Abstract
CC Chemokine Receptor 5 (CCR5) is an important mediator of chemotaxis and the primary coreceptor for HIV-1. A recent report by other researchers suggested that primary T cells harbor pools of intracellular CCR5. With the use of a series of complementary techniques to measure CCR5 expression (antibody labeling, Western blot, quantitative reverse transcription polymerase chain reaction), we established that intracellular pools of CCR5 do not exist and that the results obtained by the other researchers were false-positives that arose because of the generation of irrelevant binding sites for anti-CCR5 antibodies during fixation and permeabilization of cells.
Introduction
The G protein–coupled receptor (GPCR) CC Chemokine Receptor 5 (CCR5) recruits and activates leukocytes by responding to its chemokine ligands.1,2 It is also the primary coreceptor for HIV-1.1,3 Like other GPCRs, CCR5 is desensitized after ligation through clathrin-dependent endocytosis leading to intracellular sequestration before receptor recycling.4,5 Receptor down-modulation is an important component of the anti-HIV activity of both native chemokines6,7 and highly potent chemokine analogues.8,9
Certain GPCRs are stored in intracellular pools to provide rapid, renewable resources for surface expression.4,10 Most studies of CCR5 suggest the receptor is predominantly localized at the cell surface.1,2,6,7 On the basis of a series of experiments in which anti-CCR5 antibodies were used to detect CCR5 in fixed and permeabilized cells, Achour et al11 recently reported that CCR5 is predominantly intracellular in T cells, proposing that receptor storage is a mechanism for maintaining sustained sensitivity of leukocytes to chemokines within tissue.11 To investigate this new concept, we studied the expression of CCR5 protein and CCR5 RNA. Like Achour et al,11 we found apparent high levels of intracellular CCR5 with the use of flow cytometry in fixed, permeabilized T cells, but these results were not consistent with the low levels of CCR5 mRNA and the results of Western blotting. We conclude that large intracellular pools of CCR5 are not present within circulating human T cells.
Methods
These studies were approved by the Institutional Review Board at University Hospitals, Case Medical Center. With informed consent in accordance with the Declaration of Helsinki, blood was drawn, and peripheral blood mononuclear cells (PBMCs) were purified. GHOST (3) cells and CCR5-transfected GHOST (3) Hi-5 cells were obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program.12
For flow cytometry, fluorochrome-conjugated anti-CCR5 2D7 and 3A9 (BD Biosciences), HEK/1/85a (BioLegend), and isotype controls were used. The monoclonal 1/85a antibody for Western blotting was obtained from AbD Serotec.
For real-time polymerase chain reaction (PCR) assays, T cells were enriched from whole blood with the use of RosetteSep T (StemCell Technologies) then sorted into CD3+CCR5− and CD3+CCR5+ populations with the use of a FacsARIA instrument (Becton Dickinson). mRNA prepared from cell lysates was quantitated by Taqman assay with the use of primers, probes, and methods as described.13
For Western blotting, cell lysates were resolved on SDS–polyacrylamide gels, transferred to nitrocellulose membranes, stained for reactivity with anti-CCR5 or anti–β-actin antibodies, and identified by chemiluminescence.
Complete methodologic details are found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
Anti-CCR5 monoclonal antibodies give positive flow cytometric signals on fixed and permeabilized T cells but also on CCR5− control cell lines
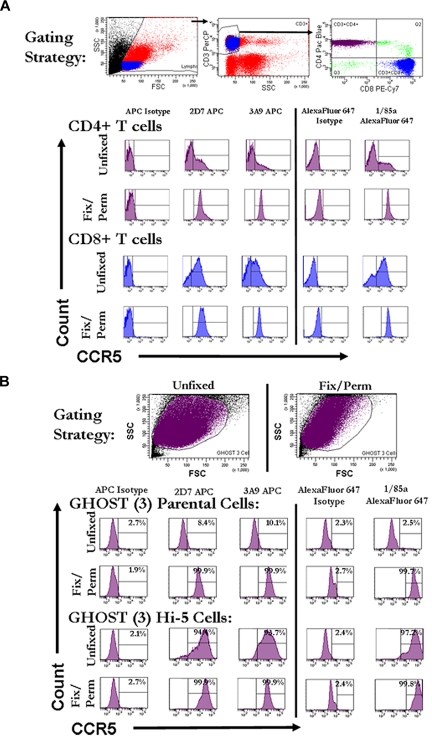
In initial flow cytometric experiments, human PBMCs were gated for expression of both CD3 and CD4 or CD8 and were stained with 3 monoclonal anti-CCR5 antibodies recognizing different domains.14–17 In agreement with the findings of Achour et al,11 we found that, although cells from each donor indicated variable but universally low levels of CCR5 staining on nonpermeabilized cells, uniformly strong signals were obtained on fixed and permeabilized cells (Figure 1A). Control GHOST (3) parental cell lines that do not express CCR5, however, also gave positive results when the same anti-CCR5 antibodies were used to stain fixed and permeabilized cells (Figure 1B).
Figure 1.
High levels of intracellular staining for CCR5 by flow cytometery in fixed, permeabilized T cells and GHOST (3) cells that do not express CCR5. (A) Representative histograms of CCR5 staining on fresh and fixed/permeabilized CD4+ and CD8+ T cells from whole PBMCs. Fresh or fixed/permeabilized cells were initially gated on forward and side scatter. Cells falling into the lymphocyte gate were further gated on being positive for CD3. CD3+ cells were further divided into CD4+ and CD8+ T cells. Histograms reflect CCR5 staining on CD4+ T cells (purple) and CD8+ T cells (blue) that were either stained unfixed or fixed and permeabilized before staining. CCR5 staining is shown on the x-axis and count on the y-axis. Histogram gates were set with the appropriate fluorochrome-linked isotype control antibody so that ∼ 2% of the population was positive for isotype. (B) Representative histograms of CCR5 staining on fresh and fixed/permeabilized GHOST (3) parental and GHOST (3) Hi-5 (CCR5-transfected) cells. Cells were initially gated on forward versus side scatter. Histograms reflect CCR5 staining on the total cell populations gated on forward and side scatter (purple). CCR5 staining is shown on the x-axis and count on the y-axis. FSC indicates forward scatter; SSC, side scatter; PE-Cy7, phycoerythrin–indocyanine 7; and APC, allophycocyanin.
Only human T cells with detectable cell surface CCR5 have CCR5 RNA by quantitative PCR and protein by Western blot
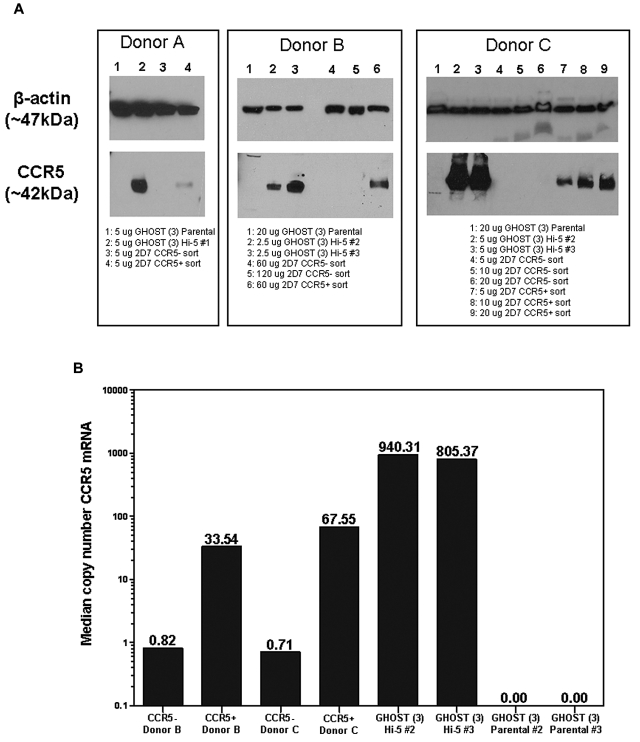
We flow-sorted PBMCs into CD3+CCR5+ and CD3+CCR5− populations, with gates set to population extremes to minimize contamination. The CCR5− population had 0.1%-2.6% contamination with surface CCR5+ cells. Consistent with this low level of contamination, median CCR5 copy number in the CCR5− population determined by quantitative PCR was between 40 and 95 times lower than that measured in the positive population. Total cell lysates were also prepared and analyzed by Western blot. As negative and positive controls, lysates were prepared from parental GHOST (3) cells and CCR5-transfected GHOST (3) cells. A band corresponding to CCR5 (∼ 42 kDa) was visible in the positive control sample but not in the negative control (Figure 2). Under the same conditions, the corresponding band was apparent in the cell surface CCR5+ but not in the cell surface CCR5− populations of sorted T cells from each of 3 donors. The cell surface CCR5− populations remained negative for CCR5 by Western blot up to the highest loaded dose tested (120 μg protein per lane; Figure 2).
Figure 2.
Western blot and quantitative PCR demonstrate that CCR5 protein and mRNA are only present in cells expressing surface CCR5. (A) Western blots of CCR5+ and CCR5− Sorted whole cell lysates. CD3+ T cells from the whole blood of 3 different donors were isolated and sorted by flow cytometry into CCR5− and CCR5+ populations. Lysates were made from the 2 populations, and CCR5 was detected by Western blot (band at ∼ 42 kDa). Lysates from parental and Hi-5 GHOST (3) cell lines were used as negative and positive controls, respectively. β-actin was used as a loading control for each lysate (band at ∼ 47 kDa). (B) CCR5 mRNA in CCR5+ and CCR5− sorted cells. Median copy number of CCR5 mRNA was normalized to median copy number of R18 mRNA, and values were multiplied by 1 000 000. Samples were run in duplicate for a given dilution, and all wells in range of the standard curve for a given sample were used to calculate the median values shown.
Fixing and permeabilization can lead to false-positive signals with anti-CCR5 antibodies
To investigate how apparently high levels of intracellular CCR5 can be detected by anti-CCR5 antibodies on fixed and permeabilized cells, we carried out labeling experiments on either unmodified Chinese hamster ovary (CHO) cells (CHO-WT) or CHO cells stably transduced to express CCR5 (CHO-CCR5).18 Under mild fixation and permeabilization conditions (see supplemental Methods), CHO-WT cells are negative for CCR5, and CHO-CCR5 cells are labeled at the cell surface (supplemental Figure 1) as has been seen previously.1,2,6,7 However, when the same cells are fixed and permeabilized under the same conditions as those used by Achour et al11 in the flow cytometric experiments, the CHO-WT cells become positive, with significant staining in the nucleus, and the CHO-CCR5 cells remain positive at the cell surface but show similar staining in the nucleus (supplemental Figure 1). Presumably this strong off-target staining, which occurs under certain fixation/permeabilization conditions, is the explanation as to why both Achour et al11 and our group (Figure 1) found intracellular CCR5 signals in fixed and permeabilized T cells.
The report of high-level intracellular stores of CCR5 in human T cells by Achour et al11 was surprising for several reasons. First, induction of CCR5 surface expression on activated CD4+ T cells is delayed for 5-8 days after activation, implying that intracellular pools are not readily mobilized to the cell surface.19,20 Second, recovery of surface CCR5 is very slow after ligand-induced receptor internalization,9 implying that surface ligation also saturates the intracellular pool that is accessible to the surface.
We conclude that intracellular pools of CCR5 in human T cells do not exist and that instead, in agreement with several previous studies,1,2,6,7 CCR5 expression is restricted to a limited population of circulating T cells and predominantly located at the cell surface. Our findings indicate that the results obtained by Achour et al11 were almost certainly because of the generation of irrelevant antibody binding sites during the fixing and permeabilization procedure, a phenomenon that is a recognized source of false-positive results in antibody-based experiments.21
Supplementary Material
Acknowledgments
The authors thank Morgan Reuter for her assistance with technical issues in electrophoresis and imaging and Fabiana Tirone for advice on detection of CCR5 by Western blot.
This research was supported by NIH (grant AI-076981), the James B. Pendleton Charitable Trust, the La Jolla Foundation for Microbicide Research, and the Swiss National Science Foundation (grant 310030_12745/1). Much of this work was performed in the Immune Function Core Facility and the Microbial Pathogenesis Core Facility of the Center for AIDS Research of Case Western Reserve University and University Hospitals/Case Medical Center (AI36219) and the Flow Cytometry Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals/Case Medical Center (P30 CA43703). Several reagents for this research were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.A.P.-C., S.F.S., O.H., and M.M.L wrote the manuscript; S.F.S., T.J.H., J.-M.E., R.O., R.S.V., D.E.M., M.R.C., J.G.K., J.A.H., R.G.C., O.H., and M.M.L. designed the research; H.A.P.-C., A.K., J.-M.E., B.C., K.M., J.K.J., and N.E.R. performed the research; H.A.P.-C., A.K., J.-M.E., B.C., K.M., J.K.J., J.G.K., V.M., and N.E.R. collected data; H.A.P.-C., S.F.S., T.J.H., A.K., R.O., R.S.V., D.E.M., B.C., K.M., J.K.J., M.R.C., J.G.K., J.A.H., R.G.C., N.E.R., V.M., O.H., and M.M.L analyzed and interpreted the data; and all authors reviewed the manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael M. Lederman, 2109 Adelbert Rd, BRB Bldg Rm 1048B, Cleveland, OH 44106; e-mail: mxl6@case.edu.
References
- 1.Lederman MM, Penn-Nicholson A, Cho M, Mosier D. Biology of CCR5 and its role in HIV infection and treatment. JAMA. 2006;296(7):815–826. doi: 10.1001/jama.296.7.815. [DOI] [PubMed] [Google Scholar]
- 2.Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal. 2004;16(11):1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors–central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20(1):111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- 4.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768(4):853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amara A, Gall SL, Schwartz O, et al. HIV coreceptor downregulation as antiviral principle: SDF-1alpha- dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186(1):139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234(2):340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 8.Mack M, Luckow B, Nelson PJ, et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187(8):1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastore C, Picchio GR, Galimi F, et al. Two mechanisms for human immunodeficiency virus type 1 inhibition by N-terminal modifications of RANTES. Antimicrob Agents Chemother. 2003;47(2):509–517. doi: 10.1128/AAC.47.2.509-517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achour L, Labbe-Jullie C, Scott MG, Marullo S. An escort for GPCRs: implications for regulation of receptor density at the cell surface. Trends Pharmacol Sci. 2008;29(10):528–535. doi: 10.1016/j.tips.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Achour L, Scott MG, Shirvani H, et al. CD4-CCR5 interaction in intracellular compartments contributes to receptor expression at the cell surface. Blood. 2009;113(9):1938–1947. doi: 10.1182/blood-2008-02-141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morner A, Bjorndal A, Albert J, et al. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol. 1999;73(3):2343–2349. doi: 10.1128/jvi.73.3.2343-2349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toossi Z, Mayanja-Kizza H, Baseke J, et al. Inhibition of human immunodeficiency virus-1 (HIV-1) by beta-chemokine analogues in mononuclear cells from HIV-1-infected patients with active tuberculosis. Clin Exp Immunol. 2005;142(2):327–332. doi: 10.1111/j.1365-2249.2005.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee B, Sharron M, Blanpain C, et al. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274(14):9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, LaRosa G, Kassam N, et al. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186(8):1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konigs C, Rowley MJ, Thompson P, et al. Monoclonal antibody screening of a phage-displayed random peptide library reveals mimotopes of chemokine receptor CCR5: implications for the tertiary structure of the receptor and for an N-terminal binding site for HIV-1 gp120. Eur J Immunol. 2000;30(4):1162–1171. doi: 10.1002/(SICI)1521-4141(200004)30:4<1162::AID-IMMU1162>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor KH, Konigs C, Rowley MJ, et al. Requirement of multiple phage displayed peptide libraries for optimal mapping of a conformational antibody epitope on CCR5. J Immunol Methods. 2005;299(1–2):21–35. doi: 10.1016/j.jim.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Hartley O, Gaertner H, Wilken J, et al. Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitors. Proc Natl Acad Sci U S A. 2004;101(47):16460–16465. doi: 10.1073/pnas.0404802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbe R, Picchio GR, Pastore C, et al. Donor- and ligand-dependent differences in C-C chemokine receptor 5 reexpression. J Virol. 2001;75(2):661–671. doi: 10.1128/JVI.75.2.661-671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mummidi S, Adams LM, VanCompernolle SE, et al. Production of specific mRNA transcripts, usage of an alternate promoter, and octamer-binding transcription factors influence the surface expression levels of the HIV coreceptor CCR5 on primary T cells. J Immunol. 2007;178(9):5668–5681. doi: 10.4049/jimmunol.178.9.5668. [DOI] [PubMed] [Google Scholar]
- 21.da Costa ES, Peres RT, Almeida J, et al. Harmonization of light scatter and fluorescence flow cytometry profiles obtained after staining peripheral blood leucocytes for cell surface-only versus intracellular antigens with the Fix & Perm reagent. Cytometry B Clin Cytom. 2010;78(1):11–20. doi: 10.1002/cyto.b.20486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.