Abstract
Src activation involves the coordinated regulation of positive and negative tyrosine phosphorylation sites. The mechanism whereby receptor tyrosine kinases, cytokine receptors, and integrins activate Src is not known. Here, we demonstrate that granulocyte colony-stimulating factor (G-CSF) activates Lyn, the predominant Src kinase in myeloid cells, through Gab2-mediated recruitment of Shp2. After G-CSF stimulation, Lyn dynamically associates with Gab2 in a spatiotemporal manner. The dephosphorylation of phospho-Lyn Tyr507 was abrogated in Shp2-deficient cells transfected with the G-CSF receptor but intact in cells expressing phosphatase-defective Shp2. Auto-phosphorylation of Lyn Tyr396 was impaired in cells treated with Gab2 siRNA. The constitutively activated Shp2E76A directed the dephosphorylation of phospho-Lyn Tyr507 in vitro. Tyr507 did not undergo dephosphorylation in G-CSF–stimulated cells expressing a mutant Gab2 unable to bind Shp2. We propose that Gab2 forms a complex with Lyn and after G-CSF stimulation, Gab2 recruits Shp2, which dephosphorylates phospho-Lyn Tyr507, leading to Lyn activation.
Introduction
Granulocyte colony-stimulating factor (G-CSF) drives the proliferation and production of granulocytes. Mice deficient in either G-CSF or the G-CSF receptor (G-CSFR) are profoundly granulocytopenic.1,2 The G-CSFR is a member of the hematopoietin/cytokine receptor superfamily.3–5 Ligand binding of the G-CSFR leads to rapid changes in protein tyrosine phosphorylation. Because it lacks an intrinsic tyrosine kinase domain, the G-CSFR must recruit non–receptor protein tyrosine kinases to transduce its signal.6 Among the different classes of non–receptor protein tyrosine kinases, members of the Janus and Src family are most likely to be involved in G-CSFR signaling.7 The predominant Src kinase expressed in granulocytes and their precursors is Lyn.6 Studies using Lyn-deficient cell lines that express the G-CSFR demonstrate a critical role for Lyn in promoting cell-cycle progression via PI3′-kinase.8 Additional studies suggest that Lyn contributes to granulocytic differentiation via a Lyn-Gab2 interaction.9 The mechanism by which engagement of the receptor leads to activation of the non–receptor protein tyrosine kinases, Lyn or Jak2, is poorly understood.
The kinase activity of Src is tightly regulated through phosphorylation of its C-terminal tyrosine site (Tyr527 for c-Src, Tyr507 for Lyn). This phosphorylation involves C-terminal Src kinase (Csk)10 and a complex of proteins localized to the plasma membrane.11 The positive tyrosine phosphorylation site in c-Src is Tyr416 (equivalent to Tyr396 for Lyn),12 known to lie within the activation loop shared by all Src family members. Phosphorylation of Lyn Tyr396 results in increased Lyn kinase activity, whereas phosphorylation of Tyr507 inhibits the kinase activity. Rapid activation and efficient attenuation of Src kinases is crucial for the normal physiology of cell signaling. This, therefore, requires the coordinated processes of Src phosphorylation at Tyr527 by Csk, its dephosphorylation by an activating tyrosine phosphatase, auto-phosphorylation at Tyr416, and its dephosphorylation by an inhibitory tyrosine phosphatase. Shp2 and Shp1 are leading candidates for the tyrosine phosphatases.13 Shp2 is expressed ubiquitously,14,15 whereas Shp1 expression is more restricted with highest levels in hematopoietic cells.16 Mice deficient in Shp1 exhibit numerous hematopoietic abnormalities including augmented production and tissue accumulation of granulocytes.17,18 In contrast, mice deficient in Shp2 die around the time of implantation from trophoblast stem cell death.19 The differentiation of homozygous Shp2−/− embryonic stem (ES) cells into erythroid and myeloid cell precursors is severely blocked.20 A deletion mutation of Shp2 severely suppresses hematopoietic cell development.21 Gain-of-function mutations, Shp2 D61A and Shp2 E76A, have been found in juvenile myelomonocytic leukemia, characterized by excess monocytes and granulocyte precursor cells.22 Recent experiments showed that expression of Shp2 D61Y and Shp2 E76K proteins in Ba/F3 cells or mice conferred a phenotype of growth factor–independent survival, suggesting that Shp2 plays a positive role in myeloid intracellular signaling.23–26
If Shp2 serves as the activating tyrosine phosphatase for G-CSF–stimulated Lyn kinase, how does it functionally interact with the G-CSFR? Gab (Grb2 associated binder) proteins comprise a distinct family of scaffolding adaptors characterized by similar overall structure organization.27 Scaffolding adaptors bind multiple signaling molecules, forming multimeric signaling complexes. Scaffolding adaptors lack catalytic activity but typically associate with one or more enzymes.27 Gab1 and Gab2 are pleckstrin homology domain–containing proteins that recruit additional SH2 or PTB domain–containing molecules. Gab1 was originally isolated as a Grb2 (growth receptor binding protein 2) binding protein from human glioma cells28 and as a c-Met substrate in yeast 2-hybrid system.29 Gab2 was originally found as a 97 kDa tyrosine-phosphorylated protein associating with Shp2, a protein tyrosine phosphatase (PTPase) in IL-3–stimulated30,31 or Bcr-Abl–transformed Ba/F3 and 32D cells.32 Gab2 family proteins, acting via Shp2, are required for full Erk activation in many signaling pathways.33,34 Studies of chimeric receptors revealed that Gab2 mutants lacking Shp2 binding sites are unable to activate Erk or downstream transcriptional reporters.35 Subsequent work showed that Gab1–Shp2 complex acts at a step upstream of Ras.36 Overexpression of mutant forms of Gab2 also can impair Erk activation in response to some stimuli.37 Moreover, mast cells and macrophages from Gab2−/− mice have decreased Erk activation in response to SCF or CSF.38,39 Cells expressing the Gab1–Shp2 fusion protein exhibit enhanced Src kinase activity, and Src inhibitors blocked the ability of the fusion protein to activate Erk.40 These findings suggest that Shp2 acts upstream of, and perhaps directly on, the negative regulatory C-terminal tyrosine phosphorylation sites of Src.
In this study, we show that Gab2 associates with and dissociates from Lyn dynamically in a spatiotemporal manner in response to G-CSF stimulation. Gab2 also associates with Shp2, and mutations of Tyr614 and Tyr643 of Gab2 abolish the binding of Shp2. Constitutively activated Shp2 E76A directs the dephosphorylation of Lyn Tyr507 in vivo, whereas Lyn was hypophosphorylated in G-CSF–stimulated Shp2−/− cells expressing the G-CSFR. Expression of a mutant form of Gab2 that cannot bind Shp2 results in loss of G-CSF–induced dephosphorylation of pTyr507 and loss of autophosphorylation of Tyr396. These studies support a mechanistic model of how a hematopoietic cytokine receptor activates a Src family kinase through the recruitment of the tyrosine phosphatase Shp2 via the scaffold protein Gab2.
Methods
Reagents
Commercially available antibodies used were phospho-SrcY416 (equivalent to LynY396), phospho-LynY507, Akt, phospho-STAT1, phospho-STAT3, and phospho-STAT5 from Cell Signaling; anti-phosphotyrosine (4G10), anti-Gab2, anti-Shp2, anti-Jak2, anti-STAT1, anti-STAT3, and anti-STAT5 from Upstate Biotechnology Inc; anti-actin, anti-Lyn, anti-Blk, anti-Hck, anti-Fgr, anti-Fyn, and anti-Src antibodies from Santa Cruz Biotechnology; and PE-conjugated anti–human GCSF receptor (CD114) antibody and its isotype IgG were purchased from BD Biosciences. The source of recombinant human G-CSF was filgrastim (Amgen). Site-directed PCR Mutagenesis Kit was purchased from Stratagene. Lipofectamine 2000 was purchased from Invitrogen.
Cell lines
Ba/F3 cells and their derivatives were grown in RPMI 1640 medium supplemented with 10% FBS, 2mM glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 ng/mL murine recombinant IL-3 (PeproTech). Wild-type and Shp2−/− murine embryonic fibroblasts (with exon 3 deletion) have been described elsewhere41 and were grown in DMEM supplemented with 10% FBS, 2mM l-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin. HEK293 cells were purchased from ATCC and grown in DMEM supplemented with 10% FBS, 2mM glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin.
Construction of plasmids and transfection
For stable transfection, the human G-CSF receptor cDNA with a hemagglutinin (HA) tag was constructed by PCR and cloned into either pcDNA3 or pcDNA3.1Zeo(+) vector (Invitrogen). Human wild-type Shp2 cDNA was cloned into the HindIII and BamHI sites of pcDNA3.1Zeo(+). Human wild-type Gab2 cDNA with a FLAG tag was cloned into HindIII and XbaI sites of pcDNA3.1Zeo(+). Site-directed PCR mutagenesis kit was used to mutate the Shp2 binding sites in Gab2 to produce Gab2Y614F, Gab2Y643F, or Gab2DM(Y614F,Y643F). Automated sequencing was performed to confirm the directed mutation(s). For glutathione-S-transferase (GST) pull-down assays, the cDNA encoding the unique domain, unique/SH3 domains, unique/SH3/SH2 domains, or SH2 domain of human Lyn were cloned into pGEX2T vector (Pharmacia LKB Biotechnology) to produce GST fusion genes. Ba/F3 cells were transfected by electroporation (Gene Pulser II; Bio-Rad). Stable cell lines were obtained by culturing in 500 μg/mL neomycin or 100 μg/mL zeocin. Murine embryonic fibroblasts (MEF), Shp2−/− MEF, and HEK293 cells were transfected by Lipofectamine 2000 according to the instructions from the manufacturer (Invitrogen).
Flow cytometry for cell surface expression of G-CSFR
From each cell line, 2 × 106 cells were harvested in 15-mL tubes and washed twice with PBS. Cells were blocked with 2% BSA in PBS for 10 minutes, centrifuged at 300g for 5 minutes at 4°C, the pellets were resuspended in 200 μL of PBS with 2% BSA and divided into two parts, one was incubated with 0.5 μg of isotype IgG, another was incubated with 0.5 μg of PE-conjugated anti–human G-CSFR (CD114) antibody on ice for 20 minutes. After incubation, cells were washed twice with PBS, resuspended in 0.5 mL of PBS, and transferred into flow tubes. Samples were analyzed on a Becton Dickinson FACScan with channel FL2. Results were analyzed with CellQuest Version 3.3 software (Becton Dickinson).
Immunoprecipitation and immunoblotting
Cell lysis with 1% NP-40 detergent was performed as described elsewhere. Protein concentration was determined using the Bradford protein assay. For immunoprecipitation, cell lysates were incubated with specific primary antibody for overnight at 4°C, then with 20 μL of protein A/G-Sepharose (Santa Cruz Biotechnology) for 1 hour on a roller system at 4°C. The beads were washed 4 times with lysis buffer. For Western blot analysis, cell lysates or immunoprecipitates were subjected to SDS-PAGE, and proteins were transferred to Immobilon-P Transfer Membrane (Millipore Corporation). The membranes were blocked for 1 hour at room temperature with blocking buffer (5% milk or 3% BSA in PBS with 0.1% Tween-20). The blots were then incubated with primary antibodies for 2-4 hours at room temperature or overnight at 4°C, followed by incubating with secondary antibodies for 1 hour at room temperature. The immunoreactive bands were visualized by enhanced chemiluminescence (Amersham).
Production of GST fusion proteins and GST pull-down assays
Recombinant GST fusion proteins were affinity purified from cell lysates prepared from plasmid-transformed Escherichia coli XL-1 Blue (Stratagene), and the fusion proteins were purified from isopropyl-1-thio-D-galactopyranoside–induced bacteria by adsorption onto glutathione-sepharose beads (Pharmacia). For GST pull-down assays, lysates from Ba/F3 cells (1.0 mg) were incubated with 20 μg of GST fusion protein coupled to glutathione beads for 4 hours at 4°C. The beads were washed 3 times with 1 mL of lysis buffer; bound proteins were released by boiling in Laemmli buffer for 10 minutes, separated on a 7.5% SDS–polyacrylamide gel, transferred to a nitrocellulose membrane, and blotted with anti-Gab2 or anti-GST antibodies. The immunoreactive bands were visualized by enhanced chemiluminescence (Amersham).
In vitro kinase assay
Ba/F3 cells that stably express the human G-CSFR were grown in 100 ng/mL G-CSF medium and lysed in NP-40 lysis buffer. The protein concentrations in the cleared lysates were measured. Equal amounts of total proteins in lysates were immunoprecipitated with rabbit polyclonal anti-Hck, Fyn, Blk, Lyn, Fgr, and Src antibodies. The immunoprecipitated samples were incubated with 2 μCi of 32P-ATP in kinase assay buffer (25mM HEPES, pH 7.7) containing 0.2mM Na3VO4, 10μM ATP, 5mM MgCl2, 3mM MnCl2, 2μM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin, and 1mM dithiothreitol) at 30°C for 30 minutes. The kinase reaction was terminated by addition of SDS-sample buffer. The sample was resolved by SDS-PAGE, dried, and autoradiographed.
In vitro phosphatase assay
The GST fusion proteins used in this study were derived by PCR amplifying and subcloning cDNA of human wild-type Shp2, constitutively activated Shp2E76A, or constitutively inactivated Shp2 C459S cDNA into BamHI and XhoI sites of pGEX4T to produce GST-Shp2, GST-Shp2E76A, or GST-Shp2C459S fusion genes, respectively. For phosphatase reactions, Lyn was immunoprecipitated overnight at 4°C from 1 × 107 starved Ba/F3 GR cells for each reaction. Protein A Sepharose (20 μL) was added and the mixture was incubated on a shaker for 1 hour at 4°C. Samples were washed twice with lysis buffer, one time with phosphatase buffer. GST fusion proteins were eluted from GST-Shp2, GST-Shp2E76A, or GST-Shp2C4595S bead complexes with 300 μL of elution buffer (10mM reduced glutathione, 50mM Tris-HCl, pH 8.0). These eluted proteins were then incubated in the indicated amount with immunoprecipitated Lyn at 37°C for 30 minutes in 200 μL of phosphatase buffer (10mM Tris-HCl, 1.0mM EDTA, 1 mg/mL BSA 0.1% 2-mercaptoethanol, 0.01% NaN3, pH 7.4). After incubation, the samples were spun down and the beads were resuspended in 30 μL of Laemmli buffer and boiled for 5 minutes. The samples were run on a 10% SDS-PAGE, transferred onto polyvinyl difluoride membrane, immunoblotted with anti-phospho-Lyn Tyr507 antibody, and reblotted with anti-Lyn antibody. The immunoreactive bands were visualized by enhanced chemiluminescence (Amersham).
siRNA silencing of Gab2
Smart pool mouse Gab2 siRNA (catalog no. M-041087-00-0010) was purchased from Dharmacon Inc and scramble control siRNA (control siRNA, catalog no. 1022076) was purchased from QIAGEN. Nucleofection of BaF3/GR cells was performed with Nucleofector Kit V from Amaxa Biosystems. Briefly, 2 × 106 cells per aliquot were harvested, and the cell pellet was resuspended in 100 μL of prewarmed Nucleofector solution V. Cell suspension was incubated with 2 μg of mouse Gab2 or scrambled siRNA and transferred into Amaxa-certified cuvettes immediately. Nucleofection was carried out according to program X-01. Cells were harvested after 24 hours and analyzed by Western blot for Gab2 levels.
shRNA lentivirus production and transduction of Ba/F3GR cells
Commercially available HIV-based shRNA constructs with green fluorescent protein (GFP) and puromycin resistance gene were obtained from GeneCopoeia. Vectors were unrelated control shRNA (CSHCTR001-HIVU6), anti–mouse Gab2 shRNA (MSH029200-HIVU6), and anti–mouse SHP2 shRNA (MSH030006-HIVU6). Lentiviral vector particles were produced by the co-transfection of 293FT cells (Invitrogen) with the lentiviral shRNA plasmid, pMDLg/p.RRE (packaging plasmid), and pCMV-VSV-G-RSV-Rev (VSV.G-Rev expressing plasmid) using Lipofectamine 2000 (Invitrogen). Vector-containing medium was collected on day 2 and day 3, and concentrated by ultracentrifugation. Concentrated lentiviral particles were added to Ba/F3GR cells, and transduced (GFP-positive) cells were selected on day 2 by a fluorescence-activated cell sorter (MoFlo; Beckman Coulter). Sorted cells were maintained in medium containing 1 μg/mL puromycin thereafter.
MTT assay
Aliquots of 3 × 103 cells in 100 μL of culture medium were grown in a 96-well plate. Viable cell number was determined on day 3 using MTT assay kit (ATCC).
Colony-forming assay
Gab2 knockout mice and wild-type littermates were created as previously described.42 Mouse bone marrow mononuclear cells were harvested using Ficoll-Paque Plus reagent (GE Healthcare). Bone marrow mononuclear cells (3 × 104) were seeded in 1 mL methylcellulose (MethoCult M3231; StemCell Technologies) supplemented with different concentrations of G-CSF (0-100 ng/mL). Colony number derived from colony-forming unit granulocyte (CFU-G) was scored on day 7.
Results
Lyn is the predominant Src kinase in Ba/F3GR cells
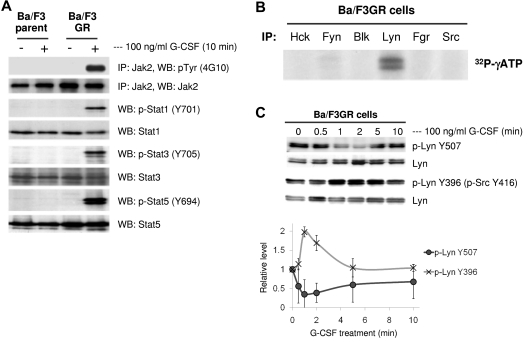
We have previously established Ba/F3 cell lines stably expressing the human G-CSFR (Ba/F3GR). To confirm that G-CSF–mediated intracellular signaling is reconstituted in this cell line, we washed Ba/F3GR cells, starved of IL-3 for 5 hours, and stimulated with 100 ng/mL G-CSF for 10 minutes. As expected, Jak2, Stat1, Stat3, and Stat5 were phosphorylated in response to G-CSF (Figure 1A). To examine whether Src family kinases are phosphorylated by G-CSF, Ba/F3GR cells were grown in medium containing 100 ng/mL G-CSF for 24 hours, and phosphorylation of Src family members (Hck, Fyn, Blk, Lyn, Fgr, and Src) were determined. After immunoprecipitation with corresponding antibodies, phosphorylation of Src family kinases was determined in an in vitro kinase assay. As shown in Figure 1B, Lyn was the predominant Src kinase phosphorylated in G-CSF–treated Ba/F3GR cells. Because the activity of Src kinases is tightly regulated by sequential tyrosine phosphorylation and dephosphorylation of a positive and negative tyrosine site, we next examined dynamic changes in the phosphorylation state of Lyn. We compared the phosphorylation status of Lyn Tyr396 (activating phosphorylation site) and Lyn Tyr507 (inhibitory phosphorylation site) in response to G-CSF stimulation. Ba/F3GR cells were starved of IL-3 for 5 hours and stimulated with 100 ng/mL G-CSF for indicated time intervals. Dephosphorylation of Lyn Tyr507 and phosphorylation Lyn Tyr396 (as detected by anti-phospho-Src Tyr416) were observed as early as 1 minute, which sustained to 5 minutes, and returned to the baseline after 10 minutes (Figure 1C). This result indicated that dephosphorylation of the negative-regulatory tyrosine and phosphorylation of the positive-regulatory tyrosine residue occurs sequentially in G-CSFR–mediated signaling.
Figure 1.
G-CSF treatment activates Lyn by the dephosphorylation of Lyn Tyr507 and phosphorylation of Lyn Tyr396. (A) Ba/F3 parent and Ba/F3GR cells were untreated or treated with 100 ng/mL G-CSF for 10 minutes; the whole cell lysates were prepared followed by immunoprecipitating Jak2, blotting with phospho-tyrosine, or by blotting with phospho-Stat1, Stat3, and Stat5, reprobing with anti-Jak2, Stat1, Stat3, and Stat5 antibodies. (B) Ten million cells were harvested from Ba/F3GR cells for each sample. The whole cell lysates were prepared and immunoprecipitated with anti-Hck, Fyn, Blk, Lyn, Fgr, and Src antibodies, respectively, kinase activity was determined by autophosphorylation in the presence of radio-labeled ATP, and the results showed that Lyn is the predominant Src kinase expressed in G-CSF receptor expressing Ba/F3 cells. (C) After 5 hours of IL-3 starvation, Ba/F3GR cells were stimulated with 100 ng/mL G-CSF for the indicated times and lysates were prepared to be blotted with anti-phospho-Lyn Tyr507 or anti-phospho Src Tyr416 (which detects phospho-Lyn Tyr396). The blot was stripped and reprobed to detect total Lyn. Three independent experiments were performed and representative data are shown (top panel). Densitometric analysis was performed from the 3 independent experiments and relative values are shown (bottom panel). Error bar denotes SE.
Dynamic association of Lyn with Gab2 in G-CSFR signaling
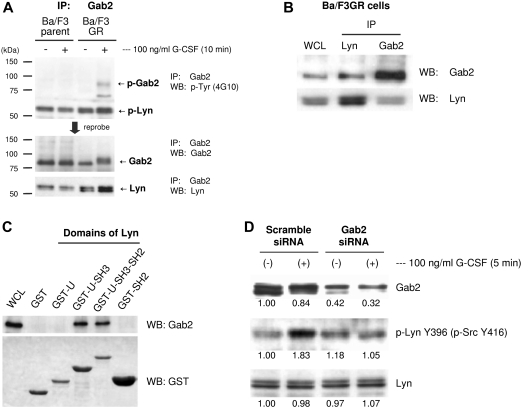
We next tried to find the pathway by which G-CSF leads to dephosphorylation of Lyn Tyr507 and phosphorylation of Lyn Tyr396. We hypothesized that an adapter protein Gab2 may be playing the role in this process. G-CSFR stimulation by G-CSF results in the tyrosine phosphorylation of cytoplasmic domain of G-CSFR. Phosphorylation of G-CSFR is believed to result in binding of Grb2 to G-CSFR, which facilitates the recruitment Gab2 to G-CSFR. On G-CSF stimulation, Gab2 is phosphorylated. Interestingly, Gab2 is reported to associate with Lyn in FcϵRI-mediated signal transduction in mast cells,43 and Gab2 is also reported to have constitutive association with Src in rat primary hepatocytes.44 Therefore, we hypothesized that G-CSFR will activate Lyn though Gab2. We treated Ba/F3GR cells with or without G-CSF, immunoprecipitated with anti-Gab2 antibody, and Western blotted using anti-phosphotyrosine, anti-Gab2, and anti-Lyn antibodies (Figure 2A). We confirmed that Gab2 is phosphorylated by G-CSF treatment. (Figure 2A top and middle panels). We also found that the phosphoprotein at 55 kDa is p53/56 isoforms of Lyn (Figure 2A top and bottom panels), suggesting the interaction between Gab2 and Lyn. The interaction between Gab2 and Lyn was further confirmed by immunoprecipitating samples using anti-Lyn antibody and blotting with anti-Gab2 antibody (Figure 2B). We therefore examined the possible mechanism underlying the association of Lyn with Gab2. GST fusion proteins containing unique domain, unique-SH3 domain, unique-SH3-SH2 domain, and SH2 domain of Lyn were purified to pull down Gab2 from lysates of Ba/F3GR cells, respectively. As shown in Figure 2C, unique-SH3 domains or unique-SH3-SH2 domains of Lyn pulled down Gab2, whereas neither the unique domain nor the SH2 domain alone pulled down Gab2. The SH3 domain of Lyn seemed to functionally interact with Gab2. To determine whether Gab2 contributes to the phosphorylation of Lyn Tyr396 by G-CSF, we knocked down Gab2 expression using siRNA, treated with or without G-CSF, and examined phosphorylation status of Lyn Tyr396. As expected, after the knockdown of Gab2, phosphorylation of Lyn Y396 was abrogated (Figure 2D).
Figure 2.
G-CSF–induced Gab2 association with Lyn. (A) Ba/F3GR and parental Ba/F3 cells were treated ± 100 ng/mL G-CSF for 10 minutes. Lysates were prepared, and proteins were immunoprecipitated with anti-Gab2 antibody. Blotting was performed with anti-phospho-tyrosine (4G10), Gab2, and Lyn antibodies. The phosphorylated double band protein is Lyn. (B) Coimmunoprecipitation assay showed that Gab2 associated with Lyn each other in BaF3GR cells. WCL indicates whole cell lysate. (C) GST pull-down results showed that SH3 domain of Lyn interacted with Gab2. Lane 1: WCL; lane 2: GST; lane 3: GST-unique domain of Lyn; lane 4: GST-unique-SH3 domain; lane 5: GST-unique-SH3-SH2 domain; lane 6: GST-SH2 domain. (D) Ba/F3GR cells were treated with either scrambled siRNA or siRNA against Gab2. Lysates were harvested at 24 hours and blotted for either total Gab2, phospho-Src Tyr416, and total Lyn. Numeric values are shown from densitometric analysis.
Gab2 associated with Shp2
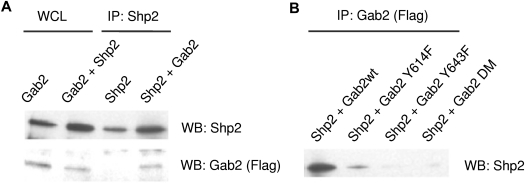
We hypothesized that Gab2 may affect Lyn kinase activity by recruiting protein tyrosine phosphatases to dephosphorylate the negative regulatory tyrosine residue of Lyn Tyr507. We reasoned that a protein phosphatase Shp2 might be playing the role. Gab2 has 2 tyrosine residues (Y614, Y643), which, when phosphorylated, provide binding sites for the SH2-domain of Shp2. Engagement of Shp2 through its SH2-phosphotyrosine interaction leads to a conformational change and activates Shp2. To confirm whether Gab2 leads to the activation of Shp2, we first transfected HEK293 cells with Gab2 and Shp2 and co-immunoprecipitated (Figure 3A). To confirm that the binding of Gab2 to Shp2 is dependent on the Gab2 phosphorylation, we transfected HEK293 cells with Shp2 as well as different types of Gab2 (wild-type or phosphorylation-defective Gab2 mutants), and immunoprecipitated with Gab2. As expected, Gab2Y614F, Gab2Y643F, and Gab2DM mutants could not bind to Shp2 (Figure 3B).
Figure 3.
Gab2 associated with Shp2. (A) After transfection of HEK293 cells with Gab2 and Shp2, proteins immunoprecipitated with anti-Shp2 antibody were blotted for Gab2. (B) HEK293 cells were transfected with FLAG-tagged Gab2 wild-type (WT) and Y to F mutations at positions 614 or 643 or both (Gab2DM). After immunoprecipitation of Gab2, proteins were blotted for Shp2. The Western blot was stripped and probed for total Gab2 using the FLAG epitope antibody.
Dephosphorylation of phospho-Lyn Tyr507 by Shp2
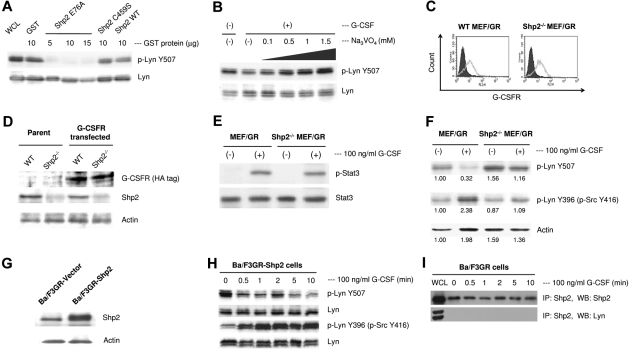
Multiple lines of evidence suggest that Shp2 plays a positive role in intracellular signaling in hematopoietic cells and lead us to hypothesize that Shp2 may dephosphorylate pTyr507 followed by autophosphorylation of Lyn Tyr396 and activate Lyn. To address this, we performed an in vitro phosphatase assay by using purified wild-type Shp2, constitutively active Shp2E76A, and constitutively inactive Shp2C459S mutants. The results showed that the constitutively active Shp2E76A dephosphorylated Lyn pTyr507, whereas constitutively inactive Shp2 did not (Figure 4A). Wild-type Shp2 did not dephosphorylate Lyn Tyr507, probably because it was not activated with any stimuli such as G-CSF in this in vitro experiment. The dephosphorylation of Lyn Tyr507 was inhibited by the treatment with a tyrosine phosphatase inhibitor, sodium orthovanadate, in a dose-dependent manner (Figure 4B). To assess whether loss of full-length Shp2 will abrogate dephosphorylation of Lyn Tyr507 in vivo, we transfected wild-type and Shp2−/− mouse embryonic fibroblasts (MEF)41 with G-CSFR (Figure 4C-D) and stimulated with G-CSF. Reconstitution of G-CSFR intracellular signaling in this system was confirmed by the phosphorylation of Stat3, the major downstream target of G-CSFR (Figure 4E). As expected, G-CSF did not induce dephosphorylation of Lyn Tyr507 and did not phosphorylate Lyn Tyr396 in Shp2−/− MEF/GR cells (Figure 4F). In contrast, when we overexpressed Shp2 in Ba/F3GR cells (Figure 4G) and treated with G-CSF, we found sustained dephosphorylation of Lyn Tyr507 and autophosphorylation of Lyn Tyr396 in Ba/F3-GR cells (Figure 4H). To determine whether Lyn activation by Shp2 requires direct binding of Shp2 to Lyn, we immunoprecipitated samples with Shp2 and blotted with Lyn. As shown in Figure 4I, there was not direct interaction, which suggests that Shp2 does not necessarily bind to Lyn to dephosphorylate Lyn.
Figure 4.
Lyn is a Shp2 substrate in vitro and in vivo. (A) Lyn protein was immunoprecipitated from IL-3–starved Ba/F3GR cells washed twice with lysis buffer and once with phosphatase buffer, then mixed with 10 μg of purified GST, GST-Shp2, GST-Shp2C459S, or 5, 10, or 15 μg of GST-Shp2E76A in 200 μL of phosphatase buffer. Western blotting was performed with anti-phospho-Lyn Tyr507 antibody and reprobed with anti-Lyn antibody. The results showed that activated form of Shp2E76A directly dephosphorylated Tyr507 of Lyn, whereas wild-type Shp2 and constitutively inactivated Shp2 C459S had no or weak ability to dephosphorylate Tyr507 of Lyn. (B) Ba/F3GR cells were starved of IL-3, pretreated with increasing concentration of phosphatase inhibitor Na3VO4 for 1 hour, and followed by stimulation with 100 ng/mL G-CSF for 5 minutes. Cell lysates were blotted with anti-phospho-LynY507 and reprobed with anti-Lyn antibody. (C) After transfection of MEF cells with HA-tagged G-CSFR, surface expression of G-CSFR was detected by flow cytometry; shaded histogram represents the staining with PE-conjugated-isotype antibody, gray outline represents the staining with PE-conjugated anti–human G-CSFR antibody. (D) The expression of HA-tagged human G-CSFR from wild-type and Shp2−/− MEF cell lysates was detected by Western blot. (E) G-CSFR signaling was reconstituted in wild-type and Shp2−/− MEF cells with the expression of G-CSFR by detection of the phosphorylation of Stat3. (F) Dephosphorylation of phospho-Lyn Tyr507 occurred in G-CSFR–expressing MEF cells in response to G-CSF, whereas it was impaired in G-CSFR–expressing Shp2−/− MEF cells. Numerical values are shown from densitometric analysis. (G) Shp2 (or empty vector as control) was stably transfected into Ba/F3GR cells, whole cell lysates were then prepared, and Western blot showed overexpression of Shp2 in Ba/F3GR cells. (H) Overexpression of Shp2 induced sustained dephosphorylation of phospho-Lyn Tyr507 and phosphorylation of Lyn Tyr396 in Ba/F3GR cells in response to G-CSF. (I) Proteins immunoprecipitated with Shp2 from G-CSF–treated Ba/F3GR cells were blotted for Lyn. Whole cell lysates (WCL) provided positive controls for Shp2 (top panel) and Lyn (bottom panel).
Gab2 phosphorylation is required for the Lyn activation
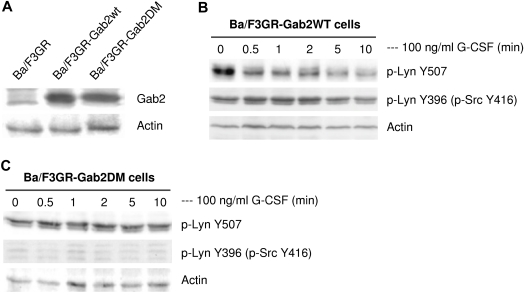
Phosphorylation of Gab2 provides binding sites for Shp2, resulting in activation of Shp2. Therefore, we hypothesized that overexpression of Gab2 will activate Lyn via Shp2 activation. To confirm this, we transfected Ba/F3GR cells with either wild-type Gab2 or Gab2DM (Figure 5A), and treated with G-CSF (Figure 5B-C). Overexpression of Gab2 enhanced the dephosphorylation of Lyn Tyr507, resulting in sustained autophosphorylation of Lyn Tyr396 (Figure 5B). In contrast, overexpression of Gab2DM, which cannot be phosphorylated, did not lead to dephosphorylation of Lyn Tyr507, and Lyn Y396 was not phosphorylated, as we expected (Figure 5C). These results support the role of Gab2 in regulating Lyn kinase activity by recruiting Shp2 to dephosphorylate Lyn Tyr507.
Figure 5.
Functional association of Gab2 with Lyn. (A) Overexpression of Gab2 WT and Gab2 DM were detected by Western blot in Ba/F3GR cells. Comparable protein levels were demonstrated by blotting for actin (bottom panel). (B) Overexpression of wild-type Gab2 induced prolonged dephosphorylation of pTyr507 but not prolonged auto-phosphorylation of Tyr396 of Lyn in response to G-CSF. Blots were stripped and reprobed with actin to demonstrate comparable protein loading. (C) Overexpression of Gab2 with mutations of Shp2 binding sites did not change phosphorylation status of Tyr507 or Tyr396 (as detected by anti-phospho Src Tyr416 antibody) of Lyn in response to G-CSF.
Hematopoietic role of the G-CSFR/Gab2/Shp2/Lyn pathway
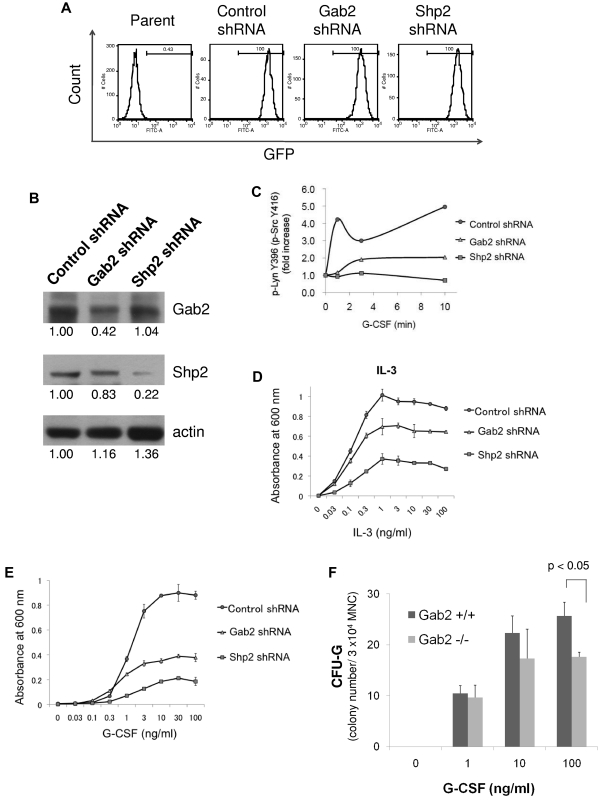
To examine whether the G-CSFR/Gab2/Shp2/Lyn pathway contributes to G-CSF–dependent proliferation, we transduced Ba/F3GR cells with Gab2 shRNA and Shp2 shRNA using lentiviral vectors, and cultured in medium containing either IL-3 or G-CSF. Before the treatment, transduced cells were sorted by GFP expression (transduction marker) using FACS. All cells (100%) were GFP-positive after the sorting (Figure 6A). Gab2 and Shp2 knockdown was confirmed by Western blot (Figure 6B). Lysates from cells treated with control, Gab2, or Shp2 shRNA were analyzed for G-CSF–induced changes in Lyn phosphorylation. Only cells treated with control shRNA demonstrated a 4- to 5-fold increase in activated Lyn following G-CSF stimulation (Figure 6C). Thus, loss of Gab2 or Shp2 resulted in a less robust activation of Lyn. This correlated with inhibition of either IL-3– or G-CSF–dependent proliferation (Figure 6D-E). The inhibition was even more significant in G-CSF–treated cells than IL-3–treated cells. These results suggested that Gab2 and Shp2 play important roles in G-CSF-induced proliferation. Using methylcellulose colony assay of mouse bone marrow mononuclear cells from wild-type and Gab2 knockout mice, we further confirmed that colony formation derived from colony-forming unit granulocyte (CFU-G) is inhibited in Gab2 knockout mice at 100 ng/mL G-CSF (Figure 6F).
Figure 6.
Hematopoietic Role of a GCSFR/Gab2/Shp2/Lyn pathway. (A) Ba/F3GR cells were transduced with lentiviral vectors containing GFP and either scrambled shRNA (control) or shRNA against murine Gab2 or Shp2. Flow sorting was performed for the GFP+ fraction. Results are shown. (B) Knockdown was confirmed by Western blotting for either Gab2 or Shp2 protein. Numerical values are shown from densitometric analysis. C) Lysates from G-CSF–stimulated Ba/F3 cells treated with control shRNA or shRNA to Gab2 or Shp2 were blotted for phospho-Src Tyr416 and total Lyn. Densitometric analysis was performed, showing a dramatic increase in phospho-Src Tyr416 (phospho-Lyn Tyr396) only in control shRNA cells. (D-E) MTT assay of Ba/F3GR cells treated with either IL-3 or G-CSF, demonstrating the functional importance of Gab2/Shp2 in proliferation. (F) Methylcellulose colony culture assays were performed on bone marrow mononuclear cells from Gab2−/− and their wild-type littermates. Bone marrow mononuclear cells (3 × 104) were seeded in methylcellulose containing different concentrations of G-CSF (0-100 g/mL). Colonies derived from colony-forming unit granulocyte (CFU-G) were scored on day 7. Error bar denotes SE (D-F).
Discussion
A small number of cytokines drive hematopoiesis by binding to receptor tyrosine kinases or hematopoietin/cytokine receptors, which lack intrinsic kinase activity. Receptor tyrosine kinases, such as those for c-Kit, M-CSF, and Flt3, recruit Src kinases to amplify their signal transduction.45 Hematopoietin/cytokine receptors, such as those for G-CSF or erythropoietin, recruit both Janus and Src families of non–receptor protein tyrosine kinases to affect signaling.7,46 Whereas Src activity is regulated by coordinated dephosphorylation/phosphorylation events, the mode by which engagement of the receptors results in Src activation is not understood. Both receptor tyrosine kinases and the hematopoietin/cytokine receptors involve similar signaling components in blood cells; we therefore hypothesized that Src regulation would involve some of those components. The predominantly expressed Src kinase in myeloid cells is Lyn. In particular, we reasoned that because dephosphorylation of the negative phosphotyrosine residue in Lyn (pTyr507) was the critical first step in activation, receptor-mediated recruitment of a tyrosine phosphatase was essential. Based on both experimental and clinical data, the most likely phosphatase is Shp2, which must itself be recruited to the receptor/cytosol region. We hypothesized that the scaffolding protein Gab2, first isolated as a binding partner for Shp2, is responsible for the recruitment of Shp2.
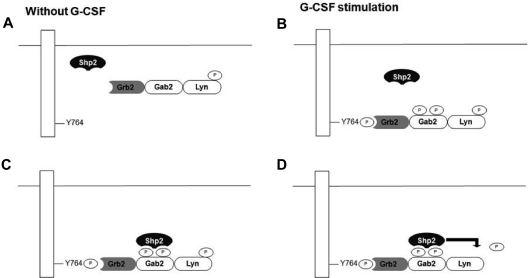
Our study suggested the following model by which G-CSF activates Lyn (Figure 7). First, Gab2 constitutively associates with Lyn via a Lyn SH3-Gab2 polyproline motif interaction. Gab2 is an important adapter protein, which is crucial for the signal transduction. Gab2 contains 2 proline-rich motifs that can bind to the Grb2 SH3 domain as well.32,47 These data suggest that Grb2, Gab2, and Lyn exist as a trimeric complex (Figure 7A). G-CSF stimulation leads to the tyrosine phosphorylation of G-CSFR (Tyr764), which provides the binding site for Grb2.48 Binding of Grb2 to G-CSFR leads to Gab2 phosphorylation (Figure 7B). Once Gab2 is phosphorylated, Shp2 binds to Gab2 through its SH2 domains (Figure 7C). Binding of Shp2 to Gab2 changes the conformation of Shp2, leading to the activation of Shp2 phosphatase activity. Activated Shp2 dephosphorylates the phospho-Lyn Tyr 507, its inhibitory regulatory site, leading to full activation of Lyn kinase (Figure 7D). In our study, Shp2−/− cells and constitutively inactive mutant of Shp2 or Gab2 could not dephosphorylate phospho-Lyn Tyr507. In contrast, overexpression of Gab2 and Shp2 enhanced the dephosphorylation of phosphor-Lyn Tyr507, leading to autophosphorylation of Lyn Tyr396 and full Lyn activation. In addition, knockdown experiments of Gab2 and Shp2 showed the inhibition of cell growth, suggesting their role in G-CSF–dependent proliferation.
Figure 7.
Proposed mechanism for Src activation by the G-CSFR through the recruitment of the tyrosine phosphatase Shp2 via the scaffolding protein Gab2. (A) Before engagement of the G-CSFR by ligand, Gab2 associates with phosphor Lyn Tyr507 through Gab2's polyproline motif interacting with Lyn's SH3 domain. Gab2 also interacts with an adaptor protein Grb2. (B) After G-CSF stimulation, tyrosine kinases such as Jak2 can phosphorylate the G-CSFR at sites such as Tyr764. The SH2 domain of Grb2 binds the G-CSFR, bringing the Gab2-Lyn complex to the receptor. (C) A tyrosyl phosphorylated form of Gab2 can then recruit Shp2 via the SH2 domain. (D) Shp2 can then dephosphorylate phosphor Lyn Tyr507, leading to Lyn's activation and autophosphorylation at the positive regulatory site Tyr396 (not shown).
One question remains as to the identity of the kinase that phosphorylates Gab2 on Tyr614 and Tyr643, thus providing docking sites for the SH2 domain of Shp2. One likely kinase is Jak2, which can phosphorylate Gab2.49 Alternatively, Gab2 sites can be phosphorylated by basal Src activity, constituting a positive feedback loop.9 Shp2 can also regulate the state of Src phosphorylation by dephosphorylating PAG and modulating Csk (C-terminal Src kinase) access to Src kinase.50 However, in resting cells, Csk is sufficiently active to phosphorylate Tyr507 of Lyn, rendering it inactive. In summary, we showed that treatment with G-CSF results in the dephosphorylation of the negative regulatory site Lyn Tyr507 and activation of Lyn, and that the binding of Shp2 to Gab2 was required for G-CSF–dependent cell proliferation. These data support a mechanistic model for how a hematopoietic/cytokine receptor can trigger a Src kinase as a signal transducer.
Acknowledgments
The authors thank Dr Michael J. Hayman for the gifts of human wild-type and mutated Shp2 plasmids and Rachel Hicklen for critical reading. They also thank Dr Hiroyuki Miyoshi (RIKEN BioResource Center, Ibaraki, Japan) and Cell Genesys for providing HIV vector constructs.
This work was supported by NIH Independent Scientist Award HL03794; grants RO1HL080052, R01CA108922 (S.J.C.), and RO1CA114945 (B.G.N.); and the AA & MDS International Foundation Young Investigator Award to M.F.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.F., Q.-s.Z., Z.L.W., L.X., and Y.K. performed the experiments; B.G.N. oversaw the construction, breeding, and harvesting of the mouse strains; M.F., Q.-s.Z., and S.J.C. designed the experiments; and G.-S.F., M.F., B.G.N., and S.J.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seth Corey, MD, Lurie 5-107, Northwestern University Feinberg School of Medicine, 303 E Superior St, Chicago, IL 60611; e-mail: s-corey@northwestern.edu.
References
- 1.Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [PubMed] [Google Scholar]
- 2.Liu F, Poursine-Laurent J, Link DC. The granulocyte colony-stimulating factor receptor is required for the mobilization of murine hematopoietic progenitors into peripheral blood by cyclophosphamide or interleukin-8 but not flt-3 ligand. Blood. 1997;90(7):2522–2528. [PubMed] [Google Scholar]
- 3.Fukunaga R, Seto Y, Mizushima S, Nagata S. Three different mRNAs encoding human granulocyte colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1990;87(22):8702–8706. doi: 10.1073/pnas.87.22.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ihle JN. Cytokine receptor signalling. Nature. 1995;377(6550):591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 5.Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354(19):2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 6.Corey SJ, Anderson SM. Src-related protein tyrosine kinases in hematopoiesis. Blood. 1999;93(1):1–14. [PubMed] [Google Scholar]
- 7.Corey SJ, Dombrosky-Ferlan PM, Zuo S, et al. Requirement of Src kinase Lyn for induction of DNA synthesis by granulocyte colony-stimulating factor. J Biol Chem. 1998;273(6):3230–3235. doi: 10.1074/jbc.273.6.3230. [DOI] [PubMed] [Google Scholar]
- 8.Grishin A, Sinha S, Roginskaya V, et al. Involvement of Shc and Cbl-PI 3-kinase in Lyn-dependent proliferative signaling pathways for G-CSF. Oncogene. 2000;19(1):97–105. doi: 10.1038/sj.onc.1203254. [DOI] [PubMed] [Google Scholar]
- 9.Zhu QS, Robinson LJ, Roginskaya V, Corey SJ. G-CSF-induced tyrosine phosphorylation of Gab2 is Lyn kinase dependent and associated with enhanced Akt and differentiative, not proliferative, responses. Blood. 2004;103(9):3305–3312. doi: 10.1182/blood-2003-06-1861. [DOI] [PubMed] [Google Scholar]
- 10.Inomata M, Takayama Y, Kiyama H, Nada S, Okada M, Nakagawa H. Regulation of Src family kinases in the developing rat brain: correlation with their regulator kinase, Csk. J Biochem (Tokyo) 1994;116(2):386–392. doi: 10.1093/oxfordjournals.jbchem.a124536. [DOI] [PubMed] [Google Scholar]
- 11.Chong YP, Mulhern TD, Cheng HC. C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)–endogenous negative regulators of Src-family protein kinases. Growth Factors. 2005;23(3):233–244. doi: 10.1080/08977190500178877. [DOI] [PubMed] [Google Scholar]
- 12.Ferracini R, Brugge J. Analysis of mutant forms of the c-src gene product containing a phenylalanine substitution for tyrosine 416. Oncogene Res. 1990;5(3):205–219. [PubMed] [Google Scholar]
- 13.Streuli M. Protein tyrosine phosphatases in signaling. Curr Opin Cell Biol. 1996;8(2):182–188. doi: 10.1016/s0955-0674(96)80064-0. [DOI] [PubMed] [Google Scholar]
- 14.Seki N, Hashimoto N, Suzuki Y, Mori S, Amano K, Saito Y. Role of SRC homology 2-containing tyrosine phosphatase 2 on proliferation of rat smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22(7):1081–1085. doi: 10.1161/01.atv.0000022878.37277.ec. [DOI] [PubMed] [Google Scholar]
- 15.Adachi M, Iwaki H, Shindoh M, et al. Predominant expression of the src homology 2-containing tyrosine phosphatase protein SHP2 in vascular smooth muscle cells. Virchows Arch. 1997;430(4):321–325. doi: 10.1007/BF01092755. [DOI] [PubMed] [Google Scholar]
- 16.Gu H, Botelho RJ, Yu M, Grinstein S, Neel BG. Critical role for scaffolding adapter Gab2 in Fc gamma R-mediated phagocytosis. J Cell Biol. 2003;161(6):1151–1161. doi: 10.1083/jcb.200212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapley P, Shevde NK, Schweitzer PA, et al. Increased G-CSF responsiveness of bone marrow cells from hematopoietic cell phosphatase deficient viable motheaten mice. Exp Hematol. 1997;25(2):122–131. [PubMed] [Google Scholar]
- 18.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4(2):124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Klaman LD, Chen B, et al. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev Cell. 2006;10(3):317–327. doi: 10.1016/j.devcel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Qu CK, Yu WM, Azzarelli B, Cooper S, Broxmeyer HE, Feng GS. Biased suppression of hematopoiesis and multiple developmental defects in chimeric mice containing Shp-2 mutant cells. Mol Cell Biol. 1998;18(10):6075–6082. doi: 10.1128/mcb.18.10.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu CK, Shi ZQ, Shen R, Tsai FY, Orkin SH, Feng GS. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17(9):5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 23.Mohi MG, Williams IR, Dearolf CR, et al. Prognostic, therapeutic, and mechanistic implications of a mouse model of leukemia evoked by Shp2 (PTPN11) mutations. Cancer Cell. 2005;7(2):179–191. doi: 10.1016/j.ccr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Schubbert S, Lieuw K, Rowe SL, et al. Functional analysis of leukemia-associated PTPN11 mutations in primary hematopoietic cells. Blood. 2005;106(1):311–317. doi: 10.1182/blood-2004-11-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan G, Kalaitzidis D, Usenko T, et al. Leukemogenic Ptpn11 causes fatal myeloproliferative disorder via cell-autonomous effects on multiple stages of hematopoiesis. Blood. 2009;113(18):4414–4424. doi: 10.1182/blood-2008-10-182626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loh ML, Vattikuti S, Schubbert S, et al. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood. 2004;103(6):2325–2331. doi: 10.1182/blood-2003-09-3287. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Rohrschneider LR. The gift of Gab. FEBS Lett. 2002;515(1–3):1–7. doi: 10.1016/s0014-5793(02)02425-0. [DOI] [PubMed] [Google Scholar]
- 28.Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379(6565):560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 29.Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384(6605):173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 30.Gu H, Griffin JD, Neel BG. Characterization of two SHP-2-associated binding proteins and potential substrates in hematopoietic cells. J Biol Chem. 1997;272(26):16421–16430. doi: 10.1074/jbc.272.26.16421. [DOI] [PubMed] [Google Scholar]
- 31.Gu H, Pratt JC, Burakoff SJ, Neel BG. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2(6):729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 32.Sattler M, Mohi MG, Pride YB, et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell. 2002;1(5):479–492. doi: 10.1016/s1535-6108(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhao C, Yu DH, Shen R, Feng GS. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J Biol Chem. 1999;274(28):19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]
- 34.Dorsey JF, Cunnick JM, Mane SM, Wu J. Regulation of the Erk2-Elk1 signaling pathway and megakaryocytic differentiation of Bcr-Abl(+) K562 leukemic cells by Gab2. Blood. 2002;99(4):1388–1397. doi: 10.1182/blood.v99.4.1388. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki S, Nishida K, Hibi M, et al. Docking protein Gab2 is phosphorylated by ZAP-70 and negatively regulates T cell receptor signaling by recruitment of inhibitory molecules. J Biol Chem. 2001;276(48):45175–45183. doi: 10.1074/jbc.M105384200. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki S, Nishida K, Yoshida Y, Itoh M, Hibi M, Hirano T. Gab1 is required for EGF receptor signaling and the transformation by activated ErbB2. Oncogene. 2003;22(10):1546–1556. doi: 10.1038/sj.onc.1206284. [DOI] [PubMed] [Google Scholar]
- 37.Yu WM, Hawley TS, Hawley RG, Qu CK. Role of the docking protein Gab2 in beta(1)-integrin signaling pathway-mediated hematopoietic cell adhesion and migration. Blood. 2002;99(7):2351–2359. doi: 10.1182/blood.v99.7.2351. [DOI] [PubMed] [Google Scholar]
- 38.Qu X, Sada K, Kyo S, Maeno K, Miah SM, Yamamura H. Negative regulation of FcepsilonRI-mediated mast cell activation by a ubiquitin-protein ligase Cbl-b. Blood. 2004;103(5):1779–1786. doi: 10.1182/blood-2003-07-2260. [DOI] [PubMed] [Google Scholar]
- 39.Nishida K, Wang L, Morii E, et al. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood. 2002;99(5):1866–1869. doi: 10.1182/blood.v99.5.1866. [DOI] [PubMed] [Google Scholar]
- 40.Cunnick JM, Meng S, Ren Y, et al. Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J Biol Chem. 2002;277(11):9498–9504. doi: 10.1074/jbc.M110547200. [DOI] [PubMed] [Google Scholar]
- 41.Shi ZQ, Lu W, Feng GS. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273(9):4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 42.Gu H, Saito K, Klaman LD, et al. Essential role for Gab2 in the allergic response. Nature. 2001;412(6843):186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 43.Xie ZH, Ambudkar I, Siraganian RP. The adapter molecule Gab2 regulates Fc epsilon RI-mediated signal transduction in mast cells. J Immunol. 2002;168(9):4682–4691. doi: 10.4049/jimmunol.168.9.4682. [DOI] [PubMed] [Google Scholar]
- 44.Kong M, Mounier C, Dumas V, Posner BI. Epidermal growth factor-induced DNA synthesis. Key role for Src phosphorylation of the docking protein Gab2. J Biol Chem. 2003;278(8):5837–5844. doi: 10.1074/jbc.M208286200. [DOI] [PubMed] [Google Scholar]
- 45.Martin GS. The road to Src. Oncogene. 2004;23(48):7910–7917. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- 46.Ward AC, Monkhouse JL, Csar XF, Touw IP, Bello PA. The Src-like tyrosine kinase Hck is activated by granulocyte colony-stimulating factor (G-CSF) and docks to the activated G-CSF receptor. Biochem Biophys Res Commun. 1998;251(1):117–123. doi: 10.1006/bbrc.1998.9441. [DOI] [PubMed] [Google Scholar]
- 47.Lock LS, Royal I, Naujokas MA, Park M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J Biol Chem. 2000;275(40):31536–31545. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- 48.de Koning JP, Schelen AM, Dong F, et al. Specific involvement of tyrosine 764 of human granulocyte colony-stimulating factor receptor in signal transduction mediated by p145/Shc/GRB2 or p90/GRB2 complexes. Blood. 1996;87(1):132–140. [PubMed] [Google Scholar]
- 49.Wang L, Xue J, Zadorozny EV, Robinson LJ. G-CSF stimulates Jak2-dependent Gab2 phosphorylation leading to Erk1/2 activation and cell proliferation. Cell Signal. 2008;20(10):1890–1899. doi: 10.1016/j.cellsig.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang SQ, Yang W, Kontaridis MI, et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell. 2004;13(3):341–355. doi: 10.1016/s1097-2765(04)00050-4. [DOI] [PubMed] [Google Scholar]