Abstract
Background
We investigated the actions of propofol and isoflurane on nociceptive responses of neurons in the spinal cord.
Methods
We determined nociceptive responses of lumbar neurons in the dorsal horn (<1200 μm) and ventral horn (>1200 μm) of decerebrate rats before and during propofol (1 effective dose, ED50) or isoflurane (1 minimum alveolar concentration) anesthesia. During recording of ventral horn neurons, we administered picrotoxin by infusion to determine whether isoflurane and propofol differed in their effects at the gamma amminobutyric acid (GABA) Type A receptors. We also determined whether decerebration altered propofol requirements to produce immobility.
Results
Decerebration did not affect propofol requirements. The ED50 for propofol was 497 ± 58 μg/kg/min in intact rats and 420 ± 65 μg/kg/min in decerebrated rats (p>0.05), with corresponding propofol blood concentrations of 8.1 ± 1.1 μg/ml and 7.3 ± 1.1 μg/ml, respectively (p>0.05). Propofol did not significantly depress dorsal horn neurons, but isoflurane depressed the responses to 56% of control (p<0.05). Propofol depressed ventral horn neurons to 47% of control, whereas isoflurane depressed ventral horn neurons to 20% of control. Picrotoxin significantly reversed the depressant effect of propofol on ventral horn neuronal responses (79% of control, not significantly different from control). Picrotoxin, however, had no effect on isoflurane’s depression of ventral horn neuronal responses (26% of control).
Conclusions
Propofol acts in the spinal cord to produce immobility. This depressive effect occurs in the ventral horn and is mediated mainly by GABAA receptors. Isoflurane also depresses neurons in the ventral horn; however, isoflurane actions at the GABAA receptor are either weak or overridden by other effects in the ventral horn.
Immobility is an end-point that is produced by all general anesthetics and is predominantly mediated by the spinal cord.1;2 Sonner et al showed that the mechanism by which 2 commonly used anesthetics, propofol and isoflurane, causes immobility is different.3 The immobilizing effects of propofol, which acts at the GABA (gamma amino butyric acid) Type A receptor,4–7 were antagonized in rats by picrotoxin, a noncompetitive GABA antagonist, while picrotoxin had lesser effects on immobility produced by isoflurane.3
Anesthetics might mediate immobility at several anatomical locations within the central nervous system. Previous studies have shown significant depression of the dorsal horn by propofol and isoflurane,8–11 leading to the hypothesis that immobility could be the result of depression at the dorsal horn. In addition, anesthetics depress neurons in the ventral spinal cord, which includes motoneurons and central pattern generators, with the latter group of neurons being particularly sensitive to anesthetics.10;12 Propofol’s sedative effect appears to occur via an action in the tuberomamillary nucleus in the hypothalamus;13 however, it is unclear whether propofol’s immobilizing action occurs there, at another supraspinal site, or in the spinal cord. Therefore, we presently investigated whether the forebrain is critical to propofol-induced immobility by determining propofol requirements in decerebrate rats.
We also studied the effects of propofol and isoflurane on spinal neuronal responses to noxious stimulation. These studies were performed in decerebrate rats to exclude any contribution of supraspinal structures and to permit us to obtain control responses in the unanesthetized animal. We were therefore able to determine the full extent of anesthetic effect in the range from no anesthesia to that needed to produce immobility. We hypothesized that isoflurane and propofol would have a similar depressing effect on ventral horn neuronal activity and that the effect of propofol, but not isoflurane, would be significantly reversed by picrotoxin.
METHODS
The experimental protocol was approved by the University of California Davis Animal Use and Care Advisory Committee. Adult male Sprague-Dawley rats weighing 402– 719 g were used for these experiments. We first determined the propofol effective dose requirements (ED50) to prevent movement in 50% of animals in separate groups of intact and decerebrate rats. In another group of rats, we determined propofol and isoflurane effects on neuronal responses to noxious stimulation.
Propofol ED50 Determination
The rats were anesthetized in a chamber with 4% isoflurane in oxygen followed by spontaneous mask ventilation with 2% isoflurane. We determined the propofol ED50 in intact and decerebrated animals. All animals were monitored and prepared as described below (Surgical and Neurophysiology Procedures) with a tracheostomy tube, mechanical ventilation, a jugular line, 0.5 mg dexamethasone IV, a carotid line and a laminectomy at T13/L1. The animals in the decerebration group (n=7) underwent a carotid ligation of the contralateral side followed by craniectomy and decerebration. The control animals (n=8) underwent a craniectomy but the brain was left intact. After a 1 h recovery time (equal for both decerebrated and non-decerebrated animals), a propofol infusion was started at either 480 μg/kg/min (≈20% below the expected ED50) or 720 μg/kg/min (≈20% above the expected ED50) for 30 min and isoflurane was turned off. The expected ED50 (600 μg/kg/min) was based on our prior data.14 Animals were investigated in ascending (n=3 control, n=3 decerebrate) and descending (n=4 control, n=5 decerebrate) dose regimens. After a 30 min equilibration time, a 10-inch hemostat was applied to the tail and oscillated at 1 Hz for 1 min or until the animal displayed gross purposeful movement with pawing or turning of the head towards the stimulus. When a positive response occurred, a 2 mg/kg propofol bolus was administered and the infusion rate increased to ≈20% above the prior infusion rate. After a 15 min equilibration time, the stimulus was repeated. When a negative response occurred, the infusion was stopped for 5 min and restarted at ≈20% below the prior infusion rate and allowed to equilibrate for 15 min before retesting. Before each application of the tail pinch, a blood sample (0.25 ml) was collected from the carotid line and analyzed for propofol concentration using gas chromatography as previously described.15 The propofol concentrations associated with the lowest infusion that prevented movement and the highest infusion that produced movement were averaged to obtain the propofol effective concentration (EC50) associated with the ED50 producing immobility.
Surgical and Neurophysiology Procedures
Rats were anesthetized with isoflurane in a chamber as described above. After tracheostomytube placement (12 gauge), positive-pressure ventilation was initiated with a pump ventilator (Harvard Apparatus, Holliston, MA) at a rate and tidal volume that maintained end-expiratory carbon dioxide between 30 and 40 mm Hg as monitored by a calibrated Ohmeda Rascal II gas analyzer (Ohmeda, Salt Lake City, UT). The body temperature was measured with a rectal temperature probe and maintained between 36.5 and 38°C with a water heated blanket. One jugular vein was cannulated with PE 50 tubing. One carotid artery was ligated while the other carotid artery was cannulated with PE 50 tubing for arterial blood pressure monitoring (PB240; Tyco PB, Pleasanton, CA) and blood sampling. The lumbar spinal cord of T13 and L1 was exposed by laminectomy.
Dexamethasone (0.5 mg) was administered IV to reduce swelling of the brainstem after decerebration.12 Dexamethasone does not affect spinal neuronal responses to noxious stimulation.16 One hour after administration of the dexamethasone the animal underwent a craniectomy to permit decerebration. The cerebral hemispheres were aspirated to expose the superior colliculi. A precollicular transection was performed with a surgical blade inserted at a 10 degree angle (angled posteriorly), and the remaining forebrain structures were removed by aspiration. Hemostasis was achieved with Surgicel (Ethicon, Cincinnati, OH) and Gelfoam (Pfizer, New York, NY) pledgets. Saline or hetastarch were infused to replace blood loss, and isoflurane administration was discontinued. The mean arterial blood pressure was maintained at 90 ± 35 mmHg (mean, standard deviation) throughout the experiments.
After recovery from the decerebration (usually 1 hour or more), as demonstrated by limb movements in response to tail clamping, the animal received pancuronium bromide IV (0.3–0.4 mg/h; Baxter, Deerfield, IL) for muscle relaxation. The animal was then placed in a stereotaxic frame (Kopf, Tujunga, CA). The cord was stabilized by vertebral clamps on the vertebral bodies of T12 and L2, and the head was secured by ear bars and an incisor bar. The spinal dura was removed and the exposed cord was covered with warm agar. A Teflon-coated tungsten microelectrode (8–11 mΩ; FHC, Bowdoinham, ME) was then advanced into the lumbar spinal cord with a hydraulic microdrive (Kopf Instruments, Tujunga, CA). The action potentials were amplified (Tucker-Davis, Alachua, FL) and filtered (band pass 300–5000 Hz). The signals were displayed by conventional means and transmitted to a computer for recording with a Powerlab interface and Chart software (AD Instruments, Grand Junction, CO) and for off-line spike discrimination and analysis. A minimum signal-to-noise ratio of 4 was standard. We searched for extracellular action potentials using tactile stimulation of the hindpaw. We isolated action potentials from neurons at 200–1200 μm and 1200–1800 μm depth in the spinal cord. Depth was estimated from the microdrive readings. We focused on neurons that responded to hindpaw pinch within the L3–L5 dermatomes. Most neurons were wide dynamic range neurons in that they responded to innocuous (brush) and noxious (pinch) mechanical stimuli with increasing firing rate, while a minority were nociceptive-specific in that they only responded to noxious stimuli. The noxious stimulus was a standardized 5-second forceps pinch at steady intensity as previously described.10 A small forceps was used which had been instrumented with a force transducer at the proximal end of the forceps. The force was maintained constant using the readout of the force transducer (Sensotec, Columbus, OH). The pinch force intensity necessary for maximal stimulation was established for each cell (between 50–100 N) and was used at that level during pinching for each individual neuron.
Data Collection
In 1 set of experiments, we studied the response of dorsal horn neurons (<1200 μm deep) to paw pinch without anesthetic (i.e., control), at either 1.3% isoflurane (i.e., 1 minimum alveolar concentration (MAC)) or 1 ED50 propofol (i.e., 2 mg/kg propofol bolus plus a 600 μg/kg/min propofol infusion with 30 min equilibration time). We studied a larger number of neurons in the propofol/dorsal horn group to insure that we did not miss a depressant effect of propofol. A power analysis revealed that we needed at least 21 neurons to detect a 15% or larger change in neuronal responses (alpha = 0.05, beta = 0.8).
In the second set of experiments, we determined nociceptive responses of neurons in the ventral horn (>1200 μm). We obtained nociceptive responses during control conditions (i.e., without anesthesia) and then during propofol (1 ED50) or isoflurane (1 MAC) anesthesia as described above. After this we concurrently administered picrotoxin (200 μg/kg/min for 30 min equilibration time) and obtained nocieptive responses again. The picrotoxin dose was chosen because this dose produced a ≈150% increase in propofol requirements based on the data of Sonner et al.3 When we initially tried a higher picrotoxin dose (400 μg/kg/min), the spinal cord neurons became so activated that the evaluation of a single neuron became virtually impossible. After completion of the experiment, the animals were euthanized by additional anesthetic and IV potassium chloride solution.
Data Analysis and Statistical Analysis
Neuronal activity was evaluated by counting the number of action potentials for 30 seconds before the pinch (spontaneous activity), during the 5-second pinch time (evoked activity) and 60 seconds after the start of the noxious pinch. The data were normalized to the control responses, i.e., the responses during anesthesia and anesthesia plus picrotoxin were divided by the control (anesthetic-free) response. Data are presented as mean and standard error. The student t-test or repeated measures analysis of variance was used for statistical comparisons as appropriate. We also performed linear correlation between neuronal depth in spinal cord and the effect of isoflurane and propofol on the neuronal response to noxious stimulation. A p-value of <0.05 was considered significant.
Results
The ED50 for propofol was 497 ± 58 μg/kg/min in intact rats and 420 ± 65 μg/kg/min in decerebrate rats (p>0.05), with corresponding propofol blood levels of 8.1 ± 1.1 μg/ml and 7.3 ± 1.1 μg/ml, respectively (p>0.05). Individual propofol blood levels are shown in Figure 1.
Figure 1.
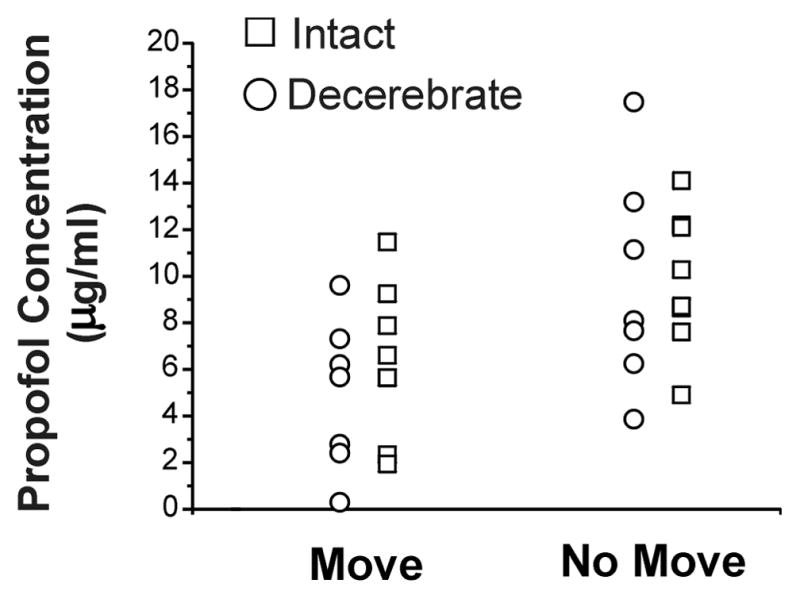
Plasma propofol concentrations. The individual plasma propofol concentrations are shown for the intact animals (□) and decerebrate animals (■) when movement (Move) and no movement (No Move) occurred.
Neuronal responses were recorded from 62 neurons in 57 rats. Recordings were made from 11 dorsal horn neurons (depth: 538 ± 57 μm) and 14 ventral horn neurons (1569 ± 65 μm) for the isoflurane group and 23 dorsal horn (490 ± 38 μm) and 14 ventral horn neurons (1563 ± 58 μm) for the propofol group.
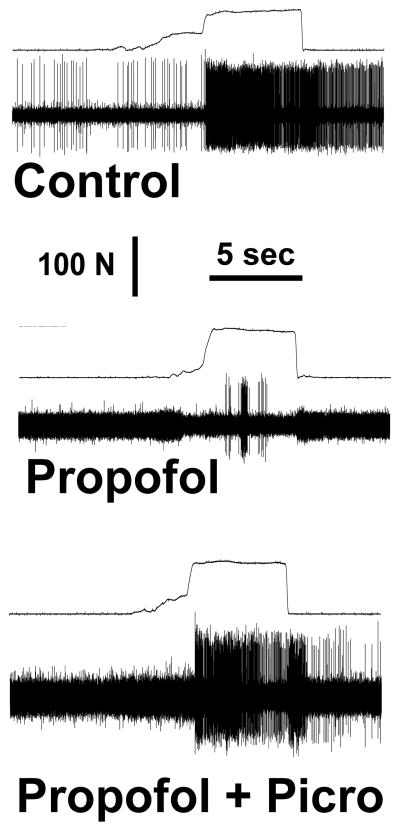
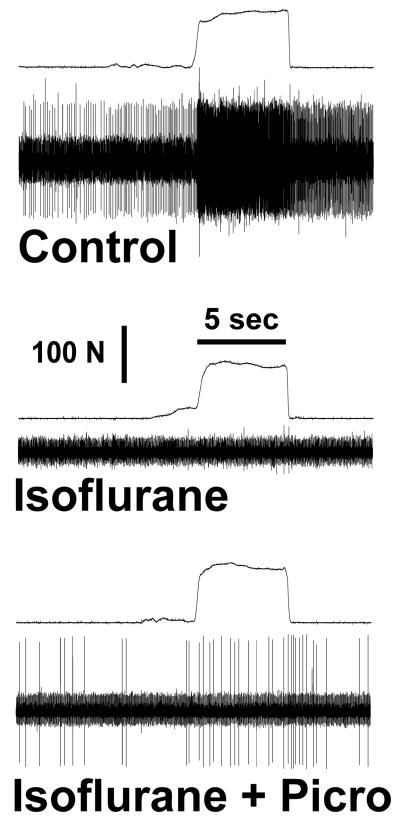
Average nociceptive responses in the absence of anesthesia (i.e., control) for the isoflurane groups (dorsal horn and ventral horn groups) and the propofol groups (dorsal horn and ventral horn groups) ranged from 283 ± 35 to 509 ± 54 impulses/5 sec (Table 1). Spontaneous activity is shown in Table 2. Figures 2 and 3 show raw examples of action potentials recorded from ventral horn neurons in the absence of anesthesia, during propofol (Figure 2) and isoflurane anesthesia (Figure 3), and with picrotoxin. Dorsal horn cells were not depressed by propofol (86 ± 8% of control, p>0.05) but were significantly depressed by isoflurane (to 56 ± 10% of control, p= 0.002; Figure 4). Ventral horn cells were significantly depressed by both propofol (47 ± 10% of control, p<0.0001) and isoflurane (20 ± 8% of control, p< 0.0001; Figure 5).
Table 1.
Control neuronal responses
| Propofol Group | Isoflurane Group | |
|---|---|---|
| Dorsal Horn | 509 ± 54 (n=23) | 456 ± 68 (n=11) |
| Ventral Horn | 283 ± 35 (n=15) * | 395 ± 54 (n=14) |
Mean and standard error of control (anesthetic-free) responses to noxious stimulation. Data are shown for the response during the 5 sec period of stimulation.
p<0.05 compared to dorsal horn value for propofol and dorsal horn value for isoflurane.
Table 2.
Control spontaneous activity
| Propofol Group | Isoflurane Group | |
|---|---|---|
| Dorsal Horn | 39 ± 10 (n=23) | 9 ± 3 (n=11)* |
| Ventral Horn | 71 ± 13 (n=15) | 98 ± 23 (n=14) |
Mean and standard error of control (anesthetic-free) spontaneous activity (5 sec). Spontaneous activity was determined in the preceding 30 sec and divided by 6 to obtain average spontaneous activity for a 5 sec period.
p<0.05 compared to dorsal horn value for propofol and ventral horn value for isoflurane.
Figure 2.
Action potentials recorded before (upper panel) and during propofol anesthesia (middle panel). Force tracings are above the action potential tracings in each panel. The variable increase in force prior to the application of the full force reflects grasping of the forceps and not pinching of skin. This neuron was located in the ventral spinal cord (1492 μm). Note that propofol depressed the neuronal response (middle panel) but that picrotoxin partially reversed the depression by propofol (lower panel).
Figure 3.
Action potentials recorded before (upper panel) and during isoflurane anesthesia (middle panel). Force tracings are above the action potential tracings in each panel. The variable increase in force prior to the application of the full force reflects grasping of the forceps and not pinching of skin. This neuron was located in the ventral spinal cord (1731 μm). Note that isoflurane completely depressed the neuronal response (middle panel). Picrotoxin minimally reversed the depression by isoflurane (lower panel).
Figure 4.
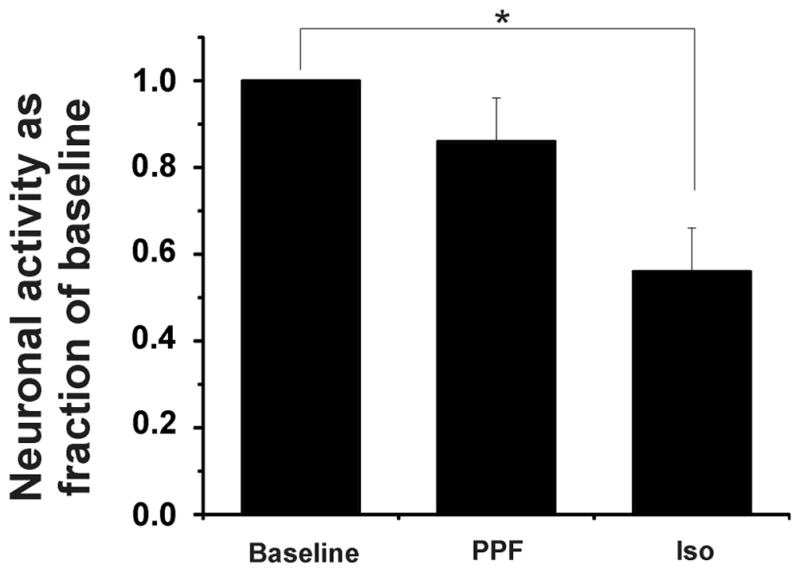
Dorsal horn neuronal responses during the 5 sec pinch stimulus as fraction of control (baseline) responses during propofol (PPF) and isoflurane (Iso) anesthesia. Propofol and isoflurane were administered at 1 effective dose-50% (ED50) and 1 minimum alveolar concentration (MAC). Note that the depression by propofol is minimal (p= NS), whereas the depression by isoflurane is significant (* p< 0.05).
Figure 5.
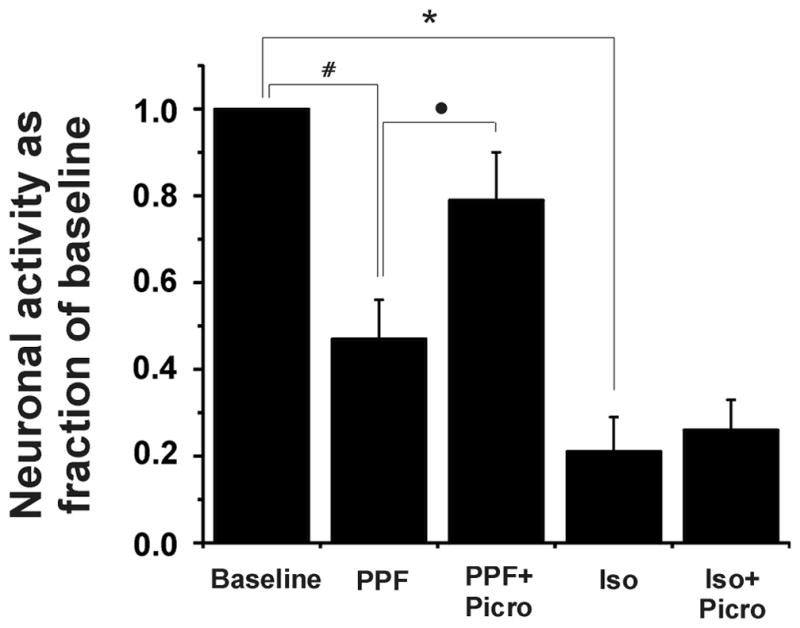
Ventral horn neuronal responses during the 5 sec pinch stimulus as fraction of control responses during propofol (PPF) and isoflurane (Iso) anesthesia and with picrotoxin (Picro). Propofol and isoflurane were administered at 1 effective dose-50% (ED50) and 1 minimum alveolar concentration (MAC). Picrotoxin was administered at 200 μg/kg/min. Note that Isoflurane and propofol both depressed these neurons (*p<0.01, #p<0.01), although isoflurane caused more depression. Also, picrotoxin reversed the depression produced by propofol (•p<0.05), but it had no effect on isoflurane’s depression.
Isoflurane depressed both the dorsal and ventral cord significantly more than did propofol (Figures 4 and 5).
The infusion of picrotoxin at 200 mcg/kg/min reversed the effects of propofol on the ventral horn neurons cord with responses increasing from 47 ± 10% of control to 79 ±12% of control, p<0.05; the latter value was not statistically significantly different from the control response. Picrotoxin did not reverse the effect of isoflurane anesthesia inasmuch as the ventral horn neurons were still significantly depressed (26 ±7% of control; p-value of <0.0001, but not significantly different from pre-picrotoxin value; Figure 4).
There was a significant linear correlation between neuronal depth in the spinal cord and the effect of isoflurane and propofol on neuronal responses to noxious stimulation. Figure 6 shows that neurons in the ventral horn were more likely to be depressed by isoflurane (Figure 6A) or propofol (Figure 6B).
Figure 6.
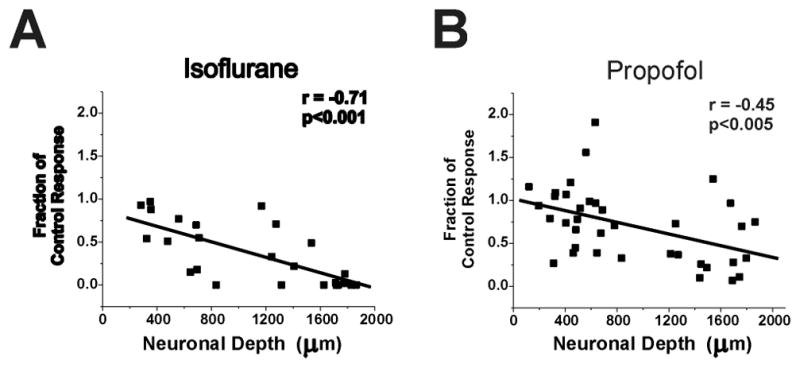
Linear correlation between neuronal depth in spinal cord and the effect of isoflurane (A) and propofol (B) on neuronal responses to noxious stimulation. Shown are neuronal responses as a fraction of the control response of each neuron. Note that neurons in deeper portions of the cord (more than 1200 μm) are more affected by isoflurane and propofol than are neurons in the superficial spinal cord. The correlation coefficients are shown in the upper right portions of each panel. The slopes of the linear correlation lines were both significantly different from zero.
Discussion
The main findings of this study were that (1) propofol acts in the spinal cord to produce immobility; (2) isoflurane, but not propofol, depressed dorsal horn neuronal responses to noxious stimulation; (3) propofol and isoflurane depressed ventral horn neurons, with isoflurane having the greater depressive effect; and (4) picrotoxin reversed (to approximately 80% of control) propofol’s depression of ventral horn neurons, while it had no significant effect on isoflurane’s depression of ventral horn neurons. We discuss these findings relative to the proposed mechanisms and sites of anesthetic-induced immobility. Although we have previously investigated effects of isoflurane and propofol on neuronal responses in the dorsal and ventral aspects of the spinal cord,10 the findings that propofol acts in spinal cord to produce immobility are novel, as are the data showing that picrotoxin reverses propofol’s depressant effect on ventral horn neurons. The sedative and immobilizing actions of propofol appear to act at different anatomic sites. Nelson et al showed that propofol’s sedative effect resulted from action in the tuberomammillary nucleus of the hypothalamus,13 part of the arousal-sleep pathway. The present data indicate that decerebration does not affect propofol requirements, thus suggesting a spinal site of action vis-à-vis immobility. This is consistent with the actions of some inhaled anesthetics, such as isoflurane and halothane. 1;2;17
Although the spinal cord is the primary site where anesthetics produce immobility, it was unclear where in the spinal cord this action occurred. Focus was initially placed on the dorsal horn; however, recent evidence suggests that neurons in the ventral horn are a more likely critical target.10 We presently determined that propofol did not significantly depress neurons in the dorsal horn, but this was not true for isoflurane. Propofol’s lack of effect in the dorsal horn turned our attention to the ventral horn. Neurons in this spinal cord area include central pattern generators and motoneurons. Data from our laboratories indicate that ventral horn neurons are more sensitive to anesthetics in the peri-MAC range compared to dorsal horn neurons and that central pattern generators are the likely neurons where anesthetics produce immobility.10;12 Data from the present study are consistent with this scenario. In our prior study, we gave propofol as a bolus to isoflurane-anesthetized animals10, so we could not exclude an interaction between isoflurane and propofol, nor could we discount the possibility that bolus administration resulted in high plasma concentrations that were well above those needed to produce immobility and that depressed dorsal horn neurons. These 2 factors were absent in the present study. We have previously found that bolus administration of propofol to the torso circulation depressed dorsal horn responses at propofol blood levels similar to those presently obtained.8 In that study, however, propofol was administered during baseline isoflurane anesthesia, so it is possible that the observed depression was due to the combination of the 2 drugs.8
Our finding that propofol’s depressive effect is reversed by picrotoxin is not surprising given that mutation of the beta-3 subunit of the GABA receptor produces resistance to propofol.4 We were presently interested in whether picrotoxin’s reversal of propofol- and isoflurane-induced depression of spinal neuronal responses mirrored the effect of picrotoxin on the ED50 for immobility. Sonner et al found that picrotoxin (at doses used in the present study) produced a ≈ 150% increase in propofol ED50, whereas it produced a 50% increase in isoflurane MAC.3 We found that picrotoxin increased spinal nociceptive responses by ≈ 100% during propofol anesthesia, whereas it produced a 20% numerical (but statistically insignificant) increase in neuronal responses during isoflurane anesthesia, a more than 3-fold difference between the 2 anesthetics.
Interestingly, Alvarez et al.18 reported that the beta-3 subunit of the GABA receptor is found in the dorsal horn and ventral horn, but not on motoneurons. How can those data be reconciled with our findings that propofol had no effect on dorsal horn neuronal responses, but decreased responses in the ventral horn? We speculate that network properties in the spinal cord might partly explain this discrepancy. Ventral horn neurons receive convergent synaptic input from neurons at multiple levels of the spinal cord, imparting these neurons with large whole-body receptive fields, making them more susceptible to inhibition. Dorsal horn neurons, however, have small excitatory receptive fields, a situation likely due to the abundant GABA receptors that produce inhibition of surrounding neurons (thereby increasing point discrimination). The finding that there are few or no beta-3 subunits on motoneurons suggests that propofol depression in the ventral horn might occur at pre-motor interneurons.
In another study, Grasshoff and Antkowiak found that sevoflurane and propofol acted at glycine and GABA receptors. These investigators used a reduced preparation, i.e., cultured spinal cord slices from embryonic rats,19 which makes it difficult to directly compare their data with ours. Changes in receptor expression and subtype during development and/or culture or changes in anesthetic immobilizing requirements in slices versus intact networks could all conceivably contribute to differences between our data and those obtained from in vitro preparations. The present study has the advantage to examine neuronal changes that occur around a known behavioral end-point (immobility) in intact spinal networks. Indeed, there is evidence that anesthetic-induced immobility is largely determined by effects on spinal locomotor networks,12;20 which are distributed and are necessarily disrupted in slice preparations.
There are some limitations to the present study. We did not determine isoflurane MAC in decerebrate rats as we did with propofol. We have previously observed that isoflurane MAC is unchanged after decerebration,12 and Rampil et al. have published similar results.1 Thus, we believe that we used isoflurane concentrations equipotent to the propofol dose. Nonetheless, we cannot exclude the possibility that decerebration might have removed sites critical to action of propofol and isoflurane. Lastly, we recorded from neurons that responded to noxious stimulation, but we did not further characterize these neurons, i.e., these neurons could have been part of central pattern generators, interneurons or motoneurons.
In summary, we found that propofol acts in the spinal cord to produce immobility. Furthermore, neurons in the ventral horn are more sensitive to propofol than are neurons in the dorsal horn. This depressant effect appears to occur by an action at the GABA-A receptor.
Acknowledgments
Supported in part by grants GM61283 (to JFA), GM47818 (to JFA) and GM78167 (to SLJ) from the NIH.
Footnotes
This work is attributed to the Department of Anesthesiology and Pain Medicine and the Section of Neurobiology, Physiology and Behavior at the University of California, Davis
Reprints will not be available from the authors.
Implications statement:
Propofol did not depress spinal dorsal horn neurons, which were depressed by isoflurane. Both anesthetics caused greater depression in the ventral horn. Picrotoxin reversed the depressive effect of propofol, but it had little or no effect on isoflurane’s depressive action. In addition, decerebration did not alter propofol requirements. These data suggest that propofol produces immobility by a GABAergic action on neurons in the ventral spinal cord.
Reference List
- 1.Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993;78:707–12. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–9. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Sonner JM, Zhang Y, Stabernack C, Abaigar W, Xing Y, Laster MJ. GABA(A) receptor blockade antagonizes the immobilizing action of propofol but not ketamine or isoflurane in a dose-related manner. Anesth Analg. 2003;96:706–12. doi: 10.1213/01.ANE.0000048821.23225.3A. table. [DOI] [PubMed] [Google Scholar]
- 4.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 5.Solt K, Forman SA. Correlating the clinical actions and molecular mechanisms of general anesthetics. Curr Opin Anaesthesiol. 2007;20:300–6. doi: 10.1097/ACO.0b013e32816678a5. [DOI] [PubMed] [Google Scholar]
- 6.Zeller A, Arras M, Jurd R, Rudolph U. Mapping the contribution of beta3-containing GABAA receptors to volatile and intravenous general anesthetic actions. BMC Pharmacol. 2007;7:2. doi: 10.1186/1471-2210-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant alpha4beta3delta and alpha4beta3 GABA(A) receptors expressed in HEK cells. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antognini JF, Wang XW, Piercy M, Carstens E. Propofol directly depresses lumbar dorsal horn neuronal responses to noxious stimulation in goats. Can J Anaesth. 2000;47:273–9. doi: 10.1007/BF03018926. [DOI] [PubMed] [Google Scholar]
- 9.Uchida H, Kishikawa K, Collins JG. Effect of propofol on spinal dorsal horn neurons. Comparison with lack of ketamine effects. Anesthesiology. 1995;83:1312–22. doi: 10.1097/00000542-199512000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Yao A, Atherely RJ, Carstens E, Jinks SL, Antognini JF. Neurons in the ventral spinal cord are more depressed by isoflurane, halothane and propofol than are neurons in the dorsal spinal cord. Anesth Analg. 2007;105:1020–6. doi: 10.1213/01.ane.0000280483.17854.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuyo T, Dutton RC, Antognini JF, Carstens E. The differential effects of halothane and isoflurane on windup of dorsal horn neurons selected in unanesthetized decerebrated rats. Anesth Analg. 2006;103:753–60. doi: 10.1213/01.ane.0000230605.22930.52. [DOI] [PubMed] [Google Scholar]
- 12.Jinks SL, Bravo M, Hayes SG. Volatile anesthetic effects on midbrain-elicited locomotion suggest that the locomotor network in the ventral spinal cord is the primary site for immobility. Anesthesiology. 2008;108:1016–24. doi: 10.1097/ALN.0b013e3181730297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–84. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 14.Orth M, Barter L, Dominguez C, Atherley R, Carstens E, Antognini JF. Halothane and propofol differentially affect electroencephalographic responses to noxious stimulation. Br J Anaesth. 2005;95:477–84. doi: 10.1093/bja/aei208. [DOI] [PubMed] [Google Scholar]
- 15.Antognini JF, Bravo E, Atherley R, Carstens E. Propofol, more than halothane, depresses electroencephalographic activation resulting from electrical stimulation in reticular formation. Acta Anaesthesiol Scand. 2006;50:993–8. doi: 10.1111/j.1399-6576.2006.01114.x. [DOI] [PubMed] [Google Scholar]
- 16.Yao A, Kim J, Atherley R, Jinks SL, Carstens E, Shargh S, Sulger A, Antognini JF. The effects of aromatic anesthetics on dorsal horn neuronal responses to noxious stimulation. Anesth Analg. 2008;106:1759–64. doi: 10.1213/ane.0b013e3181732ee3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antognini JF, Carstens E, Atherley R. Does the immobilizing effect of thiopental in brain exceed that of halothane? Anesthesiology. 2002;96:980–6. doi: 10.1097/00000542-200204000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez FJ, Taylor-Blake B, Fyffe RE, De Blas AL, Light AR. Distribution of immunoreactivity for the beta 2 and beta 3 subunits of the GABAA receptor in the mammalian spinal cord. J Comp Neurol. 1996;365:392–412. doi: 10.1002/(SICI)1096-9861(19960212)365:3<392::AID-CNE5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Grasshoff C, Antkowiak B. Propofol and sevoflurane depress spinal neurons in vitro via different molecular targets. Anesthesiology. 2004;101:1167–76. doi: 10.1097/00000542-200411000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Jinks SL, Atherley RJ, Dominguez CL, Sigvardt KA, Antognini JF. Isoflurane disrupts central pattern generator activity and coordination in the lamprey isolated spinal cord. Anesthesiology. 2005;103:567–75. doi: 10.1097/00000542-200509000-00020. [DOI] [PubMed] [Google Scholar]