Abstract
Pharmacologic induction of fetal hemoglobin (HbF) expression is an effective treatment strategy for sickle cell disease (SCD) and β-thalassemia. Pomalidomide is a potent structural analog of thalidomide and member of a new class of immunomodulatory drugs. Recent reports demonstrated that pomalidomide reduced or eliminated transfusion requirements in certain hematologic malignancies and induced HbF ex vivo in CD34+ progenitor cells from healthy and SCD donors. We investigated the effects of pomalidomide on erythropoiesis and hemoglobin synthesis in a transgenic mouse model of SCD. We found that 8 weeks of treatment with pomalidomide induced modest increases of HbF with similar efficacy as hydroxyurea. However, in stark contrast to hydroxyurea's myelosuppressive effects, pomalidomide augmented erythropoiesis and preserved bone marrow function. Surprisingly, combinatory therapy with both drugs failed to mitigate hydroxyurea's myelotoxic effects and caused loss of HbF induction. These findings support further evaluation of pomalidomide as a novel therapy for SCD.
Introduction
Substantial experimental and clinical evidence supports the development of targeted therapies for the induction of fetal hemoglobin (HbF) in patients with sickle cell disease (SCD).1–6 Hydroxyurea, a ribonucleotide reductase inhibitor, is the most thoroughly investigated and only Food and Drug Administration-approved treatment for adult patients with SCD that augments HbF expression and reduces clinical complications.7,8 Despite this progress, there is an unabated need for targeted HbF-inducing therapies because many patients fail to respond to hydroxyurea. Pomalidomide and lenalidomide are proprietary thalidomide analogs, which belong to a novel class of immunomodulatory drugs. Both compounds recently emerged from clinical trials as highly promising treatments for various hematologic cancers and chronic inflammatory conditions.9–11 Surprisingly, both immunomodulatory drug compounds were also found to restore erythropoiesis and to reduce or eliminate blood transfusion dependency in patients with multiple myeloma and myelodysplastic syndromes.12–15 Furthermore, a recent ex vivo study demonstrated that pomalidomide and lenalidomide not only stimulated the proliferation of CD34+ progenitor cells and total hemoglobin production but also up-regulated HbF synthesis by a transcriptional mechanism.16 In this system, pomalidomide enhanced HbF synthesis more potently than lenalidomide and hydroxyurea and acted synergistically in combination with hydroxyurea. These results prompted the current study to evaluate pomalidomide's hematologic properties in a relevant in vivo model of SCD.
Methods
Mice
Knockout-transgenic sickle cell mice were bred at Georgia Health Sciences University according to institutional guidelines.17 Treatment groups consisted of vehicle (saline; n = 8), pomalidomide (10 mg/kg; n = 9), hydroxyurea (100 mg/kg; n = 7), pomalidomide (10 mg/kg) plus high-dose hydroxyurea (100 mg/kg; n = 8), and pomalidomide (10 mg/kg) plus low-dose hydroxyurea (10 mg/kg; n = 8). Pomalidomide (Celgene) and hydroxyurea (Sigma-Aldrich) were mixed in saline and injected intraperitoneally daily (Monday through Friday) for 8 weeks. C57BL/6 mice were used for specific control experiments. Details of the mouse model, drug preparation, and dose selection are included in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The study was approved by Georgia Health Sciences University's Animal Care and Use Committee.
Pharmacokinetic analysis
Detailed information is contained in supplemental Methods.
Complete blood count and RBC indices
Blood was collected by intracardiac puncture from ketamine/xylazine-anesthetized mice in vacutainer EDTA tubes (BD Biosciences) and complete blood count analyzed with the CBC-Diff Veterinary Hematology System (Heska Corporation). Reticulocyte counts were determined by supravital staining with methylene blue.
HbF analysis
Hemoglobin analysis from mouse hemolysates was done by analytic high performance liquid chromatography using a weak cation-exchange column SynChropak CM-300 (Eprogen) on the Waters Empower 32 high performance liquid chromatography system (Millipore).
F cells
F cells were analyzed by flow cytometry using the Caltag HbF test kit (Caltag Laboratories) according to the manufacturer's instructions. Flow cytometry was performed using the BD FACSCalibur system (BD Biosciences). HbF/F cell (picograms per cell) was calculated using mean corpuscular hemoglobin × % HbF/% F cell.
Histology
Formalin-fixed bone marrow sections (4 μm) of the proximal femur were stained with hematoxylin and eosin (Richard-Allan Scientific) and analyzed by 2 blinded investigators for the myeloid to erythroid ratio (M:E).
Statistics
Data are mean ± SEM. Groups were analyzed by 1-way ANOVA followed by Student-Newman-Keuls test. A P value < .05 was considered significant.
Results and discussion
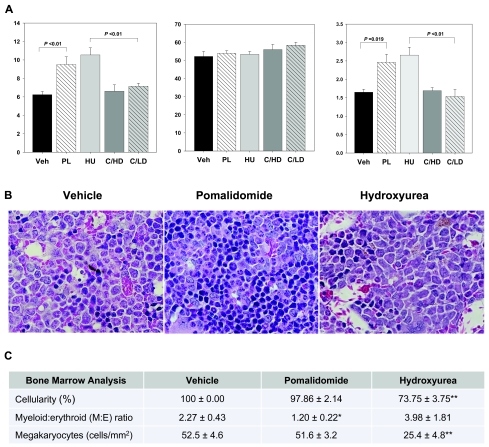
Pomalidomide was well tolerated by sickle cell mice without signs of toxicity and with similar absorption and elimination properties compared with BL/6 control mice (supplemental Figure 2). Pomalidomide increased the level of HbF expression from 6.24% at baseline to 9.51% after 8 weeks of treatment, which was comparable with hydroxyurea (Figure 1A) and similar to the magnitude of HbF induction observed in adult sickle cell patients treated with hydroxyurea in the Multicenter Study of Hydroxyurea trial.7,8 It is important to note that the human β-globin cluster transgene in our model only encodes for the Aγ-globin gene sequence. Considering that pomalidomide is known to transcriptionally activate both Aγ- and Gγ-globin genes, this transgene design may have resulted in underreporting of pomalidomide's in vivo HbF activity.16,17 The increase in HbF expression in the pomalidomide and hydroxyurea groups was accompanied by a higher HbF content per F-cell without parallel increases in the F-cell percentage (Figure 1A). This lack of an F-cell response is probably related to the high pretreatment F-cell values of ≥ 50%, which is in agreement with the observed inverse relationship between the F-cell response to hydroxyurea and pretreatment F-cell values in a clinical trial of young patients with SCD.18 Motivated by the synergistic HbF-inducing activity of pomalidomide and hydroxyurea in ex vivo CD34+ progenitor cells, we conducted combinatory treatments and surprisingly observed a virtual loss of HbF induction above control levels (Figure 1A). We tested a combination of pomalidomide with a lower dose of hydroxyurea (10 mg/kg) to rule out compound toxicity as the cause of HbF inhibition. Interestingly, this regimen recovered bone marrow function but continued to block HbF production. The reason for this loss of HbF activity in the combined treatment groups is unclear but could be related to the greater complexity of regulatory signals in the in vivo microenvironment or differences between the β-globin gene clusters in the two systems.
Figure 1.
Pomalidomide regulates HbF synthesis and erythropoiesis in transgenic sickle cell mice. Homozygous sickle cell mice were treated with vehicle, pomalidomide, or hydroxyurea for 8 weeks by intraperitoneal injection and killed for analysis. (A) HbF protein levels as a percentage of total hemoglobin were determined by high performance liquid chromatography at the end of the study to avoid artifactual increases of HbF from repeated blood draws. F cells were analyzed by flow cytometry after immunolabeling formalin-fixed RBCs with anti–human fluorescein isothiocyanate-conjugated HbF antibody. (B) Representative images depicting the M:E ratio in hematoxylin and eosin-stained bone marrow sections of the proximal femur. Erythroid cells are recognized by their round, dense, and deeply basophilic nuclei. All images were collected using a Zeiss Axioplan 2 microscope and a Plan-Apochromat 63×/1.4 oil objective. (C) Analysis of bone marrow cellularity, M:E ratio, and megakaryocyte counts. Cellularity in the active treatment groups was determined in reference to the 100% cellular marrow in vehicle-treated sickle cell mice. Megakaryocytes were counted in 5 low magnification optical fields per animal and converted to megakaryocytes/mm2. All sections were analyzed by 2 blinded investigators. Veh indicates vehicle; PL, pomalidomide; HU, hydroxyurea; C/HD, combination high-dose treatment (10 mg/kg pomalidomide, 100 mg/kg hydroxyurea); and C/LD, combination low-dose treatment (10 mg/kg pomalidomide, 10 mg/kg hydroxyurea). *Significantly different from Veh and HU (P < .05). **Significantly different from Veh and PL (P < .01).
Ineffective erythropoiesis is a contributory factor to anemia in SCD, albeit to a much lesser extent than in β-thalassemia syndromes. We found that pomalidomide, in addition to modulating HbF expression, expanded the erythron and improved the efficiency of erythropoiesis as evidenced by a trend toward higher reticulocyte counts (Figure 1B-C; Table 1). Because of the physical constraints of the mouse bone marrow compartment, the spleen in sickle mice functions as the major hematopoietic organ and becomes massively enlarged. Pomalidomide significantly raised the peripheral red blood cell (RBC) count, caused further increases in spleen weight, and decreased the M:E ratio in bone marrow and spleen. Plasma free hemoglobin levels in the pomalidomide group were not different from controls, indicating that gains in the peripheral RBC counts were not secondary to a protective effect of HbF production on F-cell survival. However, we noted that expansion of the erythroid lineage was associated with significantly reduced RBC mean corpuscular volumes and only small increases in total hemoglobin levels. These findings suggest residual defects in hemoglobin production possibly secondary to iron-restricted erythropoiesis or the mild β-thalassemic phenotype in this model. In contrast, hydroxyurea treatment was associated with sharply lower reticulocyte counts, a significant increase in the M:E ratio in both hematopoietic organs, and a reduction of spleen weights to less than one-half of control values. Bone marrow megakaryocyte counts appeared unaffected by pomalidomide but were significantly reduced by hydroxyurea. Compared with hydroxyurea, pomalidomide had no statistically significant effect on the total white blood cell count but caused a significant reduction in the monocyte fraction, which could have additional beneficial treatment effects because of the proinflammatory role of sickle monocytes in SCD.
Table 1.
Hematologic parameters after 8 weeks of treatment with pomalidomide and/or hydroxyurea in transgenic sickle cell mice
| Parameter | WT (C57BL/6) | Vehicle | Pomalidomide | Hydroxyurea HD | Pomalidomide + hydroxyurea (C/HD) | Pomalidomide + hydroxyurea (C/LD) |
|---|---|---|---|---|---|---|
| Peripheral blood | ||||||
| RBCs, × 106/μL | 8.9 ± 0.1*† | 4.8 ± 0.1 | 5.5 ± 0.2‡ | 4.5 ± 0.5 | 4.3 ± 0.4*‡ | 5.5 ± 0.2‡ |
| Total Hb, g/dL | 13.7 ± 0.2*† | 7.2 ± 0.4 | 7.7 ± 0.3 | 6.1 ± 0.5 | 5.7 ± 0.5*‡§ | 7.7 ± 0.3 |
| Hct, % | 46.6 ± 1.58*† | 28.2 ± 1.1 | 28.9 ± 1.2 | 23.6 ± 3.0 | 23.9 ± 2.0 | 31.7 ± 1.0 |
| Reticulocytes, % | 3.5 ± 0.2*† | 36.8 ± 5.8 | 40.5 ± 4.7 | 16.4 ± 3.9*†§ | 14.7 ± 2.4*†§ | 38.5 ± 2.3 |
| WBC, × 103/μL | 7.1 ± 0.2*‡ | 14.4 ± 2.7 | 14.2 ± 3.5 | 6.9 ± 0.8*‡ | 10.0 ± 1.9 | 19.1 ± 4.2 |
| Neutrophils, % WBCs | 15.0 ± 2.8 | 13.1 ± 2.6 | 19.0 ± 3.4 | 12.1 ± 1.6 | 11.0 ± 1.6 | 33.9 ± 4.4*‡ |
| Lymphocytes, % WBCs | 78.3 ± 3.7 | 80.1 ± 3.7 | 76.3 ± 3.7 | 84.3 ± 1.8 | 87.5 ± 1.5 | 60.7 ± 4.5*†‡§ |
| Monocytes, % WBCs | 5.0 ± 1.4 | 7.1 ± 1.0†§ | 4.1 ± 0.4*† | 3.0 ± 0.5*† | 1.7 ± 0.3*†§ | 3.7 ± 1.1*‡ |
| Platelets, × 103/μL | 1023 ± 126*† | 523.3 ± 83 | 652.6 ± 166 | 430.4 ± 73 | 384.3 ± 24 | 612.0 ± 99 |
| RBC indices | ||||||
| MCV, fL | 52.2 ± 1.0 | 58.1 ± 1.9‡§ | 52.8 ± 1.8*‡ | 52.3 ± 1.6*‡ | 55.9 ± 1.8 | 57.7 ± 2.1 |
| MCH, pg | 15.4 ± 0.1‡§ | 14.9 ± 1.0 | 14.0 ± 0.6 | 13.5 ± 0.8 | 13.5 ± 0.7 | 14.1 ± 0.7 |
| MCHC, g/dL | 29.6 ± 0.7 | 25.9 ± 2.2 | 27.0 ± 1.8 | 24.8 ± 0.8 | 24.0 ± 0.5 | 24.3 ± 0.5 |
| Plasma | ||||||
| Free Hb, mg/dL | 26.6 ± 0.2*† | 82.4 ± 11.4 | 81.0 ± 11.3 | NT | NT | NT |
| Extramedullary organs | ||||||
| Spleen weight, % body weight | 0.34 ± 0.04*† | 4.1 ± 0.3*‡ | 4.9 ± 0.2*† | 2.8 ± 0.3*† | 2.0 ± 0.1*† | 4.1 ± 0.2‡§ |
Data are mean ± SEM. Complete blood counts were measured from whole blood of 14-week-old mice after treatment with the indicated agent(s).
Hb indicates hemoglobin; Hct, hematocrit; WBC, white blood cell count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; C/HD, combination high-dose treatment (10 mg/kg pomalidomide, 100 mg/kg hydroxyurea); C/LD, combination low-dose treatment (10 mg/kg pomalidomide, 10 mg/kg hydroxyurea); and NT, not tested.
Versus vehicle.
P < .01.
P < .05.
Versus pomalidomide.
A potential limitation of this study in mice is the difficulty of extrapolating an equivalent HbF-inducing dose of pomalidomide in humans because of the large interspecies differences in drug metabolism. Studies in rats with [14C]-pomalidomide demonstrated that metabolism makes only a minor contribution to drug clearance, whereas monkeys and humans metabolized the compound extensively. Although further experiments are required to identify pomalidomide's mechanism of HbF induction, our study revealed that pomalidomide is a safe and effective HbF-inducing agent unaccompanied by the cytotoxic effects of hydroxyurea in mice with SCD. These results and pomalidomide's immunomodulatory properties, which are the subject of ongoing research in our laboratory, warrant further exploration of this compound as a novel therapy for patients with SCD and other β-hemoglobinopathies.
Supplementary Material
Acknowledgments
The authors thank Drs Tim Townes and Tom Ryan for the gift of knockout-transgenic sickle cell mice and Kimberly Smith and Doris Cawley for technical assistance.
S.E.M. was supported by the Celgene Corporation and National Institutes of Health (Roadmap Initiative in Nanomedicine, Nanomedicine Development Center award 1PN2EY018244).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.E.M. was responsible for the overall study, designed the research, analyzed the data, and wrote the manuscript; M.W. performed the research, analyzed the data, and assembled the figures; F.K. performed the HbF analysis; S.D.Y. analyzed the data; Y.X. conducted the pharmacokinetic analysis; L.A.M. and L.G.C. discussed the study and provided vital reagents; P.S.S. contributed to the writing of the manuscript; and A.K. conceived the idea and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: S.E.M., M.W., S.D.Y., P.S.S., and A.K. received funding from Celgene. Y.X. and L.G.C. are employees of Celgene, which has a financial interest in pomalidomide. L.A.M. was an employee of Celgene at the time of this study. F.K. declares no competing financial interests.
Correspondence: Steffen E. Meiler, Department of Anesthesiology and Perioperative Medicine, Georgia Health Sciences University, 1120 15th St., BIW 2144, Augusta, GA 30912-2700; e-mail: smeiler@georgiahealth.edu.
References
- 1.Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A. 1979;76(2):670–672. doi: 10.1073/pnas.76.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel RL, Erlingsson S, Fabry ME, et al. The Senegal DNA haplotype is associated with the amelioration of anemia in African-American sickle cell anemia patients. Blood. 1991;77(6):1371–1375. [PubMed] [Google Scholar]
- 3.Pace BS, Zein S. Understanding mechanisms of gamma-globin gene regulation to develop strategies for pharmacological fetal hemoglobin induction. Dev Dyn. 2006;235(7):1727–1737. doi: 10.1002/dvdy.20802. [DOI] [PubMed] [Google Scholar]
- 4.Perrine SP. Fetal globin stimulant therapies in the beta-hemoglobinopathies: principles and current potential. Pediatr Ann. 2008;37(5):339–346. doi: 10.3928/00904481-20080501-10. [DOI] [PubMed] [Google Scholar]
- 5.Stamatoyannopoulos G, Wood WG, Papayannopoulou T, Nute PE. A new form of hereditary persistence of fetal hemoglobin in blacks and its association with sickle cell trait. Blood. 1975;46(5):683–692. [PubMed] [Google Scholar]
- 6.Steinberg MH, Rodgers GP. Pharmacologic modulation of fetal hemoglobin. Medicine (Baltimore) 2001;80(5):328–344. doi: 10.1097/00005792-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia: Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 10.Crane E, List A. Immunomodulatory drugs. Cancer Invest. 2005;23(7):625–634. doi: 10.1080/07357900500283101. [DOI] [PubMed] [Google Scholar]
- 11.Wiernik PH. Treatment of hematologic neoplasms with new immunomodulatory drugs (IMiDs). Curr Treat Options Oncol. 2009;10(1):1–15. doi: 10.1007/s11864-008-0077-x. [DOI] [PubMed] [Google Scholar]
- 12.Mesa RA, Pardanani AD, Hussein K, et al. Phase1/-2 study of pomalidomide in myelofibrosis. Am J Hematol. 85(2):129–130. doi: 10.1002/ajh.21598. [DOI] [PubMed] [Google Scholar]
- 13.Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 14.Tefferi A, Cortes J, Verstovsek S, et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood. 2006;108(4):1158–1164. doi: 10.1182/blood-2006-02-004572. [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A, Verstovsek S, Barosi G, et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009;27(27):4563–4569. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moutouh-de Parseval LA, Verhelle D, Glezer E, et al. Pomalidomide and lenalidomide regulate erythropoiesis and fetal hemoglobin production in human CD34+ cells. J Clin Invest. 2008;118(1):248–258. doi: 10.1172/JCI32322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278(5339):873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 18.Maier-Redelsperger M, de Montalembert M, Flahault A, et al. Fetal hemoglobin and F-cell responses to long-term hydroxyurea treatment in young sickle cell patients: the French Study Group on Sickle Cell Disease. Blood. 1998;91(12):4472–4479. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.