Abstract
Objective
We examined the associations among physical activity, neurological disability, and cardiorespiratory fitness in two studies of individuals with multiple sclerosis (MS).
Method
Study 1 included 25 women with relapsing-remitting MS (RRMS) who undertook an incremental exercise test for measuring peak oxygen (V̇O2peak) consumption, wore an accelerometer during a 7-day period, and completed the Godin Leisure-Time Exercise Questionnaire (GLTEQ). Study 2 was a follow-up of Study 1 and included 24 women with RRMS who completed the self-reported Expanded Disability Status Scale (EDSS), undertook an incremental exercise test, wore an accelerometer during a 7-day period, and completed the GLTEQ.
Results
Study 1 indicated that V̇O2peak was significantly correlated with accelerometer counts (pr = 0.69) and GLTEQ scores (pr = 0.63) even after controlling for age and MS duration. Study 2 indicated that V̇O2peak was significantly correlated with accelerometer counts (pr = 0.50), GLTEQ scores (pr = 0.59), and EDSS scores (pr = −0.43) even after controlling for age and MS duration; there was a moderate partial correlation between accelerometer counts and EDSS scores (pr = −0.43). Multiple linear regression analysis indicated that both accelerometer counts (β = 0.32) and EDSS scores (β = −0.40) had statistically significant associations with V̇O2peak.
Conclusion
The findings indicate that physical inactivity and neurological disability might represent independent risk factors for reduced levels of cardiorespiratory fitness in this population.
Keywords: deconditioning, mobility, neurological disease, exercise, fitness
The maintenance of cardiorespiratory fitness is important for ensuring independence in daily life among persons with chronic disabling conditions such as multiple sclerosis (MS; 1). Cardiorespiratory fitness or peak oxygen consumption (V̇O2peak) is defined as the highest rate of oxygen uptake attained during physical work (2) and is a product of the peak cardiac output and the peak difference in arteriovenous oxygen (3). There is evidence for a reduction in cardiorespiratory fitness among persons with MS (4) and this is likely associated with physical inactivity and neurological disability (1).
Physical inactivity is one factor that may contribute to the reduction of cardiorespiratory fitness in persons with MS. Physical inactivity is prevalent in persons with MS (5, 6), but there have been few examinations of an association between lifestyle physical activity and cardiorespiratory fitness in this population. One study reported no significant association between self-reported physical activity and V̇O2peak in persons with MS (7). We are not aware of other studies that have examined objectively measured physical activity as a correlate of V̇O2peak in this population.
Neurological disability is another factor that might be associated with reduced cardiorespiratory fitness. To date, there is inconsistent evidence of an association between neurological disability and cardiorespiratory fitness in the MS population. Some studies have reported an inverse association between cardiorespiratory fitness and neurological disability (8, 9), whereas other studies have reported no association between cardiorespiratory fitness and neurological disability (10).We further note that researchers have not examined the possibility that neurological disability is associated with cardiorespiratory fitness independently of physical activity. This is important as physical inactivity has been associated with neurological disability in persons with MS (11, 12) and it might be associated with cardiorespiratory fitness.
This article includes two studies that examined the association between physical activity and cardiorespiratory fitness (Study 1) and the associations among physical activity, neurological disability, and cardiorespiratory fitness (Study 2) in persons with MS. Study 1 examined the association between physical activity and cardiorespiratory fitness given there is minimal and conflicting evidence regarding physical inactivity as a factor that may contribute to the reduction of cardiorespiratory fitness in persons with MS. Study 2 was a follow-up that examined the associations among physical activity, neurological disability, and cardiorespiratory fitness given that researchers have not examined the independent associations of physical inactivity and neurological disability with reductions in cardiorespiratory fitness in persons with MS. Importantly, physical activity was measured in both studies by objective and self-reported instruments that have been validated in persons with MS (13, 14). We hypothesized that physical inactivity would be associated with reduced levels of cardiorespiratory fitness in the first study, and that physical inactivity and neurological disability would be independently associated with reduced levels of cardiorespiratory fitness in the second study.
Study 1
Methods
Participants
The sample in Study 1 consisted of 25 women with relapsing-remitting MS (RRMS). The diagnosis of RRMS was confirmed by a neurologist and participants had been relapse-free during the previous 30 days. Participants did not have any contraindications for performing incremental exercise testing (e.g. cardiovascular disease) and provided written physician approval for undergoing the exercise test. The mean height and weight were 167.7 cm (SD = 5.9) and 76.0 kg (SD = 18.6), respectively. The mean age of the participants was 42.0 years (SD = 9.4) and the mean duration of MS was 8.9 years (SD = 6.2). All participants were ambulatory without an assistive device, and 15 of the 25 participants were on a disease-modifying agent (Betaseron, 47%; Axonex, 40%; Copaxon, 13%). This study focused on the association between physical activity and cardiorespiratory fitness, and, as such, did not include the Expanded Disability Status Scale (EDSS) as a marker of neurological disability.
Measures – Cardiorespiratory fitness
Cardiorespiratory fitness was measured as V̇O2peak using an incremental exercise test on an electronically braked, computer-driven cycle ergometer (Lode BV, Groningen, The Netherlands). Initially, participants were fitted to the cycle ergometer, and the test procedures along with instructions for providing ratings of perceived exertion (RPE) were described by an investigator who then answered participant questions. After inserting a mouthpiece for collecting expired gases, the participants performed a 5-min warm-up at 0 W. The initial work rate for the exercise test was 0 W, and the work rate continuously increased at a rate of 15 W/min until the participant reached the point of volitional termination. Using an open-circuit spirometry system (TrueOne, Parvo Medics, Sandy, UT, USA), oxygen consumption (V̇O2peak), carbon dioxide production (V̇O2peak), ventilation (VE) and respiratory exchange ratio (RER) were measured every 20 s. Heart rate (HR) was displayed using a Polar HR monitor (Polar Electro Oy, Kempele, Finland); HR and RPE were recorded every minute during the test. V̇O2peak was defined as the highest recorded V̇O2peak value expressed in ml/kg/min when two of three criteria were satisfied: RER ≥ 1.10; peak HR within 10 beats/min of age-predicted maximum (i.e. ~1 SD); or peak RPE ≥ 17.
Physical activity
Physical activity was measured using ActiGraph accelerometers (model 7164 version; Health One Technology, Fort Walton Beach, FL, USA) and the Godin Leisure-Time Exercise Questionnaire (GLTEQ; 15) because both measures have evidence of validity among persons with MS (13, 14). The ActiGraph accelerometers were calibrated mechanically by the manufacturer and provided an objective measure of physical activity that is not plagued by recall bias. Participants recorded the time that the accelerometer was worn on a log, and this was verified by our inspection of the minute-by-minute accelerometer data. We further examined the accelerometer data for long periods of continuous zeros as a check of compliance with wearing the device and we used a criterion of 60 min of continuous zeros for non-compliance. We based the judgment of a valid day of measurement based on 10 h of wear time during the waking hours (16), defined as the moment upon getting out of bed in the morning through the moment of getting into bed in the evening. We considered the data to be spurious when counts exceeded 20,000/min (16), and we required that participants have seven valid days of data for a reliable estimate of weekly physical activity. Downloaded data from the accelerometers were entered into Microsoft Excel for processing, and the movement counts for each day were summed and then averaged across the 7-day period. This resulted in accelerometer data expressed in total counts per day (i.e. usual physical activity). The GLTEQ is a self-administered two-part measure of usual physical activity; we only included the first part in this study consistent with previous research (13, 14). The first part has three items that measure the frequency of strenuous (e.g. jogging), moderate (e.g. fast walking), and mild (e.g. easy walking) exercise for periods of more than 15 min during one’s free time in the previous week. The weekly frequencies of strenuous, moderate, and mild activities were multiplied by 9, 5, and 3 metabolic equivalents (MET), respectively, and summed to form a measure of total leisure activity in MET/min/week.
Procedure
The procedure was approved by an Institutional Review Board and all testing was conducted in the Exercise Neuroscience Laboratory on the University of Illinois campus. The participants initially completed a screening for contraindications over the telephone and were then sent a form that verified physician approval for exercise testing. After returning the physician’s approval form, the participants were scheduled for exercise testing. On the day of exercise testing, all participants first provided written informed consent and then undertook an incremental exercise test to volitional exhaustion as a method of measuring V̇O2peak. After the incremental exercise test, the participants were provided with instructions and a log for wearing the accelerometer during a 7-day period as well as the GLTEQ. The participants returned the accelerometer along with the GLTEQ after the 7-day period using the US postal service along with prestamped, preaddressed envelopes provided by the research team. Participants received $20 remuneration.
Data analysis
All analyses were conducted using SPSS for Windows, Version 16.0. Descriptive statistics are presented in tables as M ± SD. Scatter plots are provided in figures as a visual illustration of the associations between V̇O2peak and accelerometer counts and GLTEQ scores. We computed bivariate correlations (r) between accelerometer counts, GLTEQ scores, and V̇O2peak. This was followed by partial correlations (pr) controlling for age and duration of MS; these variables represent possible confounders. Effect size guidelines of 0.1, 0.3, and 0.5 were used for judging the magnitude of the correlations as small, moderate, and large, respectively (17).
Results
Descriptive statistics
Descriptive statistics along with range of scores are provided in Table 1. The mean accelerometer count and GLTEQ score were comparable with previous research on persons with MS (13). We further note that the mean GLTEQ score in this sample was lower than the normative mean value of 45.8 reported for healthy adults (15) and the difference was large in magnitude (d = −0.71; 17). The mean V̇O2peak value was comparable with previous research that included women with MS and a similar incremental exercise test on a cycle ergometer (7), but lower than the normative value for healthy adult women between 40 and 49 years of age (18).
Table 1.
Descriptive statistics for primary study variables in sample consisting of 25 women with relapsing-remitting multiple sclerosis (Study 1)
| Measure | Mean | SD | Range of scores |
|---|---|---|---|
| Accelerometer (average counts/day) | 258,341 | 131,483 | 75,978–514,090 |
| GLTEQ (MET/min/week) | 29.8 | 22.5 | 0–71 |
| Cardiorespiratory fitness (ml/kg/min) | 21.4 | 6.2 | 11.1–32.5 |
GLTEQ, Godin Leisure-Time Exercise Questionnaire.
Correlation analysis
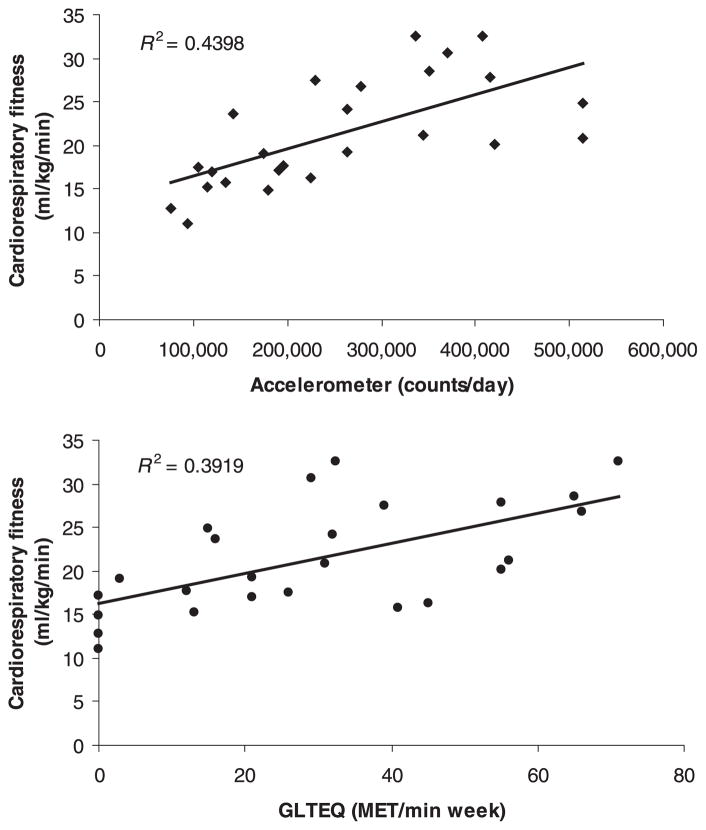
Scatter plots of the associations between V̇O2peak and accelerometer counts and GLTEQ scores are provided in Fig. 1. There were strong, statistically significant correlations between V̇O2peak and accelerometer counts (r = 0.66, P = 0.0001) and GLTEQ scores (r = 0.63, P = 0.001). Accelerometer counts and GLTEQ scores were strongly correlated (r = 0.51, P = 0.01). Partial correlation analysis indicated that the correlations between V̇O2peak and accelerometer counts (pr = 0.69, P = 0.0001) and GLTEQ scores (pr = 0.63, P = 0.001) were unchanged when controlling for age and duration of MS.
Figure 1.
Scatter plots of the associations between objective and self-reporting measures of physical activity with cardiorespiratory fitness in a sample of 25 women with relapsing-remitting multiple sclerosis (Study 1).
Study 2
Methods
Participants
The sample in Study 2 consisted of 24 women with a neurologist-confirmed diagnosis of RRMS; there was minimal overlap between subjects in Study 1 and Study 2 (n = 6) as the studies were separated by nearly 1 year in time. The participants had been relapse-free during the previous 30 days. Participants did not have any contraindications for performing incremental exercise testing and provided physician approval as in Study 1. The mean height and weight were 167.2 cm (SD = 6.4) and 70.9 kg (SD = 15.0), respectively. The mean age of the participants was 44.1 years (SD = 7.4) and the mean duration of MS was 7.5 years (SD = 4.8). Participants were either independently ambulatory or ambulatory with a cane, and 14 individuals were on a disease-modifying therapy (Betaseron, 50%; Axonex, 43%; Copaxon, 7%).
Measures – Cardiorespiratory fitness
V̇O2peak was measured using an incremental exercise test on an electronically braked, computer-driven cycle ergometer as described in the Methods section for Study 1.
Physical activity
Physical activity was measured using an ActiGraph accelerometer (model 7164 version; Health One Technology) and the GLTEQ (15) as described in the Methods section for Study 1.
Disability
Disability was measured using the self-reporting version of the Kurtzke EDSS (19). This scale contains 17 self-rated items that reflect the components of a physician-administered EDSS. Scores from this version of the EDSS have strongly correlated (r = 0.92) with scores from a physician-administered Kurtzke EDSS scale (19).
Procedure
The procedure was approved by the same Institutional Review Board, performed in the same laboratory, and was nearly identical with that of Study 1. The primary di3erence was that participants completed the EDSS after providing written informed consent on the day of exercise testing.
Data analysis
Analyses were conducted using SPSS for Windows, Version 16.0. Descriptive statistics are presented in tables as M ± SD. Scatter plots are provided in figures as a visual illustration of the associations between V̇O2peak and accelerometer counts, GLTEQ scores, and EDSS scores. We computed bivariate correlations (r) between accelerometer counts, GLTEQ scores, EDSS scores, and V̇O2peak. This was followed by partial correlations (pr) controlling for age and duration of MS as possible confounders. Effect size guidelines of 0.1, 0.3, and 0.5 were again used for judging the magnitude of the correlations as small, moderate, and large, respectively (17). We examined the independent contributions of physical activity and disability for explaining variance in cardiorespiratory fitness using multiple linear regression analysis. The multiple linear regression analysis involved direct, simultaneous entry of accelerometer counts/GLTEQ scores and EDSS scores as predictors of V̇O2peak. The resulting standardized beta-coefficients reflect the independent contribution of each of the two variables for explaining variation in V̇O2peak, but the associations are not independent of other possible predictors that were not included in the analysis.
Results
Descriptive statistics
Mean scores, standard deviations, and range of scores for all measures are provided in Table 2. Independent samples t-tests indicated that there were no di3erences between samples in Studies 1 and 2 in accelerometer counts, t(47) = 0.59, P = 0.56, GLTEQ scores, t(47) = 0.71, P = 0.48, and V̇O2peak, t(47) = 0.16, P = 0.88. Again, the mean accelerometer count and GLTEQ score were comparable with previous research on persons with MS (13). The mean GLTEQ score in this sample was lower than the normative mean value of 45.8 for healthy adults (15) and the difference in GLTEQ scores was large in magnitude (d = −1.15) (17). The mean V̇O2peak value was comparable with previous research that included women with MS and a similar incremental exercise test on a cycle ergometer (7), but lower than the normative value for healthy adult women between 40 and 49 years of age (18). The mean EDDS score was comparable with previous research examining the associations among cardio-respiratory fitness, neurological disability, and physical activity in women with MS (7).
Table 2.
Descriptive statistics for primary study variables in sample consisting of 24 females with relapsing-remitting multiple sclerosis (Study 2)
| Measure | Mean | SD | Range of scores |
|---|---|---|---|
| Accelerometer (average counts/day) | 238,330 | 105,385 | 95,501–490,801 |
| GLTEQ (MET/min/week) | 25.7 | 17.5 | 0–70 |
| Cardiorespiratory fitness (ml/kg/min) | 21.7 | 5.9 | 12.9–33.9 |
| EDDS (median) | 2.4 (1.5) | 1.6 | 0–6 |
GLTEQ, Godin Leisure-Time Exercise Questionnaire; EDDS, Expanded Disability Status Scale.
Correlation analysis
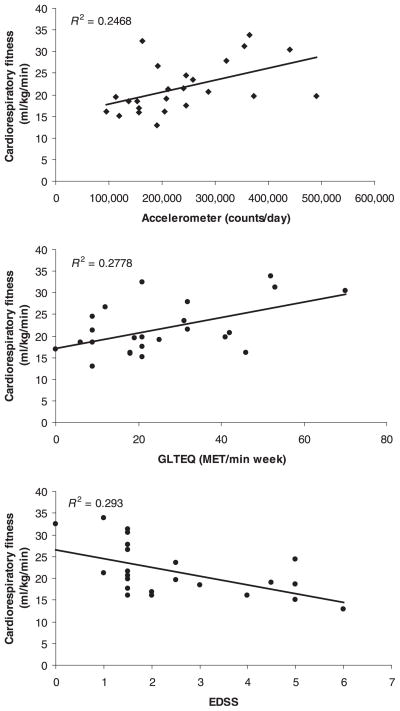
Scatter plots of the associations between V̇O2peak and accelerometer counts, GLTEQ scores, and EDSS scores are provided in Fig. 2. There were strong, statistically significant correlations between V̇O2peak and accelerometer counts (r = 0.50, P = 0.01), GLTEQ scores (r = 0.53, P = 0.01), and EDSS scores (r = −0.54, P = 0.005). We further note that EDSS scores were moderately and inversely correlated with accelerometer counts (r = −0.43, P = 0.05) and GLTEQ scores (r = −0.32, P = 0.13) and there was a strong correlation between accelerometer counts and GLTEQ (r = 0.64, P = 0.001). Partial correlation analysis indicated that the correlations between V̇O2peak and accelerometer counts (pr = 0.50, P = 0.05), GLTEQ scores (pr = 0.59, P = 0.05), and EDSS scores (pr = −0.43, P = 0.05) were relatively unchanged when controlling for age and MS duration. The correlations between EDSS scores and accelerometer counts (pr = −0.43, P = 0.05), EDSS and GLTEQ scores (pr = −0.35, P = 0.12), and accelerometer counts and GLTEQ scores (pr = 0.60, P = 0.005) were relatively unchanged when controlling for age and MS duration in the partial correlation analysis.
Figure 2.
Scatter plots of the associations between objective and self-reporting measures of physical activity, Expanded Disability Status Scale scores, and cardiorespiratory fitness in a sample of 24 women with relapsing-remitting multiple sclerosis (Study 2).
Multiple linear regression analysis
We first regressed V̇O2peak (i.e. outcome) on accelerometer counts and EDSS scores (predictors). The regression analysis was significant, F(2,21) = 6.40, P = 0.007, and explained 38% of variance in V̇O2peak (R2 = 0.38). Accelerometer counts (β = 0.32) and EDSS scores (β = −0.40) had statistically significant associations with V̇O2peak in the first model. We then regressed V̇O2peak on GLTEQ and EDSS scores. The regression analysis was significant, F (2,21) = 8.00, P = 0.003, and explained 43% of variance in V̇O2peak (R2 = 0.43). GLTEQ scores (β = 0.39) and EDSS scores (β = −0.42) had statistically significant associations with V̇O2peak in this second model.
Discussion
This article included two studies that examined the association between physical activity and cardio-respiratory fitness (Study 1) and the associations among physical activity, neurological disability, and cardiorespiratory fitness (Study 2) in persons with RRMS. The first study indicated that self-reporting and objective measurements of physical activity were similarly and strongly associated with V̇O2peak whereby those individuals with MS who were less physically active had lower levels of cardiorespiratory fitness. The second study indicated that self-reported and objective measurements of physical activity were associated with V̇O2peak, even after accounting for neurological disability in the multiple linear regression model. We further note that neurological disability was independently and negative associated with V̇O2peak such that those individuals with MS who had more neurological disability had lower levels of cardiorespiratory fitness. The findings support our hypothesis that physical inactivity and neurological disability are independently associated with reduced levels of cardiorespiratory fitness in persons with MS. We note that the associations were not independent of other possible influences of cardiorespiratory fitness that were not included in the analysis. Accordingly, both physical inactivity and neurological disability might represent independent risk factors for reduced levels of cardiorespiratory fitness in persons with MS. The reduction in cardiorespiratory fitness is in-and-of-itself a risk factor for loss of independent living as individuals with low levels of fitness have reduced functional reserve for performing activities of daily living.
We are aware of one previous study that reported a non-significant association between self-reported physical activity and V̇O2peak consumption in persons with MS (7). By comparison, we reported statistically significant associations between both objectively measured and self-reported physical activity with V̇O2peak consumption in two samples of persons with MS. One obvious reason for this difference is that this study included self-reporting and objective measures of physical activity with established evidence of score validity among persons with MS, whereas previous research included a self-reporting measure of physical activity with unknown psychometric properties in persons with MS (7). This is the most likely explanation for the difference in results between our two studies and the null results reported in previous research (7). This underscores the importance of researchers using a consistent set of measures with established validity in future studies of physical activity in MS.
There has been some inconsistency in the association between cardiorespiratory fitness and neurological disability in persons with MS. Indeed, some studies have reported an inverse association between cardiorespiratory fitness and neurological disability (8, 9), whereas other studies have reported no association between cardiorespiratory fitness and neurological disability (10). Our results supported a statistically significant inverse association between neurological disability and cardiorespiratory fitness in persons with MS, and this association was significant even after controlling for physical inactivity in the multiple linear regression analyses. Overall, this growing body of research would largely support neurological disability as a correlate of reduced cardiorespiratory fitness in MS, and our results would further support the possibility that this association was independent of physical activity behavior.
There might be a biological basis for the independent associations of both physical inactivity and neurological disability with reduced cardiorespiratory fitness in persons with MS. Physical inactivity is likely associated with a reduction of cardiorespiratory fitness through peak cardiac output and the peak di3erence in arteriovenous oxygen. Physical inactivity is associated with reductions in peak stroke volume, mitochondrial density, and muscle oxidative enzymes, and those factors contribute to reductions in peak cardiac output and di3erence in arteriovenous oxygen. By comparison, neurological disability might a3ect cardiorespiratory fitness through impairments in central motor command signals that result in early fatigue and volitional termination during an incremental exercise test or through alterations in the autonomic nervous system. Such arguments provide a plausible biological foundation for physical activity and neurological disability as independent correlates of reduced cardiorespiratory fitness in persons with MS.
Our research underscores the critical importance of promoting physical activity behavior in persons with MS as a means to prevent reductions in cardiorespiratory fitness. The maintenance of cardiorespiratory fitness is important as persons with low levels of fitness have reduced functional reserve and capacity for performing daily activities and this ultimately can result in the loss of independent living. Future research should explore approaches for maintaining and increasing the rate of physical activity among persons with MS. Another implication of our work is that neurological disability is an independent correlate of cardiorespiratory fitness in MS. Although we do not have a strong basis for the causal direction between variables, this might suggest that fitness, in addition to disease progression, is associated with markers of neurological disability in MS. Researchers might consider approaches for partitioning the contribution of fitness from neurological disability in studies of disease progression in MS.
There are important limitations of the two pilot studies included in this article. One limitation is that both studies included samples of women with RRMS and our results should not be generalized among those with progressive forms of MS or men with any form of MS, and might be specific for our selected group. Another limitation is that both studies examined the cross-sectional associations among variables and future research should consider studying reductions in physical activity and increases in neurological disability as predictors of change in cardiorespiratory fitness across time in persons with MS. An additional limitation is that we did not measure physical activity and cardiorespiratory fitness in a sample of age- and gender-matched healthy controls that would allow for direct comparisons between women with and without MS. However, published and normative data available in the literature allowed for some inferences about our sample and the results relative to those with and without MS. One final limitation is that we included a self-reported version of the EDSS and, although this scale correlates well with a neurologist-administered Kurtzke EDSS, future researchers might consider including a neurologist-administered EDSS scale in examinations of physical activity, neurological disability, and cardiorespiratory fitness in persons with MS.
Despite these limitations, the findings from the two studies in this article support our hypotheses that physical inactivity is associated with reduced levels of cardiorespiratory fitness (Study 1) and that physical inactivity and neurological disability are independently associated with reduced levels of cardiorespiratory fitness (Study 2). By extension, both physical inactivity and neurological disability represent risk factors for reduced levels of cardiorespiratory fitness in persons with MS. The importance of our findings is underscored by the observation that the maintenance of cardiorespiratory fitness is important for ensuring independence among persons with chronic disabling conditions such as MS (1).
References
- 1.Durstine JL, Painter P, Franklin BA, Morgan D, Pitetti KH, Roberts SO. Physical activity for the chronically ill and disabled. Sports Med. 2000;30:207–19. doi: 10.2165/00007256-200030030-00005. [DOI] [PubMed] [Google Scholar]
- 2.Åstrand RO, Rodahl K. Textbook of work physiology. New York, NY: McGraw-Hill; 1977. [Google Scholar]
- 3.Brooks GA, Fahey TD, White TP. Exercise physiology: human bioenergetics and its applications, 2nd edn. Mountain View, CA: Mayfield Publishing Company; 1996. [Google Scholar]
- 4.Ponichtera-Mulcare JA. Exercise and multiple sclerosis. Med Sci Sports Exerc. 1983;25:451–65. [PubMed] [Google Scholar]
- 5.Motl RW. Physical activity and its measurement and determinants in multiple sclerosis. Minerva Med. 2008;99:157–65. [PubMed] [Google Scholar]
- 6.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11:459–63. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 7.Romberg A, Virtanen A, Aunola S, Karppi SL, Karanko H, Ruutiainen J. Exercise capacity, disability, and leisure physical activity of subjects with multiple sclerosis. Mult Scler. 2004;10:212–18. doi: 10.1191/1352458504ms1001oa. [DOI] [PubMed] [Google Scholar]
- 8.Koseoglu BF, Gokkaya NKO, Ergun U, Inan L, Yesiltepe E. Cardiopulmonary and metabolic functions, aerobic capacity, fatigue and quality of life in patients with multiple sclerosis. Acta Neurol Scand. 2006;114:261–7. doi: 10.1111/j.1600-0404.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 9.Ponichtera-Mulcare JA, Mathews T, Glaser RM, Gupta SC. Change in aerobic fitness of patients with multiple sclerosis during a 6-month training program. Sports Med Trail Rehabil. 1997;7:265–72. [Google Scholar]
- 10.Foglio K, Clini E, Fasshetti D, et al. Respiratory muscle function and exercise capacity in multiple sclerosis. Eur Respir J. 1994;7:23–8. doi: 10.1183/09031936.94.07010023. [DOI] [PubMed] [Google Scholar]
- 11.Gulick EE, Goodman S. Physical activity among people with multiple sclerosis. Int J MS Care. 2006;8:121–9. [Google Scholar]
- 12.Motl RW, Snook EM, Wynn DR, Vollmer T. Physical activity correlates with neurological impairment and disability in multiple sclerosis. J Nerv Ment Dis. 2008;196:492–5. doi: 10.1097/NMD.0b013e318177351b. [DOI] [PubMed] [Google Scholar]
- 13.Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis: validity of self-report and objective measures. Fam Community Health. 2007;30:144–50. doi: 10.1097/01.fch.0000264411.20766.0c. [DOI] [PubMed] [Google Scholar]
- 14.Motl RW, McAuley E, Snook EM, Scott JA. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disabil Rehabil. 2006;28:1151–6. doi: 10.1080/09638280600551476. [DOI] [PubMed] [Google Scholar]
- 15.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 16.Mâsse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on selected outcome variables. Med Sci Sports Exerc. 2005;11:S544–54. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J. Statistical power analysis for the behavioral sciences. 2. Mahwah, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 18.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7. Baltimore, MD: Lippincott, Williams, & Wilkins; 2006. [Google Scholar]
- 19.Goodin DS. A questionnaire to assess neurological impairment in multiple sclerosis. Mult Scler. 1998;4:444–51. doi: 10.1177/135245859800400508. [DOI] [PubMed] [Google Scholar]