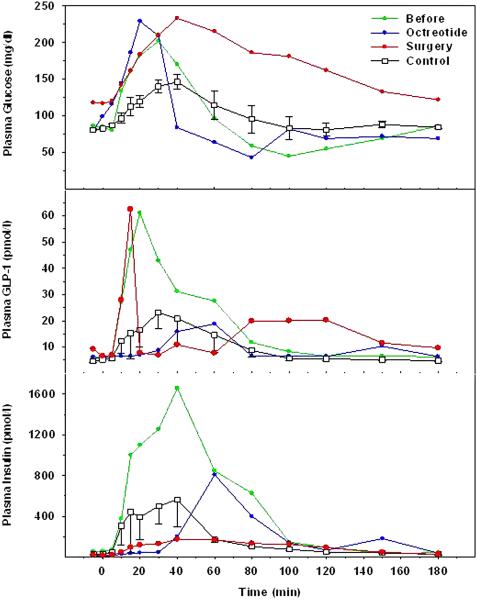
In the first subject, the fasting blood glucose level at initial evaluation was 80 mg/dl and rose to a peak level of 200 mg/dl at 40 minutes following ingestion of Ensure Plus; it then fell and reached a value of 50 mg/dl at 100 minutes, after which it slowly rose and reached a level of 90 mg/dl at 180 minutes (figure 1). The STM plasma glucose profile during octreotide treatment, was essentially unchanged from the initial study. During the first week of octreotide therapy hypoglycemic events did not occur at all. However, subsequently the patient described symptoms that were consistent with low blood glucose levels, which became more frequent as time progressed. These occurred while she was walking or after eating. One profound episode of hypoglycemia occurred in the early morning hours, and resulted in emergency resuscitation with intravenous glucose when loss of consciousness was found to be accompanied by a blood glucose level of 25 mg/dl. After 85% distal pancreatectomy, the fasting blood glucose level was 125 mg/dl and the peak level following Ensure ingestion reached 223 mg/dl at 40 minutes ,after which it slowly fell to a level of 120 mg/dl at 180 minutes. Fasting GLP-1 and insulin levels during the initial evaluation were similar to those seen in a control group of 5 non-hypoglycemic patients studied 12 months after RYGB. Plasma GLP-1 levels following STM ingestion were clearly elevated during the initial study in our patient, however, and reached levels at least 2.5 fold greater than those seen in non-hypoglycemic post-RYGB controls. Plasma insulin levels during the initial STM were similarly elevated compared to controls (figure 1). During octreotide treatment, plasma GLP-1 and insulin responses to the STM were markedly suppressed, and appeared to be delayed by approximately 30 minutes. Following 85% distal pancreatectomy, plasma insulin responses to the STM were decreased, similar to those levels seen during octreotide treatment, although plasma GLP-1 levels remained elevated, similar to those seen during the initial evaluation.
Figure 1.
Plasma glucose (upper panel), GLP-1 (middle panel) and insulin (lower panel) after the ingestion of STM in control subjects (squares) and the patient with hyperinsulinemic hypoglycemia before (green circles), during octreotide treatment (blue circles) and after partial pancreatectomy (red circles).
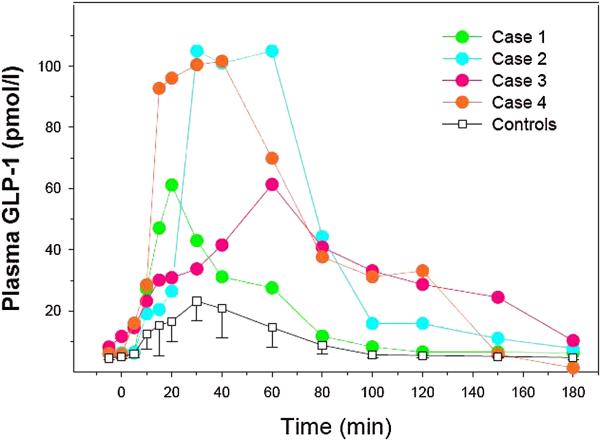
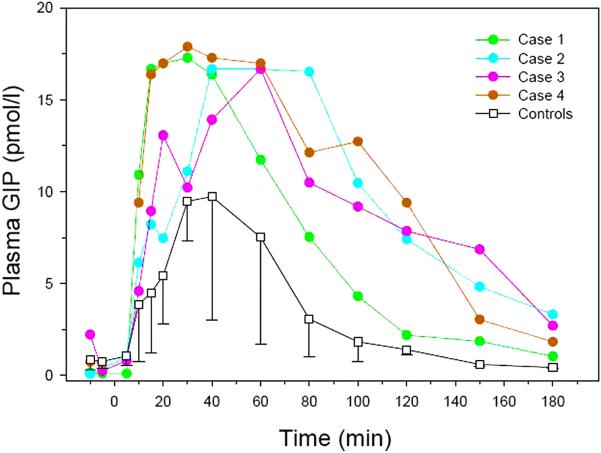
The plasma GLP-1 responses to the STM in the other three patients were similar to the subject described above and were considerably higher than our control group (figure 2). Following STM, total GIP responses of all four patients were also elevated compared to the control group of non-hypoglycemic RYGB patients (figure 3).
Figure 2.
Plasma glucose (upper panel), GLP-1 (middle panel) and insulin (lower panel) during a 2hour of hyperglycemia with GLP-1 infusion during the second hour in control subjects (squares) and the patient with hyperinsulinemic hypoglycemia before ( green circles), during octreotide treatment (blue circles) and after partial pancreatectomy (red circles).
Figure 3.
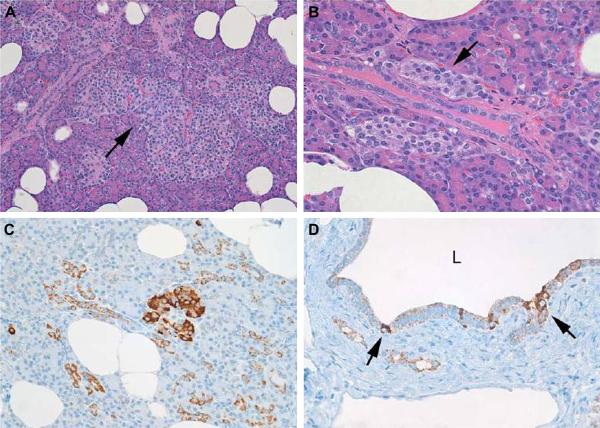
Histopathologic and immunocytochemical features of nesidioblastosis in this patient. A (upper left); variably sized islets and enlarged (450 micrometers greatest dimension) and lobulated islet (arrow). Hematoxylin and Eosin stain x40. B (upper right); Ductulo-insular complexes. Small collections of islet cells abutting a pancreatic ductule. Hematoxylin and Eosin stain x100. C (lower left); Insulin-positive cells and cell clusters scattered in the pancreatic parenchyma. Insulin immunostain x 40. D (lower right); pancreatic ductule with insulin-positive cells in the mucosa. The lumen (L) of the duct is at the top of the photomicrograph. Insulin immunostain x 40.
Glucose, Insulin, and Glucagon Responses During Hyperglycemic Clamp
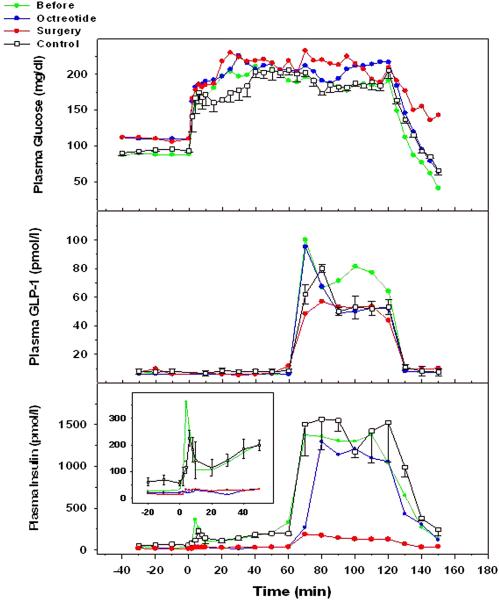
During each hyperglycemic clamp study, blood glucose levels were raised and maintained at approximately 98 mg/dL above basal (figure 4). Fasting glucose levels were noted to be slightly elevated during octreotide treatment and after 85% distal pancreatectomy compared to the initial study, but were similar to control subjects. At 120 minutes, the glucose infusion required to maintain stable hyperglycemia was stopped, and the fall in blood glucose level was followed for at least 30 minutes. During the initial study, blood glucose levels fell after cessation of glucose infusion to a level of 40 mg/dl, which was associated with mild symptoms of dizziness and which necessitated further infusion of glucose to restore euglycemia. During octreotide treatment, blood glucose fell to a level of 60 mg/dl at 150 minutes, during which the patient remained asymptomatic. Following 85% distal pancreatectomy, blood glucose fell to 100 mg/dL, and the patient remained asymptomatic. During hyperglycemia alone (0-60 min) plasma insulin levels during the initial study were similar to those seen in a group of non-hypoglycemic post-RYGB controls. Total glucose metabolism rates (M) were low-normal and similar to controls. Plasma insulin responses to hyperglycemia during octreotide treatment and after 85% distal pancreatectomy were reduced compared to the initial study for both first-phase and second-phase insulin release. During GLP-1 infusion, plasma GLP-1levels were similar in all visits: plasma insulin levels in response to combined hyperglycemia and elevated GLP-1 levels during the initial visit were robust, but similar to the control group, and M was markedly increased during GLP-1 infusion. Octreotide treatment had little effect on the insulin response to combined hyperglycemia and elevated GLP-1 levels, and M during the period of combined hyperglycemia and GLP-1 infusion remained high. After 85% distal pancreatectomy, the insulin response to combined hyperglycemia and GLP-1 infusion was markedly reduced, as was M. Plasma glucagon levels were suppressed during the hyperglycemic clamp in all studies. However during the fall of blood glucose levels after cessation of glucose infusion, a counter-regulatory increase in glucagon was not observed despite very low glycemic levels in the initial study.
Figure 4.
Standardized Test Meal in controls and four hypoglycemic post RYGB patients.
Histopathology and Immuno-histochemistry of Pancreas
Extensive gross and microscopic examination of specimen excluded the presence of an insulinoma. The histologic features of the pancreas (Fig 5) satisfied published criteria (11-16) for nesidioblastosis: irregular, variably sized and lobulated islets, enlarged and hyperchromatic beta-cell nuclei, large islets, ductulo-insular complexes (close association of islet cell clusters with small pancreatic ducts) and scattered insulin-positive cells throughout the pancreatic parenchyma. Results of morphometric study of 50 randomly selected islets showed a median islet size of 250 micrometers with a range of 100 to 450 micrometers and a median of 220 micrometers. The median islet size of our patient with nesideoblastosis was in accord with data presented by Service et al(3) and by Anlauf et al(11).
Figure 5.
Characteristic histologic and immunocytochemical features of nesidioblastosis. A. Extreme variation in islet size and the presence of large, lobulated islets (center of the panel). B. Ductuloinsular complexes. Endocrine cell clusters are intimately connected with a small pancreatic duct. C. Insulin positive endocrine cell clusters are scattered throughout the acinar parenchyma. D. Insulin positive cells are seen scattered amongst the pancreatic duct epithelium.
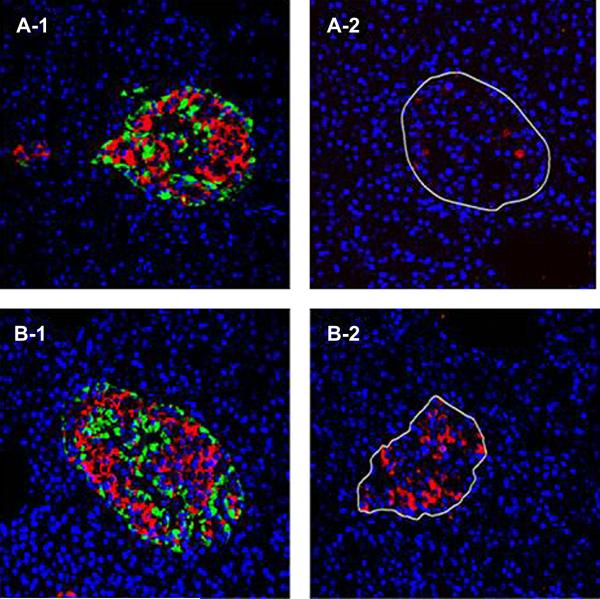
Immuno-histologic examination of pancreatic tissue was performed with additional antisera directed at insulin, glucagon, pancreatic polypeptide, and PDX-1, and comparisons were made against a normal pancreas from a patient of similar age, BMI and gender. There was no apparent increase in beta cell mass compared to control. However, there was pronounced increase in the number of alpha cells present, such that the ratio of alpha to beta cells was 65/35. PP cells were normal and present in all parts of the pancreas. There was an obvious increase in PDX-1 staining in all areas of the pancreas (Figure 6).
Figure 6.
A representative of fluorescent immune-histochemistry staining of islets (insulin in red and glucagon in green)from the control pancreas (A-1) and patient pancreas (B-1). PDX-1 staining of the control pancreas (A-2) and the patient pancreas (B-2). The right panels clearly show the increased expression of PDX-1 in the patient’s pancreas.
Discussion
Severe hypoglycemia is now recognized as a rare consequence of RYGB surgery. The prevalence of this syndrome is unknown, and it appears to be observed only after diversion of nutrients into the mid-small bowel. There is a single report in which 12 patients with this condition were treated, successfully, with low-carbohydrate diet(19). In that report, 11 patients were identified from a registry of 3082 RYGB procedure, which suggests an incidence of less than 1%. Symptoms usually do not occur for at least one year after the surgery. In a single case report, the condition occurred within three months after surgery in a patient in whom an islet-cell tumor was present in the tail of the pancreas(17), but most other reports have not implicated insulinoma formation. The most common treatment options for this condition include dietary alteration, a trial of the beta-cell antagonist, diazoxide, octreotide or acarbose. When the medical treatment options fails, surgery has been advocated. The extent of the surgical procedure can be determined using a sequential injection of calcium stimulating test. Partial, sub-total, and total pancreatic resection have been reported to treat refractory hypoglycemia, and subsequent (type 3) diabetes is a frequent consequence of pancreatectomy. Recently, a case of hyperinsulinemic hypoglycemia post-RYGB has been treated successfully with use of enteral feedings through a gastrostomy tube inserted into the remnant stomach(18). Whether the cause can be unequivocally attributed to increased beta-cell mass, or “nesidioblastosis”, is controversial. Pancreatic tissues from the study of Service et al(3), which were described as “hypoglycemia with nesidioblastosis”, were re-examined by Meier et al(4) and were not found to be consistent with beta-cell hypertrophy. The latter study deomonstrated that there was increased beta-cell nuclear diameter, which correlated with pre-operative BMI, suggesting beta-cell hyperactivity secondary to obesity. Histologic evidence of an expanded beta cell mass can be observed in normo-glycemic conditons, such as normal pregnancy, as well as in conditions of hyperinsulinism and hypoglycemia. Therefore the histologic finding and diagnosis of nesideoblastosis remains a morphologic description rather than a functional diagnosis of pancreatic endocrine dysfunction, which can be subjective based on pathologist interpretation.
It has been suggested that beta-cell hyperactivity following RYGB may be due to increased GLP-1 secretion, and our subjects demonstrated strikingly elevated GLP-1 levels following STM. GLP-1 has been shown to act as a growth factor on beta cells through a phosphatidylinositol 3 kinase pathway. It also increases transcription factor, pancreatic and duodenal homebox gene 1 (PDX-1) gene expression and binding activity. PDX-1 expression occurs predominantly in the beta and delta cells of the endocrine pancreas and mutations of the gene results in defective beta cell development(20). Thus PDX-1 is necessary for development and maturation of beta cells, and increased expression of PDX-1 could result in increased production and secretion of insulin.
In our longitudinal assessment of GLP-1 responses following RYGB in five patients who have not displayed hyperinsulinemia or hypoglycemia, we observed hypersecretion of GLP-1 at 3 and 6 months (57.3±26.4 pmol/l) after the operation which normalized to levels observed in non-obese individuals by 9 to 12 months after operation (23.2±6.2 pmol/l)(21). In all four subjects studied here, GLP-1 levels were at least three fold higher than those observed in control subjects approximately one year after RYGB surgery. Total GIP levels in all four subjects were also at least two fold higher than in controls. Thus the increased secretion of incretins could be responsible, at least in part, for similarly high insulin release during the STM. In our patient in whom had a clamp studies performed before therapy (octerotide or pancreatectomy, infusion of GLP-1 which produced a square wave of GLP-1 level similar to that achieved in our non-hypoglycemic patients, was not accompanied by an exaggerated insulin response (figure 4). The same observation with respect to insulin response was made in a second patient with hypoglycemic symptoms who had a clamp performed with the infusion of GLP-1 (data not shown). Thus, the robust insulin release during the STM was likely secondary to an exaggerated GLP-1 response to nutrients, but was not due to an enhanced beta-cell sensitivity to GLP-1.
Other beta-cell stimulatory factors (incretins) such as GIP may also play a role in the exaggerated insulin response to nutrients. GLP-1 hypersecretion has been proposed as a cause of post-RYGB hypoglycemia(8) although the incidence and pattern of this gut hormonal response to RYGB remains undefined. It has been suggested that a longer length of roux limb may be responsible for excess GLP-1 secretion, and subsequent hypoglycemia, as GLP-1 secreting “L” cells are found in highest concentration is the distal small intestine.
We are not aware of any prior report in which post-RYGB patients with hypoglycemia were examined with the clamp methodology. During the clamp, we did not observe an exaggerated insulin response either to glucose alone or to glucose and GLP-1, which refuted the possibility of autogenous hyperinsulinism. Furthermore, the rate of total (net) glucose metabolism observed during the clamp was consistent with values observed in other non-hypoglycemic patients, which refuted the possibility of enhanced insulin sensitivity. However, the fall of glucose after cessation of glucose and GLP-1 infusion was very robust. We hypothesize that the increased glucose uptake both during the STM and when GLP-1 is infused may be due in part to the established insulinomimetic properties of GLP-1(22). In addition, the lack of any glucagon response to frank hypoglycemia can be attributed to the known glucagonostatic effect of (elevated) GLP-1 levels(23). Despite abundant alpha cells in the pancreas of our patient, the failure of a counter-regulatory response to hypoglycemia appeared to play a significant role in the pathophysiology of this condition.
GLP-1 has been shown to promote PDX-1 expression(20), and our finding of increased PDX-1 expression is further evidence of the effect of excess GLP-1 levels. Although we did not assess nuclear content of the beta-cells, the finding of increased PDX-1 in the pancreas of our patient, accompanied by morphologic signs of increased beta and alpha cell activity, is consistent with our conclusion that persistently elevated GLP-1 levels had a functional effect on the pancreatic endocrine function. Restoration of normal GLP-1 and GIP secretion can presumably be achieved by reversal of the diversion of gastric contents into the mid-small bowel. Our surgical patient declined reversal of her RYGB over concern of weight gain, and it remains conjectural whether conversion to a restrictive type of bariatric procedure would maintain weight loss but avoid or reverse the consequences of persistently elevated incretin secretion. Conversion to an alternative form of bariatric procedure would nonetheless appear to be an attractive alternative than the creation of pancreatogenic (type 3) diabetes as a means to avoid hypoglycemia in these patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, Hanto DW, Callery M, Arky R, Nose V, Bonner-Weir S, Goldfine AB. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 2.Clancy TE, Moore FD, Jr., Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10:1116–1119. doi: 10.1016/j.gassur.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 4.Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care. 2006;29:1554–1559. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 5.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 6.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. The American surgeon. 2004;70:1–4. discussion 4-5. [PubMed] [Google Scholar]
- 7.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. International journal of obesity. 2009;33(Suppl 1):S33–40. doi: 10.1038/ijo.2009.15. (2005) [DOI] [PubMed] [Google Scholar]
- 8.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 9.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. International journal of obesity. 2009;33:786–795. doi: 10.1038/ijo.2009.79. (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elahi D, McAloon-Dyke M, Fukagawa N, Meneilly G, Sclater A, Minaker K, Habener J, Andersen D. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Reg Peptides. 1994;51:63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 11.Anlauf M, Wieben D, Perren A, Sipos B, Komminoth P, Raffel A, Kruse ML, Fottner C, Knoefel WT, Monig H, Heitz PU, Kloppel G. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, and characterization of beta-cell changes. The American journal of surgical pathology. 2005;29:524–533. doi: 10.1097/01.pas.0000151617.14598.ae. [DOI] [PubMed] [Google Scholar]
- 12.Hong R, Choi DY, Lim SC. Hyperinsulinemic hypoglycemia due to diffuse nesidioblastosis in adults: a case report. World J Gastroenterol. 2008;14:140–142. doi: 10.3748/wjg.14.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloppel G, Anlauf M, Raffel A, Perren A, Knoefel WT. Adult diffuse nesidioblastosis: genetically or environmentally induced? Human pathology. 2008;39:3–8. doi: 10.1016/j.humpath.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 14.McElroy MK, Lowy AM, Weidner N. Case report: focal nesidioblastosis (“nesidioblastoma”) in an adult. Human pathology. 41:447–451. doi: 10.1016/j.humpath.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Raffel A, Krausch MM, Anlauf M, Wieben D, Braunstein S, Kloppel G, Roher HD, Knoefel WT. Diffuse nesidioblastosis as a cause of hyperinsulinemic hypoglycemia in adults: a diagnostic and therapeutic challenge. Surgery. 2007;141:179–184. doi: 10.1016/j.surg.2006.04.015. discussion 185-176. [DOI] [PubMed] [Google Scholar]
- 16.Rumilla KM, Erickson LA, Service FJ, Vella A, Thompson GB, Grant CS, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis: histologic features and growth factor expression. Mod Pathol. 2009;22:239–245. doi: 10.1038/modpathol.2008.169. [DOI] [PubMed] [Google Scholar]
- 17.Abellan P, Camara R, Merino-Torres JF, Perez-Lazaro A, del Olmo MI, Ponce JL, Rayon JM, Pinon F. Severe hypoglycemia after gastric bypass surgery for morbid obesity. Diabetes Res Clin Pract. 2008;79:e7–9. doi: 10.1016/j.diabres.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 95:1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg TA, Bantle JP, Leslie DB, Redmond JB, Slusarek B, Swan T, Buchwald H, Ikramuddin S. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4:492–499. doi: 10.1016/j.soard.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Habener J, Stoffers D, Elahi D, Hani E, Froguel P. Role of homeodomain transcription factor IPF-1 in the pathogenesis of diabetes mellitus. In: Matschinsky FMM, editor. Frontier in Diabetes. Karger; Basel: 2000. pp. 197–208. MA. [Google Scholar]
- 21.Rabiee A, Magnuson T, Andersen DK, Elahi D. Resolution of Type 2 Diabetes Mellitus (T2DM) Following Bariatric Surgery: What Happens First?. 2009 Mid-Atlantic Diabetes Research Symposium; Bethesda, MD. 2009. 2009. [Google Scholar]
- 22.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–586. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]