Abstract
The limited durability of resin-dentin bonds severely compromises the lifetime of tooth-colored restorations. Bond degradation occurs via hydrolysis of suboptimally polymerized hydrophilic resin components and degradation of water-rich, resin-sparse collagen matrices by matrix metalloproteinases (MMPs) and cysteine cathepsins. This review examined data generated over the past three years on five experimental strategies developed by different research groups for extending the longevity of resin-dentin bonds. They include: (1) increasing the degree of conversion and esterase resistance of hydrophilic adhesives; (2) the use of broad-spectrum inhibitors of collagenolytic enzymes, including novel inhibitor functional groups grafted to methacrylate resins monomers to produce anti-MMP adhesives; (3) the use of cross-linking agents for silencing the activities of MMP and cathepsins that irreversibly alter the 3-D structures of their catalytic/allosteric domains; (4) ethanol wet-bonding with hydrophobic resins to completely replace water from the extrafibrillar and intrafibrillar collagen compartments and immobilize the collagenolytic enzymes; and (5) biomimetic remineralization of the water-filled collagen matrix using analogs of matrix proteins to progressively replace water with intrafibrillar and extrafibrillar apatites to exclude exogenous collagenolytic enzymes and fossilize endogenous collagenolytic enzymes. A combination of several of these strategies should result in overcoming the critical barriers to progress currently encountered in dentin bonding.
Keywords: bottom-up, biomimetic remineralization, chlorhexidine, collagen, cross-linking agents, cysteine cathepsins, degradation, esterase, ethanol wet-bonding, extracellular matrix proteins, hydrolysis, matrix metalloproteinases, resin monomers, top-down, water sorption
Introduction
Over the past two decades, bonding of resin-based composite restorations has been revolutionized by continuing advances in dentin bonding technology. Within the history of contemporary dentin adhesives, minimally invasive tooth preparations have become possible in daily clinical practice. Esthetic tooth-colored restorations have become very popular worldwide. However, resin-dentin bonds are less durable than resin-enamel bonds (Loguercio et al., 2008), because dentin bonding relies on organic components. Even though moisture is essential for successful bonding, it also adversely affects long-term bonding results. Although the immediate bond strengths of contemporary adhesives have been shown to be quite high, substantial decreases in resin-dentin bond strength occurred after aging (Breschi et al., 2008), with continuous loss of bonded restorations over time for both etch-and-rinse and self-etch adhesive systems (van Dijken et al., 2007). Bond durability is critical for the longevity of restoratives, because degradation can weaken adhesion and lead to gaps between teeth and restoratives (Amaral et al., 2007). The NIDCR 2009-2013 strategic plan on tooth-colored resin restorations reported that the average replacement time of these restorations is only 5.7 years (NIDCR, 2009-2013). Replacing defective dental fillings costs about five billion dollars per year in the US alone (Jokstad et al., 2001).
Resin-dentin bonding is a unique form of tissue engineering in which a demineralized dentin collagen matrix is used as the scaffold for resin infiltration, to produce a hybrid layer that couples adhesives/resin composites to the underlying mineralized dentin. To date, resin-dentin bonds created by infiltration of hydrophilic resin monomers into demineralized dentin matrix are imperfect (Breschi et al., 2008; Vaidyanathan and Vaidyanathan, 2009). One of the reasons for incomplete permeation of resin monomers within these hybrid layers is fluid movement within the dentinal tubule anastomosis complex during resin infiltration (Hashimoto et al., 2009). Thus, the exposed collagen fibrils resulting from incomplete resin infiltration cannot be protected against denaturation challenges and are susceptible to creep (Pashley et al., 2003) or cyclic fatigue rupture (Fung et al., 2009) after prolonged function. These denuded collagen matrices are also filled with water, which serves as a functional medium for the hydrolysis of resin matrices by esterases and collagen by endogenous and exogenous collagenolytic enzymes. Even when water-rich, resin-sparse collagen fibrils can be prevented from degradation, the mechanical properties of these denuded collagen fibrils are far inferior to those of resin-infiltrated collagen or mineralized collagen (Sano et al., 1995). These issues are the critical barriers to future progress in dentin bonding.
The limited durability of dentin bonding severely shortens the lifespan of tooth-colored resin composites. There is a compelling need to explore the underlying mechanisms involved in preventing the degradation of resin-dentin bonds and extend their longevity. This paper reviews the factors compromising dentin bonding and the experimental strategies available to prevent degradation of resin-dentin bonds.
Factors that Compromise the Durability of Resin-Dentin Bonds
Hydrolytic Degradation by Water Sorption
The water “wet bonding” technique was introduced in the early 1990s to prevent the problem of collagen collapse after acid-etching and resulted in improved resin infiltration into acid-etched dentin. In this bonding technique, acid-etched dentin is kept fully hydrated throughout the bonding procedure. Two-hydroxyethyl methacrylate (HEMA) was incorporated into many dentin adhesives to serve as a solvent for non-water-compatible resin monomers, to reduce phase separation of those monomers after evaporation of the volatile solvents (Spencer and Wang, 2002), to enhance the wetting properties of those adhesives on acid-etched dentin (Marshall et al., 2010), and for its purported affinity to demineralized collagen (Sharrock and Grégoire, 2010).
In the evolution of dentin adhesives, manufacturers have incorporated increasing concentrations of hydrophilic and ionic monomers to make these adhesives more compatible for bonding to intrinsically moist, acid-etched dentin (Van Landuyt et al., 2007). Increasing the hydrophilic nature of the adhesive-dentin interface has several disadvantages (Tay and Pashley, 2003a) and creates a weak link in the bonded assembly (Spencer et al., 2010). Hydrophilic and ionic resin monomers are vulnerable to hydrolysis, due to the presence of ester linkages (Ferracane, 2006). Inclusion of high concentrations of monomers in these adhesives lowers the vapor pressure of bonding solvents, including water. High concentrations of resin monomers in an adhesive may therefore hamper water evaporation, the entrapment of which deteriorates mechanical properties of the resulting polymer (Van Landuyt et al., 2008; Ye et al., 2009a). Previous studies have correlated the incorporation of hydrophilic and acidic resin monomers in adhesive blends with decreased longevity of resin-dentin bonds (Peumans et al., 2005), because these hydrophilic resins expedite water sorption (Malacarne et al., 2006). This, in turn, results in plasticization of polymers, which decreases the mechanical properties of resins (Ito et al., 2005; Chiaraputt et al., 2008). Even the inclusion of small amounts of water may result in nano-phase separation of the adhesive components in the form of nanoscopic worm-like structures between the polymerized hydrophilic and hydrophobic resin phases (Ye et al., 2008, 2009b). Nano-phase separation decreases the dynamic mechanical properties of the polymerized adhesives (Park et al., 2010) and increases their susceptibility to esterase-catalyzed hydrolysis (Kostoryz et al., 2009).
Dentin bonds created by contemporary hydrophilic adhesives are also susceptible to fluid permeation induced by pulpal pressure (Tay et al., 2005; Cadenaro et al., 2005; Sauro et al., 2006), which further promotes their hydrolysis and decreases bond durability (Armstrong et al., 2004; De Munck et al., 2004). Adhesive hydrophilicity, water sorption, and subsequent hydrolytic degradation have been considered as highly correlative, because hydrolytic degradation occurs only in the presence of water (Carrilho et al., 2005). Hydrolysis of methacrylate ester bonds caused either by the increase in acidity of monomer components (Aida et al., 2009) or by salivary esterases (Shokati et al., 2010) can break covalent bonds between the polymers by the addition of water to the ester bonds. Moreover, problems associated with water-based, strongly acidic single-bottle self-etching adhesives arise from both the hydrolytic instability of the methacrylate monomers used in those systems and the side-reaction of the applied initiator components (Moszner et al., 2005). These resin formulation issues raise concerns on whether current adhesives have become too hydrophilic (Tay and Pashley, 2003a).
Incomplete Infiltration of Resin Monomers
There is common consensus that the final goal of dentin bonding is complete infiltration of resin monomers into demineralized collagen fibrils exposed by acid-etching or self-etch adhesives (Breschi et al., 2008). The discrepancy between dentin demineralization and resin infiltration results in silver-nitrate- or fluorescent-dye-detectable nanoleakage within the water-rich zones of the hybrid layer (Reis et al., 2007; Sauro et al., 2009a) and the adhesive layer (Tay and Pashley, 2003b). The critical barrier to progress in contemporary dentin bonding is that both etch-and-rinse and self-etch approaches in establishing retention in dentin are hampered by their inability to completely replace free and loosely bound water from the internal and external water compartments of collagen fibrils (Kim YK et al., 2010a).
Apart from water, the interfibrillar spaces in acid-etched dentin also contain highly hydrated negatively charged proteoglycans that form a hydrogel within that space (Scott and Thomlinson, 1998). If these hydrogels remain hydrated in interfibrillar spaces, they may be responsible for “molecular sieving” of larger dimethacrylates like BisGMA, allowing only smaller molecules such as HEMA to penetrate the base of the hybrid layers. Since HEMA forms a linear polymer that does not cross-link, HEMA-rich regions of hybrid layers may experience large strains during function that result in fatigue failure of collagen fibrils. To date, complete replacement of lost apatites by resin within the intrafibrillar spaces has never been demonstrated. Thus, apatite-depleted, resin-sparse collagen fibrils within the hybrid layers become susceptible to degradation, compromising the longevity of resin-dentin bonds (Breschi et al., 2008; Hashimoto, 2010).
Collagenolysis by Endogenous MMPs and Cysteine Cathepsins
Mineralized dentin contains matrix metalloproteinases (MMPs) such as MMP-2, -3, -8, and -9 (Sulkala et al., 2002, 2007; Mazzoni et al., 2007, 2009; Boukpessi et al., 2008; Santos et al., 2009; Toledano et al., 2010). These host-derived proteases are a group of zinc- and calcium-dependent enzymes that contribute to the breakdown of collagen matrices in the pathogenesis of dentinal caries (Tjäderhane et al., 1998; van Strijp et al., 2003; Chaussain-Miller et al., 2006) and periodontal disease (Hannas et al., 2007). In addition, non-collagen-bound MMPs are also present in saliva (Tjäderhane et al., 1998; Sulkala et al., 2001), in dentinal tubules, and, presumably, in dentinal fluid (Sulkala et al., 2002; Boushell et al., 2008). Degradation of resin-sparse collagen fibrils in aged bonded dentin in vivo has also been correlated with the activation of collagen-bound MMPs and/or salivary MMPs by application of components of etch-and-rinse adhesives (Mazzoni et al., 2006; De Munck et al., 2009). The acid-etching procedure was initially thought to release and activate pro-MMPs trapped within the mineralized dentin (Pashley et al., 2004), resulting in collagenolytic and gelatinolytic activities identified within the hybridized dentin. However, phosphoric acid used in the etch-and-rinse technique is highly acidic (pH 0.7 to -1) and denatured MMPs due to its low acidity (Pashley et al., 2004). The acidic resin components incorporated into etch-and-rinse adhesives (Mazzoni et al., 2006) and self-etch adhesives (Nishitani et al., 2006a) have subsequently been shown to increase the collagenolytic and gelatinolytic activities of completely or partially demineralized collagen matrices. These studies, which utilized dentin powder and an internally quenched fluorescent collagen/gelatin substrate, could identify only collagen degradation activities, but were non-specific in terms of whether MMPs, or which type of MMP, are involved in the degradation process. The results were more recently confirmed with MMP-2 and MMP-9 specific assays for both etch-and-rinse and self-etch adhesives (Breschi and Mazzoni, unpublished observations). In addition, a self-etch adhesive has been shown to increase MMP-2 synthesis in human odontoblasts (Lehmann et al., 2009), possibly increasing MMP-2 penetration into the hybrid layer via dentinal fluid. Mildly acidic resin monomers can activate MMPs by inhibiting tissue inhibitor of metalloproteinases-1 (TIMP-1, Ishiguro et al., 1994) in TIMP-MMP complexes, thereby producing active MMPs (Tjäderhane et al., 1998; Sulkala et al., 2001). Alternatively, these acidic resin monomers may activate latent forms of MMPs (pro-MMPs) via the cysteine-switch mechanism that exposes the catalytic domain of these enzymes that were blocked by pro-peptides (Tallant et al., 2010).
Cysteine cathepsins are papain-like endopeptidases that participate in intracellular proteolysis within the lysosomal compartments of living cells (Dickinson, 2002). However, they also exist as exopeptidases and participate in extracellular matrix degradation involving the breakdown of type I collagen and proteoglycans (Obermajer et al., 2008). Cathepsins B, L, and S cleave the non-helical telopeptide extensions of collagen molecules, while cathepsin K cleaves the collagen molecules along their triple helix region. Unlike the collagenolytic MMPs (MMP-1, -2, -8, and -13) that cleave type I collagen within the triple helix at a single site (between amino acids 775 and 776 from the first GXY triplet of the triple helix domain) into a ¾ N-terminal fragment and ¼ C-terminal fragment, cathepsin K cleaves collagen molecules at multiple sites within the triple helix, thereby generating fragments of various sizes (Garnero et al., 1998). Cysteine cathepsins have recently been reported to be expressed by mature human odontoblasts and present in intact dentin (Tersariol et al., 2010), but were more abundantly (approximately 10-fold) identified from carious dentin (Nascimento and Tjäderhane, unpublished observations). Similar to MMPs, cysteine cathepsins may be activated in mildly acidic environments. Acid activation of dentin-bound cathepsins may further result in activation of matrix-bound MMPs. Moreover, glycosaminoglycans (GAGs) can accelerate conversion of the latent forms of the cathepsin enzyme family into their mature forms at neutral pH (Obermajer et al., 2008). Thus, GAG-cathepsin activation permits active cathepsins to be functional even in neutral pH environments. The presence of cysteine cathepsins in dentinal tubules (Tersariol et al., 2010) indicates that they are derived from the dental pulp via the dentinal fluid and may be activated by mildly acidic adhesive resin monomers. They may also interact with GAGs in the dentinal fluid or the collagen matrix after bonding and neutralization of the acidic monomers and participate with salivary MMPs in the degradation of resin-dentin bonds.
Etch-and-Rinse vs. Self-etch Adhesives
Analysis of data from a recent systematic review on Class V clinical data indicated that the annual failure rates of these bonded restorations are in decreasing order: 1-step self-etch > 2-step etch-and-rinse > 2-step self-etch (aggressive) > 3-step etch-and-rinse > 2-step self-etch (mild and moderately aggressive) (Van Meerbeek et al., 2010). The better durability of the mild 2-step self-etch adhesives may be attributed to the use of a separate, relatively more hydrophobic resin layer on top of the hydrophilic self-etch primer, which renders the interface less susceptible to water sorption, and partial dissolution of the apatite minerals which appear to exert a protective effect on collagen degradation. The latter was demonstrated recently by the minimal involvement of endogenous MMP-2 and MMP-9 in interfaces bonded by the mild two-step self-etch adhesives (De Munck et al., 2010). Nevertheless, mild two-step self-etch adhesives are not totally immune to bond degradation, since they are still hydrophilic, and enzymatic hydrolysis of ester bonds may still occur over time. Failure of water to be completely removed is probably another reason why bond degradation may occur in this class of adhesives, as shown by the presence of silver nanoleakage in the interface of these adhesives. Also, there is no evidence showing that water in the intrafibrillar compartments of these adhesives is completely replaced by resin.
Experimental Strategies to Prevent Degradation of Resin-Dentin Bonds
Increasing the Degree of Conversion and Esterase Resistance of Hydrophilic Adhesives
Resin degradation is directly related to water sorption, since hydrolytic attack on ester linkages by increased acidity/basicity of resin components or by salivary esterases can both occur in the presence of water. The use of a hydrophobic photoinitiator such as camphorquinone for hydrophilic adhesives in the presence of water also does not result in optimal polymerization of those adhesives (Ye et al., 2009a). Human saliva contains sufficient amounts of cholesterol esterase and pseudocholinesterase, which act synergistically to degrade dimethacrylates (Finer et al., 2004). These challenges provide the rationale for the development of water-compatible and esterase-resistant dentin adhesives (Spencer et al., 2010)
The degrees of conversion of hydrophilic adhesive components may be improved by the use of hydrophilic photoinitiators such as QTX [2-hydroxy-3-(3, 4-dimethyl-9-oxo-9H-thio-xanthen-2-yloxy)-N,N,N-trimethyl-1-propanaminium chloride] and compatible accelerators, as well as novel bulky/branched esterase-resistant hydrophilic urethane-modified resin monomers (Hayakawa et al., 2005; Ye et al., 2009a; Park et al., 2008, 2010). Other groups have also used water-soluble photoinitiators such as TPO [diphenyl(2,4,6-trimethylbenzoyl)-phosphine oxide; Cadenaro et al., 2010] and sodium acylphosphine oxide containing crown ether (Ikemura et al., 2009) to improve the polymerization of hydrophilic adhesives in the presence of water. Secondary cross-linking of polar functional groups on methacrylate side-chains may increase the hydrophobic nature of the hybrid layer after initial infiltration of the hydrophilic resin monomers into the partially or completely demineralized collagen matrix. Increasing the degree of conversion of mono- and dimethacrylate resins may also reduce the susceptibility of the polymerized adhesive layer and resins that infiltrated the hybrid layer to esterase hydrolysis by reducing the number of unreacted pendant functional groups.
The use of these novel photoinitiators, accelerators, and cross-linking resin monomers is likely to increase the initial dynamic mechanical properties of the resin-dentin interface, as well as reducing the heterogeneity and nanophase separation of polymer matrices when the latter is polymerized in the presence of water (Park et al., 2010). These strategies, however, will not result in a quantum increase in the ability of adhesive resin monomers to infiltrate a demineralized collagen matrix completely, particularly when water is present within the internal water compartments of the collagen fibrils (Fig. 1A).
Figure 1.

Schematics on the use of experimental adhesives or MMP inhibitors to prevent the degradation of resin-dentin bonds. (A) A schematic depicting the use of experimental adhesives with increased degree of conversion and esterase resistance for bonding to acid-etched dentin. However, such adhesives may not completely infiltrate the entire depth of the hybrid layer. Left side depicts denuded collagen fibrils being degraded by matrix metalloproteinases (MMPs) and cathepsins. Right side depicts cleaved, degraded collagen fragments prevented from dissociating from the collagen molecule via intermolecular and intramolecular cross-links. Because of these cross-links, measuring the amount of hydroxyproline from a degraded dentin collagen matrix is likely to underestimate the extent of collagen degradation. M, MMP; K, cathepsin K. (B) A schematic depicting the use of MMP inhibitors or MMP-inhibitor-conjugated adhesives for bonding to acid-etched dentin. Left side: Unlike MMP-8, MMP-2 is thought to function by unwinding the triple collagen helix prior to scission of the tropocollagen molecules. Right side: The catalytic domain of MMPs is blocked in the presence of a broad-spectrum MMP inhibitor. M, MMP; K, cathepsin K.
Inhibitors of Collagenolytic Enzymes
Matrix metalloproteinases contribute to the degradation of collagen fibrils within incompletely resin-infiltrated hybrid layers (Zhang and Kern, 2009) and the loss of quasi-static mechanical properties of the collagen matrix (Carrilho et al., 2009; Tezvergil-Mutluay et al., 2010a). Thus, application of MMP inhibitors to the demineralized collagen matrix prior to the application of dentin adhesives appears to be a rational approach for extending the longevity of resin-dentin bonds (Pashley et al., 2004) (Fig. 1B). There is a major difference between the selection of appropriate non-specific MMP inhibitors for preventing the degradation of hybrid layers and the design of specific MMP inhibitors for the treatment of diseases – the issue of inhibitor selectivity.
MMPs are associated with a variety of pathological conditions such as cancer, arthritis, and other diseases associated with tissue remodeling. Despite intensive interests to develop synthetic MMP inhibitors capable of potently and selectively blocking the uncontrolled activity of these enzymes under disease conditions, only a few selective and effective drugs with the desired properties have emerged for inhibiting individual MMPs (Li and Wu, 2010). The structural homology of the catalytic domains among members of the MMP family becomes an advantage in the quest for non-specific inhibitors for dentin matrix-bound MMPs and salivary MMPs. Since the application of such inhibitors to acid-etched dentin is analogous to a topical application method and involves nanogram quantities, the issue of systemic toxicity becomes less critical, albeit still important. There are unique problems associated with this use of MMP inhibitors – the issues of substantivity and its release from polymerized resin matrices, as will be discussed below. Chlorhexidine (CHX), a biguanide antimicrobial agent, has been successfully applied to inhibit the activities of MMP-2, MMP-8, and MMP-9 (Gendron et al., 1999). Investigators have used CHX as a non-specific MMP-inhibitor during adhesive application (Pashley et al., 2004; Hebling et al., 2005; Carrilho et al., 2007a,b; De Munck et al., 2009). For etch-and-rinse adhesives, CHX may be applied to the demineralized dentin directly or incorporated into an acid conditioner prior to the application of adhesives, which has been shown to be effective for reducing degradation of resin-dentin bonds after in vivo aging (Hebling et al., 2005; Carrilho et al., 2007b; Brackett et al., 2009; Ricci et al., 2010). Another non-specific MMP inhibitor, GM 6001 (galardin), is often used as a reference inhibitor in generic MMP assay kits. Galardin has been used as an experimental primer on acid-etched dentin prior to the application of an etch-and-rinse adhesive and prevented degradation of the hybrid layer after 12 months (Breschi et al., 2010). Others have shown that polyvinylphosphonic acid (Tezvergil-Mutluay et al., 2010b) and benzalkonium chloride (Pashley, unpublished observations) also possess generic anti-MMP activities. Chemically modified tetracyclines (i.e., tetracyclines that lack antibiotic activities but retain their anti-MMP activities) are effective non-specific MMP inhibitors and have been used as MMP inhibitors in experimental caries (Sulkala et al., 2001) and periodontal disease models (Tjäderhane et al., 2007). They have not been used to prevent the degradation of hybrid layers, since they may stain teeth with a purple hue after photo-oxidation of the tetracycline.
One of the benefits of using CHX as an antimicrobial agent is that it possesses substantivity by binding to mineralized dentin for at least 12 weeks (Mohammadi and Abbott, 2009). It has been recently shown that demineralized dentin can bind more CHX than mineralized dentin (Kim J, 2010b). Although the binding is electrostatic in nature and is reversible, CHX is not de-bound by HEMA and may remain bound to demineralized dentin after bonding (Kim J, 2010b). This may be the reason for the long-term efficacy of CHX as a MMP inhibitor in resin-dentin bonds. Relatively large amounts of CHX remain bound to partially and completely demineralized dentin incubated in phosphate-buffered saline for at least 8 weeks, and no de-binding occurred after the first half-hour of incubation (Carrilho et al., 2010). Since the binding mechanism is only electrostatic (Blackburn et al., 2007), there is concern that CHX may eventually be displaced by competing cations derived from dentinal fluid or saliva. This may account for the recent observation that bonds made to CHX pre-treated acid-etched dentin with commercial adhesives and water-wet bonding were preserved after 9 months but not after 18 months, with severe hybrid layer degradation at 18 months (Sadek et al., 2010b). Such a concern provides the rationale for chemically grafting CHX to resin monomers to create CHX-methacrylates (Luthra and Sandhu, 2005) and incorporating these resin monomers with anti-MMP potentials into dentin adhesives.
Another group of antimicrobial agents, the quaternary ammonium salts, also possesses anti-MMP activities. One of the best-known commercially available quaternary ammonium methacrylates, 12-methacryloyloxydodecylpyridinium bromide (MDPB; Imazato et al., 2007), was also found to possess anti-MMP activities (Tezvergil-Mutluay et al., unpublished observations). This could have accounted, in hindsight, for the in vitro and in vivo observations that resin-dentin bonds degraded after one year when Clearfil SE Bond (Kuraray Medical Inc., Tokyo, Japan) was used as the self-etching primer, while bonds created in the same study with the MDPB-containing self-etching primer Clearfil Protect Bond (Kuraray) were well preserved after one year (Donmez et al., 2005). The recent discovery of more quaternary ammonium methacrylate resin monomers with various degrees of anti-MMP activities (Tezvergil-Mutluay et al., unpublished observations) has brought on a new fervor for incorporating these resin monomers into dentin adhesives to circumvent the displacement of electrostatically bound CHX and other potential non-resin-conjugated MMP inhibitors from denuded collagen matrices of the hybrid layers during aging (Fig. 2A). Copolymerization of CHX-methacrylates or quaternary ammonium methacrylates with other dimethacrylate resin monomers prevents CHX from de-binding and diffusing out of the incompletely resin-infiltrated hybrid layers.
Figure 2.
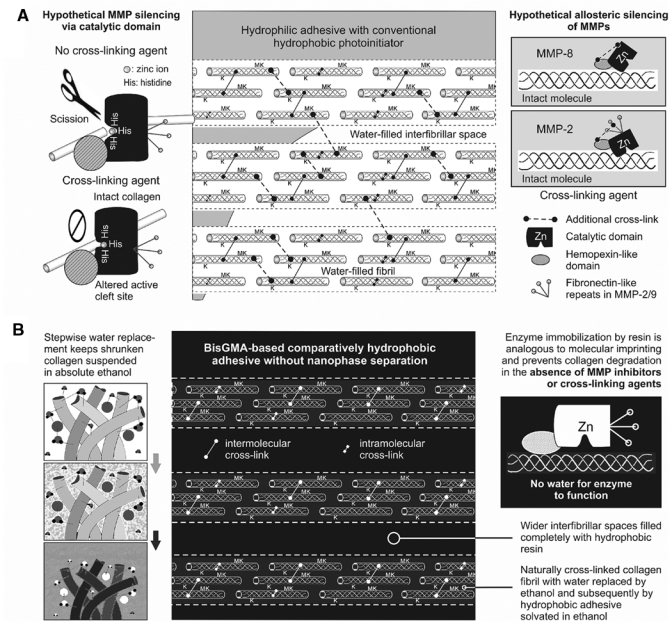
Schematics on the use of cross-linking agents or ethanol wet-bonding to prevent the degradation of resin-dentin bonds. (A) A schematic depicting the use of cross-linking agents for silencing the collagenolytic activities in bonded acid-etched dentin. Cross-linking may cause conformational changes in the active site, catalytic domain, and/or binding site of MMPs, eliminating the enzymes’ ability to degrade the substrate. Left side depicts hypothetical MMP via its catalytic domain. Right side depicts allosteric inhibition of MMPs via their other non-catalytic domains. M, MMP; K, cathepsin K. (B) A schematic depicting the use of the ethanol wet-bonding technique for bonding hydrophobic adhesives to acid-etched dentin. Both apatite-depleted extrafibrillar and intrafibrillar spaces are infiltrated by hydrophobic adhesive without nanophase separation. Left side depicts progressive water replacement of collagen matrix by ethanol, with the shrunken fibrils suspended in ethanol. Right side depicts immobilization of MMP by resin that is analogous to “molecular printing” but without removal of the enzyme. M, matrix metalloproteinase; K, cathepsin K.
For self-etch adhesives, chlorhexidine was incorporated directly into primers (De Munck et al., 2009; Zhou et al., 2010). However, there are severe limitations when CHX is directly incorporated into polymerizable resin monomer formulations. Although incorporation of 1-2% CHX directly into resin blends does not affect their degree of conversion significantly, such a process adversely affects the mechanical properties of the polymerized resins. For example, addition of even 1% CHX to a variety of resin blends with different hydrophilicity resulted in reducing the modulus of elasticity (i.e., stiffness) of the polymerized resins by 27-48% (Cadenaro et al., 2009a). This inhibition effect may also be weakened over time as CHX release from the resins is related to water-induced swelling (Hiraishi et al., 2008). Release of CHX from a polymer matrix is also pH-dependent, with more being released at lower pH values (Anusavice et al., 2006). Chlorhexidine also interacts with calcium fluoride that may be simultaneously incorporated into the resin matrix (Shen et al., 2010).
Since cathepsin K is highly expressed in osteoclasts and is the primary enzyme involved in type I collagen degradation in bone resorption, much work has been done on designing drugs that mimic natural cystatin inhibitors of cathepsins (Liu et al., 2010) as well as specific nitrile-based orally applicable cathepsin K inhibitors (Teno and Masuya, 2010). Little is known about whether these drugs that are targeted at inhibiting specific cathepsins are useful for direct application to acid-etched dentin or incorporation into self-etch adhesives for preventing the degradation of type I dentin collagen scaffolds. Conversely, CHX has been reported to be effective against cysteine proteinases produced by Porphyromonas gingivalis (Houle et al., 2003; Sela et al., 2009) and human recombinant cysteine cathepsins (Scaffa et al., 2010). Thus, it is worth examining if CHX will serve as a non-specific inhibitor for both MMPs and cysteine cathepsins associated with intact and carious dentin.
MMP and Cathepsin Silencing via the Use of Cross-linking Agents
Over the last few years, the experimental use of cross-linking agents to increase the longevity of resin-dentin bonds has taken on a life of its own, with various attempts to use agents such as glutaraldehyde, genipin, proanthrocyanidin, and carbodiimide for long time periods (generally > 1 hr) to introduce additional cross-links to acid-demineralized dentin collagen (Al-Ammar et al., 2009; Macedo et al., 2009; Bedran-Russo et al., 2009, 2010; Castellan et al., 2010a,b). These in vitro studies demonstrated that the use of cross-linking agents improved the short-term mechanical properties of dentin collagen, reduced the susceptibility of additionally cross-linked dentin collagen to enzymatic degradation by collagenases, and increased the stability of the resin-dentin interface.
It is beyond doubt that the use of cross-linking agents will improve the resistance of uncross-linked or mildly cross-linked collagen matrices to degradation by bacterial collagenases (Avila and Navia, 2010; Ma et al., 2010). Dentin collagen is strengthened by native inter- and intramolecular cross-links, which increase its resistance to thermal denaturing and enzymatic degradation (Kuboki and Mechanic, 1982). Since dentin collagen is already highly cross-linked, it is doubtful if the increase in resin-dentin bond longevity can be explained by augmentation in cross-linking density alone. Proanthrocyanidin, for example, is a potential inhibitor of MMP-2 and MMP-9 (Matchett et al., 2005). Current literature suggests that this MMP resistance may be attributed to an alternative mechanism – silencing of MMPs and probably other exogenous collagen degradation enzymes via conformational changes in the enzyme 3-D structure (Busenlehner and Armstrong, 2005). Theoretically, this may be achieved via irreversible changes induced within the catalytic domain or allosteric inhibition of other modular domains that co-participate in collagen degradation (Sela-Passwell et al., 2010) (Fig. 2A).
Natural TIMPs inhibit MMPs by recapitulating the thiol-zinc interaction between the pro-peptide and catalytic domains of pro-MMPs via insertion of a conserved peptide anchor into the catalytic domain. The molecular anchor coordinates a catalytic zinc ion with a cysteine residue along the N-terminal of the TIMP molecule (Gomis-Ruth et al., 1997). Many non-specific, small-molecule MMP-inhibitors exert their inhibitory function by coordinating with the histidine-coupled zinc ion to produce MMP-inhibitor complexes (Tallant et al., 2010). Conversely, the use of cross-linking agents may create multiple cross-links between amino acids within the catalytic site that irreversibly alter the 3-D conformation or flexibility of the cleft-like catalytic domain and prevent its optimal recognition and complexing with the type I collagen substrate (O’Farrell et al., 2006). Although there is no evidence that the catalytic domain of collagenolytic MMPs can be cross-linked to inactivate their functions, oxidative cross-linking of adjacent tryptophan and glycine residues in the catalytic domain of Matrilysin (MMP-7) by hypochlorous acid released by myeloperoxidase resulted in silencing of the catabolic activity of this enzyme (Fu et al., 2004).
The use of cross-linking agents may also contribute to MMP silencing via allosteric control of non-catalytic domains. For example, the catalytic domains in collagenolytic MMPs can cleave non-collagen substrates, but the hemopexin-like domain of these enzymes is essential for them to initially unwind and subsequently cleave the three triple-helical fibrillar elements of the collagen molecule in succession (Lauer-Fields et al., 2009). For MMP-8, binding and orientation of the triple helix through the hemopexin-like domain results in cleavage of the α1(1) and β2(1) chains without discrimination, while the gross conformation of the triple helix is preserved (Gioia et al., 2007). For MMP-2, there are three fibronectin-like repeats that form a domain for binding to collagen or gelatin substrates. This collagen-binding domain binds preferentially to the α1(1) chain and mediates local unwinding and gross alteration of the triple helix prior to the cleavage of the β2(1) chain (Gioia et al., 2007). Regardless of which of the two collagen-binding mechanisms is involved, cross-linking of either the hemopexin-like or fibronectin-like domains may contribute to inactivation of the associated MMPs and reduction in their collagenolytic efficacy. This hypothesis appears to be supported by the results of a study in which MMP-2 and -9 activities of native bovine and porcine pericardium were completely abolished by cross-linking with glutaraldehyde or diphenylphosphorylazide. The use of carbodiimide was less effective, but was more efficacious in reducing MMP-9 than MMP-2 (Calero et al., 2002). These results have recently been confirmed in our laboratory; the use of carbodiimide for 1 min on a thiopeptolide from a generic MMP assay kit completely abolished the lytic activity of purified human recombinant MMP-9. Moreover, treating completely demineralized dentin with carbodiimide for only 1 min resulted in a significant decrease in the amount of free hydroxyproline derived from the degraded collagen after 30 days when compared with untreated demineralized dentin (Tezvergil-Mutluay et al., unpublished observations). Analysis of these data indicates that cross-linking agents can inactivate MMP, which, in turn, is responsible for the reduction of collagen degradation.
Cathepsin K, which breaks down the non-telopeptide triple helix of the collagen molecules, is also allosterically regulated by modifiers such as sulphated glycosaminoglycans (GAGs) outside of its active catalytic site (Novinec et al., 2010). Binding of heparin to cathepsin K induces allosteric conformational changes that increase the collagenolytic activity of the enzyme. Thus, GAGs act as natural allosteric modifiers of cathepsin K. It is possible that the use of cross-linking agents may alter the GAG-cathepsin allosteric interaction and “trap” the enzyme in a certain conformation that inactivates its collagenolytic activity. Cross-linking may also affect MMP activities known to be modified by non-collagenous proteins (Malla et al., 2008). In dentin, MMP activities and resistance to degradation may be regulated by fetuin-A (Ray et al., 2003) and the SIBLINGs Bone Sialoprotein (BSP) and Dentin Matrix Protein-1 (DMP-1) (Fedarko et al., 2004), all of them being present in dentin. Thus, cross-linking of these non-collagenous proteins may indirectly silence MMPs via inactivation of the functional domains of these glycoproteins.
Similar to the use of non-specific inhibitors, the major drawback in the use of cross-linking agents to inactivate MMPs and cysteine cathepsins is that a water-rich, resin-sparse collagen matrix with poor mechanical properties is retained within the hybrid layer. Demineralized dentin collagen has a modulus of elasticity of less than 8 MPa (Carrilho et al., 2009). Even if these demineralized collagen fibrils can be stiffened 50X with cross-linking agents, the resulting modulus of elasticity (ca. 0.4 GPa) is still far inferior to that of resin-infiltrated dentin (ca. 3-5 GPa; Ito et al., 2005; Chiaraputt et al., 2008) and mineralized dentin (ca. 20 GPa; Kinney et al., 2003). Moreover, chemical cross-linking of collagen does not alter the intrinsic collagen molecular stiffness (Liao et al., 2005). Thus, these very flaccid collagen fibrils are susceptible to creep and subsequent fatigue rupture after prolonged function (Fung et al., 2009). This highlights the need for additional strategies that enable the dynamic mechanical properties of denuded collagen matrices to be improved or regained as a mechanism to prevent the degradation of resin-dentin bonds.
Ethanol Wet-bonding with Hydrophobic Resins
During dentin bonding with contemporary hydrophilic etch-and-rinse adhesives, the polar solvents used in these adhesives remove water and cause shrinkage of the collagen matrix (Pashley et al., 2007). After solvent evaporation, the matrix will further shrink, with a higher solvent concentration resulting in greater matrix shrinkage upon evaporation of the solvent. Pre-treatment of water-saturated collagen matrix with 100% alcohol achieves similar matrix shrinkage but prevents phase separation of hydrophobic resin monomers such as BisGMA. That is, wet-bonding with ethanol instead of water provides an opportunity for coaxing hydrophobic monomers into a demineralized collagen matrix without sacrificing any additional matrix shrinkage. Infiltration of hydrophobic monomers into a collagen matrix decreases water sorption/solubility, resin plasticization, and enzyme-catalyzed hydrolytic cleavage of collagen (Hosaka et al., 2009; Sadek et al., 2010b), thereby creating more durable resin bonds.
A demineralized collagen matrix may be rendered more hydrophobic by replacing the water with ethanol, while keeping the collagen matrix suspended in absolute ethanol. Likewise, a hydrophobic resin blend consisting of BisGMA and TEGDMA may be rendered more hydrophilic by dissolving these comonomers in ethanol. These sequential steps allow for improved miscibility of the solvated adhesive and the collagen matrix (Tay et al., 2007), thereby enabling the ethanol-solvated hydrophobic resin blend to infiltrate an ethanol-saturated collagen matrix. Whereas infiltration of resin monomers invariably results in a diffusion gradient of resin infiltration within the collagen matrix, recent studies with two-photon laser confocal microscopy (Sauro et al., 2009a) and micro-Raman spectral analysis (Shin et al., 2009) indicated that a relatively homogeneous distribution of hydrophobic resins within the hybrid layer could be achieved with ethanol wet-bonding (Fig. 2B).
There are two versions of the ethanol wet-bonding technique. In the simplified technique, absolute ethanol is applied to water-saturated acid-etched dentin for 1 min prior to the application of ethanol-solvated hydrophobic resin comonomer blends (Nishitani et al., 2006b; Sauro et al., 2010). The original rationale for this simplified technique was to provide a method of application of hydrophobic resin comonomers to acid-etched dentin within a clinically relevant time frame. However, this technique is extremely technique-sensitive and does not completely reduce dentin permeability or replace the water caused by outward fluid flow without the use of adjunctive tubular occlusion agents (Sadek et al., 2007; Cadenaro et al., 2009b; Sauro et al., 2009b), even with three absolute ethanol applications (Sadek et al., 2010a). In the progressive ethanol replacement version of the technique, water is gradually removed from the collagen matrix via a series of ascending ethanol concentrations (Sadek et al., 2007, 2010b). However, this technique version is time-consuming and impractical for clinical application. For both techniques, when ethanol replacement is not meticulously performed to prevent water-saturated collagen from exposure to air, the surface tension present along the air-collagen interface can easily result in collapse of the collagen matrix and prevent optimal infiltration of the adhesive monomers (Osorio et al., 2010).
Since hydrophobic resins are considerably difficult to apply to dentin, an alternative version of the ethanol wet-bonding technique is to apply hydrophilic adhesives to ethanol-saturated demineralized dentin. Wet-bonding with ethanol achieved higher bond strengths even with hydrophilic resins than were possible with water-saturated matrices (Nishitani et al., 2006b). When hybrid layers created with commercially available etch-and-rinse adhesive using water wet-bonding or ethanol wet-bonding were compared, significantly less micropermeability of the fluorescent tracer could be seen in hybrid layers created with ethanol wet-bonding (Sauro et al., 2009a). The results suggest that ethanol wet-bonding is capable of increasing resin uptake and producing better sealing of the collagen matrix, even with the use of hydrophilic adhesives. The presence of ethanol probably also increases the degree of conversion of the hydrophilic adhesives. Nevertheless, incomplete removal of ethanol from the hydrophilic adhesives may render the polymerized matrix more susceptible to water sorption when compared with the use of hydrophobic adhesives (Malacarne-Zanon et al., 2009). Thus, the use of less-hydrophilic resins in dental adhesives may create more reliable and durable bonds to dentin.
Ethanol wet-bonding is generally conceived to be a bonding philosophy rather than a bonding technique, due to its clinical impracticality. However, it represents a major contribution to adhesive density, since the philosophy behind it reveals the critical barrier to progress in dentin bonding with etch-and-rinse and self-etch adhesives. Hybrid layers created by ethanol wet-bonding were found to contain collagen fibrils with reduced fibrillar diameter and increased interfibrillar spaces (Tay et al., 2007; Hosaka et al., 2009) (Fig. 3). While this permits high resin uptake, such a phenomenon has never been observed in hybrid layers created during water-wet-bonding with hydrophilic etch-and-rinse adhesives, or bonded with water-containing self-etch adhesives. Milder versions of self-etch adhesives are generally more capable of infiltrating the interfibrillar spaces of a partially demineralized collagen matrix via the mechanism of simultaneous etching and resin infiltration. Nevertheless, both self-etch and etch-and-rinse adhesives are incapable of removing water from the intrafibrillar compartments of the collagen fibrils. Hence, the phenomena of fibrillar shrinkage and widened interfibrillar spaces could not be detected in hybrid layers created by these two classes of commercially available adhesives when they were bonded according to the manufacturers’ instructions. With the use of these adhesive techniques, apatite crystallites are removed from both the extrafibrillar and intrafibrillar compartments and subsequently replaced by water. If intrafibrillar spaces are replaced by resin during infiltration, there should be no additional room for filling with additional material. Conversely, intrafibrillar spaces created by both classes of adhesives are amenable to remineralization by apatite crystallites (Kim J et al., 2010c). Such an observation provides indirect evidence that these water-filled intrafibrillar spaces are not completely replaced by resins. However, when ethanol wet-bonding was meticulously performed, neither nanoleakage (Sadek et al., 2008) nor intrafibrillar remineralization (Kim J et al., 2010a) could be detected. This indicates that resin can enter the intrafibrillar compartment if it contains ethanol, but not if it contains water.
Figure 3.
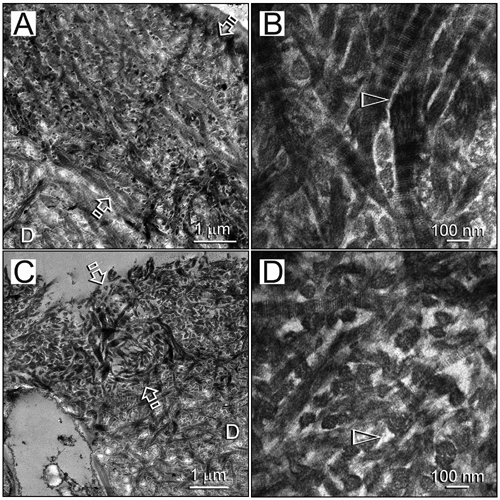
Stained transmission electron micrographs comparing the thickness of hybrid layers (low magnification, between open arrows) and dimensions of the collagen fibrils and interfibrillar spaces (high magnification, open arrowheads). (A,B) Resin-dentin interface bonded with a hydrophilic adhesive by the water wet-bonding technique. (C,D) Resin-dentin interface bonded with an experimental hydrophobic adhesive by the ethanol wet-bonding technique. D, laboratory-demineralized intact dentin.
A recent study showed that resin-dentin bonds created by ethanol wet-bonding with an experimental hydrophobic adhesive did not degrade after 18 months of water storage in the absence of MMP inhibitors (Sadek et al., 2010b). This is an important observation, since it addresses the issue that MMPs are not capable of collagenolysis in the absence of water as a functional medium. During collagen mineralization, bulk water and loosely bound water are progressively removed from the internal compartments of the collagen fibrils and replaced by apatite crystallites (Chesnick et al., 2008). Encapsulating MMPs and cathepsins with hydrophobic resins represents another innovative way of immobilizing the catalytic and allosteric domains of these enzymes to inactivate their functional activities. Such a method is similar to the technique of molecular imprinting to produce abiotic polymers with enzymatic functions (Takeuchi and Hishiya, 2008), with the exception that the enzyme substrate is not removed to expose their functional sites (Fig. 2B). Even if hydrophilic adhesives may infiltrate a collagen matrix to immobilize collagenolytic enzymes initially, resin hydrolysis via esterases and water sorption may subsequently result in re-activation of those immobilized enzymes. Hard tissue fossils from the late Cretaceous era (65 million years ago) were well-preserved at the microstructural and molecular levels and responded to collagenase digestion (Avci et al., 2005). Analysis of these data suggests that MMP-bound collagen must have been functionally immobilized by apatite crystallites for the integrity of those collagen fibrils to be preserved. Conversely, when apatites are replaced by water in dentin bonding, the longevity of these resin-dentin bonds has seldom been shown to last for more than 10 years (van Dijken et al., 2007). Thus, recapitulating the mechanism of molecular immobilization of the functional activity of collagenolytic enzymes created during evolution appears to be a logical biomimetic strategy for extending the longevity of man-made resin-dentin bonds. This may be achieved with a biomimetic mineralization strategy for remineralizing resin-dentin bonds.
Biomimetic Remineralization of Resin-Dentin Bonds
Apart from increases in mechanical properties (Kinney et al., 2003; Balooch et al., 2008), a major role played by the hierarchical deposition of apatite in mineralized collagen is the exclusion of molecules larger than water (ca. 18 Da) from the mineral-protein biocomposite (Lees and Page, 1992). Whereas even ethanol (ca. 46 Da) cannot solvate air-dried mineralized dentin, demineralized, water-filled collagen is susceptible to penetration by larger molecules. Molecules larger than 40 kDa are completely excluded from the internal water compartments of type I collagen, while molecules smaller than 6 kDa can diffuse into all of the water compartments within the collagen fibril (Toroian et al., 2007). The physical exclusion of exogenous collagenolytic enzymes (bacterial collagenase, 68-130 kDa; activated MMP-2, 67 kDa; activated MMP-9, 85 kDa; activated cathepsin K, 27 kDa) by apatite forms the tenet of the “enzyme exclusion” mechanism that protects archeological collagen from degradation (Nielsen-Marsh et al., 2000). Experiments on collagenase hydrolysis of dentin have also confirmed the protective role played by the mineral phase on collagen degradation (Klont and ten Cate, 1991). Similar to a host of growth factors and signaling molecules, endogenous MMPs and cathepsins become “fossilized” (Smith, 2003) within dentin by the apatite minerals, but retain their biologic activities after they are incorporated into the mineralized dentin matrix. These molecules assume their biologic activities upon removal of the mineral phase, provided that the demineralization agent is not strong enough to denature these molecules. As collagen mineralizes, free and loosely bound water is progressively replaced by apatite. This physiologic dehydration mechanism (Chesnick et al., 2008) ensures that the internal environment of the mineralized fibril remains relatively dry to preserve the integrity of the entrapped bioactive molecules.
In dentin bonding, the mineral phase of dentin is intentionally removed by acids, chelating agents, or acidic resin monomers to expose the collagen for creating micromechanical retention of resins. Unfortunately, it seems that contemporary etch-and-rinse and self-etch adhesives are incapable of completely replacing water from the extrafibrillar and intrafibrillar collagen compartments with resin monomers. This can be seen by the number of publications on nanoleakage within hybrid layers. Biomimetic mineralization is a proof-of-concept strategy that utilizes nanotechnology principles to mimic what occurs in biomineralization (Tay and Pashley, 2008). This strategy replaces water from resin-sparse regions of the hybrid layer with apatite crystallites that are small enough to occupy the extrafibrillar and intrafibrillar compartments of the collagen matrix, and has been adopted for remineralization of resin-dentin bonds (Tay and Pashley, 2009). By restoring the enzyme exclusion and fossilization properties of mineralized dentin, this proof-of-concept strategy is capable of preserving the longevity of resin-dentin bonds (Kim YK et al., 2010a).
Remineralization of apatite-depleted, partially demineralized dentin is not new. Reports on remineralization of carious dentin appeared in the dental literature more than half a century ago. The dental literature abounds with reports on the use of fluoride-releasing glass-ionomer cements (Ngo, 2010) and calcium-phosphate-based composites (Peters et al., 2010) for remineralizing partially demineralized carious dentin. Intact, non-denatured collagen is remineralizable as long as seed crystallites are present as nidi for heterogeneous nucleation of calcium phosphate phases (Koutsoukos and Nancollas, 1981). Remineralization by epitaxial growth is a thermodynamically favorable process that overcomes the energy barrier of homogeneous nucleation (Jiang and Liu, 2004). In nanotechnology terminology, remineralization techniques currently used in dentistry represent a top-down approach (Wong et al., 2009). This approach creates materials using scaled down versions of a bulk material that incorporates nanoscale details of the original material. Partial demineralization of mineralized collagen matrix by acids derived from bacteria or dentin-bonding procedures creates the seed crystallites necessary for this top-down remineralization approach. The orientation of those remineralized crystalline lattices is pre-determined by the lattice of the original seed crystallites. However, remineralization does not occur in locations where seed crystallites are absent, as demonstrated with the use of a strontium-based glass-ionomer cement (Kim YK et al., 2010b). This limitation severely restricts the apatite-sparse hybrid layers created by etch-and-rinse and moderately aggressive self-etch adhesives to be remineralized with a conventional top-down approach.
During biomineralization, there are no apatite seed crystallites present in an organic scaffold. Consequently, biomineralization has to proceed via an alternative pathway that involves homogeneous nucleation. One of the mechanisms of homogeneous nucleation involves sequestration of the amorphous mineral phase by polyanionic extracellular matrix protein molecules. Additional acidic matrix phosphoproteins are utilized as templates to induce mineral nucleation and growth within the organic scaffold (George and Veis, 2008). Fluoride, which has been shown to enhance conventional remineralization via the top-down approach, is not a functional motif for biomineralization. Homogeneous nucleation is not as thermodynamically favorable and involves alternative kinetically driven protein/polymer-modulated pathways for lowering the activation energy barrier for crystal nucleation via sequential steps of phase transformation (Wang and Nancollas, 2009). In nanotechnology terminology, such pathways are examples of a bottom-up approach (Wong et al., 2009), wherein nanoscale materials are created by a particle-based self-assembly process (Xu et al., 2007; Gower, 2008). This process is compatible with the observation of amorphous calcium phosphate phases in the biomineralization of hard tissues (Mahamid et al., 2010).
Although it is possible to utilize recombinant matrix proteins as nucleating agents for in situ mineralization, the cost of production of these molecules renders their use in restorative dentistry prohibitive. Accordingly, scientists have resorted to the use of poly(anionic) acid molecules to mimic the functional domains of naturally occurring mineralization-promoting proteins. Biomimetic remineralization of resin-dentin bonds adopts the bottom-up biomineralization approach by using two polyanionic analogs of acidic matrix proteins to separately mimic the sequestration and templating functional motifs that are present in naturally occurring matrix protein molecules (Fig. 4A). This approach does not rely on the presence of remnant dentin matrix proteins within a demineralized collagen matrix and can mineralize reconstituted collagen that is devoid of mineralization-promoting proteins (Kim YK et al., 2010c). A polycarboxylic acid such as polyacrylic acid (Girija et al., 2004) was used as a sequestration agent to simulate the aspartic acid and serine-rich mineralization-promoting C-terminal domain (ASARM) of cleaved DMP-1 (Gericke et al., 2010) to stabilize amorphous calcium phosphate into fluidic nanoprecursor particles (Gower, 2008; Nudelman et al., 2010). The latter has the ability to infiltrate the microfibrillar spaces within collagen fibrils and produce initially amorphous, intertwining mineral strands within the fibril (Kim J et al., 2010c). A phosphorus-based analog of matrix phosphoproteins (George and Veis, 2008), such as polyvinylphosphonic acid, is used as a collagen-binding template to induce the nucleation and growth of apatite from the initial amorphous mineral phase (Gajjeraman et al., 2008). In the past, sequestration or templating analogs had been separately used for biomineralization, without success in producing intrafibrillar apatites. For example, when only polyaspartic acid was used as the sequestration analog, infiltration of amorphous calcium phosphate nanoprecursors into reconstituted collagen fibrils produced intrafibrillar mineralization that apparently lacked the hierarchical order of apatite arrangement in natural mineralized collagen (Deshpande and Beniash, 2008). Conversely, when only a templating analog was used, only large extrafibrillar mineral spheres were deposited around the collagen matrix (Li and Chang, 2008). Thus, two biomimetic analogs (sequestration and templating) must be utilized in concert to reproduce the dimension and hierarchy of the apatite crystallites that are found in natural mineralized dentin.
Figure 4.
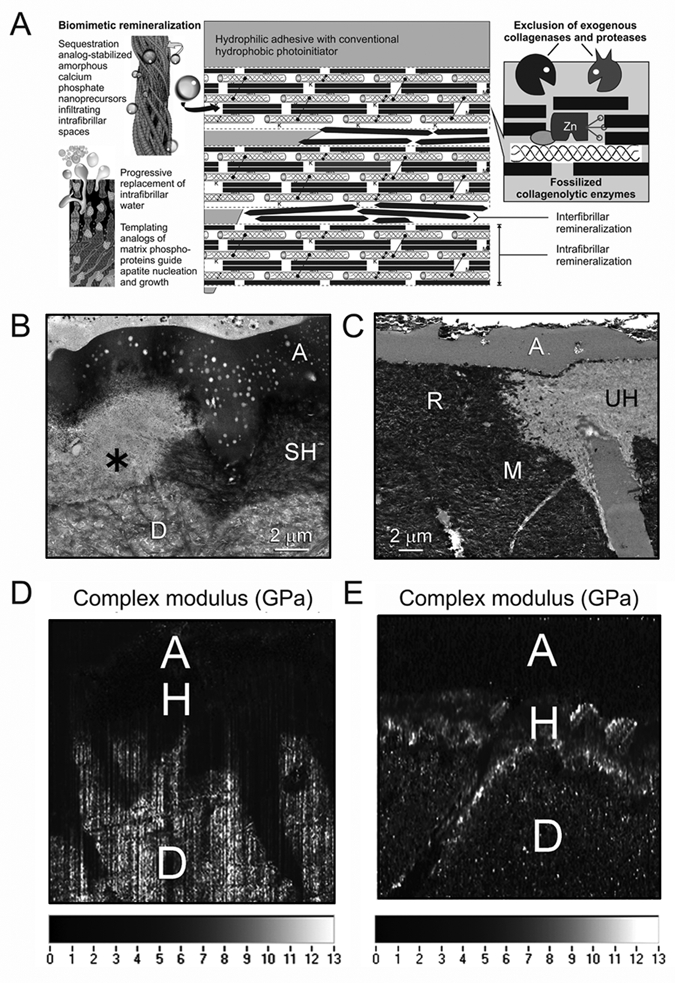
Biomimetic remineralization of resin-dentin bonds as a potential means of preventing the degradation of resin-dentin bonds. (A) A schematic depicting the use of a biomimetic remineralization mechanism for refilling water-rich, resin-sparse regions of the hybrid layer with apatite for exclusion of exogenous collagenolytic enzymes and fossilization of endogenous collagenolytic enzymes. Left side depicts the action of a sequestration analog such as polyaspartic acid or polyacrylic acid in stabilizing amorphous calcium phosphate nanoprecursors, and the use of a templating analog such as sodium trimetaphosphate to initiate the nucleation and growth of apatite within the intrafibrillar spaces of a collagen fibril. Right side depicts apatite crystallites fossilizing the collagen molecules, protecting them from exogenous and endogenous collagen-degrading enzymes. M, MMP; K, cathepsin K. (B) Stained transmission electron micrograph showing the degradation of collagen fibrils within the hybrid layer (asterisk) after aging. SH, stained, intact hybrid layer; A, dentin adhesive; D, laboratory-demineralized intact dentin. (C) Unstained transmission electron micrograph of the resin-dentin interface that has undergone 3 months of biomimetic remineralization with a remineralizing composite and in the presence of sequestration and templating biomimetic analogs present in the incubation medium. Note the striking similarity between the remineralized part of the hybrid layer (R) and the degraded part of the hybrid layer shown in Fig. 4B. UH, unstained, non-degraded part of the hybrid layer; A, adhesive; M, mineralized intact dentin. (D) Nanoscopic dynamic mechanical analysis showing the distribution of complex modulus (i.e., loss modulus/storage modulus) across the resin-dentin interface after 3 months of immersion in a control medium. A, adhesive; H, hybrid layer; D, intact dentin. Note the complete lack of high modulus (i.e., light-colored material within the hybrid layer or resin tag). (E) Nanoscopic dynamic mechanical analysis showing the distribution of complex modulus (i.e., loss modulus/storage modulus) across the resin-dentin interface after 3 months of biomimetic remineralization. A, adhesive; H, hybrid layer; D, intact dentin. Note the presence of high-modulus material throughout the hybrid layer and resin tag.
Both hybrid layers created by etch-and-rinse adhesives (Mai et al., 2009, 2010) and self-etch adhesives (Kim J et al., 2010c,d) have been shown to be remineralizable with a biomimetic mineralization approach. For etch-and-rinse adhesives, apatite crystallites can be detected in both extrafibrillar and intrafibrillar spaces, since denuded collagen matrices are present within those hybrid layers. It is amazing how strikingly similar the remineralized part of the hybrid layer is when compared with hybrid layers that have undergone degradation after aging (Figs. 4B, 4C). For self-etch adhesives, apatite deposition is almost exclusively identified from the intrafibrillar spaces, indicating that the extrafibrillar spaces are better infiltrated by adhesive resins. In addition, water-filled spaces within the adhesive layers of some adhesives are also filled by apatite nanocrystals (Kim J et al., 2010a; Kim YK et al., 2010a). Unlike the conventional top-down remineralization approach that proceeds rapidly via epitaxial growth over existing seed crystallites, biomimetic remineralization is a slower process, since it involves at least two kinetically driven pathways and usually takes 3-4 months to complete. Thus, it is important to prevent the collagen matrix from degrading while it is being remineralized. In a proof-of-concept biomimetic remineralization strategy, polyvinylphosphonic acid has been shown to possess MMP-inhibiting properties that prevent collagen degradation during remineralization (Tezvergil-Mutluay et al., 2010b).
A critical problem encountered with the use of the experimental strategies described in the previous four sections is that the water-filled denuded collagen fibrils remain flaccid and exhibit weak mechanical properties, even with preservation of their integrity (Bertassoni et al., 2009). The modulus of elasticity of resin-infiltrated dentin beams increased by 55-118% after biomimetic remineralization as a result of intrafibrillar remineralization of the collagen matrices (Gu et al., 2010). A limitation of that study, however, is that the resin-infiltrated dentin beams (macro-hybrid layers) were evaluated en masse by three-point bending. Since remineralization does not occur in locations of the collagen matrices that are occupied by resins, three-point bending is insensitive for evaluating the localized increases in modulus of elasticity of the remineralized collagen. Due to the complexity of these regions, we have resorted to the use of nanoscopic dynamic mechanical analysis (nano-DMA) for characterizing the viscoplastic mechanical behavior of resin-infiltrated dentin before and after biomimetic remineralization. This is achieved with scanning probe microscopy (SPM) attached to a tribo-indentor to perform rasterized imaging and nanoindentations across an area of interest (Arola et al., unpublished observations). We have developed a method of scanning these hybrid layers under hydrated conditions, preventing water from evaporating. Analysis of our preliminary data from use of the combined nanoDMA and SPM approach indicates that biomimetic remineralization is capable of restoring the storage modulus, loss modulus, and complex modulus of water-rich, resin-sparse regions of the hybrid layer to those values characteristic of intact mineralized dentin (Figs. 4D, 4E).
To date, studies published on biomimetic remineralization of resin-dentin bonds represent a proof-of-concept for that approach. Remineralization was performed via a lateral diffusion mechanism by the immersion of specimen slabs in a remineralizing medium containing dissolved biomimetic analogs. Experiments are under development for translating this proof-of-concept strategy into a clinically applicable technique. For example, an experimental calcium-silicate-based hydrophilic composite has been developed for the sustained release of calcium and hydroxyl ions (Kim YK et al., 2010c). While this method is useful for remineralizing resin-dentin interfaces with thin adhesive layers (Fig. 4C), remineralization is hampered when a thick adhesive layer is encountered (Dickens and Flaim, 2008). Thus, alternative strategies are being developed to deliver calcium, hydroxyl, and phosphate ions to the base of the hybrid layers created by etch-and-rinse adhesives, where remineralization is most needed. Additional work is also needed to deliver sequestration and templating analogs to the collagen matrix. It is at the nanotechnology level that one would expect the greatest development of translational strategies for biomimetic remineralization as a means to preserve the longevity of resin-dentin bonds.
Conclusions
Contemporary dentin bonding is not as durable as has previously been assumed. Two major mechanisms are involved in the degradation of resin-dentin bonds over time. One mechanism is the slow hydrolysis of resin components caused by water sorption and/or esterases, which are promoted by the increased hydrophilicity of contemporary adhesive formulations. The other is degradation of water-rich, resin-sparse collagen fibrils within hybrid layers by the activation of host-derived MMPs and possibly cysteine cathepsins during bonding procedures. Complete replacement of free and loosely bound water within the collagen water compartments and inactivation or silencing of collagenolytic enzymes appear to be the ultimate goals for improving the durability of resin-dentin bonds. During the past five years, the knowledge acquired from experimental techniques to increase the service life of resin-based bonding procedures has been nothing short of phenomenal. This pool of knowledge is summarized into five experimental strategies that attempt to address different problems encountered in dentin bonding. While each strategy has its merits and limitations, it is probably the amalgamation and translation of several strategies into a single treatment approach that may overcome the critical barriers currently encountered in bonding to dentin.
Footnotes
This study was supported by Grant R21 DE019213-02 (PI: Franklin R. Tay) and Grant R01 DE015306-06 (PI: David H. Pashley) from the National Institute of Dental and Craniofacial Research . The authors are grateful to Michelle Barnes for secretarial support.
References
- Aida M, Odaki M, Fujita K, Kitagawa T, Teshima I, Suzuki K, et al. (2009). Degradation-stage effect of self-etching primer on dentin bond durability. J Dent Res 88:443-448 [DOI] [PubMed] [Google Scholar]
- Al-Ammar A, Drummond JL, Bedran-Russo AK. (2009). The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res B Appl Biomater 91:419-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral FL, Colucci V, Palma-Dibb RG, Corona SA. (2007). Assessment of in vitro methods used to promote adhesive interface degradation: a critical review. J Esthet Restor Dent 19:340-353 [DOI] [PubMed] [Google Scholar]
- Anusavice KJ, Zhang NZ, Shen C. (2006). Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res 85:950-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, et al. (2004). Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Oper Dent 29:705-712 [PubMed] [Google Scholar]
- Avci R, Schweitzer MH, Boyd RD, Wittmeyer JL, Terán Arce F, Calvo JO. (2005). Preservation of bone collagen from the late Cretaceous period studied by immunological techniques and atomic force microscopy. Langmuir 21:3584-3590 [DOI] [PubMed] [Google Scholar]
- Avila MY, Navia JL. (2010). Effect of genipin collagen crosslinking on porcine corneas. J Cataract Refract Surg 36:659-664 [DOI] [PubMed] [Google Scholar]
- Balooch M, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. (2008). Mechanical properties of mineralized collagen fibrils as influenced by demineralization. J Struct Biol 162:404-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. (2009). Mechanical properties of tannic-acid-treated dentin matrix. J Dent Res 88:807-811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran-Russo AK, Vidal CM, Dos Santos PH, Castellan CS. (2010). Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J Biomed Mater Res B Appl Biomater 94:250-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni LE, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. (2009). Biomechanical perspective on the remineralization of dentin. Caries Res 43:70-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn RS, Harvey A, Kettle LL, Manian AP, Payne JD, Russell SJ. (2007). Sorption of chlorhexidine on cellulose: mechanism of binding and molecular recognition. J Phys Chem B 111:8775-8784 [DOI] [PubMed] [Google Scholar]
- Boukpessi T, Menashi S, Camoin L, ten Cate JM, Goldberg M, Chaussain-Miller C. (2008). The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials 29:4367-4373 [DOI] [PubMed] [Google Scholar]
- Boushell LW, Kaku M, Mochida Y, Bagnell R, Yamauchi M. (2008). Immunohistochemical localization of matrixmetalloproteinase-2 in human coronal dentin. Arch Oral Biol 53:109-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackett MG, Tay FR, Brackett WW, Dib A, Dipp FA, Mai S, et al. (2009). in vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Oper Dent 34:379-383 [DOI] [PubMed] [Google Scholar]
- Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. (2008). Dental adhesion review: aging and stability of the bonded interface. Dent Mater 24:90-101 [DOI] [PubMed] [Google Scholar]
- Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. (2010). Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater 26:571-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busenlehner LS, Armstrong RN. (2005). Insights into enzyme structure and dynamics elucidated by amide H/D exchange mass spectrometry. Arch Biochem Biophys 433:34-46 [DOI] [PubMed] [Google Scholar]
- Cadenaro M, Antoniolli F, Sauro S, Tay FR, Di Lenarda R, Prati C, et al. (2005). Degree of conversion and permeability of dental adhesives. Eur J Oral Sci 113:525-530 [DOI] [PubMed] [Google Scholar]
- Cadenaro M, Breschi L, Rueggeberg FA, Agee K, Di Lenarda R, Carrilho M, et al. (2009a). Effect of adhesive hydrophilicity and curing time on the permeability of resins bonded to water vs. ethanol-saturated acid-etched dentin. Dent Mater 25:39-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenaro M, Pashley DH, Marchesi G, Carrilho M, Antoniolli F, Mazzoni A, et al. (2009b). Influence of chlorhexidine on the degree of conversion and E-modulus of experimental adhesive blends. Dent Mater 25:1269-1274 [DOI] [PubMed] [Google Scholar]
- Cadenaro M, Antoniolli F, Codan B, Agee K, Tay FR, Dorigo Ede S, et al. (2010). Influence of different initiators on the degree of conversion of experimental adhesive blends in relation to their hydrophilicity and solvent content. Dent Mater 26:288-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero P, Jorge-Herrero E, Turnay J, Olmo N, López de Silanes I, Lizarbe MA, et al. (2002). Gelatinases in soft tissue biomaterials. Analysis of different crosslinking agents. Biomaterials 23:3473-3478 [DOI] [PubMed] [Google Scholar]
- Carrilho MR, Carvalho RM, Tay FR, Yiu C, Pashley DH. (2005). Durability of resin-dentin bonds related to water and oil storage. Am J Dent 18:315-319 [PubMed] [Google Scholar]
- Carrilho MR, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, et al. (2007a). Chlorhexidine preserves dentin bond in vitro/ . J Dent Res 86:90-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrilho MR, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjäderhane L, et al. (2007b). In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res 86:529-533 [DOI] [PubMed] [Google Scholar]
- Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, et al. (2009). Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater 90:373-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrilho MR, Carvalho RM, Sousa EN, Nicolau J, Breschi L, Mazzoni A, et al. (2010). Substantivity of chlorhexidine to human dentin. Dent Mater 26:779-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. (2010a). Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater 26:968-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan CS, Pereira PN, Viana G, Chen SN, Pauli GF, Bedran-Russo AK. (2010b). Solubility study of phytochemical cross-linking agents on dentin stiffness. J Dent 38:431-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. (2006). The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res 85:22-32 [DOI] [PubMed] [Google Scholar]
- Chesnick IE, Mason JT, Giuseppetti AA, Eidelman N, Potter K. (2008). Magnetic resonance microscopy of collagen mineralization. Biophys J 95:2017-2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaraputt S, Mai S, Huffman BP, Kapur R, Agee KA, Yiu CKY, et al. (2008). Changes in resin-infiltrated dentin stiffness after water storage. J Dent Res 87:655-660 [DOI] [PubMed] [Google Scholar]
- De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Suzuki K, Lambrechts P. (2004). Four-year water degradation of a resin-modified glass-ionomer adhesive bonded to dentin. Eur J Oral Sci 112:73-83; erratum in Eur J Oral Sci 112:205, 2004 [DOI] [PubMed] [Google Scholar]
- De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G. (2009). Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res 88:1101-1106 [DOI] [PubMed] [Google Scholar]
- De Munck J, Mine A, Van den Steen PE, Van Landuyt KL, Poitevin A, Opdenakker G, et al. (2010). Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. Eur J Oral Sci 118:494-501 [DOI] [PubMed] [Google Scholar]
- Deshpande AS, Beniash E. (2008). Bio-inspired synthesis of mineralized collagen fibrils. Cryst Growth Des 8:3084-3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens SH, Flaim GM. (2008). Effect of a bonding agent on in vitro biochemical activities of remineralizing resin-based calcium phosphate cements. Dent Mater 24:1273-1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DP. (2002). Cysteine peptidases of mammals: their biological roles and potential effects in the oral cavity and other tissues in health and disease. Crit Rev Oral Biol Med 13:238-275 [DOI] [PubMed] [Google Scholar]
- Donmez N, Belli S, Pashley DH, Tay FR. (2005). Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res 84:355-359; erratum in J Dent Res 85:384, 2006 [DOI] [PubMed] [Google Scholar]
- Fedarko NS, Jain A, Karadag A, Fisher LW. (2004). Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J 18:734-736 [DOI] [PubMed] [Google Scholar]
- Ferracane JL. (2006). Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater 22:211-222 [DOI] [PubMed] [Google Scholar]
- Finer Y, Jaffer F, Santerre JP. (2004). Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials 25:1787-1793 [DOI] [PubMed] [Google Scholar]
- Fu X, Kao JL, Bergt C, Kassim SY, Huq NP, d’Avignon A, et al. (2004). Oxidative cross-linking of tryptophan to glycine restrains matrix metalloproteinase activity: specific structural motifs control protein oxidation. J Biol Chem 279:6209-6212 [DOI] [PubMed] [Google Scholar]
- Fung DT, Wang VM, Laudier DM, Shine JH, Basta-Pljakic J, Jepsen KJ, et al. (2009). Subrupture tendon fatigue damage. J Orthop Res 27:264-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajjeraman S, He G, Narayanan K, George A. (2008). Biological assemblies provide novel templates for the synthesis of hierarchical structures and facilitate cell adhesion. Adv Funct Mater 18:3972-3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, et al. (1998). The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem 273:32347-32352 [DOI] [PubMed] [Google Scholar]
- Gendron R, Grenier D, Sorsa T, Mayrand D. (1999). Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol 6:437-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A, Veis A. (2008). Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev 108:4670-4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, et al. (2010). Different forms of DMP1 play distinct roles in mineralization. J Dent Res 89:355-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia M, Monaco S, Fasciglione GF, Coletti A, Modesti A, Marini S, et al. (2007). Characterization of the mechanisms by which gelatinase A, neutrophil collagenase, and membrane-type metalloproteinase MMP-14 recognize collagen I and enzymatically process the two alpha-chains. J Mol Biol 368:1101-1113 [DOI] [PubMed] [Google Scholar]
- Girija EK, Yokogawa Y, Nagata F. (2004). Apatite formation on collagen fibrils in the presence of polyacrylic acid. J Mater Sci Mater Med 15:593-599 [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth FX, Maskos K, Betz M, Bergner A, Huber R, Suzuki K, et al. (1997). Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature 389:77-81 [DOI] [PubMed] [Google Scholar]
- Gower LB. (2008). Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev 108:4551-4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu LS, Huffman BP, Arola DD, Kim YK, Mai S, Elsalanty ME, et al. (2010). Changes in stiffness of resin-infiltrated demineralized dentin after remineralization by a bottom-up biomimetic approach. Acta Biomater 6:1453-1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. (2007). The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand 65:1-13 [DOI] [PubMed] [Google Scholar]
- Hashimoto M. (2010). A review—micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. J Biomed Mater Res B Appl Biomater 92:268-280 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Fujita S, Endo K, Ohno H. (2009). Effect of dentinal water on bonding of self-etching adhesives. Dent Mater J 28:634-641 [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kikutake-Sugiyama K, Nemoto K. (2005). Efficacy of water-soluble photoinitiator on the adhesion of composite resin to bovine teeth in all-in-one bonding system. Dent Mater J 24:213-218 [DOI] [PubMed] [Google Scholar]
- Hebling J, Pashley DH, Tjäderhane L, Tay FR. (2005). Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo . J Dent Res 84:741-746 [DOI] [PubMed] [Google Scholar]
- Hiraishi N, Yiu CK, King NM, Tay FR, Pashley DH. (2008). Chlorhexidine release and water sorption characteristics of chlorhexidine-incorporated hydrophobic/hydrophilic resins. Dent Mater 24:1391-1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K, Nishitani Y, Tagami J, Yoshiyama M, Brackett WW, Agee KA, et al. (2009). Durability of resin-dentin bonds to water- vs. ethanol-saturated dentin. J Dent Res 88:146-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle MA, Grenier D, Plamondon P, Nakayama K. (2003). The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. FEMS Microbiol Lett 221:181-185 [DOI] [PubMed] [Google Scholar]
- Ikemura K, Ichizawa K, Fuchigami K, Ito S, Endo T. (2009). Design of a new dental adhesive—effect of a water-soluble sodium acylphosphine oxide with crown ether on adhesion to dental hard tissues. Dent Mater J 28:267-276 [DOI] [PubMed] [Google Scholar]
- Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. (2007). An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent Mater 23:170-176 [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Yamashita K, Nakagaki H, Iwata K, Hayakawa T. (1994). Identification of tissue inhibitor of metalloproteinases-1 (TIMP-1) in human teeth and its distribution in cementum and dentine. Arch Oral Biol 39:345-349 [DOI] [PubMed] [Google Scholar]
- Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. (2005). Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 26:6449-6459 [DOI] [PubMed] [Google Scholar]
- Jiang H, Liu XY. (2004). Principles of mimicking and engineering the self-organized structure of hard tissues. J Biol Chem 297:41286-41293 [DOI] [PubMed] [Google Scholar]
- Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. (2001). Quality of dental restorations. FDI Commission Projects 2-95. Int Dent J 51:117-158 [DOI] [PubMed] [Google Scholar]
- Kim J, Arola DD, Gu L, Kim YK, Mai S, Liu Y, et al. (2010a). Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach. Acta Biomater 6:2740-2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Gu L, Breschi L, Tjäderhane L, Choi KK, Pashley DH, et al. (2010b). Implication of ethanol wet-bonding in hybrid layer remineralization. J Dent Res 89:575-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, et al. (2010c). Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater 26:771-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Vaughn RM, Gu L, Rockman RA, Arola DD, Schafer TE, et al. (2010d). Imperfect hybrid layers created by an aggressive one-step self-etch adhesive in primary dentin are amendable to biomimetic remineralization in vitro . J Biomed Mater Res A 93:1225-1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Gu LS, Bryan TE, Kim JR, Chen L, Liu Y, et al. (2010a). Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials 31:6618-6627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Mai S, Mazzoni A, Liu Y, Tezvergil-Mutluay A, Takahashi K, et al. (2010b). Biomimetic remineralization as a progressive dehydration mechanism of collagen matrices—Implications in the aging of resin-dentin bonds. Acta Biomater 6:3729-3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Yiu CK, Kim JR, Gu L, Kim SK, Weller RN, et al. (2010c). Failure of a glass ionomer to remineralize apatite-depleted dentin. J Dent Res 89:230-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. (2003). The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res 82:957-961 [DOI] [PubMed] [Google Scholar]
- Klont B, ten Cate JM. (1991). Susceptibility of the collagenous matrix from bovine incisor roots to proteolysis after in vitro lesion formation. Caries Res 25:46-50 [DOI] [PubMed] [Google Scholar]
- Kostoryz EL, Dharmala K, Ye Q, Wang Y, Huber J, Park JG, et al. (2009). Enzymatic biodegradation of HEMA/bisGMA adhesives formulated with different water content. J Biomed Mater Res B Appl Biomater 88:394-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoukos PG, Nancollas GH. (1981). Crystal growth of calcium phosphates—epitaxial considerations. J Cryst Growth 53:10-19 [Google Scholar]
- Kuboki Y, Mechanic GL. (1982). Comparative molecular distribution of cross-link in bone and dentin collagen. Structure-function relationships. Calcif Tissue Int 34:306-308 [DOI] [PubMed] [Google Scholar]
- Lauer-Fields JL, Chalmers MJ, Busby SA, Minond D, Griffin PR, Fields GB. (2009). Identification of specific hemopexin-like domain residues that facilitate matrix metalloproteinase collagenolytic activity. J Biol Chem 284:24017-24024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees S, Page EA. (1992). A study of some properties of mineralized turkey leg tendon. Connect Tissue Res 28:263-287 [DOI] [PubMed] [Google Scholar]
- Lehmann N, Debret R, Roméas A, Magloire H, Degrange M, Bleicher F, et al. (2009). Self-etching increases matrix metalloproteinase expression in the dentin-pulp complex. J Dent Res 88:77-82 [DOI] [PubMed] [Google Scholar]
- Li X, Chang J. (2008). Preparation of bone-like apatite-collagen nanocomposites by a biomimetic process with phosphorylated collagen. J Biomed Mater Res A 85:293-300 [DOI] [PubMed] [Google Scholar]
- Li X, Wu JF. (2010). Recent developments in patent anti-cancer agents targeting the matrix metalloproteinases (MMPs). Recent Pat Anticancer Drug Discov 5:109-141 [DOI] [PubMed] [Google Scholar]
- Liao J, Yang L, Grashow J, Sacks MS. (2005). Molecular orientation of collagen in intact planar connective tissues under biaxial stretch. Acta Biomater 1:45-54 [DOI] [PubMed] [Google Scholar]
- Liu YH, Han YP, Li ZY, Wei J, He HJ, Xu CZ, et al. (2010). Molecular cloning and characterization of cystatin, a cysteine protease inhibitor, from Angiostrongylus cantonensis . Parasitol Res 107:915-922 [DOI] [PubMed] [Google Scholar]
- Loguercio AD, Moura SK, Pellizzaro A, Dal-Bianco K, Patzlaff RT, Grande RH, et al. (2008). Durability of enamel bonding using two-step self-etch systems on ground and unground enamel. Oper Dent 33:79-88 [DOI] [PubMed] [Google Scholar]
- Luthra AK, Sandhu SS. (2005). Methods and clinical devices for the inhibition or prevention of mammalian cell growth. United States Patent 6,929,818. URL available at: http://www.freepatentsonline.com/6929818.html (accessed 10/13/2010).
- Ma DH, Lai JY, Cheng HY, Tsai CC, Yeh LK. (2010). Carbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cells. Biomaterials 31:6647-6658 [DOI] [PubMed] [Google Scholar]
- Macedo GV, Yamauchi M, Bedran-Russo AK. (2009). Effects of chemical cross-linkers on caries-affected dentin bonding. J Dent Res 88:1096-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li C, Siegel S, et al. (2010). Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc Natl Acad Sci USA 107:6316-6321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S, Kim YK, Toledano M, Breschi L, Ling JQ, Pashley DH, et al. (2009). Phosphoric acid esters cannot replace polyvinylphosphonic acid as phosphoprotein analogs in biomimetic remineralization of resin-bonded dentin. Dent Mater 25:1230-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S, Kim YK, Kim J, Yiu CK, Ling J, Pashley DH, et al. (2010). In vitro remineralization of severely compromised bonded dentin. J Dent Res 89:405-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, et al. (2006). Water sorption/solubility of dental adhesive resins. Dent Mater 22:973-980 [DOI] [PubMed] [Google Scholar]
- Malacarne-Zanon J, Pashley DH, Agee KA, Foulger S, Alves MC, Breschi L, et al. (2009). Effects of ethanol addition on the water sorption/ solubility and percent conversion of comonomers in model dental adhesives. Dent Mater 25:1275-1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla N, Sjøli S, Winberg JO, Hadler-Olsen E, Uhlin-Hansen L. (2008). Biological and pathobiological functions of gelatinase dimers and complexes. Connect Tissue Res 49:180-184 [DOI] [PubMed] [Google Scholar]
- Marshall SJ, Bayne SC, Baier R, Tomsia AP, Marshall GW. (2010). A review of adhesion science. Dent Mater 26:e11-e16 [DOI] [PubMed] [Google Scholar]
- Matchett MD, MacKinnon SL, Sweeney MI, Gottschall-Pass KT, Hurta RA. (2005). Blueberry flavonoids inhibit matrix metalloproteinase activity in DU145 human prostate cancer cells. Biochem Cell Biol 83:637-643 [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. (2006). Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials 27:4470-4476 [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. (2007). Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res 86:436-440 [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Tay FR, Gobbi P, Orsini G, Ruggeri A, Jr, et al. (2009). Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: correlative FEI-SEM/TEM analysis. J Biomed Mater Res A 88:697-703 [DOI] [PubMed] [Google Scholar]
- Meldrum FC, Cölfen H. (2008). Controlling mineral morphologies and structures in biological and synthetic systems. Chem Rev 108:4332-4432 [DOI] [PubMed] [Google Scholar]
- Mohammadi Z, Abbott PV. (2009). Antimicrobial substantivity of root canal irrigants and medicaments: a review. Aust Endod J 35:131-139 [DOI] [PubMed] [Google Scholar]
- Moszner N, Salz U, Zimmermann J. (2005). Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater 21:895-910 [DOI] [PubMed] [Google Scholar]
- Ngo H. (2010). Glass-ionomer cements as restorative and preventive materials. Dent Clin North Am 54:551-563 [DOI] [PubMed] [Google Scholar]
- NIDCR Strategic Plan 2009-2013. URL available at http://www.nidcr.nih.gov/Research/ResearchPriorities/StrategicPlan (accessed 10/13/2010).
- Nielsen-Marsh CM, Hedges REM, Mann T, Collins MJ. (2000). A preliminary investigation of the application of differential scanning calorimetry to the study of collagen degradation in archaeological bone. Thermochim Acta 365:129-139 [Google Scholar]
- Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, et al. (2006a). Effects of resin hydrophilicity on dentin bond strength. J Dent Res 85:1016-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. (2006b). Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci 114:160-166 [DOI] [PubMed] [Google Scholar]
- Novinec M, Kovacic L, Lenarcic B, Baici A. (2010). Conformational flexibility and allosteric regulation of cathepsin K. Biochem J 429:379-389 [DOI] [PubMed] [Google Scholar]
- Nudelman F, Pieterse K, George A, Bomans PH, Friedrich H, Brylka LJ, et al. (2010). The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater 9:1004-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell TJ, Guo R, Hasegawa H, Pourmotabbed T. (2006). Matrix metalloproteinase-1 takes advantage of the induced fit mechanism to cleave the triple-helical type I collagen molecule. Biochemistry 45:15411-15418 [DOI] [PubMed] [Google Scholar]
- Obermajer N, Jevnikar Z, Doljak B, Kos J. (2008). Role of cysteine cathepsins in matrix degradation and cell signalling. Connect Tissue Res 49:193-196 [DOI] [PubMed] [Google Scholar]
- Osorio E, Toledano M, Aguilera FS, Tay FR, Osorio R. (2010). Ethanol wet bonding technique sensitivity assessed by AFM. J Dent Res 89:1264-1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Ye Q, Topp EM, Misra A, Kieweg SL, Spencer P. (2010). Effect of photoinitiator system and water content on dynamic mechanical properties of a light-cured bisGMA/HEMA dental resin. J Biomed Mater Res A 93:1245-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Ye Q, Topp EM, Kostoryz EL, Wang Y, Kieweg SL, et al. (2008). Preparation and properties of novel dentin adhesives with esterase resistance. J Appl Polym Sci 107:3588-3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashley DH, Agee KA, Wataha JC, Rueggeberg F, Ceballos L, Itou K, et al. (2003). Viscoelastic properties of demineralized dentin matrix. Dent Mater 19:700-706 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, et al. (2007). From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent 20:7-20 [PubMed] [Google Scholar]
- Peters MC, Bresciani E, Barata TJ, Fagundes TC, Navarro RL, Navarro MF, et al. (2010). In vivo dentin remineralization by calcium-phosphate cement. J Dent Res 89:286-291 [DOI] [PubMed] [Google Scholar]
- Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. (2005). Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater 21:864-881 [DOI] [PubMed] [Google Scholar]
- Ray S, Lukyanov P, Ochieng J. (2003). Members of the cystatin superfamily interact with MMP-9 and protect it from autolytic degradation without affecting its gelatinolytic activities. Biochim Biophys Acta 1652:91-102 [DOI] [PubMed] [Google Scholar]
- Reis AF, Giannini M, Pereira PN. (2007). Long-term TEM analysis of the nanoleakage patterns in resin-dentin interfaces produced by different bonding strategies. Dent Mater 23:1164-1172 [DOI] [PubMed] [Google Scholar]
- Ricci HA, Sanabe ME, de Souza Costa CA, Pashley DH, Hebling J. (2010). Chlorhexidine increases the longevity of in vivo resin-dentin bonds. Eur J Oral Sci 118:411-416 [DOI] [PubMed] [Google Scholar]
- Sadek FT, Pashley DH, Ferrari M, Tay FR. (2007). Tubular occlusion optimizes bonding of hydrophobic resins to dentin. J Dent Res 86:524-528 [DOI] [PubMed] [Google Scholar]
- Sadek FT, Pashley DH, Nishitani Y, Carrilho MR, Donnelly A, Ferrari M, et al. (2008). Application of hydrophobic resin adhesives to acid-etched dentin with an alternative wet bonding technique. J Biomed Mater Res A 84:19-29 [DOI] [PubMed] [Google Scholar]
- Sadek FT, Mazzoni A, Breschi L, Tay FR, Braga RR. (2010a). Six-month evaluation of adhesives interface created by a hydrophobic adhesive to acid-etched ethanol-wet bonded dentine with simplified dehydration protocols. J Dent 38:276-283 [DOI] [PubMed] [Google Scholar]
- Sadek FT, Braga RR, Muench A, Liu Y, Pashley DH, Tay FR. (2010b). Ethanol wet bonding challenges current anti-degradation strategy. J Dent Res 89:1499-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Takatsu T, Ciucchi B, Russell CM, Pashley DH. (1995). Tensile properties of resin-infiltrated demineralized human dentin. J Dent Res 74:1093-1102 [DOI] [PubMed] [Google Scholar]
- Santos J, Carrilho M, Tervahartiala T, Sorsa T, Breschi L, Mazzoni A, et al. (2009). Determination of matrix metalloproteinases in human radicular dentin. J Endod 35:686-689 [DOI] [PubMed] [Google Scholar]
- Sauro S, Watson TF, Tay FR, Chersoni S, Breschi L, Bernardi F, et al. (2006). Water uptake of bonding systems applied on root dentin surfaces: a SEM and confocal microscopic study. Dent Mater 22:671-680 [DOI] [PubMed] [Google Scholar]
- Sauro S, Watson TF, Mannocci F, Miyake K, Huffman BP, Tay FR, et al. (2009a). Two-photon laser confocal microscopy of micropermeability of resin-dentin bonds made with water or ethanol wet bonding. J Biomed Mater Res B Appl Biomater 90:327-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauro S, Watson TF, Mannocci F, Tay FR, Pashley DH. (2009b). Prevention of water contamination of ethanol-saturated dentin and hydrophobic hybrid layers. J Adhes Dent 11:271-278 [PMC free article] [PubMed] [Google Scholar]
- Sauro S, Toledano M, Aguilera FS, Mannocci F, Pashley DH, Tay FR, et al. (2010). Resin-dentin bonds to EDTA-treated vs. acid-etched dentin using ethanol wet-bonding. Dent Mater 36:368-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffa P, Barros N, Vidal C, Tersariol IL, Pashley D, Tjäderhane L, et al. (2010). Chlorhexidine inhibits cysteine cathepsins [abstract]. J Dent Res (Spec Iss):4554 URL available at: http://iadr.confex.com/iadr/2010barce/webprogramcd/Paper140222.html (accessed 10/13/2010).
- Scott JE, Thomlinson AM. (1998). The structure of interfibrillar proteoglycan bridges (‘shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat 192(Pt 3):391-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela MN, Babitski E, Steinberg D, Kohavi D, Rosen G. (2009). Degradation of collagen-guided tissue regeneration membranes by proteolytic enzymes of Porphyromonas gingivalis and its inhibition by antibacterial agents. Clin Oral Implants Res 20:496-502 [DOI] [PubMed] [Google Scholar]
- Sela-Passwell N, Rosenblum G, Shoham T, Sagi I. (2010). Structural and functional bases for allosteric control of MMP activities: Can it pave the path for selective inhibition? Biochim Biophys Acta 1803:29-38 [DOI] [PubMed] [Google Scholar]
- Sharrock P, Grégoire G. (2010). HEMA reactivity with demineralized dentin. J Dent 38:331-335 [DOI] [PubMed] [Google Scholar]
- Shen C, Zhang NZ, Anusavice KJ. (2010). Fluoride and chlorhexidine release from filled resins. J Dent Res 89:1002-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin TP, Yao X, Huenergardt R, Walker MP, Wang Y. (2009). Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dent Mater 25:1050-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokati B, Tam LE, Santerre JP, Finer Y. (2010). Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface. J Biomed Mater Res B Appl Biomater 94:230-237 [DOI] [PubMed] [Google Scholar]
- Smith AJ. (2003). Vitality of the dentin-pulp complex in health and disease: growth factors as key mediators. J Dent Educ 67:678-689 [PubMed] [Google Scholar]
- Spencer P, Wang Y. (2002). Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res 62:447-456 [DOI] [PubMed] [Google Scholar]
- Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O. (2010). Adhesive/dentin interface: the weak link in the composite restoration. Ann Biomed Eng 38:1989-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkala M, Wahlgren J, Larmas M, Sorsa T, Teronen O, Salo T, et al. (2001). The effects of MMP inhibitors on human salivary MMP activity and caries progression in rats. J Dent Res 80:1545-1549 [DOI] [PubMed] [Google Scholar]
- Sulkala M, Larmas M, Sorsa T, Salo T, Tjäderhane L. (2002). The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J Dent Res 81:603-607 [DOI] [PubMed] [Google Scholar]
- Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. (2007). Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol 52:121-127 [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Hishiya T. (2008). Molecular imprinting of proteins emerging as a tool of protein recognition. Org Biomol Chem 6:2459-2467 [DOI] [PubMed] [Google Scholar]
- Tallant C, Marrero A, Gomis-Rüth FX. (2010). Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta 1803:20-28 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2003a). Have dentin adhesives become too hydrophilic? J Can Dent Assoc 69:724-731 [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2003b). Water treeing—a potential mechanism for degradation of dentin adhesives. Am J Dent 16:6-12 [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2008). Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 29:1127-1137 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2009). Biomimetic remineralization of resin-bonded acid-etched dentin. J Dent Res 88:719-724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CK. (2005). Water treeing in simplified dentin adhesives—déjà vu? Oper Dent 30:561-579 [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Kapur RR, Carrilho MR, Hur YB, Garrett LV, et al. (2007). Bonding BisGMA to dentin—a proof of concept for hydrophobic dentin bonding. J Dent Res 86:1034-1039 [DOI] [PubMed] [Google Scholar]
- Teno N, Masuya K. (2010). Orally bioavailable cathepsin K inhibitors with pyrrolopyrimidine scaffold. Curr Top Med Chem 10:752-766 [DOI] [PubMed] [Google Scholar]
- Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. (2010). Cysteine cathepsins in human dentin-pulp complex. J Endod 36:475-481 [DOI] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, et al. (2010a). The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent Mater 26:1059-1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Agee KA, Hoshika T, Tay FR, Pashley DH. (2010b). The inhibitory effect of polyvinylphosphonic acid on functional matrix metalloproteinase activities in human demineralized dentin. Acta Biomater 6:4136:4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. (1998). The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res 77:1622-1629 [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Hotakainen T, Kinnunen S, Ahonen M, Salo T. (2007). The effect of chemical inhibition of matrix metalloproteinases on the size of experimentally induced apical periodontitis. Int Endo J 40:282-289 [DOI] [PubMed] [Google Scholar]
- Toledano M, Nieto-Aguilar R, Osorio R, Campos A, Osorio E, Tay FR, et al. (2010). Differential expression of matrix metalloproteinase-2 in human coronal and radicular sound and carious dentine. J Dent 38:635-640 [DOI] [PubMed] [Google Scholar]
- Toroian D, Lim JE, Price PA. (2007). The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem 282:22437-22447 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan TK, Vaidyanathan J. (2009). Recent advances in the theory and mechanism of adhesive resin bonding to dentin: a critical review. J Biomed Mater Res B Appl Biomater 88:558-578 [DOI] [PubMed] [Google Scholar]
- van Dijken JW, Sunnegårdh-Grönberg K, Lindberg A. (2007). Clinical long-term retention of etch-and-rinse and self-etch adhesive systems in non-carious cervical lesions. A 13 years evaluation. Dent Mater 23:1101-1107 [DOI] [PubMed] [Google Scholar]
- Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. (2007). Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 28:3757-3785 [DOI] [PubMed] [Google Scholar]
- Van Landuyt KL, Snauwaert J, Peumans M, De Munck J, Lambrechts P, Van Meerbeek B. (2008). The role of HEMA in one-step self-etch adhesives. Dent Mater 24:1412-1419 [DOI] [PubMed] [Google Scholar]
- Van Meerbeek B, Peumans M, Poitevin A, Mine A, Van Ende A, Neves A, et al. (2010). Relationship between bond-strength tests and clinical outcomes. Dent Mater 26:e100-e121 [DOI] [PubMed] [Google Scholar]
- van Strijp AJ, Jansen DC, DeGroot J, ten Cate JM, Everts V. (2003). Host-derived proteinases and degradation of dentine collagen in situ . Caries Res 37:58-65 [DOI] [PubMed] [Google Scholar]
- Wang L, Nancollas GH. (2009). Pathways to biomineralization and biodemineralization of calcium phosphates: the thermodynamic and kinetic controls. Dalton Trans 15:2665-2672 [DOI] [PubMed] [Google Scholar]
- Wong TS, Brough B, Ho CM. (2009). Creation of functional micro/nano systems through top-down and bottom-up approaches. Mol Cell Biomech 6:1-55 [PMC free article] [PubMed] [Google Scholar]
- Xu A-W, Ma Y, Cölfen H. (2007). Biomimetic mineralization. J Mater Chem 7:415-449 [Google Scholar]
- Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. (2008). In vitro performance of nano-heterogeneous dentin adhesive. J Dent Res 87:829-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Park J, Topp E, Spencer P. (2009a). Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dent Mater 25:452-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Wang Y, Spencer P. (2009b). Nanophase separation of polymers exposed to simulated bonding conditions. J Biomed Mater Res B Appl Biomater 88:339-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC, Kern M. (2009). The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int J Oral Sci 1:163-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Tan J, Yang X, Cheng C, Wang X, Chen L. (2010). Effect of chlorhexidine application in a self-etching adhesive on the immediate resin-dentin bond strength. J Adhes Dent 12:27-31 [DOI] [PubMed] [Google Scholar]


