Abstract
Defining the T helper functions impaired by programmed death–1 (PD-1) is crucial for understanding its role in defective HIV control and determining the therapeutic potential of targeting this inhibitory pathway. We describe here the relationships among disease stage, levels of PD-1 expression, and reversibility of CD4 T-cell impairment. PD-L1 blockade in vitro enhanced HIV-specific production of Th0 (IL-2), Th1 (IFN-γ), Th2 (IL-13), and TFH (IL-21) cytokines by CD4 T cells. PD-L1 blockade caused an early increase in cytokine transcription and translation that preceded cell proliferation. Although the impact of PD-L1 blockade on cytokine expression and, to a lesser extent, cell proliferation was associated with markers of disease progression, restoration of cytokine secretion was also observed in most subjects with undetectable viremia. PD-L1 blockade restored cytokine secretion in both PD-1intermediate and PD-1high sorted CD4 T-cell subsets. Compared with PD-1high HIV-specific CD8 T cells, PD-1high HIV-specific CD4 T cells showed lower expression of the inhibitory molecules CD160 and 2B4, demonstrating marked differences in expression of inhibitory receptors between T-cell subsets. These data show that PD-1 impairs HIV-specific T helper responses both by limiting expansion of these cells and by inhibiting effector functions of multiple differentiated CD4 T-cell subsets.
Introduction
T-cell exhaustion, defined as the progressive loss of functions caused by ongoing antigen exposure, is a major factor leading to defective pathogen clearance in chronic viral infections.1,2 Studies in the murine lymphocytic horizomeningitis virus (LCMV) model identified programmed death-1 (PD-1) as a critical mediator of this immune impairment.3,4 Blockade of the PD-1 pathway is considered a promising approach in both infectious diseases and cancer,5,6 as illustrated by studies in SIV-infected macaques.7,8 PD-1 is a member of the B7:CD28 family that has 2 ligands: PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273). PD-1 inhibits T-cell activation by interfering with T-cell receptor signaling9–11 and by up-regulating the transcription factor BATF.12
Several studies have shown that PD-1 inhibits HIV-specific T cells in humans. The majority of these reports focused on cytotoxic T lymphocyte (CTL) responses,13–16 and less is known on the role of PD-1 in HIV-specific T helper impairment.14,17,18 Studies in animal models and humans suggest that CD4 T-cell help is important for immune control of HIV replication.19–22 PD-1 is up-regulated on HIV-specific CD4 T cells,17,18 and its expression correlates with viremia.17 Blockade of the PD-1 pathway with a PD-L1–blocking antibody increased HIV-specific CD4 T-cell proliferation, with significant variability among the small cohorts of subjects investigated.14,17,18 An important unresolved issue is whether the effect of PD-L1 blockade is limited to increased expansion of virus-specific CD4 T cells or also leads to qualitative changes in CD4 T-cell function independent of cell proliferation. In the perspective of potential therapeutic interventions targeting the PD-1 pathway, the categories of subjects probable to respond to PD-L1 blockade by improved HIV-specific CD4 T-cell function need to be defined. It is crucial to determine the impact of blockade of the PD-1 pathway in persons with suppressed viral load (VL) on antiretroviral therapy (ART), which corresponds to the aim of current clinical care.
To define the role of the PD-1 pathway in HIV-specific CD4 T-cell impairment, we examined the impact of PD-L1 blockade on several T helper functions in different cohorts of HIV-infected subjects. Our results show that anti–PD-L1 not only improves CD4 T-cell proliferation, but also enhances effector CD4 T-cell responses by increasing secretion of cytokines produced by distinct T helper subsets. Although the impact of PD-L1 blockade in vitro correlates with VL in vivo, inhibition of the PD-1 pathway still significantly enhances cytokine secretion, but not proliferation, in most persons with controlled viremia. Within the same subjects, abrogation of the PD-1 signal increases cytokine secretion by CD4 T cells presenting a wide range of PD-1 levels. HIV-specific CD4 T cells show higher PD-1 expression than HIV-specific CTLs in the same persons but strikingly lower levels of the coinhibitory molecules 2B4 (CD244) and CD160. These findings illustrate differences in the coregulation of molecules associated with exhaustion between 2 arms of the adaptive cellular immune response. Our results suggest that PD-1 blockade with or without vaccine administration may have a role in HIV infection, even if viral replication is optimally controlled by ART.
Methods
Human subjects
Peripheral blood was obtained from HIV-infected persons at the Massachusetts General Hospital, Boston. Untreated chronic progressors (CPs) were defined as persons with VL between 2000 and 150 000 RNA copies/mL. Treated persons were patients on ART with VL < 50 RNA copies/mL (ART-controlled [ARTC]). Elite controllers (ECs) were defined as persons with VL < 50 copies/mL in the absence of ART. Viremic controllers (VCs) were defined as untreated subjects with a VL > 50 and < 2000 copies/mL. The MGH Institutional Review Boards approved these studies, and blood was collected from enrolled subjects after written informed consent was obtained in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density centrifugation.
Quantitation of cytokine production by quantitative RT-PCR and Luminex arrays
To assess cytokine production by HIV-specific CD4 T cells, 106 CD8 T cell-depleted PBMCs (RosetteSep CD8 depletion reagents, Stem Cell Technologies) were incubated with an HIV Gag peptide pool (1 μg/mL/peptide), or left unstimulated in the presence of PD-L1 blocking antibody (clone 29E.2A3, 10 μg/mL23) or IgG2b isotype control. For kinetic analysis of cytokine mRNA production and protein secretion, cell pellets and supernatants were collected at specified time points.
For assessment of the transcriptional regulation of IL-2, IL-13, IL-21, and IFN-γ in response to PD-L1 blockade, cDNA was synthesized (Promega) after RNA extraction (RNeasy Mini kit, QIAGEN) according to the manufacturer's instructions. Quantitative PCR (Stratagene MX3005P, Agilent Technologies) was performed as described24 (supplemental Table 1, primer sequences; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cytokine secretion was measured in supernatants using the Milliplex High sensitivity Kit (Millipore) on a Bio-plex 200 (Bio-Rad).
Proliferation assays
Proliferation of CD4 T cells was assessed by CFSE assay on CD8-depleted PBMCs.18 PBMCs were labeled with 1.25μM CFSE dye (Invitrogen) and incubated with either 10 μg/mL PD-L1 blocking antibody or isotype control. Cells were either left unstimulated or incubated with recombinant HIV p24 protein (5 μg/mL; Protein Sciences). After 7 days of incubation, cells were stained with antibodies against CD3, CD4, and CD8 (BD Biosciences PharMingen) and analyzed by flow cytometry (LSRII; BD Biosciences).
Intracellular cytokine staining and phenotyping
Two panels of antibodies were used to phenotype HIV-specific T cells identified by intracellular cytokine staining (ICS).18 The first panel was used to assess PD-1 expression among different cohorts of HIV-infected persons, and the second panel was used to determine coexpression of inhibitory receptors. PBMCs were incubated for 6 hours (first panel) or overnight (second panel) with an HIV Gag peptide pool, or a cytomegalovirus (CMV) lysate and CMV pp65 peptide pool in the presence of 5 μg/mL brefeldin A. Unstimulated cells were used as a control. Cells were then stained with blue viability dye (Invitrogen) and fluorescent antibodies against CD14 and CD19 (excluded populations), CD3, CD4, CD8, PD-1 (clone EH12.2H7; BioLegend), and, for the second panel only, antibodies against 2B4 (clone C1.7; BioLegend), CD160 (clone BY55; BioLegend), and LAG-3 (polyclonal antibody; R&D Systems). After fixation and permeabilization (Fix and Perm kit; Invitrogen), cells were stained with anti–IL-2 and anti–IFN-γ. All antibodies were from BD Biosciences unless otherwise stated. Cells were acquired on an LSR II (BD Biosciences) for the first panel and an LSR Fortessa cytometer for the second panel. Fluorescence minus one staining was used to define the cut-off for positivity.
Combined CFSE and ICS assays with PD-L1 blockade
A total of 2 million CD8-depleted PBMCs were stained with CFSE and stimulated with HIV Gag peptide pool in the presence of blocking anti–PD-L1 antibody (10 μg/mL) or isotypic control. Cells were collected at 48, 72, and 96 hours after stimulation, stained with a dead cell dye and antibodies against CD14, CD19, CD3, CD4, CD8, IFN-γ, and IL-2 following the ICS procedure described in “Intracellular cytokine staining and phenotyping” and acquired on an LSR Fortessa.
PD-L1 blockade on sorted CD4 T-cell subsets expressing different PD-1 levels
CD8-depleted PBMCs were treated with anti–PD-L1 antibody (10 μg/mL) or isotypic control and stimulated with HIV Gag peptide pool (1 μg/mL/peptide). After a 16-hour incubation, cells were stained with anti–PD-1 (BioLegend) and a lineage exclusion channel (CD8, CD14, CD19, and CD56). CD4 T cells were defined as lineage-negative cells in the lymphocyte gate. Subsets of CD4 T cells identified by PD-1 level as PD-1low, PD-1intermediate, and PD-1high were live-sorted (FACSAria; BD Biosciences). The same numbers of cells were incubated at an equal concentration for 48 hours before measurement of cytokines in supernatants by Luminex assays.
Statistical analysis
Flow cytometric data were analyzed with FlowJo Version 7.5.5 (TreeStar). Statistical analyses were performed using Prism Version 4.0 (GraphPad). Pair-wise comparisons for cytokine secretion were verified using the Wilcoxon matched-pairs test. Comparisons of cytokine secretion or PD-1 expression among cohorts were made using the Kruskal-Wallis and Dunn post-test. Distribution of PD-1 expression (mean fluorescence intensity) among groups was analyzed with analysis of variance and Tukey post-test. Correlation coefficients were calculated using the Spearman rank-sum test. We used repeated-measures analysis of variance and Tukey posttest to compare expression of inhibitory receptors among either the CD4 or the CD8 T-cell populations. Comparison of inhibitory receptor expression between the CD4 and CD8 T cells as well as between the HIV- and CMV-specific cells in the same persons was performed using Wilcoxon matched-pairs test. All tests were 2-tailed, and P values < .05 were considered significant.
Results
PD-1 blockade restores multiple HIV-specific CD4 T cell effector functions
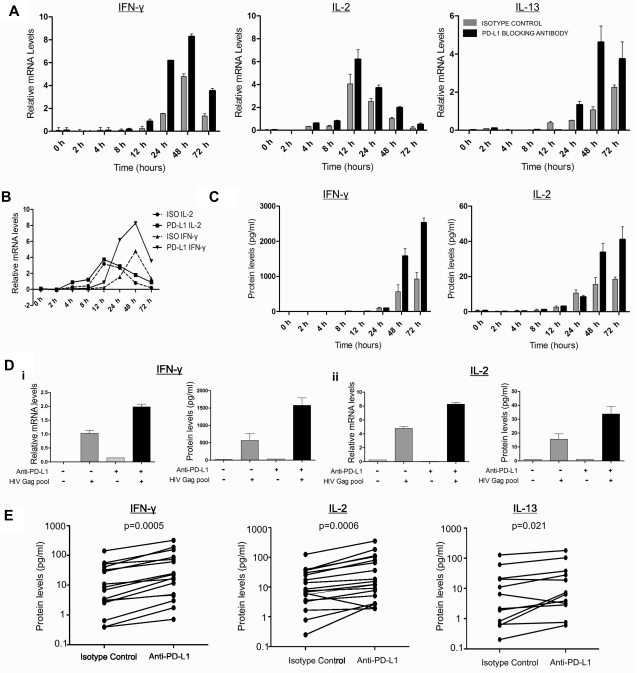
To define the impact of PD-1 on effector functions mediated by HIV-specific CD4 T cells, we first examined whether PD-L1 blockade regulated the transcription and release of Th0-, Th1-, and Th2- types cytokines in vitro. We selected IL-2 as a Th0 cytokine, IFN-γ as a Th1 cytokine, and IL-13 as a representative Th2 cytokine. IL-13 has been associated with slower disease progression25 and shown to improve HIV-specific T-cell responses in vitro by enhancing antigen-presenting cell function of monocytes.26 We incubated freshly isolated, CD8-depleted PBMCs from HIV-infected subjects with anti–PD-L1 or isotype control antibody in the presence of an HIV-Gag peptide pool. Kinetic analysis performed on 3 CP persons showed increased IL-2 mRNA levels as early as 4 hours after stimulation (Figure 1A). Whereas IL-2 mRNA levels peaked 12 hours after stimulation, a slower increase was observed for IFN-γ and IL-13 mRNA levels. Compared with isotype control, PD-L1 blockade resulted in greater production of IL-2, IL-13, and IFN-γ mRNA at all time points tested (Figure 1A). The early effect of PD-L1 blockade on cytokine transcription shows that this initial increase is not the result of proliferation (Figure 1B) and that anti–PD-L1 enhances effector functions of already differentiated T helper cells. This may have important implications in vivo in peripheral tissues, as PD-1 blockade could restore functions of differentiated T cells that have lost their proliferative capacity.
Figure 1.
PD-1 blockade restores HIV-specific CD4 T-cell effector functions. (A-C) Kinetic analysis of cytokine production by CD8-depleted PBMCs stimulated with HIV Gag peptide pool in the presence of PD-L1 blocking antibody or isotype control antibody. (A) mRNA levels of IL-2, IFN-γ, and IL-13 were measured by quantitative RT-PCR and normalized to the housekeeping gene GAPDH. (B) Representation of the transcription kinetics of IL-2 and IFN-γ mRNA. (C) Cytokine secretion as measured in the supernatants by Luminex bead arrays. (D) Representative example of the mRNA and the secreted levels of IL-2 and IFN-γ at 48 hours after stimulation. (E) Statistical analysis of data on a cohort of 16 untreated subjects indicated a significant increase in IL-2 (median fold increase = 2.26, P = .0006) and IFN-γ (median fold increase = 2.83, P = .0005) in the presence of PD-L1 blocking antibody. For IL-13 analysis, we used 12 untreated subjects (median fold increase = 1.74, P = .021; Wilcoxon matched-pairs test).
With a delay of a few hours relative to mRNA levels, PD-L1 blockade also enhanced IL-2 and IFN-γ protein secretion in supernatants (Figure 1C). Based on kinetics of protein expression, we then used the 48-hour time point to observe the effect of blockade in 16 CP subjects. PD-L1 blockade significantly increased secretion of IL-2, IFN-γ, and IL-13 (Figure 1E). Consistent with data obtained by flow cytometry,27 we did not identify HIV-specific Th17 CD4 T-cell responses in the presence of isotype control antibody, and such responses did not appear on PD-L1 blockade. Finally, transcriptional analysis showed that PD-L1 blockade increased IL-21 mRNA levels in 7 of 10 viremic subjects (supplemental Figure 1). Thus, PD-1 blockade enhances effector functions of various CD4 T-cell subsets that are considered important components of T-cell help.
Enhancement of HIV-specific CD4 T-cell function by PD-L1 blockade correlates with disease status but is also observed in most aviremic persons
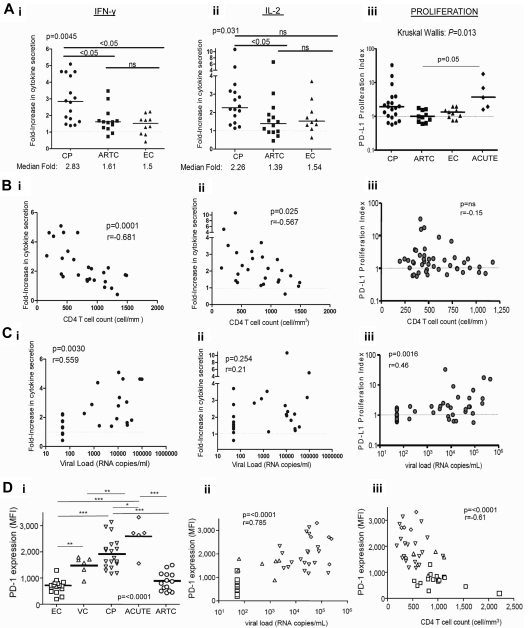
We next investigated the relationship between the effect of anti–PD-L1 on cytokine secretion by HIV-specific CD4 T cells and disease stage. We performed experiments on PBMCs from subjects belonging to 3 different cohorts (CP with uncontrolled viremia, ARTC with undetectable VL on ART, and EC with spontaneous viral control). The effect of PD-L1 blockade on IL-2 and IFN-γ secretion by HIV Gag-specific CD4 T cells was greater in CP than in ARTC and EC (Figure 2Ai-ii). In contrast, no significant difference was observed between ARTC and EC subjects.
Figure 2.
Capacity of PD-L1 blockade to restore HIV-specific CD4 T-cell functions correlates with HIV disease stage. (Ai-iii) Statistical comparison of impact of PD-L1 blockade on IFN-γ (i) and IL-2 (ii) secretion and HIV-specific CD4 T-cell proliferation (iii) in subjects with different disease status; horizontal bars represent the median fold increase; statistical analysis was performed with the Kruskall Wallis test, followed by Dunn post-test for paired comparisons. (B-C) Correlation of the effect of PD-L1 blockade on IFN-γ (i), IL-2 (ii), and proliferation (iii), from untreated subjects (CP and EC), with the VL and CD4 count, respectively. Statistical analysis used the Spearman rank-sum test. (Di) PD-1 expression on HIV-specific CD4 T cells from CP (n = 18), ARTC (n = 14), VC (n = 6), EC (n = 15), and acutely infected subjects (ACUTE; n = 5; Kruskall Wallis test, followed by Dunn post-test for paired comparisons). (Dii-iii) Statistical analysis of the correlation of the PD-1 mean fluorescence intensity on the HIV-specific CD4 T cells with the VL and CD4 count. Statistical analysis used the Spearman rank sum test. Symbols for values: *P < .0; **P < .01; ***P < .001.
Our previous studies and results from others14,17,18,28 have shown that PD-L1 blockade can enhance HIV-specific CD4 T-cell proliferation. However, the categories of subjects responsive to PD-L1 blockade have not been defined. We thus performed a cross-sectional study of 45 HIV-infected persons (CP, ARTC, EC, and VC and persons with acute/early infection). Consistent with previous data,14,17,18 a wide distribution in the responsiveness to PD-L1 blockade was observed (Figure 2Aiii). In the presence of control isotype antibody and as previously reported,29 HIV-specific CD4 T-cell proliferative responses were weaker in CP than in subjects with suppressed viral replication (data not shown), but viremic subjects also showed better response to PD-L1 blockade, consistent with the results we observed for cytokine secretion by HIV-specific CD4 T cells. However, in contrast to the reliable effect of PD-L1 observed on IFN-γ secretion, some CP subjects did not respond to PD-L1 blockade in the proliferation assay, and the differences among chronically infected groups did not reach statistical significance. Persons with acute/early HIV infection presented robust enhancement of HIV-specific CD4 T-cell responses on PD-L1 blockade. Again in contrast with the cytokine data, none of the subjects with undetectable viremia (ARTC and EC) showed a robust improvement in HIV-specific CD4 T-cell proliferative capacity on anti–PD-L1 blockade.
The effect of PD-L1 blockade on IFN-γ secretion by HIV-specific CD4 T cells correlated positively with VL (Figure 2Ci) and negatively with CD4 count (Figure 2Bi). Similarly, the effect of PD-L1 blockade on IL-2 secretion showed a negative correlation to CD4 counts (Figure 2Bii). However, we did not observe a correlation between the effect of PD-L1 on IL-2 secretion and viremia (Figure 2Cii). We also found a positive correlation between VL and enhanced HIV-specific CD4 T-cell proliferation after PD-L1 blockade (Figure 2Ciii), but no correlation was found with CD4 count (Figure 2Biii). Therefore, data on CD4 T-cell proliferation show both similarities and differences with cytokine secretion, which suggests that restoration of cytokine and T-cell proliferation may affect different CD4 T-cell subpopulations.
PD-1 expression correlates with markers of disease progression but does not differ between spontaneous and ART-induced control of viremia
Because PD-L1 blockade experiments had the strongest impact in viremic subjects, we determined PD-1 expression on HIV-specific CD4 T cells in 60 HIV-infected persons belonging to 5 different cohorts (Figure 2Di). Using HIV class II tetramers, we first demonstrated that PD-1 was expressed on HIV-specific CD4 T cells and did not change during the first 6 hours after stimulation (supplemental Figure 2). In addition, there was no significant difference between PD-1 levels on cells examined by ICS after 6 hours compared with overnight incubation (data not shown). Thus, measurement of PD-1 by ICS is representative of PD-1 expression before encounter with the cognate antigen. Levels of PD-1 expression on IFN-γ–producing HIV-specific CD4 T cells significantly differed among groups (Figure 2Di). HIV-specific IFN-γ+ CD4 T cells from EC and ARTC subjects expressed similar levels of PD-1 that were lower than those observed in VC and CP. The expression of PD-1 on HIV-specific CD4 T cells was found to be highest in acutely infected subjects. Similar patterns of PD-1 were observed on IL-2+ Gag-specific CD4 T cells, although virus-specific CD4 T cells producing only IL-2 expressed less PD-1 than cells producing both IL-2 and IFN-γ or IFN-γ alone (supplemental Figure 3). We next assessed the relationship between PD-1 levels and differentiation stage (supplemental Figure 4). We observed that within both the central memory (CM, CD27+ CD45RA−) and effector memory (EM, CD27−CD45RA−) HIV-specific IFN-γ+ CD4 T-cell subsets, CP expressed higher levels of PD-1 than ARTC and EC subjects. In each cohort, EM cells expressed more PD-1 than CM cells. These data are consistent with the fact that PD-1 expression is also related to memory maturation of T cells. In contrast, there was no significant difference among the 5 groups for CMV-specific CD4 T-cell responses, apart from a small difference between CP and ARTC persons (data not shown), showing that these differences are HIV-specific. PD-1 expression on HIV-specific CD4 T cells showed a strong positive correlation with VL and a negative correlation with CD4 counts (Figure 2Dii-iii). As expression of PD ligands will probably also affect responsiveness to PD-1 blockade, we determined expression of PD-L1 on various PBMC subsets (supplemental Figure 5). Whereas monocytes and B cells expressed far more PD-L1 than the other subsets investigated, we did not find statistically significant differences among the cohorts investigated.
Blockade of the PD-1 pathway enhances cytokine secretion by both proliferating and nonproliferating HIV-specific CD4 T cells
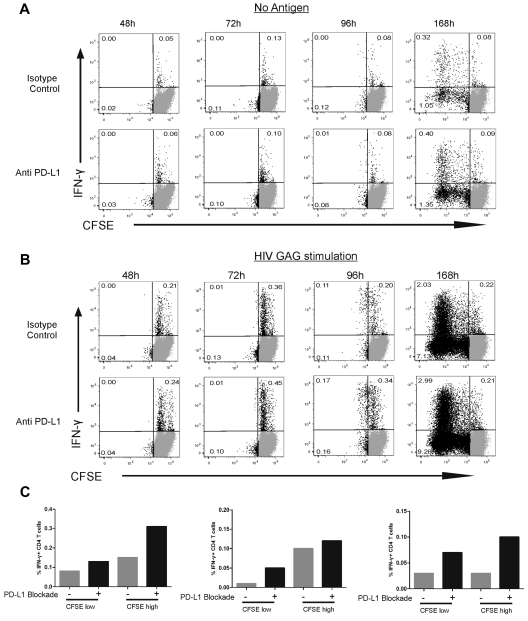
Two important issues raised by these results are whether (1) early proliferation contributes to the enhanced cytokine secretion we observed on PD-L1 blockade and (2) CD4 T-cell subpopulations responding to PD-L1 blockade by increased cytokine secretion are the same or distinct from the ones undergoing enhanced expansion. We therefore optimized an assay combining CFSE and ICS techniques and used it to determine the kinetics of HIV-specific CD4 T-cell responses (Figure 3A-B). A first cycle of cell division was visible at 96 hours after Gag stimulation (Figure 3B) but was not yet detectable at the 48- and 72-hour time points. These data were confirmed in a group of 5 persons (Figure 3B). Further expansion of HIV-specific CD4 T cells occurred beyond 96 hours, as shown by the 168-hour time point in a representative person (Figure 3B). To rule out that the CFSE assays missed the first cycle of proliferation, we confirmed these data with an Edu (5-ethynyl-2′-deoxyuridine) incorporation assay (supplemental Figure 6): increased DNA synthesis in dividing HIV-specific CD4 T cells was present 72 hours after stimulation, one day before detection of cell division by CFSE assays. These data demonstrate that no significant proliferation of HIV-specific CD4 T cells has yet occurred at the 48-hour time point used to determine the impact of PD-L1 blockade on cytokine secretion. We then used the 96-hour time point to compare the impact of PD-L1 blockade on dividing and nondividing cells. In all 5 persons studied, PD-L1 blockade increased the frequency of IFN-γ–secreting HIV-specific CD4 T cells in both nondividing (CFSEhigh) and dividing (CFSElow) cells (Figure 3C). As PD-1 is known to decrease cell survival,30 we measured cell numbers, expression of active caspase 3, and fraction of dead cells as defined by staining with dead cell dye at different time points. These parameters were not significantly affected by PD-L1 blockade (supplemental Figure 7).
Figure 3.
Blockade of the PD-1 pathway augments the fraction of HIV-specific IFN-γ+ CD4 T cells in both proliferating and nonproliferating subsets. (A-B) CFSE-labeled, CD8-depleted PBMCs were incubated with no antigen (A) or HIV Gag peptide (B) in the presence of PD-L1 blocking antibody or isotype control antibody. Intracellular cytokine staining was detected at 48, 72, 96, and 168 hours after stimulation. (C) The effect of PD-L1 blockade on IFN-γ+ CD4 T cells at 96 hours after stimulation in 3 representative subjects.
PD-L1 blockade affects CD4 T-cell subsets expressing different levels of PD-1
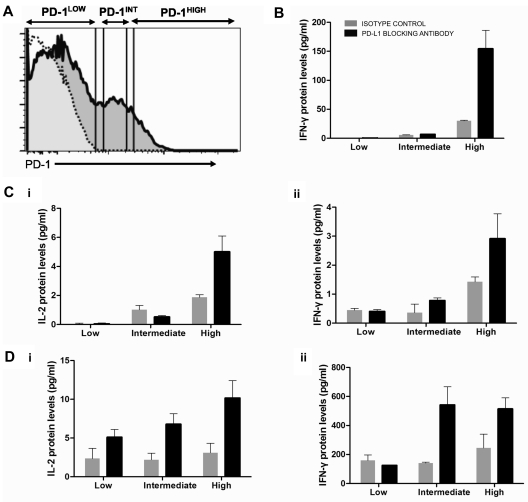
The tight correlation between PD-1 expression levels and viremia (Figure 2D) contrasted with the weaker correlation we observed between VL and responsiveness to PD-L1 blockade in functional assays. Adoptive transfer experiments in the LCMV model30 demonstrated that virus-specific CTL subsets expressing intermediate levels of PD-1 responded better to PD-L1 blockade than terminally differentiated, PD-1high CTLs. To determine whether, within the same person, HIV-specific CD4 T cells expressing different levels of PD-1 responded differentially to PD-L1 blockade, we stimulated PBMCs with HIV Gag in the presence or absence of PD-L1 blockade, sorted CD4 T cells into 3 subsets according to level of PD-1 expression, and measured cytokine secretion in supernatants (Figure 4A). In all 3 subjects investigated (Figure 4B-D), we observed that the main cytokine-secreting population was the PD-1high subset, consistent with the fact that this subset was enriched in HIV-specific CD4 T cells. The effect of PD-L1 blockade was not limited to the PD-1intermediate population but also seen in the PD-1high subset. These data suggest that, at least for some functions, the putatively more exhausted cells PD-1high cells can still be revived by inhibition of PD-1 signaling.
Figure 4.
PD-L1 blockade enhances cytokine secretion by PD-1high as well as PD-1intermediate HIV-specific CD4 T cells. CD8-depleted PBMCs from 3 chronically infected, untreated subjects were incubated with an HIV Gag peptide pool or left unstimulated for 16 hours in the presence of isotype control or PD-L1 blocking antibody. (A) CD4 T cells were then negatively selected by lineage exclusion on the lymphocyte gate and sorted according to their level of PD-1 expression into PD-1low, PD-1intermediate, and PD-1high subsets and isolated by live cell sorting. (B-D) IL-2 and IFN-γ secretion in the different cell subsets in the presence or absence of PD-L1 blockade. No significant IL-2 secretion was detected in the first subject.
HIV specific CD4 and CD8 T cells show different patterns of inhibitory coreceptor expression
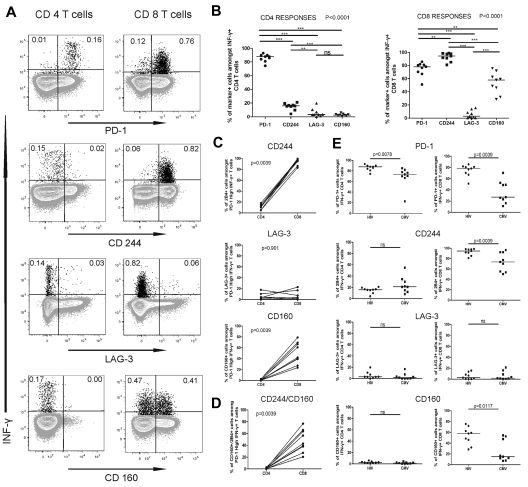
The robust restoration of functions of PD-1high HIV-specific CD4 T cell we observed differed from what was previously reported for PD-1high CTL in the LCMV model.30 Progressive accumulation of inhibitory molecules, such as 2B4, LAG-3, and CD160 on exhausted CTLs31 increases their dysfunction and probably contributes to the poor responsiveness of LCMV-specific PD-1high CTL to PD-L1 blockade. Thus, a question raised by our data is whether PD-1high HIV-specific CD4 and CD8 T cells differ in their pattern of inhibitory molecules. We used ICS to determine the expression of PD-1, 2B4, LAG-3, and CD160 on HIV-specific IFN-γ+ CD4 and CD8 T cells of 9 CP persons. The results indicated that HIV-specific CD4 T cells had a markedly different expression profile compared with HIV-specific CD8 T cells (Figure 5A-B). The majority of IFN-γ+ HIV-specific CD4 T cells expressed PD-1, but not 2B4, CD160, or LAG-3, whereas they expressed high levels of CTLA-4, consistent with our previous studies18 (supplemental Figure 8). Interestingly, 2B4 was detectable almost exclusively coexpressed with PD-1 and CTLA-4. In contrast, the majority of HIV-specific CTLs expressed 2B4 and PD-1, and a significant fraction also expressed CD160 (Figure 5B). Only a small percentage of HIV-specific T cells expressed LAG-3. When we focused the analysis on PD-1high HIV-specific T cells, we found again significant differences between the CD4 and CD8 T cells (Figure 5C). We did not find significant differences in the fractions of CD160+ and 2B4+ IFN-γ+ cells between the PD-1intermediate and PD-1high HIV-specific CD4 and CD8 T cells (supplemental Figure 9). The percentage of CD160+2B4+ IFN-γ+ CD8 T cells was greater in PD-1high HIV-specific CD8 T cells compared with HIV-specific CD4 T cells (Figure 5D).
Figure 5.
HIV-specific CD4 T cells express lower levels of the inhibitory molecules 2B4 and CD160 than HIV-specific CD8 T cells. (A) Expression of PD-1, 2B4, and CD160 on IFN-γ-producing HIV Gag-specific CD4 and CD8 T cells from an HIV viremic subject. (B) Statistical comparison of frequencies of HIV Gag-specific CD4 (i) and CD8 (ii) T cells that express the receptors described in panel A; analysis was performed using repeated-measures analysis of variance and Tukey post-test for pair-wise comparison. (C) Comparison of the percentage of cells that express 2B4 (i), LAG-3 (ii), and CD160 (iii) between the PD-1high CD4 and CD8 T cells. (D) Comparison of the frequency of cells expressing 2B4 and CD160 between the PD-1high CD4 and CD8 T cells. (E) Analysis of the expression of the inhibitory receptors on HIV- and CMV-specific CD4 and CD8 T cells; all statistics for the comparisons in panel E were performed using the Wilcoxon matched-pairs test.
To determine whether these patterns were virus-specific, we compared the phenotypes of HIV-specific and CMV-specific T cells in the same persons. HIV-specific and CMV-specific CD4 T-cell responses presented fairly similar patterns of coinhibitors dominated by PD-1 expression (Figure 5E), although the fraction of PD-1+ cells was lower on the CMV-specific than the HIV-specific CD4 T cells. In contrast, we observed striking differences for CTL responses, with lower percentages of PD-1+, 2B4+, and CD160+ CMV-specific CTLs compared with HIV-specific CTLs. Combinatorial analyses by boolean gating showed that, although 2B4 is usually coexpressed with PD-1 on HIV-specific CTLs, a large fraction of the CMV-specific CTLs express only 2B4 (supplemental Figure 10). These data indicate that CD4 and CD8 T cells specific for chronic viral infections are governed by complex patterns of inhibitory molecules that differ between these 2 arms of the immune response. Mechanisms of CD4 T-cell exhaustion cannot be simply inferred from those of CTL impairment and need to be investigated in separate studies.
Discussion
Our data demonstrate that multiple effector functions of HIV-specific CD4 T cells are inhibited by PD-1 and can be restored by PD-L1 blockade. Whereas previous studies had shown that proliferation of HIV-specific CD4 T cells could be enhanced by anti-PD-L1, our findings clarify the role of PD-1 in impaired cytokine secretion by exhausted T helper cell subsets, including Th0 (IL-2), Th1 (IFN-γ), Th2 (IL-13), and IL-21, a cytokine preferentially produced by follicular (TFH) helper cells that is crucial for CD4 help to CTL in the murine LCMV model32,33 and probably also plays an important role in HIV infection.34–36 Measurements of transcription by quantitative RT-PCR and protein secretion in culture supernatants by bead arrays proved to be sensitive and specific tools to assess the impact of PD-L1 blockade on production of these cytokines. This contrasts with the lack of significant effect observed by us and others with 6- or 12-hour ICS assays.14,15,17 The increased cytokine secretion previously detected on PD-L1 blockade after a 6-day incubation13,14 did not establish whether this effect was only the result of cell proliferation and the subsequent increased number of specific T cells. Our data provide evidence that PD-L1 blockade enhances cytokine secretion before T cells divide. First, kinetic analysis of IFNγ, IL-2, and IL-13 levels showed that anti-PD-L1 increased cytokine transcription < 12 hours and protein secretion < 24 hours after stimulation of HIV-specific CD4 T cells. Second, combined CFSE and ICS assays showed that IFN-γ secretion by HIV-specific CD4 T was enhanced on PD-L1 blockade in both the nondividing and dividing populations. Third, Edu incorporation assays confirmed that no quantitatively significant proliferation occurred during the first 2 days after stimulation, whereas our cytokine measurements were performed at 48 hours. These results demonstrate that anti–PD-L1 can restore secretion of an array of cytokines by differentiated HIV-specific T helper cells independently of cell proliferation.
Restoration of cytokine secretion by HIV-specific CD4 T cells correlated with viremia but was also observed in most HIV-infected persons with undetectable VL (ARTC and EC). As levels of PD-1 and its ligands are higher in lymphoid tissues than in peripheral blood,17,37 the activity of the PD-1 pathway in other compartments may be stronger than shown by our experiments on PBMCs and will need to be determined in further studies. Whereas the impact of PD-L1 blockade on IL-2 is well documented,38 the observed effect of PD-L1 blockade on other cytokines is more intriguing. To our knowledge, there is no evidence of a direct modulation of IFN-γ, IL-13, or IL-21 transcription, translation, or release by PD-1. Although limited experiments did not show evidence of reduced apoptosis on PD-L1 blockade, further studies are needed to define the potential role of enhanced cell survival. Whereas several studies have assessed IFN-γ and IL-2 secretion by HIV-specific CD4 T cells, less is known on the impact of IL-13 and IL-21 in HIV pathogenesis. IL-13 promotes humoral responses and augments expression of integrins on monocytes/macrophages under physiologic conditions.39 In HIV infection, higher IL-13 secretion was associated with lower viremia.25 IL-13 improved antigen presentation in vitro40 and enhanced proliferative T-cell responses against HIV p24.26 Of particular interest is the increased production of IL-21 on PD-L1 blockade. IL-21 plays a crucial role in maintaining CD8 T-cell function41 and regulating B-cell differentiation.42,43 Recent studies found that higher serum IL-21 levels34,44 and IL-21 production by HIV-specific CD4 T cells35,36 were associated with better viral control. Addition of IL-21 to PBMCs restored suppression of viral replication by HIV-specific CTLs.35 Thus, blockade of the PD-1 pathway enhances the secretion of cytokines that may be important for viral control.
Although previous studies by our group and others have demonstrated that PD-L1 blockade can restore HIV-specific CD4 T-cell proliferation, the heterogeneity of the responses and the limited size of the cohorts precluded the identification of patterns among different disease stages. We show here, that although PD-L1 blockade enhances cytokine secretion in the large majority of viremic persons, a significant fraction of subjects with uncontrolled VL failed to respond to inhibition of the PD-1 signal by increased proliferation. This discrepancy is at least in part explained by our finding that these functions are mediated by partially different CD4 T-cell subsets.
In line with previous studies,17 we found that HIV-specific CD4 T cells from CP expressed more PD-1 than those of ARTC subjects, which is consistent with a higher activity of the PD-1 pathway in subjects with high antigen load. PD-1 levels on HIV-specific CD4 T cells correlate with viremia and do not discriminate between spontaneous and ART-induced viral control, in contrast to what we previously showed for CTLA-4.18 The majority of the HIV-specific CD4 T cells in all cohorts express PD-1. Thus PD-1 expression is not restricted to exhausted T cells in humans.45,46 Additional studies will be necessary to determine whether the PD-1 has significant activity on virus-specific T cells in vivo once viral replication is suppressed by therapy.
Our data show that higher viremia is associated with increased PD-1 expression on HIV-specific CD4 T cells, and greater impact of PD-L1 blockade. Whether this correlation is explained by the PD-1 levels on T cells or defined by other factors, including ligand availability on antigen-presenting cells, remains to be better defined. The levels of PD-L1 expression on B cells and monocytes were similar among the cohorts investigated. An additional factor that may contribute to the effects observed is the use of an anti–PD-L1 and not an anti–PD-1 blocking antibody, thus also abrogating the PD-L1–CD80 interaction.47 The cytokine secretion assays on CD4 T-cell subsets sorted according to PD-1 levels suggest that CD4 T cells with a wide range of PD-1 expression are responsive to PD-L1 blockade. Although these results seem to differ from data in the LCMV model showing a preferential rescue of PD-1intermediate virus-specific CTLs, but not of more exhausted, terminally differentiated PD-1high cells,30 several factors may explain this discrepancy: (1) the experimental models are different; (2) persistent viruses may lead to different patterns of T-cell exhaustion; (3) PD-L1 blockade could differentially affect cytokine secretion and other T-cell functions; and (4) CD4 and CD8 T cells may be governed by different mechanisms of T-cell exhaustion, which could in turn affect the responsiveness of these subsets to blockade of the PD-1 pathway alone.
To explore this last hypothesis, we phenotyped HIV-specific CD4 and CD8 T cells and compared expression of PD-1 and the coinhibitory molecules 2B4, CD160, and LAG-3, which have been shown to regulate CTL exhaustion in the LCMV model.31,48 We found that there was a good correlation between PD-1 expression on HIV-specific CD4 T cells and PD-1 expression on HIV-specific CTLs but that levels of PD-1 quantitated by mean fluorescence intensity was 2.5-fold higher on HIV-specific CD4 than CD8 T cells, indicating some differential regulation of PD-1 between the 2 subsets. In contrast to this modest difference in PD-1 expression, we observed strikingly lower expression of 2B4 and CD160 on HIV Gag-specific CD4 T cells than on HIV-Gag-specific CTLs within the same subjects. Our data are in agreement with recent studies showing that HIV- and HCV-specific CD8 T cells express mainly PD-1, 2B4, and CD160 but not the LAG-3 receptor.49,50 Consistent with our previous data,18 CTLA-4 was preferentially expressed by HIV-specific CD4 T cells, and the minority of HIV-specific CD4 T cells expressing 2B4 did so along with CTLA-4 and PD-1. Our results illustrate that, although HIV-specific CD4 and CD8 T cells share some mechanisms of exhaustion, they are also governed by different subsets of inhibitory receptors, and restoration of their respective functions may require subset-specific immune interventions.
In these studies, we have therefore defined in different categories of HIV-infected subjects the potential of PD-L1 blockade to restore functions of several HIV-specific T helper subsets, an arm of the immune system that is impaired early in the course of HIV infection and provides critical help to CTL and B cells. Although the magnitude of the effect seems relatively modest in the assays used (typically in the 2-fold range for a given function), this is comparable to results obtained in LCMV and SIV models in which PD-1 blockade in vivo had significant impact on immune responses and/or VLs.3,7 The impact on effector functions may be critical for the therapeutic potential of immune interventions blocking the PD-1 pathway, as they could enhance efficacy of tissue-infiltrating T cells lacking proliferative potential. Our results support the rationale for careful assessment of immunotherapeutic strategies targeting the PD-1 pathway in HIV infection. To mimic the situation of HIV-infected persons receiving optimal care, we think that a critical next step will be to determine in an animal model whether PD-1 blockade, possibly as a vaccine adjuvant,4 has a positive impact on immune responses when administered after control of viremia by antiviral therapy.
Supplementary Material
Acknowledgments
The authors thank Rafi Ahmed, Bruce Walker, and Sylvie Le Gall for their input on this manuscript; the clinical and laboratory staff at the Massachusetts General Hospital and that of the International HIV Controllers Study (www.hivcontrollers.org); and all study participants for their invaluable role in this project.
This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (RO1 HL-092565; D.E.K.), the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (P0 AI-080192, D.E.K., D.G.K., and G.J.F.; and P0 AI056299, G.J.F.), the Concerned Parents for AIDS Research Foundation (D.E.K.), and the Mark and Lisa Schwartz Foundation (gift).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.E.K. was responsible for the overall design and conduct and provided supervision; F. Porichis, D.S.K., M.A.B., M.R.-G., G.J.F., D.G.K., and D.E.K. provided intellectual input and contributed to the experimental design; F. Porichis, D.S.K., J.Z., D.P.T., A.M., D.F.P., and D.E.K. performed experiments; F. Pereyra provided clinical samples; G.J.F. provided novel reagents; and F. Porichis and D.E.K. wrote the paper.
Conflict-of-interest disclosure: G.J.F. has patents and receives patent royalties on the PD-1 pathway. The remaining authors declare no competing financial interests.
Correspondence: Daniel E. Kaufmann, Massachusetts General Hospital East, Ragon Institute of MGH, MIT and Harvard, Rm 5239, 149 13th St, Charlestown, MA 02129; e-mail: dkaufmann@partners.org.
References
- 1.Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol Rev. 2008;223:317–333. doi: 10.1111/j.1600-065X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182(10):5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 4.Ha SJ, Mueller SN, Wherry EJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205(3):543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 7.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finnefrock AC, Tang A, Li F, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182(2):980–987. doi: 10.4049/jimmunol.182.2.980. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 10.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T-cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quigley M, Pereyra F, Nilsson B, et al. Transcriptional analysis of HIV-specific CD8(+) T cells shows that PD-1 inhibits T-cell function by upregulating BATF. Nat Med. 2010;16(10):1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 14.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 15.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203(10):2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JY, Zhang Z, Wang X, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109(11):4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza M, Fontenot AP, Mack DG, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179(3):1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8(11):1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 19.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sette A, Moutaftsi M, Moyron-Quiroz J, et al. Selective CD4+ T-cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28(6):847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day CL, Walker BD. Progress in defining CD4 helper cell responses in chronic viral infections. J Exp Med. 2003;198(12):1773–1777. doi: 10.1084/jem.20031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munier ML, Kelleher AD. Acutely dysregulated, chronically disabled by the enemy within: T-cell responses to HIV-1 infection. Immunol Cell Biol. 2007;85(1):6–15. doi: 10.1038/sj.icb.7100015. [DOI] [PubMed] [Google Scholar]
- 23.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T-cell activation and cytokine production. J Immunol. 2003;170(3):1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 24.Porichis F, Morou A, Baritaki S, Spandidos DA, Krambovitis E. Activation-induced cell death signalling in CD4+ T cells by staphylococcal enterotoxin A. Toxicol Lett. 2008;176(1):77–84. doi: 10.1016/j.toxlet.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Bailer RT, Holloway A, Sun J, et al. IL-13 and IFN-gamma secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. J Immunol. 1999;162(12):7534–7542. [PubMed] [Google Scholar]
- 26.Papasavvas E, Sun J, Luo Q, et al. IL-13 acutely augments HIV-specific and recall responses from HIV-1-infected subjects in vitro by modulating monocytes. J Immunol. 2005;175(8):5532–5540. doi: 10.4049/jimmunol.175.8.5532. [DOI] [PubMed] [Google Scholar]
- 27.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassu A, Marcus RA, D'Souza MB, et al. Regulation of virus-specific CD4+ T-cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol. 2010;185(5):3007–3018. doi: 10.4049/jimmunol.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T-cell responses associated with control of viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 30.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105(39):15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T-cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iannello A, Boulassel MR, Samarani S, et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010;184(1):114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- 35.Chevalier MF, Julg B, Pyo A, et al. HIV-1-specific IL-21+ CD4+ T-cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T-cell function. J Virol. 2011;85(2):733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue FY, Lo C, Sakhdari A, et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol. 2010;185(1):498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- 37.Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J Virol. 2010;84(4):2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111(4):677–690. doi: 10.1067/mai.2003.1333. quiz 691. [DOI] [PubMed] [Google Scholar]
- 40.Montaner LJ, Doyle AG, Collin M, et al. Interleukin 13 inhibits human immunodeficiency virus type 1 production in primary blood-derived human macrophages in vitro. J Exp Med. 1993;178(2):743–747. doi: 10.1084/jem.178.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201(1):139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175(12):7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 43.Kuchen S, Robbins R, Sims GP, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179(9):5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 44.Iannello A, Tremblay C, Routy JP, Boulassel MR, Toma E, Ahmad A. Decreased levels of circulating IL-21 in HIV-infected AIDS patients: correlation with CD4+ T-cell counts. Viral Immunol. 2008;21(3):385–388. doi: 10.1089/vim.2008.0025. [DOI] [PubMed] [Google Scholar]
- 45.Duraiswamy J, Ibegbu CC, Masopust D, et al. Phenotype, function, and gene expression profiles of programmed death-1hi CD8 T cells in healthy human adults. J Immunol. 2011;186(7):4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauce D, Almeida JR, Larsen M, et al. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS. 2007;21(15):2005–2013. doi: 10.1097/QAD.0b013e3282eee548. [DOI] [PubMed] [Google Scholar]
- 47.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T-cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 49.Bengsch B, Seigel B, Ruhl M, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6(6):e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto T, Price DA, Casazza JP, et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood. 2011;117(18):4805–4815. doi: 10.1182/blood-2010-11-317297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.