Abstract
Objective
To describe the utility of twin studies for attention-deficit/hyperactivity disorder (ADHD) research and demonstrate their potential for the identification of alternative phenotypes suitable for genomewide association, developmental risk assessment, treatment response, and intervention targets.
Method
Brief descriptions of the classic twin study and genetic association study methods are provided, with illustrative findings from ADHD research. Biometrical genetics refers to the statistical modeling of data gathered from one or more group of known biological relation; it was apparently coined by Francis Galton in the 1860s and led to the “Biometrical School” at the University of London. Twin studies use genetic correlations between pairs of relatives, derived using this theoretical framework, to parse the individual differences in a trait into latent (unmeasured) genetic and environmental influences. This method enables the estimation of heritability, i.e., the percentage of variance due to genetic influences. It is usually implemented with a method called structural equation modeling, which is a statistical technique for fitting models to data, typically using maximum likelihood estimation. Genetic association studies aim to identify those genetic variants that account for the heritability estimated in twin studies. Measurements other than those used for the clinical diagnosis of the disorder are popular phenotype choices in current ADHD research. It is argued that twin studies have great potential to refine phenotypes relevant to ADHD.
Results
Prior studies have consistently found that the majority of the variance in ADHD symptoms is due to genetic factors. To date, genomewide association studies of ADHD have not identified replicable associations that account for the heritable variation. Possibly, the application of genomewide association studies to these alternative phenotypic measurements will assist in identifying the pathways from genetic variants to ADHD.
Conclusion
Power to detect associations should be improved by the study of highly heritable endophenotypes for ADHD and by reducing the number of phenotypes to be considered. Therefore, twin studies are an important research tool in the development of endophenotypes, defined as alternative, more highly heritable traits that act at earlier stages of the pathway from genes to behavior. Although genetic variation in liability to ADHD is likely polygenic, the proposed approach should help to identify improved alternative measurements for genetic association studies.
Keywords: twin studies, ADHD, genomewide association, endophenotype, translational research
Biometrical genetic studies of attention-deficit/hyperactivity disorder (ADHD) in twins consistently support the hypothesis that there is genetic variation in liability to this disorder.1–3 That the heritability of ADHD is so universally established and accepted might suggest that twin studies of ADHD have fulfilled their scientific utility and that research should focus on identifying the actual genetic variants underlying ADHD. However, after reviewing basic twin study methodology, we argue that the failure of molecular genetic research to live up to its initial promise requires additional twin studies aimed at developing and validating more promising measurements for molecular genetic research.
BACKGROUND TO THE CLASSIC TWIN STUDY
Biometric genetic studies of human populations typically analyze data on monozygotic (MZ) and dizygotic (DZ) twin pairs reared together to partition the variation of a trait within a sample of individuals into constituent variance components (VCs). These VCs typically consist of additive genetic (A), nonadditive genetic (D), or shared environmental (C) and child-specific environmental (E) influences. This last component also subsumes measurement error. Four key assumptions are involved in this approach: MZ twins are genetically identical, whereas DZ twins share on average 50% of their segregating alleles; MZ and DZ twin pairs share external environmental influences to the same extent; there is no correlation between members of twin pairs for E influences; and the total variance is the same in all individuals. Assuming a purely additive genetic model (D = 0), these assumptions predict the following statistics: phenotypic variance, A + C + E; MZ covariance, A + C; and DZ covariance, 0.5A + C. Estimates of these VCs may be obtained by using structural equation modeling (SEM) software, typically through maximum likelihood estimation.4,5 Differences in the relative impact of these VCs predict different patterns of the MZ and DZ correlations. Accordingly, examination of MZ and DZ twin correlations can be used to infer the relative magnitude of the VCs. Thus, MZ and DZ correlations of zero would imply no influence of A, C, or D. If the ratio of MZ to DZ twin correlations is between 1 and 2, A and C are implicated, and ratios greater than this suggest D. Variance due to sibling interaction, where the behavior of one twin affects the behavior or the rating of the other twin, is implicated if MZ and DZ variances for a trait differ.6,7
SUMMARY OF VC ESTIMATES ON ADHD SYMPTOMS MEASURED USING BEHAVIORAL RATINGS SCALE DATA
Meta-analyses of behavioral ratings of ADHD symptoms in the general population have concluded that variation is largely genetic (60% to 76%).1–3 Different methodologic approaches to meta-analysis may account for the differences across the three studies. In 2005 an influential review of behavioral ratings scale data concluded that 76% of the variance was due genetic influences, with the rest being attributable to E.2 However, this review used an average of “broad sense” heritability estimates across just 20 studies, unweighted for sample sizes. A more recent weighted meta-analysis using SEM methodology to combine the results of individual biometrical genetic studies drew a similar conclusion: 70% of the variance was due to A + D, and the rest to E. This result was somewhat surprising because all other behaviors associated with childhood psychopathology indicated a significant influence of C.1 It is, however, possible that this finding was affected by methodologic limitations of meta-analytic biometrical genetics, including a lack of power to detect sibling interaction, the confounding of C and D in genetic models using only MZ and DZ twins reared together, and the correction used for contrast effects (a form of rater bias, see below). Any one of these factors could lead to an overestimate of heritability. A more recent analysis, taking the unweighted average approach of Faraone et al.,2 which took further account of these limitations, concluded that 60% of the variance was due to genetic factors with the rest equally split between C and E.3
MOLECULAR GENETIC STUDIES ON BEHAVIORAL RATINGS SCALE DATA
Consistent with the dimensional approach to studying ADHD, the quantitative trait locus model typically used in gene mapping studies assumes that the genetic variance (captured by the heritability estimates from twin studies) in ADHD symptoms is likely to be accounted for by multiple genetic variants, each conferring a relatively small risk for disease (or trait) susceptibility.8–10 A recent meta-analysis concluded that significant associations could be identified only for the dopamine transporter gene (DAT1), D4 and D5 receptor genes, the serotonin transporter and receptor genes, and the synaptosomal-associated protein 25 gene.11 Although findings across genetic association studies of ADHD (as with all psychiatric disorders) remain inconsistent, one clear conclusion does emerge: the estimated effect sizes of individual genetic variants are small, with odds ratios in the range of 1.12 to 1.33.11 Very large samples within powerful designs will be needed to detect odds ratios of this magnitude, given the observed minor allele frequencies for current ADHD-risk alleles. Genome-wide association studies (GWAS), in which “a dense set of SNPs [single nucleotide polymorphisms] across the genome is genotyped to survey the most common genetic variation for a role in disease or to identify the heritable quantitative traits that are risk factors for disease,”12 offer promise due to their increased power to detect such small effect sizes. However, currently no genomewide significance levels have been reached for ADHD traits (see Franke et al.13 for a review). Thus, to date, molecular genetic studies have accounted for less than 5% of the estimated heritability in ADHD symptoms,14,15 and although this is common to many neuropsychiatric and other phenotypes, it has lead some researchers to conclude that there is a disparity between molecular and quantitative approaches to understanding the genetic etiology of ADHD.
PROBLEMS WITH PHENOTYPE DEFINITION IN ADHD
Although ADHD is a dichotomous diagnostic category in the Diagnostic and Statistical Manual of Mental Disorders (the disorder is considered present or absent), twin studies have focused on the heritability of continuously distributed traits that contribute to the diagnosis (see Wood et al.3 for a summary of measurements used). This dimensional model approach assumes that a diagnosis of ADHD is given when individuals pass a clinically relevant threshold on these behaviors. Under this model, twin studies on such behaviors as “inattentiveness” and “overactivity” in the general population can be generalized to the clinical diagnosis of the disorder. This approach is supported by research studies that have found similar heritability estimates for dichotomous (reflecting the presence or absence of the disorder) and dimensional measurements of ADHD. In addition, using a regression-based method (extreme group),16 it is possible to compare heritability estimates in different parts of the distribution of the trait. ADHD behaviors yield similar heritability estimates regardless of where the threshold is placed to define the two groups.17
This use of the dimensional model in the study of ADHD has facilitated the collection of data from larger samples, because face-to-face interviews are not required. However, without recourse to a clinical category, defining the ADHD phenotype for genetic studies remains a contemporary and controversial issue.18,19 Interrater correlations for ADHD behaviors between parents are teachers are typically around 0.3.18,20,21 This low correlation has been attributed to many sources, and problems arise for genetic studies when differences in these assessments reflect sources of variance other than the ADHD trait of interest. These alternative sources of variance may include conflation with other correlated behaviors (such as oppositional behaviors, known as halo effects).22–24 Also possible are “contrast effects,” where the behavior of one child affects the perception and rating of the other child within a twin pair.25–28 Contributions from these other sources of variance to ADHD behavioral assessments decrease the precision with which ADHD symptoms measure the underlying genetic liability for ADHD. In association and linkage studies, statistical power increases with the effect size of the quantitative trait locus.29,30 Thus a precise definition of ADHD, which measures as accurately as possible the trait of interest, remains a crucial issue in increasing the success of molecular genetic studies.31
ENDOPHENOTPYES IN ADHD RESEARCH
One proposed approach to addressing the phenotype definition issues is to employ alternative measurements of ADHD symptoms instead of traditional behavioral rating scale data. This approach has been subject of much recent research and debate. Endophenotypes have been defined as stable, heritable measurements that are more proximal to the biological etiology of a disorder than the clinical diagnosis itself32 and thus partly a better measurement of the underlying genetic liability for a disorder. In ADHD research, this approach is seen as especially valuable because it eliminates rater effects. Further, whereas ADHD is a complex phenotype encompassing many different behaviors, endophenotypes measure simpler traits that may be influenced by a smaller number of genetic loci. In consequence, each genetic variant may account for a larger proportion of the variance and may be detected with smaller samples. There is controversy over what constitutes a measurement that is biologically closer to the underlying susceptibility, and some researchers differentiate “intermediate” and “objective” phenotypes from endophenotypes. In this article we consider ADHD endophenotypes to be those measurements that are designed to assess a heritable, genetically simpler construct, which covaries with ADHD outcomes.
There is a lack of consensus regarding the necessary and sufficient steps for empirically validating a measurement as a suitable candidate for an ADHD endophenotype.32–37 However, there is almost unanimous agreement on the following four criteria. One, the measurement needs to have good metric properties; scores should be continuously distributed without obvious floor or ceiling effects, they should show high internal consistency (the components that make up the measurement should correlate highly with each other), and test-retest reliability should be high. Two, it must be correlated with the disorder or its behavioral symptoms. Three, the measurement must be heritable. Four, at least a portion of this heritable variance is shared with the genetic liability for ADHD. This fourth criterion is traditionally examined by “means comparisons analysis” on groups of ADHD probands, unaffected siblings of the probands, and controls. If familial factors contribute to the endophenotype, then the means should be ordered as follows: controls ← unaffected siblings ← probands. This approach has been successful in other fields of neuropsychiatric research suc as schizophrenia.38,39 However, initial failures to substantiate measurements of sustained attention, cognitive flexibility, encoding, impulsivity,40 memory,40,41 visual information processing,42 and executive functions41 as potential ADHD endophenotypes may have contributed to a relative lag in the study of this disorder.43 Fortunately, recent research is more promising, with stronger evidence from means comparisons analysis for specific cognitive measurements such as reaction time (RT) data, response inhibition (typically indexed by commission errors), and sustained attention (usually indexed by omission errors44–48), motor measurements,49 and activity level measurements.50 However, means comparison analyses have failed to consistently validate some measurements, such as delay aversion and motivation.51 Although several promising endophenotype candidates have not produced convincing results in molecular genetic studies, several significant associations have been reported: Actigraph data (a mechanical assessment of activity level) is associated with the 7-repeat allele of the DRD4 receptor gene52; measurements of response inhibition with the 10/10 repeat genotype on DAT153; RT data and the absence of the ADHD-risk allele on the DRD4 gene54–56; and homozygosity for the 10-repeat allele of DAT1 with left-sided inattention and enhanced methylphenidate response.57,58 A significant genomewide linkage signal was also reported for motor timing on 2q21.1 and digit span on 13q12.11.59 However, these studies need replication (e.g., Manor et al.55).
Despite these seemingly positive findings, some studies have found associations in the opposite direction to that predicted, i.e., the risk allele for ADHD seems to confer a protective effect on the endophenotype.55–57 These conflicting results and failures to replicate (see Kebir et al.60 for a comprehensive review and meta-analysis) have led to “a failure to establish a consistent pattern of findings on the modes of action of known risk genes [in] the current literature.”43 This situation strongly emphasizes the need to consider and refine the methodologic approach to selecting endophenotypes for molecular genetic research. Although means comparison analyses can be easily employed by nonstatisticians and have certain intuitive appeal, they are subject to several limitations: neither the familial variance underlying the measurement nor the covariance with ADHD can be parsed into separable genetic and environmental components;61 the amount of familial sharing is not explicitly quantified; and it is difficult to compare between measurements. SEM of twin data addresses all three of these problems. To the authors’ knowledge, only one study has directly compared the results of SEM and means comparison approaches in the selection of candidate endophenotypes.50 This study compared different measurements of “overactivity” and found that the two methodologic approaches yielded similar results because they validated the same measurements as candidate endophenotypes. However, the SEM approach quantified the amount of familial sharing of each measurement with ADHD, allowing future research to explicitly choose those measurements sharing the most familial variance with ADHD. Therefore, twin studies appear to be a valuable strategy for endophenotype research.
TWIN RESEARCH AND ENDOPHENOTYPE DEVELOPMENT
Several research groups have started to employ twin studies to identify endophenotypes for ADHD. Initially, samples were too small to draw robust conclusions from parameter estimates gained from biometric genetic analyses,37 but recent analyses on larger-scale twin studies have been promising. For example, the heritability of RT data was estimated at 50% to 80%,4,62 with lower estimates increasing to around 70% when corrected for measured test-retest unreliability.63 This estimate is close to the average heritability of ADHD (~60% to 70%).1,3 Other measurements such as commission errors show somewhat lower estimates in the range of 18% to 48%, although these increase to 68% when also corrected for test-retest unreliability.15 Nevertheless, it is important to realize that biometrical genetic studies, including SEM analyses of twin data, transcend simple estimation of heritability. Such studies can address additional issues in the search for endophenotypes, in particular the identification and selection of appropriate endophenotypes from the many potential candidates.
ADVANTAGES OF ANALYSIS OF DATA COLLECTED FROM RELATIVES
It is not widely recognized that factor analysis of data from relatives confers several advantages over factor analysis from unrelated individuals. One benefit is that it is possible to analyze data from structured interviews, in which certain questions are asked if and only if a previous item has been responded to affirmatively. For example, in substance-use research it is possible to factor analyze jointly the initiation of substance use and subsequent symptoms of abuse or dependence.64 In the context of developing ADHD endophenotypes, it is possible to analyze commission errors for contributing characteristics and to ascertain how much they reflect biological characteristics specific to ADHD and how much they may reflect more generalized cognitive deficits.62 Second, when data have been collected from relatives and the factors correlate between relatives, it becomes possible to identify a greater number of factors than is the case with data from unrelated individuals. The data to identify a larger number of factors come from the cross-relative, cross-phenotype correlations. Third, given a genetically informative design, it is possible to partition variation in the latent factors into genetic and environmental components. For example, Figure 1 shows a path diagram of a model with three latent factors and seven observed variables, comprising three endophenotype measurements (End1 to End3, e.g., Actigraph data) and four behavioral measurements (Beh1 to Beh4). Application of this model to such a dataset might yield a general factor (F1) with substantial loadings on all measures, a factor F2 with large loadings on the endophenotypes but little effect on the behavioral measurements, and a third factor F3, which has the opposite pattern to F2. Under these circumstances, interest might center on F1 because both domains of measurement are influenced by it. Factor scores for all persons in the sample could be derived by maximum likelihood, and this could be used in, e.g., GWAS to identify genetic factors that contribute to the endophenotype and the behavioral components of ADHD. Without data from twins or other genetically informative studies of relatives, it is not possible to know in advance whether there is any evidence for genetic factors influencing the common factor F1. The addition of twin data enables examination of the impact of genes on variation in each of the common factors, as shown in Figure 2. Here, variation in factors F1, F2, and F3 has been partitioned into genetic and environmental components, and the same separation is applied to the residual VCs specific to each measure. The parameters of this model can be freely estimated with data from a study of MZ and DZ twins. Possibly, the common factor F1 would have substantial genetic variation (a1 is large relative to c1 and e1). If the covariance between the endophenotypes and the behavioral measurements were entirely due to genetic factors, then c1 = e1 = 0 (which can be empirically tested with twin data), and maximum likelihood estimates of the individual F1 factor scores would be an especially promising candidate for GWAS.
FIGURE 1.
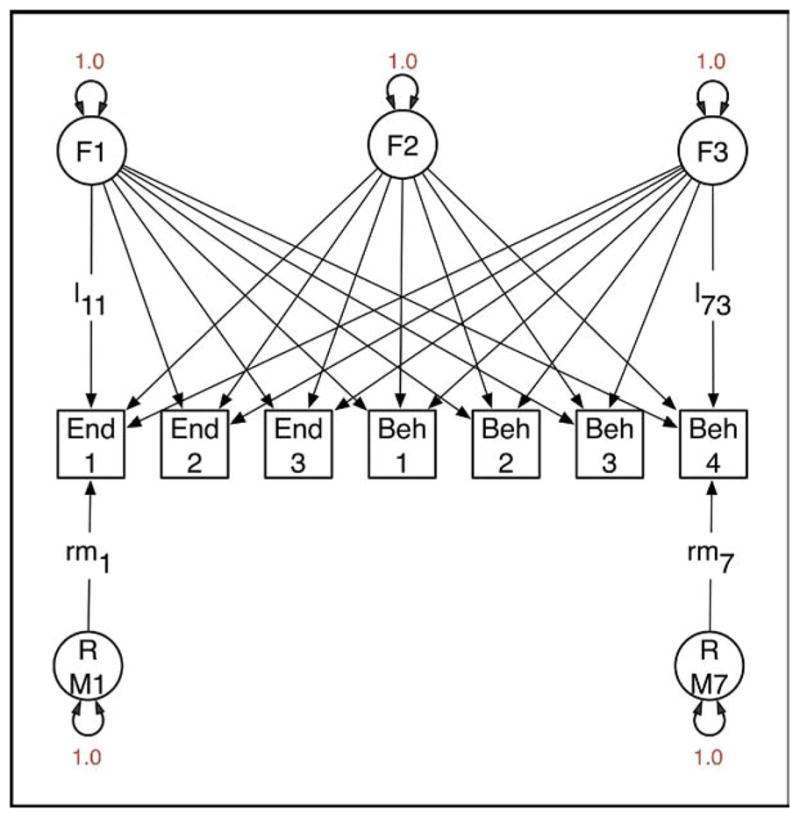
Factor model for data collected from unrelated individuals. Three factors (F1 to F3) are hypothesized to generate covariance between three endophenotypes (End1 to End3) and four behavioral measurements (Beh4 to Beh7). Causal paths from the factors to the observed variables are drawn as single-direction arrows (e.g., l11, l73). All latent factors (F1 to F3) and residual variance components (RE1 to RB7) are specified to have unit variance. Variation in each observed measurement is thus partitioned into four components: that due to each of the factors and that due to residual variance (including measurement error) specific to each measurement.
FIGURE 2.
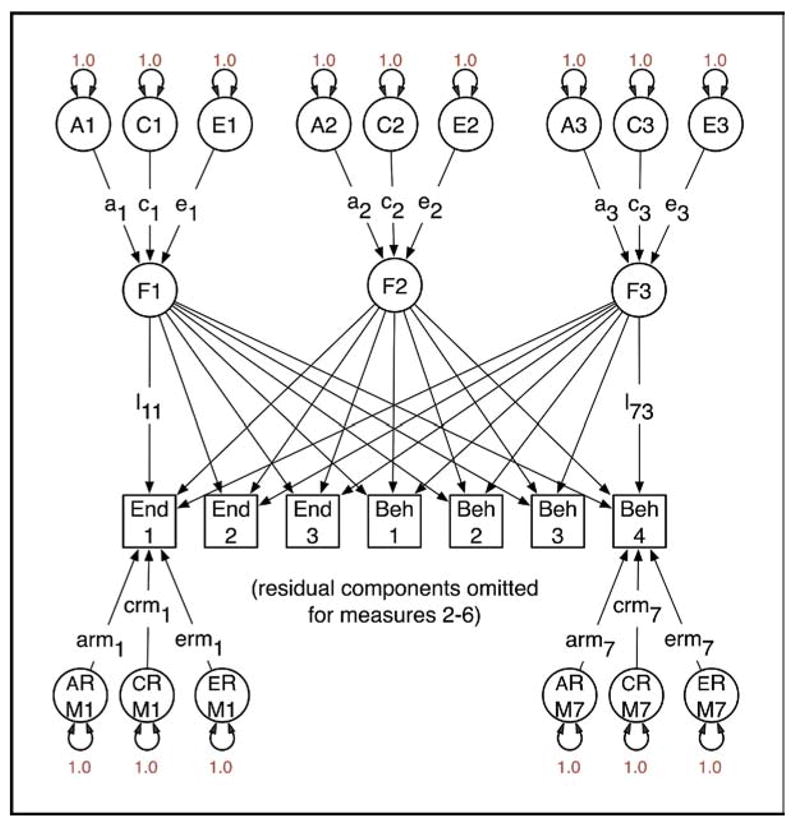
Factor model for genetically informative data from relatives assessed on seven measures (M1 to M7). Variation in the latent factors (F1 to F3) and residual components is partitioned into additive genetic (A1 to A3 and ARM1 to ARM7), shared or common environment (C1 to C3 and CRM1 to CRM7), and specific environment (E1 to E3 and ERM1 to ERM7) components. The regressions on each of these sources are estimated as free parameters (l1 to l7, a1 to a3, c1 to c3, e1 to e3, arm1 to arm7, crm1 to crm7, erm1 to erm7). If estimates for a particular factor appear to be largely genetic (e.g., a1 is large but c1 and e1 are small), it would be a good candidate for genomewide association studies. Beh = behavioral measurement; End = endophenotypes.
It is not uncommon to find that a model in which some factors are designated as purely additive genetic (and therefore correlate 1.0 between MZ twins and 0.5 between DZ twins), whereas others are purely environmental, fits the data well. In this situation, estimating latent genetic factor scores can be particularly valuable, although there is no barrier to estimating latent factor scores even when a factor is highly, but not completely, heritable. This latent factor score method was successful in a study of anxiety disorders,65 in which Diagnostic and Statistical Manual of Mental Disorders diagnoses and neuroticism trait scores were analyzed jointly in a multivariate twin model to derive a genetic latent trait factor score. The same approach could be applied to joint analyses of objective measurements of ADHD-related phenotypes such as RT or Actigraph measurements and more subjective but widely accepted ratings of attention and hyperactivity.
Of particular note is the potential of twin studies to distinguish between different models for the covariation between an endophenotype and an outcome variable. Models for comorbidity such as those described by Klein and Riso66 and implemented statistically for twin data by Neale and Kendler67 (see also Rhee et al.68) are potentially very valuable in this context. Interest in an endophenotype is usually diminished if it is a consequence of the ADHD phenotype as opposed to a cause of it. A more promising possibility is that the endophenotype and the outcome variable share certain liability risk factors. Bivariate analyses of data from twins permit some resolution between these alternative hypotheses.
TWIN STUDIES CAN MAXIMIZE POWER IN FUTURE MOLECULAR GENETIC STUDIES
Maximizing power remains a key issue for molecular genetic studies, even with the endophenotype approach. Twin studies can increase power in endophenotype association studies (or GWAS) by decreasing the number of phenotypes to be tested for molecular genetic associations. This decrease may be achieved in three main ways. First, they may help determine which measurements may be dropped; second, they may determine which measurements may be combined; and third, they can help determine which measurements are most likely to have the power to result in successful gene-hunting.
Twin studies have helped to decrease the number of phenotypes by showing that, although closely related to the behavioral disorder, not all phenotypes are familial. For example, when collected in a laboratory setting, only mechanical motion sensor data from the waist, and not the leg, show significant familial overlap with ADHD, although both body loci show a significant within-person correlation.50
Studies of twins and families also provide an empirical basis for combining data across measurements, which decreases the number of phenotypes to be analyzed. This decrease mitigates the loss of power incurred when correcting for multiple testing. Recent analyses have highlighted that, although phenotypic correlations across RT data collected across different tasks may be low, genetic correlations can approach unity.62 Findings for activity-level data are similar: mechanical assessments of activity level across situations show modest phenotypic correlations, in the region of 0.5 to 0.6, but the genetic factors underlying laboratory-based tests and those from a “free play” session are very highly correlated, indicating that the two situations measure the same underlying genetic liability.69,70 Twin and factor analysis further indicate that mean RT and RT variability measure the same underlying construct or liability in the general population,62 indicating that only one cognitive construct need be analyzed in gene-finding studies. Thus twin studies can identify which measurements can be dropped, or preferably combined, because, despite modest phenotypic correlations, the genetic factors underlying the measurements may be largely identical.
After data reduction, further phenotype selection may be necessary. If data cannot be aggregated, selecting those measurements with the highest heritabilities may increase the power of genetic association studies; this has been highlighted as a key avenue of research in recent studies (e.g., Doyle et al.71). Selection may be at the individual measurement level (e.g., measurements of RT variability show higher heritabilities than do measurements of mean RT62) or at the aggregation level, with twin analysis having indicated higher heritabilities (or higher familial variance) for latent factor scores over mean measurements.62,72 Whether this approach will translate into a “real-world effect” remains an empirical question. Similarly, understanding the etiology of the covariance between endophenotype measurements and ADHD will help researchers to select measurements that covary with ADHD for reasons other than being due to a general underlying deficit. This is a newer line of research, but data from larger-scale twin studies, for example, have indicated that the covariance between RT data and ADHD scores in the general population is independent of the covariation between ADHD scores and lowered IQ.62 To date, a multivariate genetic approach with twin data has not been employed in the development of endophenotypes for ADHD, and replication remains a key outstanding issue. Nevertheless, the potential value of twin studies to endophenotype and phenotype definitions seems clear given these interesting and promising initial results.
TRANSLATIONAL IMPLICATIONS
Zerhouni73 identified translational studies as a key priority for the National Institute of Health, and the importance of twin studies of endophenotypes for clinicians and those outside the genetic field should not be overlooked. Translational research is normally taken to mean “from science to bedside.”74 However, with research that does not involve clinical trials or direct clinical interventions, such as quantitative genetic studies, it is important to take a broader view. The road from science to bedside may involve many steps, and it is necessary to understand the role of research findings to yield individual health benefits. Much endophenotype research is concerned with identifying objective measurements that can be used as measurements of ADHD. Such assessments and their biological markers may provide powerful phenotypes for future studies that will enhance the lives of those with ADHD. For example, molecular genetic and functional magnetic resonance imaging studies may improve the characterization of biological pathways between genes, brain, and behavior. This improvement may in turn help to identify more homogenous clinical subgroups that differ in their responses to treatment. In addition, although the research discussed in this review might be thought to be focused on identifying, and maximizing, heritable variance, it does, of course, follow that the nonheritable variance can be refined in a similar fashion. This, too, can help target future research, by portioning the environmental variance into shared and nonshared environmental factors, indicating where epidemiologic studies should be directed. Better still, multivariate twin studies have the potential to clarify the role of putative environmental risk factors by assessing whether they affect ADHD behaviors directly or moderate the influence of genetic or other environmental sources of variation. Further, multivariate SEM of twin data reveals environmental causes of covariation, indicating where clinicians can direct target treatment in, for example, addressing comorbid oppositional and ADHD behaviors. This is particularly valuable where twin studies establish that the same behaviors in different settings (e.g., across tasks or situations) do not share the same environmental factors. The validation of increased ADHD correlates in unaffected relatives of probands suggests that ADHD behaviors, throughout the lifespan, are associated with disruptions in interpersonal relationships, mood instability, employment problems, and chaotic living arrangements.75 If relatives of ADHD probands share these characteristics, the stress-diathesis hypothesis would suggest that this would create a disruptive environment for the individual with high ADHD liability, which in turn would give clinicians an important area for targeting intervention or symptom management.
Studies of twins and other family relatives continue to offer a great deal to ADHD research. Univariate analyses provide a “target” of genetic variation to be accounted for by molecular genetic studies, but this is a small fraction of the potential value of multivariate analyses of twin and family data. The latter offer a clear route to the identification of a small number of latent, substantially or entirely genetic factors. Estimation of individual factor scores of these latent traits—which may underlie variation in objective and subjective measurements of ADHD phenotypes—is a straightforward statistical procedure. These scores in turn seem likely to prove valuable in the identification of single nucleotide polymorphisms that generate individual differences in liability to ADHD. A further advantage is that twin studies permit some resolution between “true” endophenotypes, which are intermediate between genotype and ADHD phenotype, and simple correlates of ADHD, which share some of their causal factors.
Footnotes
This article is one of several articles published in the August and September issues of the Journal of the American Academy of Child and Adolescent Psychiatry that explores the intersection of genetics and mental health disorders in children and adolescents. The editors invite the reader to investigate the additional articles on this burgeoning area of developmental psychopathology.
Disclosure: Dr. Neale has received financial support from the National Institute on Drug Abuse (grant DA-18673). Dr. Wood reports no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Alexis C. Wood, University of Alabama at Birmingham
Dr. Michael C. Neale, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond
References
- 1.Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull. 2009;135:608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- 2.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Wood AC, Buitelaar J, Rijsdijk F, Asherson P, Kuntsi J. Rethinking shared environment as a source of variance underlying attention-deficit/hyperactivity disorder symptoms: comment on Burt (2009) Psychol Bull. 2010;136:331–340. doi: 10.1037/a0019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luciano M, Wright M, Smith GA, Geffen GM, Geffen LB, Martin NG. Genetic covariance among measures of information processing speed, working memory, and IQ. Behav Genet. 2001;31:581–592. doi: 10.1023/a:1013397428612. [DOI] [PubMed] [Google Scholar]
- 5.Pearl J. Causality: Models, Reasoning, and Inference. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 6.Eaves LJ. A model for sibling effects in man. Heredity. 1976;36:205–214. doi: 10.1038/hdy.1976.25. [DOI] [PubMed] [Google Scholar]
- 7.Rietveld MJ, Posthuma D, Dolan CV, Boomsma DI. ADHD: sibling interaction or dominance: an evaluation of statistical power. Behav Genet. 2003;33:247–255. doi: 10.1023/a:1023490307170. [DOI] [PubMed] [Google Scholar]
- 8.Asherson P. attention-deficit hyperactivity disorder in the post-genomic era. Eur Child Adolesc Psychiatry. 2004;13(suppl 1):I50–I70. doi: 10.1007/s00787-004-1006-6. [DOI] [PubMed] [Google Scholar]
- 9.Kirley A, Hawi Z, Daly G, et al. Dopaminergic system genes in ADHD: toward a biological hypothesis. Neuropsychopharmacology. 2002;27:607–619. doi: 10.1016/S0893-133X(02)00315-9. [DOI] [PubMed] [Google Scholar]
- 10.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 11.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 12.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 13.Franke B, Neale BM, Faraone SV. Genome-wide association studies in ADHD. Hum Genet. 2009;126:13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiMaio S, Grizenko N, Joober R. Dopamine genes and attention-deficit hyperactivity disorder: a review. J Psychiatry Neurosci. 2003;28:27–38. [PMC free article] [PubMed] [Google Scholar]
- 15.Kuntsi J, Neale BM, Chen W, Faraone SV, Asherson P. The IMAGE project: methodological issues for the molecular genetic analysis of ADHD. Behav Brain Funct. 2006;2:27. doi: 10.1186/1744-9081-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Defries JC, Fulker DW. Multiple regression analysis of twin data. Behav Genet. 1985;15:467–473. doi: 10.1007/BF01066239. [DOI] [PubMed] [Google Scholar]
- 17.Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Thapar A, Harrington R, Ross K, McGuffin P. Does the definition of ADHD affect heritability? J Am Acad Child Adolesc Psychiatry. 2000;39:1528–1536. doi: 10.1097/00004583-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Thapar A, Langley K, O’Donovan M, Owen M. Refining the attention deficit hyperactivity disorder phenotype for molecular genetic studies. Mol Psychiatry. 2006;11:714–720. doi: 10.1038/sj.mp.4001831. [DOI] [PubMed] [Google Scholar]
- 20.Saudino KJ, Ronald A, Plomin R. The etiology of behavior problems in 7-year-old twins: substantial genetic influence and negligible shared environmental influence for parent ratings and ratings by same and different teachers. J Abnorm Child Psychol. 2005;33:113–130. doi: 10.1007/s10802-005-0939-7. [DOI] [PubMed] [Google Scholar]
- 21.Wood AC, Rijsdijk F, Asherson P, Kuntsi J. Hyperactive-impulsive symptom scores and oppositional behaviors reflect alternate manifestations of a single liability. Behav Genet. 2009c;39:447–460. doi: 10.1007/s10519-009-9290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abikoff H, Courtney M, Pelham WE, Jr, Koplewicz HS. Teachers’ ratings of disruptive behaviors: the influence of halo effects. J Abnorm Child Psychol. 1993;21:519–533. doi: 10.1007/BF00916317. [DOI] [PubMed] [Google Scholar]
- 23.Schachar R, Sandberg S, Rutter M. Agreement between teachers’ ratings and observations of hyperactivity, inattentiveness, and defiance. J Abnorm Child Psychol. 1986;14:331–345. doi: 10.1007/BF00915450. [DOI] [PubMed] [Google Scholar]
- 24.Stevens J, Quittner AL, Abikoff H. Factors influencing elementary school teachers’ ratings of ADHD and ODD behaviors. J Clin Child Psychol. 1998;27:406–414. doi: 10.1207/s15374424jccp2704_4. [DOI] [PubMed] [Google Scholar]
- 25.Eaves LJ, Rutter M, Silberg JL, Shillady L, Maes HH, Pickles A. Genetic and environmental causes of covariation in interview assessments of disruptive behavior in child and adolescent twins. Behav Genet. 2000;30:321–334. doi: 10.1023/a:1026553518272. [DOI] [PubMed] [Google Scholar]
- 26.Neale MC, Stevenson J. Rater bias in the EASI Temperament Scales: a twin study. J Pers Soc Psychol. 1989;56:446–455. doi: 10.1037//0022-3514.56.3.446. [DOI] [PubMed] [Google Scholar]
- 27.Saudino KJ, Cherny SS, Plomin R. Parent ratings of temperament in twins: explaining the ‘too low’ DZ correlations. Twin Res. 2000;3:224–233. doi: 10.1375/136905200320565193. [DOI] [PubMed] [Google Scholar]
- 28.Simonoff E, Pickles A, Hervas A, Silberg JL, Rutter M, Eaves L. Genetic influences on childhood hyperactivity: contrast effects imply parental rating bias, not sibling interaction. Psychol Med. 1998;28:825–837. doi: 10.1017/s0033291798006886. [DOI] [PubMed] [Google Scholar]
- 29.Fulker DW, Cherny SS, Sham PC, Hewitt JK. Combined linkage and association sib-pair analysis for quantitative traits. Am J Hum Genet. 1999;64:259–267. doi: 10.1086/302193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sham PC, Cherny SS, Purcell S, Hewitt JK. Power of linkage versus association analysis of quantitative traits, by use of variance-components models, for sibship data. Am J Hum Genet. 2000;66:1616–1630. doi: 10.1086/302891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes J, Payton A, Barrett J, et al. Association of DRD4 in children with ADHD and comorbid conduct problems. Am J Med Genet. 2002;114:150–153. doi: 10.1002/ajmg.10149. [DOI] [PubMed] [Google Scholar]
- 32.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 33.Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. Am J Med Genet. 2001;105:42–44. [PubMed] [Google Scholar]
- 34.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 35.Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am J Med Genet. 2001;105:11–15. [PubMed] [Google Scholar]
- 36.Doyle AE, Biederman J, Seidman LJ, Reske-Nielsen JJ, Faraone SV. Neuropsychological functioning in relatives of girls with and without ADHD. Psychol Med. 2005;35:1121–1132. doi: 10.1017/s0033291705004496. [DOI] [PubMed] [Google Scholar]
- 37.Waldman ID. Statistical approaches to complex phenotypes: evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1347–1356. doi: 10.1016/j.biopsych.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Braff D, Schork NJ, Gottesman II. Endophenotyping schizophrenia. Am J Psychiatry. 2007;164:705–707. doi: 10.1176/ajp.2007.164.5.705. [DOI] [PubMed] [Google Scholar]
- 39.Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy KR, Barkley RA. Parents of children with attention-deficit/hyperactivity disorder: psychological and attentional impairment. Am J Orthopsychiatry. 1996;66:93–102. doi: 10.1037/h0080159. [DOI] [PubMed] [Google Scholar]
- 41.Seidman LJ, Biederman J, Monuteaux MC, Weber W, Faraone SV. Neuropsychological functioning in nonreferred siblings of children with attention deficit/hyperactivity disorder. J Abnorm Psychol. 2000;109:252–265. doi: 10.1037/0021-843X.109.2.252. [DOI] [PubMed] [Google Scholar]
- 42.Asarnow RF, Nuechterlein KH, Subotnik KL, et al. Neurocognitive impairments in nonpsychotic parents of children with schizophrenia and attention-deficit/hyperactivity disorder: the University of California, Los Angeles Family Study. Arch Gen Psychiatry. 2002;59:1053–1060. doi: 10.1001/archpsyc.59.11.1053. [DOI] [PubMed] [Google Scholar]
- 43.Rommelse NN. Endophenotypes in the genetic research of ADHD over the last decade: have they lived up to their expectations? Expert Rev Neurother. 2008;8:1425–1429. doi: 10.1586/14737175.8.10.1425. [DOI] [PubMed] [Google Scholar]
- 44.Andreou P, Neale BM, Chen W, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson KA, Wiersema JR, Kuntsi J. What would Karl Popper say? Are current psychological theories of ADHD falsifiable? Behav Brain Funct. 2009;5:15. doi: 10.1186/1744-9081-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Uebel H, Albrecht B, Asherson P, et al. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. J Child Psychol Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Schachar RJ, Crosbie J, Barr CL, et al. Inhibition of motor responses in siblings concordant and discordant for attention deficit hyperactivity disorder. Am J Psychiatry. 2005;162:1076–1082. doi: 10.1176/appi.ajp.162.6.1076. [DOI] [PubMed] [Google Scholar]
- 50.Wood AC, Asherson P, Rijsdijk F, Kuntsi J. Is overactivity a core feature in ADHD? Familial and receiver operating characteristic curve analysis of mechanically assessed activity level. J Am Acad Child Adolesc Psychiatry. 2009;40:1027–1037. doi: 10.1097/CHI.0b013e3181b54612. [DOI] [PubMed] [Google Scholar]
- 51.Bidwell LC, Willcutt EG, Defries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langley K, Marshall L, Van den Bree M, et al. Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry. 2004;161:133–138. doi: 10.1176/appi.ajp.161.1.133. [DOI] [PubMed] [Google Scholar]
- 53.Cornish KM, Manly T, Savage R, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10:686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- 54.Bellgrove MA, Hawi Z, Lowe N, Kirley A, Robertson IH, Gill M. DRD4 gene variants and sustained attention in attention deficit hyperactivity disorder (ADHD): effects of associated alleles at the VNTR and −521 SNP. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:81–86. doi: 10.1002/ajmg.b.30193. [DOI] [PubMed] [Google Scholar]
- 55.Manor I, Tyano S, Eisenberg J, Bachner-Melman R, Kotler M, Ebstein RP. The short DRD4 repeats confer risk to attention deficit hyperactivity disorder in a family-based design and impair performance on a continuous performance test (TOVA) Mol Psychiatry. 2002;7:790–794. doi: 10.1038/sj.mp.4001078. [DOI] [PubMed] [Google Scholar]
- 56.Swanson J, Oosterlaan J, Murias M, et al. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proc Natl Acad Sci U S A. 2000;97:4754–4759. doi: 10.1073/pnas.080070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellgrove MA, Hawi Z, Kirley A, Fitzgerald M, Gill M, Robertson IH. Association between dopamine transporter (DAT1) genotype, left-sided inattention, and an enhanced response to methylphenidate in attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2005;30:2290–2297. doi: 10.1038/sj.npp.1300839. [DOI] [PubMed] [Google Scholar]
- 58.Loo SK, Specter E, Smolen A, Hopfer C, Teale PD, Reite ML. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:986–993. doi: 10.1097/01.CHI.0000046890.27264.88. [DOI] [PubMed] [Google Scholar]
- 59.Rommelse NN, Arias-Vasquez A, Altink ME, et al. Neuropsychological endophenotype approach to genome-wide linkage analysis identifies susceptibility loci for ADHD on 2q21.1 and 13q12.11. Am J Hum Genet. 2008;83:99–105. doi: 10.1016/j.ajhg.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kebir O, Tabbane K, Sengupta S, Joober R. Candidate genes and neuropsychological phenotypes in children with ADHD: review of association studies. J Psychiatry Neurosci. 2009;34:88–101. [PMC free article] [PubMed] [Google Scholar]
- 61.Kendler KS, Neale MC. “Familiality” or heritability. Arch Gen Psychiatry. 2009;66(4):452–453. doi: 10.1001/archgenpsychiatry.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood AC, Asherson P, van der Meere JJ, Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychol Med. 2009;40:1–11. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuntsi J, Rogers H, Swinard G, et al. Reaction time, inhibition, working memory and ‘delay aversion’ performance: genetic influences and their interpretation. Psychol Med. 2006;36:1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neale MC, Aggen SH, Maes HH, Kubarych TS, Schmitt JE. Methodological issues in the assessment of substance use phenotypes. Addict Behav. 2006;31(6):1010–1034. doi: 10.1016/j.addbeh.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 65.Hettema JM, An SS, Neale MC, et al. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol Psychiatry. 2006;11:752–762. doi: 10.1038/sj.mp.4001845. [DOI] [PubMed] [Google Scholar]
- 66.Klein DN, Riso LP. Psychiatric disorders: problems of boundaries and comorbidity. In: Costello CG, editor. Basic Issues in Psychopathology. New York: Guilford; 1994. pp. 19–66. [Google Scholar]
- 67.Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. Am J Hum Genet. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- 68.Rhee SH, Hewitt JK, Lessem JM, Stallings MC, Corley RP, Neale MC. The validity of the Neale and Kendler model-fitting approach in examining the etiology of comorbidity. Behav Genet. 2004;34(3):251–265. doi: 10.1023/B:BEGE.0000017871.87431.2a. [DOI] [PubMed] [Google Scholar]
- 69.Saudino KJ, Zapfe JA. Genetic influences on activity level in early childhood: do situations matter? Child Dev. 2008;79:930–943. doi: 10.1111/j.1467-8624.2008.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood AC, Saudino KJ, Rogers H, Asherson P, Kuntsi J. Genetic influences on mechanically-assessed activity level in children. J Child Psychol Psychiatry. 2007;48:695–702. doi: 10.1111/j.1469-7610.2007.01739.x. [DOI] [PubMed] [Google Scholar]
- 71.Doyle AE, Faraone SV, Seidman LJ, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J Child Psychol Psychiatry. 2005;46:774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 72.Wood AC, Rijsdijk F, Saudino KJ, Asherson P, Kuntsi J. High heritability for a composite index of children’s activity level measures. Behav Genet. 2008;38:266–276. doi: 10.1007/s10519-008-9196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zerhouni E. Medicine. The NIH roadmap. Science. 2003;302:63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- 74.Rutter M, Plomin R. Pathways from science findings to health benefits. Psychol Med. 2009;39:529–542. doi: 10.1017/S003329170800398X. [DOI] [PubMed] [Google Scholar]
- 75.Harpin VA. The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch Dis Child. 2005;90(suppl 1):i2–i7. doi: 10.1136/adc.2004.059006. [DOI] [PMC free article] [PubMed] [Google Scholar]


