Abstract
Walking impairment is a ubiquitous feature of multiple sclerosis (MS) and the O2 cost of walking might quantify this dysfunction in mild MS. This paper examined the difference in O2 cost of walking between persons with MS who have mild disability and healthy controls and the correlation between the O2 cost of walking and disability. Study 1 included 18 persons with mild MS and 18 controls and indicated that the O2 cost of walking was significantly higher in MS than controls and that disability was significantly associated with the O2 cost of slow, moderate, and fast treadmill walking. Study 2 included 24 persons with mild MS and indicated that disability was significantly correlated with O2 cost of comfortable, fast, and slow over-ground walking. We provide evidence that the O2 cost of walking is an indicator of walking dysfunction in mildly disabled persons with MS and should be considered in clinical research and practice.
Keywords: Multiple sclerosis, Validity, Walking
Introduction
Walking impairment is a ubiquitous feature of disability in multiple sclerosis (MS). For example, both the expanded disability status scale (EDSS) and multiple sclerosis functional composite (MSFC) capture walking impairment as a facet of disease progression. Walking impairment is measured in the EDSS by the 500-m walk and the MSFC using the Timed 25 Foot Walk (T25FW) [1]. Unfortunately, both the 500-m walk and T25FW have limited ability to distinguish mildly disabled persons with MS from controls [2]. Mildly disabled persons with MS typically perform at a comparable level to controls on those measures [3, 4] and demonstrate minimal change in the early disease course [5]. Additional work has evaluated the 6-min walk (6MW) as a measure of walking impairment in persons with MS and again reported that mildly disabled persons with MS performed similarly to healthy controls [2]. We believe that although mildly impaired persons with MS are able to perform the walking challenges at a level that is comparable to healthy controls, it is done so at a higher energetic cost (i.e., less economy of movement). This underscores the importance of a measure that could capture and quantify the energetic cost of walking and detect early change and progression in mildly disabled persons with MS.
The oxygen cost (O2 cost) of walking measured in milliliters of O2 consumed per kilogram of body weight per meter traveled (ml kg−1 m−1) is a promising candidate for quantifying ambulatory impairments in persons with MS, particularly those with mild disability. The O2 cost of walking is a physiological parameter that is influenced by subclinical and clinical manifestations of pathologic gait abnormalities [6] and provides an objective measure of the interaction between walking distance/speed and energy expenditure. A change in O2 cost of walking reflects an increase in O2 consumption (i.e., energy expenditure) with normal walking speed or a reduction in walking speed to maintain a lower rate of O2 consumption. Existing evidence demonstrates that the O2 cost of walking is associated with the degree of pathologic gait abnormality in persons with neurologic and orthopedic conditions [6], and researchers have reported that the O2 cost of walking on a treadmill is elevated in persons with MS who have moderate disability compared with controls [7]. Such observations support an examination of O2 cost of walking as a physiological marker of ambulatory impairment in persons with MS who have mild disability.
Consequently, we examined the O2 cost of walking and its association with disability in two studies of individuals with MS who had mild disability [i.e., patient-determined disease steps (PDDS) score ≤4.0] [8]. The first study measured the O2 cost of walking in individuals with MS who had mild disability (median PDDS score = 1, range = 0–4) and controls during three 6-min periods of slow (54 m min−1), moderate (80 m min−1), and fast (107 m min−1) treadmill walking. The advantage of using a treadmill is that walking speed can be easily controlled, particularly between groups. We first examined expected differences in the O2 cost of walking on a treadmill between persons with MS who had mild disability and controls and then examined the expected strong association between disability and the O2 cost of walking across the three speeds in those with MS. The second study measured the O2 cost of walking in individuals with MS who had mild disability (median PDDS score = 1, range = 0–4) during three 6-min periods of over-ground walking. The advantage of over-ground walking is that it is more ecologically valid (i.e., representative of the real world) than walking on a treadmill. The first 6-min period was performed at the participant’s comfortable walking speed (CWS) and the second and third 6-min periods were undertaken above (faster walking speed, FWS) or below (slower walking speed, SWS) the participant’s CWS. We again examined the expected strong association between disability and the O2 cost of over-ground walking across the three speeds of walking in persons with mild MS.
Study 1
Methods
Participants
The sample of participants in Study 1 was recruited from the local community via telephone and e-mail messages from a member of the research team. This initial contact was followed by a telephone call for describing the study and its procedures, answering all questions, and conducting a screening interview for inclusion and exclusion criteria. The inclusion criteria included (a) being ambulatory with minimal assistance (i.e., maximum of early cane use); (b) having the visual ability to read 14 point font; and (c) being 18–64 years of age. We excluded all individuals who reported more than one risk factor for maximal exercise testing (i.e., family history of coronary heart disease, cigarette smoking, hypertension, high cholesterol, diabetes, and obesity). The sample consisted of 36 subjects, 18 individuals (14 women) with a definite diagnosis of MS that was confirmed by a neurologist and 18 individuals without MS (14 women) who were similar in age [t(34) = 0.69, p = 0.49], height [t(34) = −1.18, p = 0.25], and weight [t(34) = −0.14, p = 0.89]. The mean (SD) age, height, and weight of those with MS were 41.9 years (12.6), 167.7 cm (13.1), and 72.1 kg (16.4), respectively. The mean (SD) age, height, and weight of the control sample were 39.1 years (11.9), 171.9 cm (7.6), and 72.8 kg (15.0), respectively. Of the 18 individuals with MS, 15 had relapsing–remitting MS, 1 had primary progressive MS, and 2 had benign MS. The mean (SD) duration of MS was 8.4 years (7.2) and the median PDDS score was 1 (range = 0–4). This median PDDS score corresponds to mild disability (i.e., noticeable, but minor symptoms) and the range of PDDS scores corresponds to normal (PDDS = 0) through early cane (PDDS = 4) [8].
Protocol
The protocol was approved by a University Institutional Review Board and all participants provided written informed consent. The participants initially completed a demographic scale and those with MS then completed the PDDS scale. We measured the participant’s height and weight using a scale-stadiometer unit (Detecto model 3P7044, Webb City, MO). The participants undertook three 6-min periods of walking on a motor-driven treadmill (Trackmaster model TMX425C, Full Vision, Inc., Newton, KS). The three 6-min periods of walking each involved a different treadmill speed and there were 6-min periods of seated rest between the periods of walking. The three treadmill speeds were 54, 80, and 107 m min−1 and the order of the treadmill speeds was randomized in both those with MS and the controls. The treadmill speed was verified by measuring belt length and the time it took to complete 25 revolutions of the belt, and the treadmill grade (0%) was verified by a digital inclinometer (Beall Tilt Box, The Beall Tool Company, Newark, OH).
Measures
Oxygen cost of walking
Oxygen consumption (V̇O2) was measured by breath-by-breath analysis using an open-circuit spirometry system (TrueOne, Parvo Medics, Sandy, UT) with participants breathing through a Hans Rudolph (Kansas City, MO) 2-way, nonrebreathable valve (Model 2700B). The O2 and CO2 analyzers of the spirometry system were calibrated using verified concentrations of gases, and the flow-meter was calibrated using a 3-L syringe (Hans Rudolph, Kansas City, MO). Steady-state V̇O2 was calculated by averaging V̇O2 values across the final 3 min (minutes 4–6) of the 6-min periods of treadmill walking. The O2 cost of walking was calculated in ml kg−1 m−1 by dividing steady-state V̇O2 in ml kg−1 min−1 by treadmill speed in m min−1 [6].
Patient-determined disease steps scale
The PDDS scale [8] is a self-report questionnaire that contains an ordinal scale ranging between 0 (normal) and 8 (bedridden) with a midpoint of 4 (early cane). This scale was developed as an inexpensive surrogate for the expanded disability status scale (EDSS) and scores from the PDDS are linearly and strongly correlated with physician-administered EDSS scores (r = 0.93) [8].
Statistical analysis
All analyses were performed using SPSS, version 16 (SPSS, Chicago, IL). Descriptive statistics are presented in text and Tables as mean (M) ± standard deviation (SD). The O2 cost of walking between the two groups and across the three treadmill speeds was analyzed with a 2 (Group: MS and controls) × 3 (speed; 54, 80, and 107 m min−1) mixed-model ANOVA based on the univariate F-statistic. Effect sizes for F-statistics were expressed as partial eta-squared ( ) and interpreted as small, medium, and large based on values of 0.01, 0.06, and 0.14, respectively [9]. Effect sizes for between group differences in mean scores were expressed as Cohen’s d and interpreted as small, medium, and large based on values of 0.20, 0.50, and 0.80, respectively [9]. We examined the association between PDDS scores and O2 cost of walking for each of the three speeds using Pearson product-moment (r) correlations as well as partial correlations (pr) controlling for body weight. Cohen’s guidelines of 0.1, 0.3, and 0.5 were used for judging the magnitude of the correlations as small, moderate, and large, respectively [9].
Results
Table 1 provides the O2 cost of walking data for the pooled sample and separate samples of persons with MS and controls. The table further contains effect sizes for mean differences in the O2 cost of walking between persons with MS and controls. The two-way mixed-model ANOVA identified statistically significant Group [F(1, 34) = 10.09, p < 0.01, ] and Speed [F(2, 68) = 48.47, p < 0.001, ] main effects on O2 cost of walking. The sample with MS had a higher O2 cost of walking than did the control sample across all three speeds and the magnitude of differences were large based on the partial eta-squared value and Cohen’s d. This indicates that the O2 cost of walking can differentiate between persons with MS who have mild disability and controls. There was a stronger quadratic [F(1, 34) = 99.01, p < 0.001, ] than linear [F(1, 34) = 22.55, p < 0.001, ] trend for speed whereby the O2 cost of walking was lower for the 80 m min−1 speed than for the 54 and 107 m min−1 speeds.
Table 1.
M ± SD for the O2 cost of walking on a treadmill as a function of speed in the overall sample and in the separate samples of individuals with multiple sclerosis (n = 18) and healthy controls (n = 18)
| Sample | 54 m min−1 | 80 m min−1 | 107 m min−1 |
|---|---|---|---|
| Overall | 0.194 ± 0.019 | 0.171 ± 0.019 | 0.181 ± 0.020 |
| Multiple sclerosis | 0.202 ± 0.023* | 0.179 ± 0.020* | 0.190 ± 0.024* |
| Healthy controls | 0.186 ± 0.010 | 0.163 ± 0.013 | 0.172 ± 0.011 |
| Cohen’s d | 0.97 | 0.97 | 1.03 |
O2 cost of walking expressed as ml kg−1 m−1
Mean score significantly different from mean of healthy controls (p < 0.05)
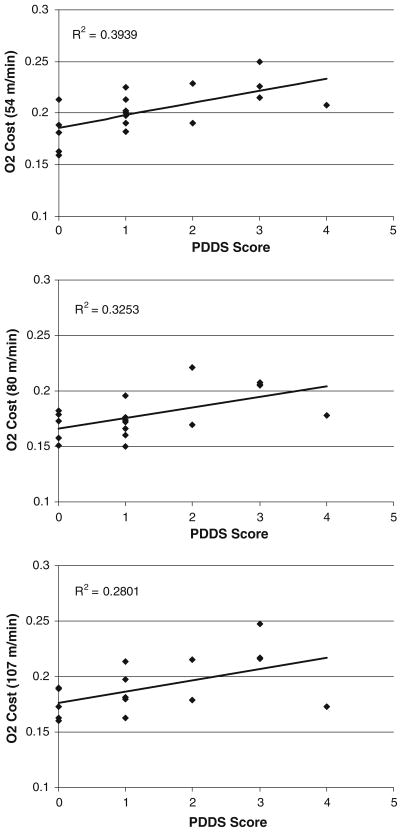
Scatter plots of the association between PDDS scores and O2 cost of walking in the sample with MS are provided in Fig. 1. There were statistically significant correlations between PDDS scores and O2 cost of walking across all three treadmill speeds: 54 m min−1 (r = 0.628, p = 0.005), 80 m min−1 (r = 0.570, p = 0.01), and 107 m min−1 (r = 0.529, p = 0.02). The correlations between PDDS scores and O2 cost of walking were unaffected when controlling for body weight: 54 m min−1 (pr = 0.643, p = 0.005), 80 m min−1 (pr = 0.622, p = 0.008), and 107 m min−1 (pr = 0.560, p = 0.02).
Fig. 1.
Scatter plot, linear trend-line, and squared multiple correlation (R2) for the association between patient-determined disease steps (PDDS) scale scores and O2 cost of treadmill walking at speeds of 54 (top panel), 80 (middle panel), and 107 (bottom panel) m min−1 in persons with MS (n = 18)
Study 2
Methods
Participants
Participants in Study 2 were again recruited from the local community and there were six participants from Study 1 who were in Study 2. Recruitment occurred via telephone and e-mail messages from a member of the research team. This was followed by a telephone call for describing the study and conducting a screening for inclusion and exclusion criteria; the criteria were the same as in Study 1. Twenty-four persons with a diagnosis of MS satisfied inclusion criteria and volunteered for participation. The mean (SD) age, height, and weight were 42.0 years (11.7), 169.5 cm (9.3), and 70.6 kg (14.7), respectively. The mean duration of time since diagnosis of MS was 11.1 years (8.5) and all participants had relapsing-remitting MS. The median PDDS score was again 1 (range = 0–4) and corresponds to mild disability (i.e., noticeable, but minor symptoms). The range of PDDS scores corresponds to normal (PDDS = 0) through early cane (PDDS = 4) [8]. The mean (SD) Godin lesiure-time exercise questionnaire (GLTEQ) score was 34.0 (29.6). This value is less than that of healthy controls [10], but not of persons with MS [11] and this observation of reduced physical activity in persons with MS is consistent with general findings reported in a meta-analysis [12].
Protocol
The protocol was approved by the same University Institutional Review Board and all participants provided written informed consent. Participants completed a demographic questionnaire and the PDSS and then were measured for weight and height on a scale-stadiometer unit (Detecto model 3P7044, Webb City, MO). The participant and one researcher then walked the course for the three 6-min periods of over-ground walking as a familiarization protocol; the course was located in a handicap-accessible, rectangular hallway that was clear of obstructions and foot traffic. We then connected the K4b2 portable metabolic unit for collecting expired gases and the participants sat and rested for a 5-min period. This was followed by three 6-min periods of walking that were interspersed with 5–10 min of rest. The first 6-min period of walking was performed at the participant’s CWS. The second and third 6-min periods of walking were performed above (FWS) and below (SWS) the participant’s CWS (i.e., ± 13 m min−1 of CWS) and the order was counter-balanced. The manipulation of walking speed in the second and third 6-min periods of walking was accomplished by having the participant follow a pacer who monitored, controlled, and recorded the speed using a measuring wheel (Stanley MW50, New Briton, CT) outfitted with a calibrated bicycle computer (Cateye Velo5, Osaka, Japan).
Measures
Oxygen cost of walking
V̇O2 was measured during the three 6-min periods of walking using a commercially available portable metabolic unit (K4b2 Cosmed, Italy). The O2 and CO2 analyzers of the portable metabolic unit were calibrated using verified concentrations of gases, and the flow-meter was calibrated using a 3-L syringe (Hans Rudolph, Kansas City, MO). Steady-state V̇O2 was calculated by averaging V̇O2 values across the final 3 min (minutes 4–6) of the 6-min periods of walking. The O2 cost of walking was expressed as ml kg−1 m−1 by dividing steady-state V̇O2 in ml kg−1 min−1 by actual walking speed in m min−1 [6].
Patient-determined disease steps scale
The PDDS scale was again used as a self-report measure of disability status in MS [8].
Godin leisure-time exercise questionnaire
The GLTEQ is a self-administered measure of usual physical activity [10] that has been validated for use in persons with MS [11]. The GLTEQ contains three items that measure the frequency of strenuous (e.g., jogging), moderate (e.g., fast walking), and mild (e.g., easy walking) exercise for periods of more than 15 min during one’s free time in a typical week. The weekly frequencies occurring over a 7-day period of strenuous, moderate, and mild activities are multiplied by 9, 5, and 3 metabolic equivalents (METs), respectively, and summed to form a measure of total leisure activity. The scores range between 0 and 119 in MET min/week, with higher values reflecting more frequent and/or intense physical activity.
Data analysis
All analyses were performed using SPSS, version 16 (SPSS, Chicago, IL). Descriptive statistics are presented as M ± SD. The differences in speed and O2 cost of walking across the three 6-min periods of walking was examined using one-way, within-subjects ANOVAs; speed was the within-subjects factor with three levels (CWS, FWS, and SWS). The effect size for the F-statistic was expressed as partial eta-squared ( ) and interpreted as small, medium, and large based on values of 0.01, 0.06, and 0.14, respectively [9]. The relationships between scores from the PDDS and O2 cost of walking were estimated using Pearson product-moment correlations (r) as well as partial correlations (pr) controlling for body weight and GLTEQ scores. Cohen’s guidelines of 0.1, 0.3, and 0.5 were used for judging the magnitude of the correlations as small, moderate, and large, respectively [9].
Results
The CWS was 76.6 ± 13.0 m min−1. The FWS and SWS were 89.0 ± 13.8 and 64.2 ± 12.3 m min−1, respectively. The difference in walking speed was statistically significant [F(2, 46) = 454.69, p < 0.0001] and this indicates a successful manipulation of speed pacing during the three 6-min periods of walking. There was a statistically significant difference in O2 cost of walking as a function of speed [F(2, 46) = 4.09, p <0.05, ]. The O2 cost of walking was 0.172 ± 0.030, 0.179 ± 0.033, and 0.167 ± 0.035 ml kg−1 m−1 for the CWS, FWS, and SWS, respectively.
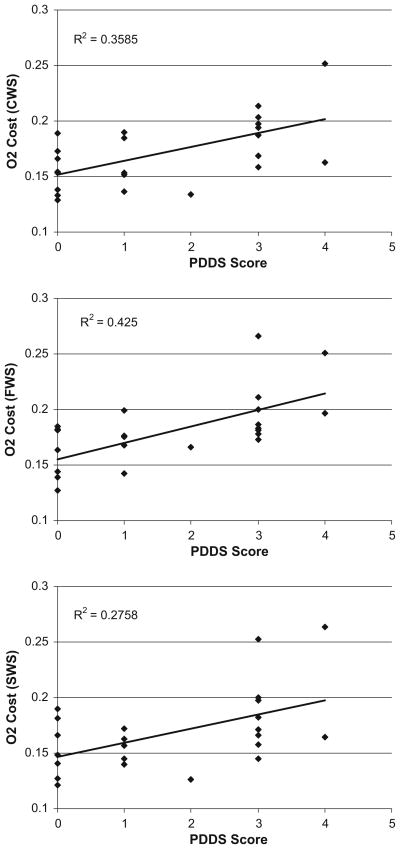
Scatter plots of the association between PDDS scores and O2 cost of walking are provided in Fig. 2. PDDS scores were significantly and strongly correlated with O2 cost of walking during the three 6-min periods of over-ground walking: CWS (r = 0.599, p = 0.001), FWS (r = 0.652, p = 0.001), and SWS (r = 0.525, p = 0.001). Again, the correlations between PDDS scores and O2 cost of walking were unaffected when controlling for body weight: CWS (pr = 0.531, p = 0.009), FWS (pr = 0.489, p = 0.018), and SWS (pr = 0.500, p = 0.015). The correlations were further unaffected when controlling for GLTEQ scores: CWS (pr = 0.575, p = 0.004), FWS (pr = 0.575, p = 0.004), and SWS (pr = 0.516, p = 0.012).
Fig. 2.
Scatter plot, linear trend-line, and squared multiple correlation (R2) for the association between patient-determined disease steps (PDDS) scale scores and O2 cost of over-ground walking performed under comfortable (top panel), faster (middle panel), and slower (bottom panel) speeds in persons with MS (n = 24)
Discussion
Current measures of walking impairment are limited by floor effects in mildly disabled persons with MS [2], and we report herein that the O2 cost of walking can differentiate between persons with mild MS and healthy controls and strongly correlates with disability status in individuals with MS who have mild disability. The first study indicated that the O2 cost of walking on a treadmill was significantly higher in persons with MS who had mild disability compared with controls and that PDDS scores were significantly and strongly associated with the O2 cost of slow, moderate, and fast treadmill walking. Importantly, the mildly disabled persons with MS completed the same paced treadmill walking as the healthy controls (i.e., performed at a level similar to that of controls on the ambulatory challenge), but the energetic cost was higher for those with MS. The second study only included persons with mild MS and further indicated that PDDS scores were significantly and strongly correlated with O2 cost of comfortable, slow, and fast over-ground walking. Such results indicate that the O2 cost of walking across different modes (over-ground and treadmill walking) and speeds (slow through fast) is associated with disability status in persons with MS. The robustness of the association between disability and O2 cost of walking combined with difference in the O2 cost of walking between persons with MS who had mild disability and healthy controls supports the consideration of O2 cost of walking as a physiological marker of locomotor impairments in persons with MS. Indeed, the degree of disability is directly associated with an increase in the energetic cost of walking in persons with MS who have mild disability. Future studies should consider the comparative sensitivity of the O2 cost of walking versus other markers of locomotor impairment as well as the association between O2 cost of walking and measures of free-living function and mobility (e.g., multiple sclerosis walking scale-12 or accelerometry) [1, 13].
One previous study has examined the energetic cost of walking on a treadmill in persons with MS versus controls [7]. That previous study included 24 persons with MS who had moderate disability (estimated median PDDS = 4 and estimated PDDS range = 2–6 based on walking aid use and neurologic features reported in Table 1 of that study) and 24 controls and examined the energy cost of treadmill walking whereby speed progressively increased in increments of 0.5 km h−1 every 3 min from an initial speed of 1.5 km h−1 through a maximal speed of 5 km h−1. Oxygen consumption was measured during the last minute of each speed and O2 cost of walking was expressed as ml kg−1 m−1. The results indicated that O2 cost of walking was between 2 and 3 times greater in those with MS than controls across four speeds of treadmill walking (2, 3, 4, and 5 km h−1). The present study similarly observed that O2 cost of walking was greater in those with MS than controls across all three speeds of treadmill walking (~3.2, 4.8, and 6.4 km h−1), but the magnitude of difference was smaller than that observed in previous research [7]. There are several possible explanations for the variability in the magnitude of the difference in O2 cost of treadmill walking between studies. One obvious difference is that our sample included persons with minimal ambulatory impairment (i.e., maximum of early cane use or single-point assistance during walking), whereas previous research included persons with greater ambulatory impairments (e.g., 33% relied upon two-point assistance during walking). This difference in ambulatory status and therefore disability likely influenced the O2 cost of treadmill walking in persons with MS as those with greater reliance upon walking aids likely had a greater O2 cost of walking than those with less reliance upon a walking aid when compared with the control sample. That explanation is consistent with the findings of our correlation analysis whereby there was a linear relationship between PDDS scores and the O2 cost of treadmill and over-ground walking. Another major difference is that only 12, 7, and 3 of the 24 persons with MS completed the 3, 4, and 5 km h−1 treadmill walking speeds, respectively, in previous research [7]. All 18 of our participants with MS completed the entire treadmill walking protocol. The drop-off in sample size as a function of speed would likely have affected the precision of the estimates of the O2 cost of treadmill walking in that previous study [7]. One final difference is that the present study included 6-min periods of treadmill walking with 3-min sampling of expired gases for measuring O2 cost of walking and the periods of walking were interspersed with seated rest. Previous research included 3-min periods of incremental walking with 1-min sampling of expired gases and no rest between walking speeds [7]. Our protocol is expected to yield more stable and accurate assessments of O2 cost of walking because of a steady-state in O2 consumption during the last 3 min of the 6-min period and minimal carry-over effects between successive periods of incremental treadmill walking. Despite the major differences, both studies observed that O2 cost of walking on a treadmill was elevated in persons with MS compared with controls, and our correlation analyses suggest that the degree of disability is associated with the O2 cost of treadmill and over-ground walking.
We examined the association between disability and the O2 cost of both treadmill and over-ground walking in two studies of persons with MS who had mild disability. The advantage of walking on a treadmill is that the speed can be tightly controlled allowing for precise comparison of O2 cost of walking between groups or across time. One limitation is that the kinematics of gait on a treadmill might differ from those of over-ground walking, particularly in persons with gait disability. The advantage of over-ground walking is that it is more ecologically valid, but there is less control over speed of movement. We note that in the current studies the O2 cost of walking was comparable between treadmill and over-ground walking. Indeed, the O2 cost of walking on a treadmill at 80 m min−1 was 0.179 ml kg−1 m−1 in the MS sample and the O2 cost of over-ground walking at the CWS of 76.6 m min−1 was 0.172 ml kg−1 m−1. This suggests that the O2 cost of walking is comparable across treadmill and over-ground modes, and we further note that the magnitude of correlations between O2 cost of walking and disability was similar across modes and speeds of walking. Such results imply that the O2 cost of walking is a marker of locomotor impairments that can be measured using treadmill and over-ground conditions, but this should be verified in a direct comparison of treadmill and over-ground walking in a single study of persons with MS.
To our knowledge, researchers have not routinely used the O2 cost of walking as a marker of locomotor impairment that coincides with disability in persons with MS. This might be linked with the perceived expense of equipment combined with a lack of expertise for measuring O2 cost of walking. We further note that the O2 cost of walking might be impractical for inclusion in epidemiological and natural history studies of disability in persons with MS. Nevertheless, the O2 cost of walking might provide a more sensitive approach for monitoring locomotor impairment that coincides with MS disease progression in clinical research and clinical care, even in mild MS. This approach, in particular, might provide novel metrics for quantifying changes in disease status during clinical trials of disease modifying medications and rehabilitation regimens, especially where the effects might be small in magnitude and below the threshold for detection of current disability measurements (EDSS, T25FW, or 6MW test). The O2 cost of walking might further provide a novel approach for monitoring disease status in a clinical setting, particularly in cases where patient-reports of daily function do not coincide with markers from a clinical examination [14]. We encourage additional research that examines the veracity and sensitivity of the O2 cost of walking as a marker of disease progression in clinical trials and practice involving persons with MS.
Importantly, the two studies are not without limitations. One limitation is the demographic composition and size of the samples. The studies included small samples of 18 and 24 individuals with MS who consisted primarily of middle-aged, Caucasian women. The demographic characteristics of the samples are consistent with those of MS [15], but we do recognize the importance of including more diverse samples in future studies. Another possible limitation is the focus on a relatively mild degree of disability in the samples of persons with MS. Future researchers should examine the association between disability and O2 cost of walking in individuals who present with greater disability. The small, heterogeneous samples of persons with mild MS clearly indicate that the results cannot be generalized for the whole MS population. Perhaps the association is even stronger across a larger range of disability. Another limitation is that we only included the PDSS rather than the best-known and more familiar expanded disability status scale (EDSS) for examining the association between disability status and the O2 cost of treadmill and over-ground walking. The inclusion of the EDSS would be an important consideration in future research. An additional limitation is the cross-sectional nature of the study and that we did not establish evidence of sensitivity and responsiveness for the O2 cost of walking based on drug (e.g., IV steroid treatment) or rehabilitation (e.g., physical therapy) interventions. The comparative evaluation of the O2 cost of walking versus PDDS and EDSS scores as well as T25FW and 6MW test performance in response to drug (e.g., IV steroid treatment) or rehabilitation (e.g., physical therapy) intervention therapies would be an interesting future direction for establishing the sensitivity and responsiveness of the O2 cost of walking.
This paper demonstrated that the O2 cost of walking differs between persons with mild MS and healthy controls and documented strong and consistent associations between disability status and the O2 cost of treadmill and over-ground walking in persons with mild MS. Such results highlight the importance of considering the O2 cost of walking as a possible physiological marker of locomotor impairments that coincide with disability progression among persons with MS. This consideration combined with additional investigations of sensitivity and responsiveness might better position research and clinical efforts in the management of disease progression in this population.
Footnotes
Conflict of interest The authors report no conflicts of interest.
Contributor Information
Robert W. Motl, Email: robmotl@illinois.edu, Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, 350 Freer Hall, 906 South Goodwin Ave., Urbana, IL 61801, USA
Yoojin Suh, Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, 350 Freer Hall, 906 South Goodwin Ave., Urbana, IL 61801, USA.
Deirdre Dlugonski, Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, 350 Freer Hall, 906 South Goodwin Ave., Urbana, IL 61801, USA.
Madeline Weikert, Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, 350 Freer Hall, 906 South Goodwin Ave., Urbana, IL 61801, USA.
Stamatis Agiovlasitis, Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, 350 Freer Hall, 906 South Goodwin Ave., Urbana, IL 61801, USA.
Bo Fernhall, Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, 350 Freer Hall, 906 South Goodwin Ave., Urbana, IL 61801, USA.
Myla Goldman, Department of Neurology, University of Virginia, PO Box 800394, Charlottesville, VA 22958, USA.
References
- 1.Pearson OR, Busse ME, van Deursen RW, Wiles CM. Quantification of walking mobility in neurological disorders. QJM. 2004;97:463–475. doi: 10.1093/qjmed/hch084. [DOI] [PubMed] [Google Scholar]
- 2.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwenhuis MM, Van Tongeren H, Sørensen PS, Ravnborg M. The six spot step test: a new measurement for walking ability in multiple sclerosis. Mult Scler. 2006;12:495–500. doi: 10.1191/1352458506ms1293oa. [DOI] [PubMed] [Google Scholar]
- 4.Romberg A, Virtanen A, Ruutiainen J, Aunola S, Karppi SL, Vaara M, Surakka J, Pohjolainen T, Seppänen A. Effects of a 6-month exercise program on patients with multiple sclerosis: a randomized study. Neurology. 2004;63:2034–2038. doi: 10.1212/01.wnl.0000145761.38400.65. [DOI] [PubMed] [Google Scholar]
- 5.Ytternerg C, Johansson S, Andersson M, Holmqvist LW, von Koch L. Variations in function and disability in multiple sclerosis: a two year prospective study. J Neurol. 2008;255:967–973. doi: 10.1007/s00415-008-0767-0. [DOI] [PubMed] [Google Scholar]
- 6.Waters S, Mulroy S. The energy expenditure of normal and pathological gait. Gait Posture. 1999;9:207–231. doi: 10.1016/s0966-6362(99)00009-0. [DOI] [PubMed] [Google Scholar]
- 7.Olgiati R, Jacquet J, Di Prampero PE. Energy cost of walking and exertional dyspnea in multiple sclerosis. Am Rev Respir Dis. 1986;134:1005–1010. doi: 10.1164/arrd.1986.134.5.1005. [DOI] [PubMed] [Google Scholar]
- 8.Hadjimichael O, Kerns RB, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007;127:35–41. doi: 10.1016/j.pain.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 10.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 11.Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis: validity of self-report and objective measures. Fam Commun Health. 2007;3:144–150. doi: 10.1097/01.fch.0000264411.20766.0c. [DOI] [PubMed] [Google Scholar]
- 12.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta analysis. Mult Scler. 2005;11:459–463. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 13.Motl RW, Snook EM. Confirmation and extension of the validity of the Multiple Sclerosis Walking Scale-12 (MSWS-12) J Neurol Sci. 2008;268:69–73. doi: 10.1016/j.jns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Hoogervorst EL, Eikelenboom MJ, Uitdehaag BM, Polman CH. One year changes in disability in multiple sclerosis: neurological examination compared with patient self report. J Neurol Neurosurg Psychiatry. 2003;74:439–442. doi: 10.1136/jnnp.74.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Eng J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]