Abstract
The thalamus is classically viewed as passively relaying information to the cortex. However, there is growing evidence that the thalamus actively regulates information transmission to the cortex and between cortical areas using a variety of mechanisms, including the modulation of response magnitude, firing mode and synchrony of neurons according to behavioral demands. We discuss how the visual thalamus contributes to attention, awareness and visually-guided actions, to present a general role for the thalamus in perception and cognition.
Keywords: lateral geniculate nucleus, pulvinar, thalamic reticular nucleus, spike timing, oscillations, burst firing
“The [cortex] must depend entirely on the thalamus for the precise nature of the sensory material which it receives indirectly from peripheral receptors. It is true that there is evidence to indicate that cortical mechanisms can modify thalamic activities by inhibitory influences, but the fact remains that […] the [cortex] from the developmental and functional point of view is to be regarded as a dependency of the thalamus and not vice versa.” (Le Gros Clark, 1932, p. 406).
Introduction
Galen (129–199/217 AD) was the first to call the mass of nuclei that constitute the diencephalon thalamos, a Greek word meaning inner room or chamber (Jones, 2007). Deep within the brain, the thalamus and surrounding cortex form a closely coupled system: the thalamus transmits information from the environment and internal processes to the cortex, while the cortex sends the output from multiple processing stages to the thalamus. The cortex critically depends on the thalamus, since it receives relatively little other input.
The thalamus has been extensively studied in terms of its anatomical organization, efferent and afferent connectivity patterns, basic neural response properties, and synaptic, biochemical, and molecular characteristics (Jones, 2007; Sherman and Guillery, 2006). However, its role in perception and cognition has remained poorly understood. Studies in awake, behaving monkeys during the last decades have focused almost exclusively on defining the roles of cortical areas in attention, memory, decision making and other cognitive processes. Similarly, human neuroimaging studies have heavily emphasized the functions of cortical rather than subcortical networks, partially due to technical limitations in terms of spatial resolution. During the last few years, we have seen the beginning of a ’renaissance’ for the study of thalamic function in perception and cognition due to the development of functional magnetic resonance imaging (fMRI) at high resolution that permitted for the first time the study of the human thalamus in some detail (reviewed in Saalmann and Kastner, 2009), followed by a renewed interest of physiologists in thalamic function in awake, behaving monkeys (e.g., McAlonan et al., 2006, 2008). In the present review, we will focus on the visual thalamus as a model system to exemplify the changing views of the thalamus’ role in perception and cognition that have begun to emerge from these studies.
The visual thalamus consists of three main nuclei, the lateral geniculate nucleus (LGN), the thalamic reticular nucleus (TRN), and the pulvinar. These three structures are characterized by differences in their efferent and afferent connectivity patterns (Jones, 2007; Sherman and Guillery, 2006). The LGN is considered a first-order thalamic nucleus because it transmits peripheral signals to the cortex, along the retino-cortical pathway. In addition to retinal afferents that form only a minority of the input to the LGN, it receives projections from multiple sources including primary visual cortex (V1), the TRN and brainstem. Thus, the LGN represents the first stage in the visual pathway at which modulatory influences from other sources could affect information processing. The TRN forms a thin shell of neurons that covers the lateral and anterior surface of the dorsal thalamus, and it receives input from branches of both thalamo-cortical and cortico-thalamic fibers. The TRN in turn sends its output exclusively to the thalamus and is positioned to provide inhibitory control over thalamo-cortical transmission. The pulvinar is the largest nucleus in the primate thalamus and is considered a higher-order thalamic nucleus because it forms input-output loops almost exclusively with the cortex. The extensive and reciprocal connectivity with the cortex suggests that the pulvinar serves in aiding cortico-cortical transmission through thalamic loops. Thus, from an anatomical perspective, the visual thalamus is ideally positioned to regulate the transmission of information to the cortex and between cortical areas, as was originally proposed more than 20 years ago (Crick, 1984; Sherman and Koch, 1986; Singer, 1977). The experimental evidence in favor of such a functional role will be reviewed in the following sections that are organized by thalamic nucleus.
LGN: Early Modulation of Visual Information
In the case of the LGN, the classical view of the thalamus as a passive relay of information from the sensory periphery to cortex may have been largely based on the high specificity of retinal afferents to the LGN and the similarity of receptive field (RF) properties of retinal ganglion cells and LGN neurons. However, by the early 1980 s, evidence was emerging that thalamic neurons operate in one of two modes, either burst or tonic firing of action potentials (Deschenes et al., 1982; Llinas and Jahnsen, 1982; Mukhametov et al., 1970). These two firing modes suggested that thalamic neurons were not simple relays, but instead were in a position to differentially transmit retinal information to visual cortex. By the mid to late 1980’s, theoretical accounts proposed active roles for the thalamus in regulating information transfer to the cortex (Crick, 1984; Sherman and Koch, 1986; Singer, 1977), but further evidence in support of such roles was not immediately forthcoming. Instead, burst firing was shown to be common during sleep (Livingstone and Hubel, 1981; Steriade et al., 1993) and thus a possible role for bursts during wakefulness was not apparent. Moreover, cognitive influences such as selective attention appeared to affect neural responses only in visual cortex, but not in the LGN, where little, if any modulation was found in early studies (Bender and Youakim, 2001; Lehky and Maunsell, 1996; Mehta et al., 2000). Consequently, this evidence led to a notion that perceptual and cognitive influences may affect neural processing only at the cortical level. This account has been revised during the last few years based on reports of attentional and perceptual modulation in the human and macaque LGN (e.g., McAlonan et al., 2008; O’Connor et al., 2002).
Anatomy and Physiology
LGN topography and the response properties of LGN neurons have been extensively studied in anesthetized non-human primates (e.g., Connolly and Van Essen, 1984; Kaas et al., 1972; Malpeli and Baker, 1975). The LGN is typically organized into six main layers, and each layer receives input from either the contra- or ipsilateral eye. The four dorsal layers contain small (parvocellular) neurons that are characterized by sustained discharge patterns and low contrast sensitivity, largely processing form and color information. The two ventral layers contain large (magnocellular) neurons that are characterized by transient discharge patterns and high contrast sensitivity, largely processing motion and depth information (Creutzfeldt et al., 1979; Derrington and Lennie, 1984; Dreher et al., 1976; Merigan and Maunsell, 1993; Shapley et al., 1981; Wiesel and Hubel, 1966). In addition, there are six thin LGN layers, located ventral to each of the parvo- and magnocellular layers, that contain very small (koniocellular) neurons, some of which carry signals from short-wavelength-sensitive (blue) cones (Hendry and Reid, 2000; Martin et al., 1997; Roy et al., 2009; Xu et al., 2001). These three LGN cell classes target different cortical layers (Figure 1A). Parvocellular and magnocellular neurons project to layer 4 and to a lesser extent to layer 6, while koniocellular cells project to layers 1 and 3 of area V1 and to extrastriate areas as well (reviewed in Callaway, 2005).
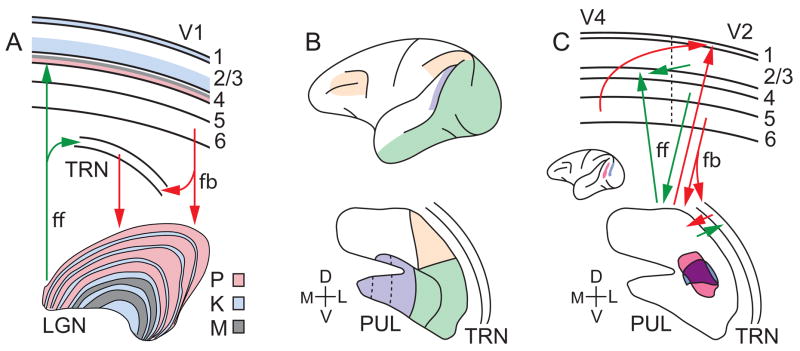
Figure 1. Thalamo-cortical connectivity.
(A) Feedforward (ff) projections from parvo-, konio- and magnocellular (P, K, M) neurons in the LGN target specific layers in V1 (color-coded). Layer 6 feedback (fb) from V1 respectively targets P, K, and M layers of the LGN. (B) Fronto-parietal (yellow), medio-temporal (violet), infero-temporal and occipital (green) cortical regions preferentially connect with different divisions of the pulvinar (PUL). The ventro-lateral and ventro-central divisions shown in green are retinotopically organized. Note that there are alternative parcellation schemes of the pulvinar based on neurochemical criteria (Gutierrez et al., 1995; Stepniewska and Kaas, 1997; Adams et al., 2000); however, there is reasonable agreement on subdivisions of the ventro-medial pulvinar (dotted lines). (C) Direct cortico-cortical connections (top) and indirect cortico-pulvino-cortical loops exemplified by V2-pulvino-V4 circuitry. Tracer injections into V2 (blue) and V4 (pink; inset) showed overlapping (purple) projection zones in the pulvinar (bottom). (C) Adapted from Fig. 8 in Adams et al. (2000).
Each LGN contains a retinotopic map of the contralateral visual hemifield. Similar to retinal ganglion cells, LGN cells have circular RFs, typically with an antagonistic center-surround organization. Despite the similarities in response characteristics between retinal ganglion and LGN cells, there are a number of signal transformations that occur across retino-geniculate synapses. For example, retinal action potentials following a short inter-spike interval are more likely to generate action potentials in LGN neurons than those following longer intervals. These relayed spikes are more highly correlated with the visual stimulus, that is, they have greater spike timing precision than non-relayed spikes. Consequently, relayed spikes carry more visual information per spike, enabling LGN neurons to improve coding efficiency (Rathbun et al., 2010; Wang et al., 2010b).
In the human LGN, the layout of the visual field representation was initially studied using postmortem anatomical analyses of degeneration patterns following retinal and cortical lesions (e.g., Hickey and Guillery, 1979). This layout was later confirmed in detail using high-resolution fMRI techniques (Figure 2A; (Schneider et al., 2004), revealing a close correspondence between the topographies of the macaque and human LGNs. Anatomical studies have also revealed laminar patterns of parvo- and magnocellular subdivisions similar to the macaque LGN (Hickey and Guillery, 1979). Although current neuroimaging techniques are insufficient to resolve single lamina within the human LGN, magno- and parvocellular-dominated regions of the LGN can be identified based on functional criteria, that is, the higher contrast sensitivity of magno- relative to parvocellular neurons (Derrington and Lennie, 1984; Sclar et al., 1990). Hence, it is possible to probe how magno- and parvocellular processing contributes to human behavior and cognition, since the LGN is the only structure in the visual system where the two pathways are sufficiently spatially segregated to be resolved using current fMRI methods.
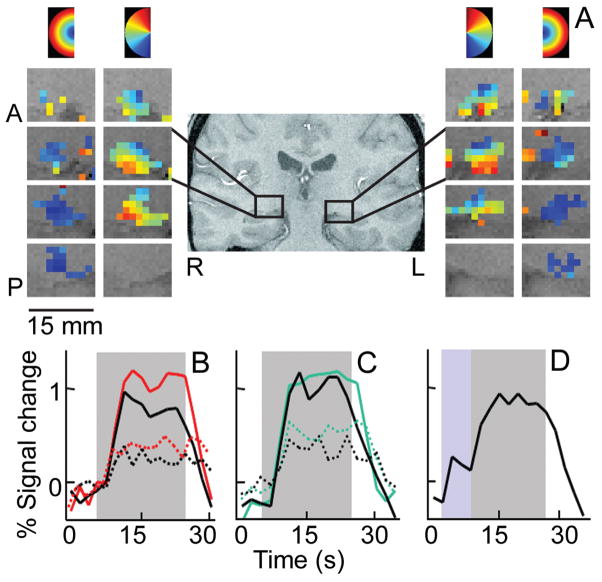
Figure 2. Attentional modulation of human LGN responses.
(A) Retinotopic organization of the LGN for a representative subject. Polar angle and eccentricity maps are shown in columns of sequential slices from anterior (A) to posterior (P), for the right (R) and left (L) LGN. The color-code at top of columns shows regions of the visual field to which LGN voxels preferentially responded. (B)–(D) Time series of fMRI signals in the LGN averaged across subjects (n=4) and hemispheres. Gray area shows period of checkerboard stimulus presentation. (B) Directed attention (red lines) enhanced responses to high (solid) and low (dashed) contrast stimuli, relative to the unattended condition (black lines). (C) Responses to high (solid) and low (dashed) contrast stimuli were suppressed during a fixation task of high attentional load (black lines) relative to a low load fixation task (green lines). (D) Attention directed to the periphery in expectation (blue area) of the visual stimulus increased baseline activity. (A) From Fig. 2 in Schneider et al. (2004); (B)–(D) from Fig. 2 in O’Connor et al. (2002).
In addition to retinal afferents, the LGN receives modulatory input from multiple sources. Cortico-thalamic feedback projections from V1 comprise about 30% of the input to the LGN, and inhibitory input from the TRN and local interneurons contributes another 30% of LGN input (Sherman and Guillery, 2006). Both V1 and TRN represent visual information in retinotopically organized maps and can thereby influence LGN responses in spatially specific ways. Moreover, V1 feedback arises from three classes of neurons, each selectively targeting parvo-, magno- or koniocellular LGN neurons (Briggs and Usrey, 2009). This finding suggests that cortico-thalamic feedback may differentially modulate information processing in parvo-, magno- and koniocellular afferent pathways, and thus be more selective than the TRN input to LGN. A third major modulatory influence that represents another 30% of input to the LGN arises from brainstem nuclei, that is, the pedunculopontine tegmentum and the parabigeminal nucleus. These cholinergic projections are more diffusely organized than the V1 and TRN projections (Bickford et al., 2000; Erisir et al., 1997) and, consequently, are likely to influence LGN responses with less spatial specificity. Due to the multiple modulatory inputs, the LGN is well positioned for early regulation of visual information transmission.
Attentional Response Modulation
Human fMRI studies provided the first compelling evidence of cognitive tasks that modulated LGN responses. In a series of attention experiments, O’Connor et al. (2002) showed that selective attention affects visual processing in at least three different ways, similar to the modulatory effects observed in visual cortex. First, LGN responses to attended visual stimuli increased relative to the same stimuli when unattended (Figure 2B). This response enhancement was specific to the attended visual field location and occurred in both parvo- and magnocellular regions of the LGN, although the attentional enhancement tended to be stronger in magnocellular regions (Schneider and Kastner, 2009). Second, neural responses to unattended stimuli were attenuated depending on the load of attentional resources engaged elsewhere (Figure 2C). This is consistent with a deoxyglucose study in macaques showing suppressed metabolic activity peripheral to the attended stimulus representation, largely in magnocellular LGN layers (Vanduffel et al., 2000). Third, baseline activity increased when participants directed attention to a location in the absence of visual stimulation and in anticipation of the upcoming stimulus (Figure 2D). All three attention effects tended to be larger in the LGN than in V1, with effects on the order of the attentional modulation typically observed in extrastriate areas such as V4. Thus, feedback from V1 may only partly contribute to the attentional modulation of LGN responses, suggesting that additional sources such as the TRN and brainstem cholinergic inputs may contribute as well.
The finding of attentional modulation in the human LGN has been corroborated by a recent single-cell recording study in the macaque LGN that provides a more space- and time-resolved view of the attention effects (McAlonan et al., 2008). The spike rate of LGN neurons increased for attended stimuli relative to unattended stimuli, with slightly stronger effects on magnocellular neurons (11% enhancement) than parvocellular neurons (9%; Figures 3A and 3B) across the population. Selective attention also influenced magnocellular neurons earlier than parvocellular neurons (the influence of attention on koniocellular neurons is not known). The attention effects varied over time, as evidenced by an early period of attentional modulation within the first 100 ms after stimulus onset, and a later period of modulation starting around 200 ms, possibly reflecting different sources of modulatory input. Based on the response patterns of TRN and V1 neurons, it is possible that the early period of attentional effects in the LGN is attributable to TRN influences, whereas the late period may reflect feedback from V1 (Figure 1A).
Figure 3. Attention effects on macaque LGN and TRN neurons.
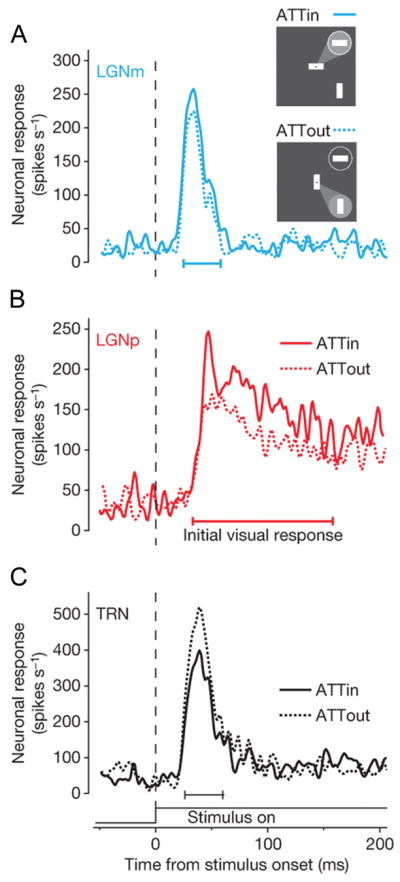
(Inset) Monkeys were cued to direct their attention to a visual stimulus inside (ATTin) or outside (ATTout) the receptive field (circle). Selective attention increased spike rates of (A) magno- and (B) parvocellular LGN neurons, but reduced spike rates of (C) TRN neurons. From Fig. 1 in McAlonan et al. (2008).
Perceptual Response Modulation
The thalamus may not only contribute to the selection of behaviorally relevant information from the environment, but also to the conscious perception or awareness of visual information. A classical task to probe visual awareness is binocular rivalry, in which dissimilar images such as gratings of orthogonal orientation are presented to the two eyes. This leads to a competition for perceptual dominance where only one image is visible at a time while the other one is suppressed (Figure 4A). Human neuroimaging studies have shown that activity in the LGN reflects the subjects’ reported percept and not necessarily the actual retinal input (Haynes et al., 2005; Wunderlich et al., 2005). For example, while a grating of different contrast and orientation was presented to each eye, LGN activity increased when participants perceived a high-contrast horizontal grating and decreased when they perceived a low-contrast vertical grating, similar to the response pattern that is evoked by physical alternations of the same stimuli (Figure 4B; Wunderlich et al., 2005). However, these findings contrast somewhat with results from macaque physiology studies. Using a generalized flash suppression task, in which a target stimulus is no longer perceived after being surrounded by randomly moving dots, there was no perceptual modulation of the spike rate of macaque LGN neurons (Wilke et al., 2009). Since mainly parvocellular neurons were studied, it is unclear how flash suppression affects magno- and koniocellular neurons. For example, it is possible that perceptual modulation is largely limited to magnocellular neurons, and thus the magnocellular LGN was driving the responses in the human fMRI studies. Another possibility is that changes in response timing and synchrony of LGN neurons contributed to the signal changes observed in the human fMRI studies, thereby raising the question of the type of neural signals that underlie hemodynamic signals measured with fMRI. FMRI signals can be reliably predicted from local field potentials (LFPs), which reflect subthreshold membrane potentials, including synaptic events, oscillatory activity and after-potentials (Logothetis and Wandell, 2004). Importantly, LGN LFPs reflect, in large part, the modulatory inputs to the LGN and subthreshold oscillatory activity that can influence the spike timing and synchrony of LGN neurons. As further elaborated below, particularly, alpha (8–13 Hz) and beta (14–30 Hz) oscillations have been reported to shape the timing of LGN responses. Interestingly, the flash suppression task modulated LFPs in the LGN in the alpha and beta frequency range. Thus, considering modulation of LGN LFPs and spike timing, rather than spike rate, may reconcile the discrepancy between monkey physiology and human fMRI studies on perceptual modulation. However, it remains to be probed whether reported perceptual dominance or suppression alters the temporal structure of LGN spiking activity.
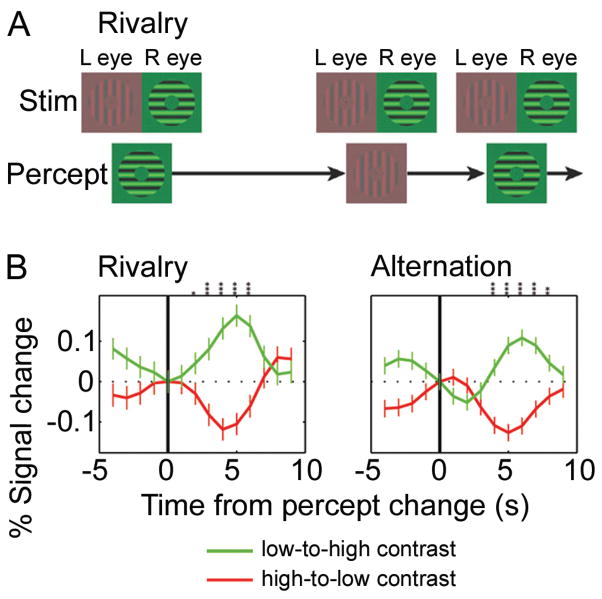
Figure 4. LGN responses reflect perceptual experience during binocular rivalry.
(A) Schematic view of the visual stimuli presented to each eye (top row) and the subject’s perceptual experience (bottom row) during the binocular rivalry task. (B) Mean fMRI signal from the LGN for all switches from low-to-high (green) or high-to-low (red) contrast percepts. Data from both hemispheres of all five subjects combined. (Left) Although the retinal stimulation did not change, LGN responses increased when subjects perceived the high contrast stimulus and decreased when they perceived the low contrast stimulus (manual report of percept at t=0). (Right) Physically alternating the visual stimuli (i.e., only presenting the high or low contrast stimulus at any one time) produced a similar pattern of LGN responses. The same response pattern during rivalry and physical alternations was also seen in V1, suggesting that the LGN/V1 circuit resolves the rivalry between simple grating stimuli. Difference between the green and red curves: t-test, *P<0.05; **P<0.01; ***P<0.001. From Figs. 1 and 2 in Wunderlich et al. (2005).
Burst and Tonic Response Modes
Modulating the response magnitude of LGN neurons is one mechanism by which information transmitted to the cortex can be influenced depending on behavioral context. Switching the response mode of LGN neurons potentially represents another important mechanism to regulate thalamo-cortical transmission. Thalamic neurons respond in one of two modes, tonic or burst firing mode, depending on a calcium current (IT) through a low threshold calcium channel (T channel). The calcium channel is inactivated when the neuron is depolarized and de-inactivated when the neuron is hyperpolarized for at least 50 ms. When the calcium current is inactivated, the neuron responds linearly to its input, with a relatively steady train of action potentials (tonic mode). When the calcium current is activated, the neuron responds to its input in a less linear fashion, with a burst of action potentials (burst mode); that is, IT activates a Ca2+-dependent spike, activating a burst of Na+ spikes (Huguenard, 1996). For example, suppressive stimuli may cause sufficiently prolonged hyperpolarization of an LGN neuron to de-inactivate low-threshold calcium channels. A subsequent depolarizing input is then more likely to induce the LGN neuron to burst fire (Alitto et al., 2005; Denning and Reinagel, 2005; Lesica and Stanley, 2004). Because bursts are more efficacious in activating thalamo-cortical synapses than tonic spikes (Swadlow and Gusev, 2001), burst firing mode may be useful for initially detecting stimuli (Fanselow et al., 2001). After stimulus detection, a switch to tonic firing mode would allow thalamic neurons to be more faithful to their retinal input, reliably transmitting information from retinal afferents to the cortex, for more detailed information processing. Such switching of firing modes has been shown in the cat LGN, in which most bursting occurred during early responses to a visual stimulus, followed by tonic firing (Guido and Weyand, 1995). The degree of vigilance also appears to influence the firing mode of thalamo-cortical neurons. LGN neurons tend to burst more when rabbits were in a low vigilance state than in an alert state; and this switch in firing mode occurred within one second of the EEG-defined state transition (Figure 5; Bezdudnaya et al., 2006). The increased bursting may allow the detection of stimuli that are relevant for ongoing behavior even when in an inattentive state. Importantly, both cortical feedback as well as cholinergic brainstem influences have been shown to depolarize LGN neurons (Scharfman et al., 1990) and thus are able to switch their firing mode from burst to tonic (Lu et al., 1993; McCormick and von Krosigk, 1992; Varela and Sherman, 2007). However, little is known about the way in which cognitive processes may impact the firing mode of thalamic neurons.
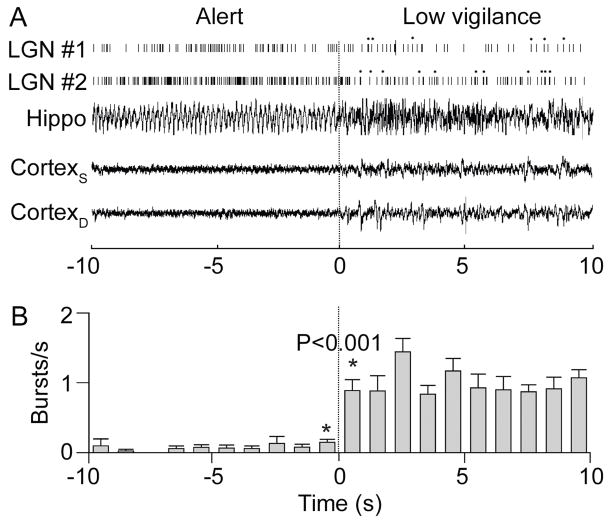
Figure 5. Burst firing of LGN neurons depends on the level of vigilance.
(A) Burst firing of two spontaneously active LGN neurons increased when the rabbit shifted from an alert (left) to a low vigilance (right) state. Asterisks denote bursts. EEG recorded from the hippocampus (Hippo) as well as the superficial (CortexS) and deep (CortexD) layers of the cortex showed the state change (at t = 0s). (B) Mean burst rate of ten spontaneously active LGN neurons before and after the change in vigilance. From Fig. 5 in Bezdudnaya et al. (2006).
Neural Synchrony and Oscillations
Thus far, we have considered influences on response magnitude and firing mode as mechanisms to modulate the efficacy of thalamic drive to the cortex. Synchronizing thalamic output represents yet a third relevant mechanism, which may be particularly effective in light of the reported low efficacy of thalamo-cortical synapses (Bruno and Sakmann, 2006). Accordingly, simultaneous recordings from the LGN and V1 in anesthetized cats have found that correlated spiking of LGN neurons increased their efficacy in driving cortical neurons (Alonso et al., 1996). Neurons with greater overlap of their RFs showed greater synchrony. A recent modeling study estimated that as few as 5 to 10 synchronized LGN cells may be sufficient to drive a cortical neuron (Wang et al., 2010a). Thus, modulating the synchrony of a group of thalamic neurons may be a potent mechanism to regulate information transmission to cortex.
Synchronizing the activity of two groups of neurons can also increase their information exchange (Gregoriou et al., 2009; Saalmann et al., 2007; Tiesinga and Sejnowski, 2009; Womelsdorf et al., 2007). Spikes are more likely to be relayed if those from presynaptic neurons arrive during periods of reduced inhibition of postsynaptic neurons. This spike timing relationship can be achieved by synchronizing oscillatory activity of pre- and postsynaptic neurons with an appropriate phase lag. Consequently, synchrony between thalamic and cortical neurons, with LGN leading, may increase the efficacy of thalamic input to cortex. Consistent with such a gain control mechanism, it has been found that attentive viewing synchronizes beta frequency oscillations of LFPs in cat LGN and V1 (Bekisz and Wrobel, 1993; Wrobel et al., 1994). Such synchrony largely seems to occur between interconnected groups of neurons in each area (Briggs and Usrey, 2007; Steriade et al., 1996), offering the possibility of spatially-specific control of information transmission.
LGN synchrony and oscillations are controlled by the areas that provide modulatory inputs to the LGN, that is, V1, TRN and cholinergic brainstem nuclei. Importantly, these sources may differentially influence different oscillation frequencies (the TRN input is discussed in its own section below). For example, evidence suggests that the cholinergic input to the thalamus regulates alpha oscillations in the LGN, as evidenced by activation of muscarinic cholinergic receptors that induce alpha oscillations of LFPs in the LGN (Lorincz et al., 2008). Thalamo-cortical cell firing appears to be correlated with these alpha oscillations, with different groups of LGN neurons firing at distinct phases of the alpha oscillation (Lorincz et al., 2009). Thus, cholinergic inputs to the LGN may influence thalamo-cortical transmission by changing the synchrony of LGN neurons (Hughes and Crunelli, 2005; Steriade, 2004). Because cholinergic tone increases with vigilance (Datta and Siwek, 2002), cholinergic influence on thalamo-cortical transmission may be modulated by behavioral context. Moreover, the thalamus is critically involved in generating cortical alpha rhythms (Hughes and Crunelli, 2005), which are linked to spatial attention bias and stimulus visibility (Mathewson et al., 2009; Romei et al., 2010; Thut et al., 2006). In comparison, feedback from V1 may influence alpha oscillations in the LGN to a lesser degree (Lorincz et al., 2009). However, feedback from V1 appears to play an important role at higher frequencies. For instance, inter-areal synchrony in the beta frequency range can help route information during selective attention (Buschman and Miller, 2007; Saalmann et al., 2007). Accordingly, feedback from V1 has been reported to modulate beta oscillatory activity in the LGN according to attentional demands (Bekisz and Wrobel, 1993).
In summary, there is growing evidence from human fMRI and macaque physiology studies that the response magnitude of LGN neurons is influenced by perceptual and cognitive tasks. Thus, the LGN may regulate information transmission from the retina to visual cortex according to behavioral context. Although the spike timing of LGN neurons is important in influencing thalamo-cortical transmission, perceptual and cognitive modulation of spike timing in the LGN of awake, behaving primates has been largely unexplored.
Pulvinar: Modulation of Information Transmission between Cortical Areas?
Despite being the largest nucleus in the primate thalamus, the pulvinar has been studied much less than the LGN. In the 1970’s, evidence started emerging for visual functions of the pulvinar, based on RF properties of its neurons and connections with visual cortex (Allman et al., 1972; Benevento and Rezak, 1976; Mathers and Rapisardi, 1973). These findings were extended in the 1980’s by monkey physiology studies demonstrating modulatory effects of attention and eye movements on responses of pulvinar neurons (Bender, 1982; Petersen et al., 1985; Robinson et al., 1986). These data, and the effects of pulvinar lesions (Chalupa et al., 1976; Ungerleider and Christensen, 1977), suggested a role for the pulvinar in visual attention. However, few experiments followed up on these initial promising results, and the pulvinar remains relatively poorly understood and understudied brain territory. We will review both the older literature and the more recent studies that have begun to characterize a novel and possibly fundamental functional role of the pulvinar in regulating cortico-cortical communication.
Anatomy and Physiology
Traditionally, the pulvinar has been divided into medial, lateral, inferior and anterior areas. However, these cytoarchitectonically-defined divisions do not correspond well with divisions based on connectivity, neurochemistry or electrophysiological properties (Adams et al., 2000; Gutierrez et al., 1995; Stepniewska and Kaas, 1997). Based on retinotopic organization and cortical connections, at least four visual areas of the pulvinar have been differentiated. There are two areas with clearly organized retinotopic maps in the lateral and inferior parts of the pulvinar, which connect with ventral visual cortex. The other two pulvinar areas do not show clear retinotopy: an inferomedial area that connects with dorsal visual cortex (areas MT, MST and FST); and a dorsal area that connects with the posterior parietal cortex (PPC) and frontal eye fields (Figure 1B). The RF size of pulvinar neurons appears to roughly correspond to that of cortical neurons to which they connect (Bender, 1982; Petersen et al., 1985). The majority of pulvinar neurons respond phasically to the onset of visual stimuli, although a number of pulvinar neurons show more tonic responses (Petersen et al., 1985). Pulvinar neurons have been reported to show broad orientation tuning and weak directional preference for moving stimuli; and a subset of neurons show color-sensitivity, including color-opponent responses (Bender, 1982; Felsten et al., 1983; Petersen et al., 1985).
The pulvinar is heavily connected to the cortex and forms cortico-thalamo-cortical pathways. As a general principle, directly-connected cortical areas will be indirectly connected via the pulvinar (Figure 1C; Sherman and Guillery, 2006; Shipp, 2003). The direct cortico-cortical feedforward connections originate in layer 3 and terminate in layer 4 in a higher cortical area (Felleman and Van Essen, 1991). In parallel, the putative feedforward pathways through the pulvinar originate in cortical layer 5 and terminate in layer 4 of the higher cortical area as well. There are also direct and indirect feedback pathways between cortical areas. The direct cortico-cortical feedback connections commonly project from layer 6 to layer 1 of the lower cortical area. Cortical layer 6 also provides feedback to the pulvinar, which itself projects to cortical layer 1 (Benevento and Rezak, 1976; Lund et al., 1975; Shipp, 2003). The fact that the direct and indirect pathways terminate in similar cortical layers presents an opportunity for the two pathways to interact.
Apart from cortico-pulvino-cortical pathways, there is a pathway that connects the superficial layers of the superior colliculus (SC) to dorsal visual cortex (MT, V3) through the inferior pulvinar (Berman and Wurtz, 2010; Glendenning et al., 1975; Lyon et al., 2010). Because the superficial SC layers receive retinal input, this pathway likely represents a second route from the retina to visual cortex that bypasses the LGN. The fast transmission time estimated between the SC and MT (Berman and Wurtz, 2010) suggests that this pulvinar pathway may be well suited to mediate motion detection, saliency processing and saccadic suppression. In addition, the pulvinar, like the LGN, receives modulatory subcortical input from the TRN and cholinergic brainstem sources (Fitzgibbon et al., 1995; Fitzpatrick et al., 1989). Interestingly, the subthalamic nucleus zona incerta provides inhibitory input to the pulvinar, but not the LGN (Power et al., 1999). Because brainstem cholinergic inputs suppress zona incerta activity (Trageser et al., 2006), increased vigilance may result in disinhibition of pulvinar neurons, including the facilitation of transmission along the colliculo-cortical pathway (Trageser and Keller, 2004). Due to the overall connectivity pattern, the pulvinar is positioned to regulate cortico-cortical transmission according to behavioral context.
Effects of Pulvinar Lesions
Arguably the most compelling evidence for the pulvinar playing an important role in visual perception and behavior comes from lesion studies in humans and monkeys. Cortical lesions involving the posterior parietal cortex (PPC) may lead to profound attentional deficits such as visuo-spatial hemineglect, a syndrome associated with a failure to direct attention to contralesional space. Neglect is not only associated with cortical lesions, but can also occur after thalamic lesions that include the pulvinar (Karnath et al., 2002; Petersen et al., 1987). More specifically, the PPC is interconnected with the dorsal pulvinar and, accordingly, inactivation of the dorsal pulvinar in monkeys leads to deficits in directing attention to contralateral space (Wilke et al., 2010). Even though thalamic neglect in humans is rare and severe attentional deficits that occur as a consequence of pulvinar lesions typically do not persist, a milder deficit that may be a residual form of thalamic neglect has been observed as a slowing of orienting responses to contralesional space (Danziger et al., 2001; Rafal and Posner, 1987).
More generally, patients with pulvinar lesions present with deficits in coding spatial information in the contralesional visual field. They have difficulty localizing stimuli in the affected visual space and these difficulties extend to the binding of visual features based on spatial information (Ward et al., 2002), which is one of the most fundamental operations that the visual system has to perform in order to integrate visual information across various feature dimensions. For example, these patients may have difficulties binding the appropriate color to each of multiple shapes that are presented simultaneously: a red square and a blue circle may be mistaken to be a blue square or red circle. Such errors in binding information from different feature dimensions that require accurate spatial coding are classically associated with PPC lesions (Friedman-Hill et al., 1995), but appear to be associated with pulvinar lesions as well (Arend et al., 2008; Ward et al., 2002). Interestingly, the spatial coding deficits have been observed in different spatial reference frames (e.g., retinotopic or object-based), thus underlining the close functional relationship between the (dorsal) pulvinar and PPC (Ward and Arend, 2007).
In accordance with its role in visual attention, patients with pulvinar lesions also show deficits in filtering distracter information. While these patients have no difficulty discriminating target stimuli when shown alone, discrimination performance is impaired when salient distracters are present that compete with the target for attentional resources, consistent with a difficulty in filtering out the unwanted information present in the visual display (Danziger et al., 2004; Snow et al., 2009). Similar filtering deficits have been observed after PPC lesions in humans (Friedman-Hill et al., 2003) and after extrastriate cortex lesions that include area V4 in humans (Gallant et al., 2000) and monkeys (De Weerd et al., 1999), suggesting that the pulvinar is part of a distributed network of brain areas that subserves visuo-spatial attention.
In monkeys, dorsal pulvinar lesions have also been shown to affect visually-guided behavior such as reaching and grasping contralesional targets (Wilke et al., 2010), similar to the optic ataxia produced after lesions to superior parietal areas that process motor intentions and represent peri-personal space (e.g., Battaglia-Mayer and Caminiti, 2002).
Taken together, lesion studies point to the critical involvement of the pulvinar in a number of fundamental cognitive functions, including orienting responses and the exploration of visual space, spatial coding of visual information necessary for feature binding, the filtering of unwanted information, and visually-guided behavior. These studies indicate that the pulvinar is an integral subcortical part of multiple large-scale networks that regulate behavior.
Behavioral Response Modulation
The findings from lesion studies are corroborated by physiology and neuroimaging studies showing that neural responses in the pulvinar reflect the behavioral relevance of stimuli. In human neuroimaging studies, modulation of responses has been shown in several different parts of the human pulvinar, including dorso-medial and inferior regions, using selective attention tasks that emphasized directing attention to a spatial location (Kastner et al., 2004), filtering of unwanted information (LaBerge and Buchsbaum, 1990) and shifts of attention across the visual field (Yantis et al., 2002). In monkey physiology studies, it has been demonstrated that spatial attention modulates the response magnitude of neurons in dorsal, lateral and inferior parts of the pulvinar (Bender and Youakim, 2001; Petersen et al., 1985). Neural responses typically increased by up to 25% or more and, in some cases, spontaneous activity was also affected. In addition to response magnitude, the timing and variability of pulvinar responses is likely to influence information transmission to the cortex. Accordingly, pulvinar neurons show reduced response variability during peripheral attention and saccade tasks (Petersen et al., 1985).
Like other thalamic cells, pulvinar neurons are able to respond in burst or tonic firing modes. Because the activity of the low threshold calcium channel depends on cell membrane potential, modulatory inputs to the pulvinar may influence the firing mode. Cholinergic inputs will likely depolarize most pulvinar neurons, switching their firing from burst to tonic mode (Varela and Sherman, 2007). However, unlike the LGN, muscarinic activation hyperpolarized about one-fifth of rat pulvinar neurons, suggesting that cholinergic inputs can induce bursting in these neurons (Varela and Sherman, 2007). In addition, inhibitory input to the pulvinar from sources such as the TRN, anterior pretectal nucleus and the zona incerta (Bokor et al., 2005; Power et al., 1999) may sufficiently hyperpolarize pulvinar neurons, to enable burst firing. Although data on the relationship between pulvinar burst firing mode and behavior is lacking, it has been shown that pulvinar neurons are more frequently in burst firing mode than LGN neurons (Ramcharan et al., 2005), and thus burst firing may play a larger role in cortico-cortical transmission than retino-cortical transmission.
Regulation of Cortico-cortical Transmission
The direct cortico-cortical pathways are commonly thought to be the major routes for the transmission of visual information between cortical areas (but see e.g., Sherman and Guillery, 2006). Given that these direct pathways are paralleled by indirect pathways through the pulvinar, it is important to ask what function these cortico-thalamo-cortical pathways may serve. In vitro studies have shown that microstimulation of the indirect pathways strongly activated cortical areas (Theyel et al., 2010). Moreover, inactivation of the thalamic projection zone that two interconnected cortical areas share led to a failure of cortico-cortical communication, raising the possibility that all cortico-cortical information transmission may strongly depend on thalamic loops (Theyel et al., 2010).
How does pulvinar output influence cortical activity? Simultaneous recordings of LFPs from the lateral posterior-pulvinar complex and visual cortex of cats performing a spatial discrimination task have demonstrated inter-areal synchrony of beta-band oscillations when the animal anticipated the visual target (Figure 6; Wrobel et al., 2007). In anesthetized cats, deactivating the pulvinar has been reported to disrupt oscillatory activity in visual cortex (Molotchnikoff and Shumikhina, 1996; Shumikhina and Molotchnikoff, 1999). Together, these results suggest that the pulvinar may facilitate oscillatory activity in visual cortical areas. Such oscillatory activity may be controlled by thalamo-cortical cells that synapse on fast-spiking (FS) inhibitory cortical neurons (Cruikshank et al., 2007; Gibson et al., 1999; Puig et al., 2008). FS neurons are known to be involved in generating oscillatory activity, that is, alternating inhibition and excitation between FS and pyramidal neurons. Thalamo-cortical inputs may initially activate a group of FS cells, setting up oscillatory activity in the gamma range (>30 Hz) for instance, which is then maintained by means of local cortical mechanisms such as recruitment of additional FS cells through chemical and electrical couplings (Puig et al., 2008).
Figure 6. Synchrony between the pulvinar complex and visual cortex.
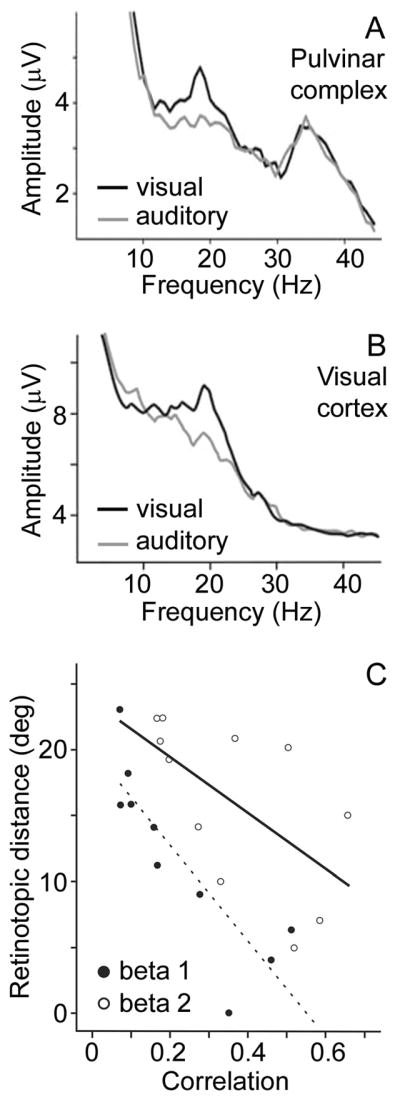
Power in the beta frequency range of the local field potential (LFP) increased in the (A) lateral posterior-pulvinar complex and (B) visual cortex (area 18) when cats performed a spatial discrimination task based on visual rather than auditory cues. (C) Synchrony between the lateral posterior-pulvinar complex and visual cortex was measured as the phase correlation between LFPs at the respective recording sites. Thalamo-cortical synchrony in the beta frequency range increased with increasing similarity of the visual field representations at the recording sites (displayed as retinotopic distance). Beta 1 = 12–19 Hz; Beta 2 = 17–25 Hz. Adapted from Figs. 2, 4 and 7 in Wrobel et al. (2007).
What may be the functional role of such oscillatory activity in cortico-thalamo-cortical communication? As previously mentioned, oscillations are able to gate input to an area, by restricting effective information transmission to times of reduced inhibition. It has been proposed that the pulvinar may synchronize oscillations between interconnected cortical areas (Jones, 2001; Saalmann and Kastner, 2009; Shipp, 2003), thereby modulating the efficacy of cortico-cortical information transfer. Initial support for such a proposal comes from pulvinar deactivation studies that have shown disrupted synchrony between two sites in area 17 or 18 of anesthetized cats (Shumikhina and Molotchnikoff, 1999). However, it is not clear how the cognitive or perceptual state might affect pulvinar influence on cortico-cortical transmission. A necessary test of the hypothesis that the pulvinar synchronizes cortical areas according to behavioral context requires simultaneous recordings from at least two interconnected cortical areas and the corresponding projection zone in the pulvinar in awake, behaving animals. In recent studies, we have simultaneously recorded from the pulvinar, V4, TEO and LIP of macaque monkeys performing a spatial attention task (Saalmann, Pinsk, Li, Kastner, 2010, Soc. Neurosci. abstract 413.10). Recording electrodes targeted pulvinar sites interconnected with the cortical areas, as determined by probabilistic tractography on diffusion tensor imaging data. Our preliminary findings suggest that the pulvinar causally influenced the cortex in the beta frequency range during selective attention and, accordingly, synchrony between the cortical areas increased at the same frequencies. Thus, the pulvinar may be able to regulate information transfer between cortical areas based on attentional demands. Because direct and indirect feedforward pathways project to cortical layer 4 and direct and indirect feedback pathways project to cortical layer 1 (Figure 1C), the pulvinar is well positioned to regulate both feedforward and feedback cortical pathways. Together, these results provide first evidence for an important role of the pulvinar in regulating cortico-cortical information transmission through the modulation of inter-areal synchrony during cognitive tasks.
In summary, lesion studies have shown that the pulvinar is critically involved in visual perception, attention and visually-guided behavior. However, it is unclear how the different subdivisions of the pulvinar contribute to these functions. Although anatomical studies have revealed basic principles of pulvino-cortical connectivity, little is known about the physiological interactions of the pulvinar and cortex. First evidence suggests a fundamental role of the pulvinar in increasing the efficacy of cortico-cortical information transmission. Studies of pulvino-cortical networks probing visual and cognitive behavior that use human neuroimaging and simultaneous neural recordings from macaque thalamus and cortex will be needed to characterize this functional role further.
TRN: Modulator and Pacemaker of Thalamo-cortical Signals?
Early accounts suggested that the TRN exerted spatially non-specific influences, largely due to its connectivity with more than one thalamic nucleus, diffuse input from the brainstem, and the extensive dendrites of TRN neurons (reviewed in Guillery and Harting, 2003). However, through the 1970s and 1980s, it became apparent that the TRN and its connections with thalamic nuclei are topographically organized (Crabtree and Killackey, 1989; Montero et al., 1977), suggesting relatively targeted and specific influences on thalamo-cortical cells. These findings were consistent with theoretical accounts proposing a role of the TRN in selective attention by gating thalamic signals (Crick, 1984; Guillery et al., 1998; Yingling and Skinner, 1976). However, compelling evidence in support of this hypothesis emerged only recently from monkey physiology studies (McAlonan et al., 2006, 2008), which we will review in this section as well as the mechanisms by which the TRN may control thalamo-cortical transmission.
Anatomy and Physiology
The TRN is subdivided into sectors, each associated with a different thalamo-cortical pathway. The visual sector of the TRN receives cortical input from layer 6 as well as thalamic input from the LGN and pulvinar in the form of collaterals from descending or ascending fibers. However, the TRN only projects to the thalamus, providing inhibitory input to the LGN and pulvinar. The TRN contains topographically organized representations of the visual field, with the RF size of many TRN neurons comparable to that of LGN neurons (McAlonan et al., 2006). The TRN input to the LGN is retinotopically organized (Crabtree and Killackey, 1989; Montero et al., 1977), suggesting that the TRN can influence thalamic processing at specific locations in the visual field. However, the TRN is unlikely to selectively modulate magno-, parvo- or koniocellular pathways, because an individual TRN axon projects to multiple LGN layers (Uhlrich et al., 2003). In contrast with the high spatial specificity of the TRN’s input to the LGN, the TRN input to the pulvinar appears to be only roughly topographically organized (Fitzgibbon et al., 1995). Tracer studies have shown that there are reciprocal connections between the TRN and the LGN or pulvinar, forming closed loops. Nonetheless, incomplete overlap in thalamic labeling after the injection of retrograde and anterograde tracers into the TRN suggests that a number of TRN neurons synapse on thalamo-cortical neurons which do not project back to the same TRN neurons, consequently forming open loops (Fitzgibbon et al., 1995; Pinault and Deschenes, 1998). Such open and closed loops offer lateral and feedback inhibition, respectively. In addition to these loops formed between the TRN and an individual thalamic nucleus, there are pathways between different thalamic nuclei via the TRN. These disynaptic, intrathalamic pathways can connect first-order and higher-order thalamic nuclei within the same modality, or connect two nuclei of different modalities. These pathways inhibit the target nucleus, thereby providing a means to facilitate information transmission through one thalamic nucleus, while suppressing another one (Crabtree et al., 1998; Crabtree and Isaac, 2002).
TRN neurons respond transiently and with short latency to visual stimuli (McAlonan et al., 2006), suggesting that the TRN can influence early evoked responses of LGN and pulvinar neurons. TRN neurons also have high spontaneous activity (McAlonan et al., 2006), consistent with a tonic inhibition of thalamic nuclei. There is growing evidence for modulation of TRN responses depending on stimulus context. For example, in anesthetized rats, TRN neurons have been reported to habituate to repetitive stimuli (Yu et al., 2009a) and to increase their response to deviant stimuli in an oddball paradigm (Yu et al., 2009b). The TRN receives input from the prefrontal cortex, visual cortex, SC and cholinergic brainstem nuclei (Kolmac and Mitrofanis, 1998; Montero, 2000; Zikopoulos and Barbas, 2006), which may enable the TRN to integrate information from various processing levels and to modulate its output according to behavioral needs.
Attentional Modulation
The TRN has been implicated in playing an important role in selective attention by regulating thalamo-cortical information transmission (e.g., Crick, 1984; Guillery et al., 1998; Yingling and Skinner, 1976). The effects of TRN lesions are consistent with such a role. For example, like in humans, the reaction times of rats to visual targets that are cued are faster than those to targets that are not. However, a unilateral TRN lesion has been shown to abolish this behavioral advantage for the cued stimulus, suggesting that the TRN normally contributes to directing attention to a cued location (Weese et al., 1999). Rat TRN lesions have also been reported to impair orienting responses and, more generally, to reduce exploratory behavior (Friedberg and Ross, 1993).
There is converging evidence from metabolic mapping and electrophysiology studies that selective attention modulates the activity of TRN neurons. Increased activity, as gauged by the number of Fos-labeled cells, has been observed in the visual sector of the rat TRN for attended visual stimuli relative to unattended stimuli (McAlonan et al., 2000). Moreover, increased deoxyglucose uptake has been demonstrated in the TRN of macaques performing a feature-based attention task (Vanduffel et al., 2000). Single-neuron recordings in macaques using cues to guide their attention, directly show specific modulatory effects of attention on TRN neuronal responses. When visual and auditory stimuli were simultaneously presented, the spike rate of neurons in the visual sector of the TRN increased when monkeys directed attention to the visual stimulus relative to when they attended to the auditory stimulus (McAlonan et al., 2006). When a monkey attended to one of two visual stimuli presented simultaneously, the spike rate of TRN neurons decreased relative to that evoked by the same stimulus when unattended (Fig. 3C; McAlonan et al., 2008). Although magnocellular LGN neurons tended to have a slightly shorter response latency to the visual stimuli, the attentional modulation started in the TRN before LGN, suggesting that the TRN contributed to the attention effects on the LGN. Interestingly, the attentional modulation of TRN responses in the intra-modal attention task differed in sign relative to that found in the cross-modal attention task. The implications of these modulatory effects on thalamo-cortical neurons will be further discussed below.
Response Modes and Oscillatory Activity
Like LGN and pulvinar neurons, TRN neurons fire in burst or tonic modes depending on the level of vigilance. Importantly, the firing mode can significantly influence the TRN response to sensory stimulation. TRN neurons reach their peak response rate more rapidly for sensory stimulation during tonic mode relative to burst mode. Considering the corresponding time courses of inhibition exerted on thalamo-cortical neurons, tonic mode may thereby facilitate rapid changes in thalamo-cortical signaling, while burst mode may permit an initially strong evoked response from thalamo-cortical neurons (Hartings et al., 2003).
TRN neurons are critically involved in initiating and sustaining thalamo-cortical oscillations. For example, a deafferented TRN is able to self-generate oscillations in the 7–15 Hz range (spindles; Steriade et al., 1987). Moreover, interactions between TRN and thalamo-cortical neurons sustain oscillations, that is, TRN neurons inhibit thalamo-cortical neurons, which rebound fire to excite TRN neurons, thereby initiating another oscillatory cycle (Steriade et al., 1993). In addition to its prominent role in spindle generation, the TRN has been shown to oscillate at lower (Amzica et al., 1992) and higher frequencies, including the beta/gamma frequency range (Pinault and Deschenes, 1992). These different oscillation frequencies manifest during different behavioral contexts. Spindles and lower frequencies commonly occur during states of low vigilance, while beta/gamma frequencies are more associated with increased vigilance (Steriade et al., 1993). It appears that spindle oscillations may contribute to reduced efficacy of information transfer across retino-thalamic synapses, by decorrelating retinal input from thalamic output (Le Masson et al., 2002). A more specific role of response modes and oscillatory TRN activity in cognitive and perceptual tasks remains to be defined.
Influences on Thalamo-cortical Transmission
TRN neurons may influence thalamo-cortical neurons of the LGN and pulvinar in a number of ways. First, TRN neurons reduce the spike rate of thalamo-cortical neurons through direct inhibition. For example, the responses of TRN neurons evoked by stimuli at unattended locations were shown to increase, while the responses of LGN neurons decreased (McAlonan et al., 2008), thus suppressing thalamo-cortical transmission of information at unattended locations. In the case of an attended visual stimulus, the converse response pattern was found. That is, responses of LGN neurons increased, while the responses of TRN neurons decreased, thus facilitating the transmission of information at attended locations. Such an inverse correlation has also been reported in anesthetized cats between simultaneously recorded neurons in the LGN and the perigeniculate nucleus, the equivalent of the TRN’s visual sector in the cat (Funke and Eysel, 1998).
Second, it is possible that TRN neurons increase the responses of thalamo-cortical neurons through disinhibition. Disinhibition of thalamo-cortical neurons has been shown to arise from TRN neurons inhibiting other TRN cells via dendrodendritic synapses (Pinault et al., 1997) or from TRN neurons synapsing on local inhibitory thalamic neurons, which constitutes about 10% of the synapses formed by TRN neurons projecting to the dorsal thalamus of the cat (Liu et al., 1995; Steriade et al., 1986). Such disinhibitory mechanisms may facilitate the thalamo-cortical transmission of relevant information (Steriade, 1999).
Third, TRN neurons may contribute to switching the firing mode of thalamo-cortical neurons. Direct TRN input hyperpolarizes thalamo-cortical cells, which typically invokes burst firing (Huguenard, 1996). Consequently, modulation of TRN activity may change the firing mode of thalamo-cortical neurons and the way information is transmitted to cortex (Yu et al., 2009b).
Finally, the TRN may impact the synchrony and oscillatory patterns of thalamic neurons. TRN inhibitory input to LGN and pulvinar neurons may constrain their spike times to time windows following periods of inhibition, thereby helping to synchronize thalamic output (Steriade et al., 1996). Furthermore, it has been argued that the TRN might function as a pacemaker of thalamo-cortical oscillations (Fuentealba and Steriade, 2005). For thalamo-cortical synchrony at spindle frequencies, cortical feedback appears to drive TRN-mediated inhibition and rebound firing of thalamic neurons. Thus, these neurons are recruited into thalamo-cortical spindle oscillations during states of low vigilance (Destexhe et al., 1998). In contrast, thalamo-cortical synchrony at higher frequencies, in the beta/gamma band, may rely more on direct cortical feedback providing excitatory input to thalamo-cortical neurons. In this case, the role of the TRN neurons may be to influence thalamo-cortical beta/gamma oscillations by resetting their phase (Pedroarena and Llinas, 1997). Such a phase reset may help to synchronize localized beta/gamma oscillations between the thalamus and cortex, thereby increasing information exchange during states of increased vigilance. This is consistent with the localized enhancement of gamma oscillations in sensory cortex that has been reported after electrical stimulation of the TRN (Macdonald et al., 1998). Such an account is also supported by a recent computational model showing that the TRN, via other thalamic nuclei, is well positioned to help synchronize areas of the cortex (Drover et al., 2010). However, a functional role of such TRN influences on thalamo-cortical synchrony and oscillations in perception and cognition remains to be determined.
In summary, the TRN forms cortico-reticular-thalamic loops that allow the TRN to influence both the LGN and pulvinar, and this may include playing the role of a pacemaker coordinating the visual thalamus. Although the empirical evidence is sparse, the TRN has a rich mechanistic infrastructure to flexibly control both thalamo-cortical and cortico-thalamic signal transmission according to behavioral context.
Conclusion
The overall evidence that has emerged during recent years suggests that the visual thalamus serves a fundamental function in regulating information transmission to the cortex and between cortical areas according to behavioral context. Selective attention and visual awareness have been shown to modulate LGN activity, thus indicating that the LGN filters visual information before it reaches the cortex. Behavioral context appears to even more strongly modulate pulvinar activity and, due to its connectivity, the pulvinar is well-positioned to influence feedforward and feedback information transmission between cortical areas. Because the TRN provides strong inhibitory input to both the LGN and pulvinar, the TRN may control and coordinate the information transmitted along both retino-cortical and cortico-cortical pathways.
The visual thalamus serves as a useful model for the thalamus in general because of common cellular mechanisms and thalamo-cortical connectivity principles across different sensorimotor domains. Specifically, the LGN and pulvinar respectively serve as models for first- and higher-order thalamic nuclei, under inhibitory control from associated sectors of the TRN. Because the pulvinar receives input from the SC to form an extra-geniculate pathway to cortex, the pulvinar also promises to be a useful model for higher-order thalamic nuclei that receive ascending sensory information from brainstem inputs, that is, nuclei exhibiting mixed first- and higher-order characteristics.
As noted in our review, there are bold question marks regarding the exact role of the visual thalamus, particularly the pulvinar and TRN, in perception and cognition, and our account of these functional roles cannot be more than an approximation based on sparse experimental evidence at this time. While there has been much study in in vitro and in anesthetized in vivo preparations of the cellular mechanisms involved in thalamo-cortical transmission, studies are missing that will link the mechanistic details to perceptual and cognitive operations. For example, it is still not clear how firing modes or oscillatory activity in the thalamus relate to perceptual and cognitive processing. Basic electrophysiology studies of the thalamus in animals performing perceptual and cognitive tasks are much needed. Moreover, although selective attention has been shown to modulate neural activity in the LGN, pulvinar and TRN, it is not clear how the TRN interacts with the LGN and pulvinar, nor how the thalamus interacts with cortex depending on behavioral context. These network properties will need investigation using simultaneous recordings from thalamic and cortical areas in awake, behaving primates.
One reason for the scarcity of studies on the visual thalamus in awake, behaving animals may be the classical view that cognition is the exclusive domain of the cortex. An additional reason is presumably methodological, such as the difficulty in targeting thalamic regions. However, this problem has been greatly reduced since structural imaging of macaque brains has become routine. Moreover, combining electrophysiology with electrical stimulation (Berman and Wurtz, 2010) or diffusion tensor imaging (Saalmann et al., SfN 2010) allows subregions of thalamic nuclei to be targeted based on connectivity.
Although there are still many unanswered questions about the role of the thalamus in perception and cognition, converging evidence from neuroimaging, physiological, anatomical and computational studies suggests that the classical view of cognitive functions exclusively depending on the cortex needs to be thoroughly revised. Only with detailed knowledge of thalamic processing and thalamo-cortical interactions will it be possible to fully understand cognition.
Acknowledgments
This work is supported by grants NEI RO1 EY017699, NIMH R01 MH064043 and NEI R21 EY021078.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Hof PR, Gattass R, Webster MJ, Ungerleider LG. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J Comp Neurol. 2000;419:377–393. doi: 10.1002/(sici)1096-9861(20000410)419:3<377::aid-cne9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Alitto HJ, Weyand TG, Usrey WM. Distinct properties of stimulus-evoked bursts in the lateral geniculate nucleus. J Neurosci. 2005;25:514–523. doi: 10.1523/JNEUROSCI.3369-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Kaas JH, Lane RH, Miezin FM. A representation of the visual field in the inferior nucleus of the pulvinar in the owl monkey (Aotus trivirgatus) Brain Res. 1972;40:291–302. doi: 10.1016/0006-8993(72)90135-7. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- Amzica F, Nunez A, Steriade M. Delta frequency (1–4 Hz) oscillations of perigeniculate thalamic neurons and their modulation by light. Neuroscience. 1992;51:285–294. doi: 10.1016/0306-4522(92)90315-s. [DOI] [PubMed] [Google Scholar]
- Arend I, Rafal R, Ward R. Spatial and temporal deficits are regionally dissociable in patients with pulvinar lesions. Brain. 2008;131:2140–2152. doi: 10.1093/brain/awn135. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Caminiti R. Optic ataxia as a result of the breakdown of the global tuning fields of parietal neurones. Brain. 2002;125:225–237. doi: 10.1093/brain/awf034. [DOI] [PubMed] [Google Scholar]
- Bekisz M, Wrobel A. 20 Hz rhythm of activity in visual system of perceiving cat. Acta Neurobiol Exp (Wars) 1993;53:175–182. [PubMed] [Google Scholar]
- Bender DB. Receptive-field properties of neurons in the macaque inferior pulvinar. J Neurophysiol. 1982;48:1–17. doi: 10.1152/jn.1982.48.1.1. [DOI] [PubMed] [Google Scholar]
- Bender DB, Youakim M. Effect of attentive fixation in macaque thalamus and cortex. J Neurophysiol. 2001;85:219–234. doi: 10.1152/jn.2001.85.1.219. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Rezak M. The cortical projections of the inferior pulvinar and adjacent lateral pulvinar in the rhesus monkey (Macaca mulatta): an autoradiographic study. Brain Res. 1976;108:1–24. doi: 10.1016/0006-8993(76)90160-8. [DOI] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH. Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci. 2010;30:6342–6354. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Ramcharan E, Godwin DW, Erisir A, Gnadt J, Sherman SM. Neurotransmitters contained in the subcortical extraretinal inputs to the monkey lateral geniculate nucleus. J Comp Neurol. 2000;424:701–717. doi: 10.1002/1096-9861(20000904)424:4<701::aid-cne11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Bokor H, Frere SG, Eyre MD, Slezia A, Ulbert I, Luthi A, Acsady L. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45:929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Cortical activity influences geniculocortical spike efficacy in the macaque monkey. Front Integr Neurosci. 2007;1:3. doi: 10.3389/neuro.07.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Parallel processing in the corticogeniculate pathway of the macaque monkey. Neuron. 2009;62:135–146. doi: 10.1016/j.neuron.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Structure and function of parallel pathways in the primate early visual system. J Physiol. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, Coyle RS, Lindsley DB. Effect of pulvinar lesions on visual pattern discrimination in monkeys. J Neurophysiol. 1976;39:354–369. doi: 10.1152/jn.1976.39.2.354. [DOI] [PubMed] [Google Scholar]
- Connolly M, Van Essen D. The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. J Comp Neurol. 1984;226:544–564. doi: 10.1002/cne.902260408. [DOI] [PubMed] [Google Scholar]
- Crabtree JW, Collingridge GL, Isaac JT. A new intrathalamic pathway linking modality-related nuclei in the dorsal thalamus. Nat Neurosci. 1998;1:389–394. doi: 10.1038/1603. [DOI] [PubMed] [Google Scholar]
- Crabtree JW, Isaac JT. New intrathalamic pathways allowing modality-related and cross-modality switching in the dorsal thalamus. J Neurosci. 2002;22:8754–8761. doi: 10.1523/JNEUROSCI.22-19-08754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JW, Killackey HP. The Topographic Organization and Axis of Projection within the Visual Sector of the Rabbit’s Thalamic Reticular Nucleus. Eur J Neurosci. 1989;1:94–109. doi: 10.1111/j.1460-9568.1989.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Lee BB, Elepfandt A. A quantitative study of chromatic organisation and receptive fields of cells in the lateral geniculate body of the rhesus monkey. Exp Brain Res. 1979;35:527–545. doi: 10.1007/BF00236770. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Danziger S, Ward R, Owen V, Rafal R. The effects of unilateral pulvinar damage in humans on reflexive orienting and filtering of irrelevant information. Behav Neurol. 2001;13:95–104. doi: 10.1155/2002/917570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger S, Ward R, Owen V, Rafal R. Contributions of the human pulvinar to linking vision and action. Cogn Affect Behav Neurosci. 2004;4:89–99. doi: 10.3758/cabn.4.1.89. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Peralta MR, 3rd, Desimone R, Ungerleider LG. Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nat Neurosci. 1999;2:753–758. doi: 10.1038/11234. [DOI] [PubMed] [Google Scholar]
- Denning KS, Reinagel P. Visual control of burst priming in the anesthetized lateral geniculate nucleus. J Neurosci. 2005;25:3531–3538. doi: 10.1523/JNEUROSCI.4417-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Roy JP, Steriade M. Thalamic bursting mechanism: an inward slow current revealed by membrane hyperpolarization. Brain Res. 1982;239:289–293. doi: 10.1016/0006-8993(82)90854-x. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol. 1998;79:999–1016. doi: 10.1152/jn.1998.79.2.999. [DOI] [PubMed] [Google Scholar]
- Dreher B, Fukada Y, Rodieck RW. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976;258:433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drover JD, Schiff ND, Victor JD. Dynamics of coupled thalamocortical modules. J Comput Neurosci. 2010;28:605–616. doi: 10.1007/s10827-010-0244-5. [DOI] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Bickford ME, Sherman SM. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: a comparison with corticogeniculate terminals. J Comp Neurol. 1997;377:535–549. [PubMed] [Google Scholar]
- Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA. 2001;98:15330–15335. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Felsten G, Benevento LA, Burman D. Opponent-color responses in macaque extrageniculate visual pathways: the lateral pulvinar. Brain Res. 1983;288:363–367. doi: 10.1016/0006-8993(83)90119-1. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon T, Tevah LV, Sefton AJ. Connections between the reticular nucleus of the thalamus and pulvinar-lateralis posterior complex: a WGA-HRP study. J Comp Neurol. 1995;363:489–504. doi: 10.1002/cne.903630311. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D, Diamond IT, Raczkowski D. Cholinergic and monoaminergic innervation of the cat’s thalamus: comparison of the lateral geniculate nucleus with other principal sensory nuclei. J Comp Neurol. 1989;288:647–675. doi: 10.1002/cne.902880411. [DOI] [PubMed] [Google Scholar]
- Friedberg EB, Ross DT. Degeneration of rat thalamic reticular neurons following intrathalamic domoic acid injection. Neurosci Lett. 1993;151:115–119. doi: 10.1016/0304-3940(93)90060-x. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Desimone R, Ungerleider LG. Posterior parietal cortex and the filtering of distractors. Proc Natl Acad Sci USA. 2003;100:4263–4268. doi: 10.1073/pnas.0730772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Treisman A. Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions. Science. 1995;269:853–855. doi: 10.1126/science.7638604. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Funke K, Eysel UT. Inverse correlation of firing patterns of single topographically matched perigeniculate neurons and cat dorsal lateral geniculate relay cells. Vis Neurosci. 1998;15:711–729. doi: 10.1017/s0952523898154111. [DOI] [PubMed] [Google Scholar]
- Gallant JL, Shoup RE, Mazer JA. A human extrastriate area functionally homologous to macaque V4. Neuron. 2000;27:227–235. doi: 10.1016/s0896-6273(00)00032-5. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Hall JA, Diamond IT, Hall WC. The pulvinar nucleus of Galago senegalensis. J Comp Neurol. 1975;161:419–458. doi: 10.1002/cne.901610309. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W, Weyand T. Burst responses in thalamic relay cells of the awake behaving cat. J Neurophysiol. 1995;74:1782–1786. doi: 10.1152/jn.1995.74.4.1782. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Lozsadi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21:28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol. 2003;463:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Yaun A, Cusick CG. Neurochemical subdivisions of the inferior pulvinar in macaque monkeys. J Comp Neurol. 1995;363:545–562. doi: 10.1002/cne.903630404. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Temereanca S, Simons DJ. State-dependent processing of sensory stimuli by thalamic reticular neurons. J Neurosci. 2003;23:5264–5271. doi: 10.1523/JNEUROSCI.23-12-05264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JD, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438:496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Hickey TL, Guillery RW. Variability of laminar patterns in the human lateral geniculate nucleus. J Comp Neurol. 1979;183:221–246. doi: 10.1002/cne.901830202. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2. New York, New York: Cambridge University Press; 2007. [Google Scholar]
- Kaas JH, Guillery RW, Allman JM. Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav Evol. 1972;6:253–299. doi: 10.1159/000123713. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Kastner S, O’Connor DH, Fukui MM, Fehd HM, Herwig U, Pinsk MA. Functional imaging of the human lateral geniculate nucleus and pulvinar. J Neurophysiol. 2004;91:438–448. doi: 10.1152/jn.00553.2003. [DOI] [PubMed] [Google Scholar]
- Kolmac CI, Mitrofanis J. Patterns of brainstem projection to the thalamic reticular nucleus. J Comp Neurol. 1998;396:531–543. [PubMed] [Google Scholar]
- LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci. 1990;10:613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros Clark WE. The structure and connections of the thalamus. Brain. 1932;55:406–470. [Google Scholar]
- Le Masson G, Renaud-Le Masson S, Debay D, Bal T. Feedback inhibition controls spike transfer in hybrid thalamic circuits. Nature. 2002;417:854–858. doi: 10.1038/nature00825. [DOI] [PubMed] [Google Scholar]
- Lehky SR, Maunsell JH. No binocular rivalry in the LGN of alert macaque monkeys. Vision Res. 1996;36:1225–1234. doi: 10.1016/0042-6989(95)00232-4. [DOI] [PubMed] [Google Scholar]
- Lesica NA, Stanley GB. Encoding of natural scene movies by tonic and burst spikes in the lateral geniculate nucleus. J Neurosci. 2004;24:10731–10740. doi: 10.1523/JNEUROSCI.3059-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Warren RA, Jones EG. Synaptic distribution of afferents from reticular nucleus in ventroposterior nucleus of cat thalamus. J Comp Neurol. 1995;352:187–202. doi: 10.1002/cne.903520203. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lorincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8–13 Hz) rhythms in sensory thalamic nuclei in vitro. J Neurosci. 2008;28:660–671. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Guido W, Sherman SM. The brain-stem parabrachial region controls mode of response to visual stimulation of neurons in the cat’s lateral geniculate nucleus. Vis Neurosci. 1993;10:631–642. doi: 10.1017/s0952523800005332. [DOI] [PubMed] [Google Scholar]
- Lund JS, Lund RD, Hendrickson AE, Bunt AH, Fuchs AF. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol. 1975;164:287–303. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Nassi JJ, Callaway EM. A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron. 2010;65:270–279. doi: 10.1016/j.neuron.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald KD, Fifkova E, Jones MS, Barth DS. Focal stimulation of the thalamic reticular nucleus induces focal gamma waves in cortex. J Neurophysiol. 1998;79:474–477. doi: 10.1152/jn.1998.79.1.474. [DOI] [PubMed] [Google Scholar]
- Malpeli JG, Baker FH. The representation of the visual field in the lateral geniculate nucleus of Macaca mulatta. J Comp Neurol. 1975;161:569–594. doi: 10.1002/cne.901610407. [DOI] [PubMed] [Google Scholar]
- Martin PR, White AJ, Goodchild AK, Wilder HD, Sefton AE. Evidence that blue-on cells are part of the third geniculocortical pathway in primates. Eur J Neurosci. 1997;9:1536–1541. doi: 10.1111/j.1460-9568.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Mathers LH, Rapisardi SC. Visual and somatosensory receptive fields of neurons in the squirrel monkey pulvinar. Brain Res. 1973;64:65–83. doi: 10.1016/0006-8993(73)90171-6. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. J Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ, Bowman EM. Thalamic reticular nucleus activation reflects attentional gating during classical conditioning. J Neurosci. 2000;20:8897–8901. doi: 10.1523/JNEUROSCI.20-23-08897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. I: distribution and timing of effects across visual areas. Cereb Cortex. 2000;10:343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Molotchnikoff S, Shumikhina S. The lateral posterior-pulvinar complex modulation of stimulus-dependent oscillations in the cat visual cortex. Vision Res. 1996;36:2037–2046. doi: 10.1016/0042-6989(95)00311-8. [DOI] [PubMed] [Google Scholar]
- Montero VM. Attentional activation of the visual thalamic reticular nucleus depends on ‘top-down’ inputs from the primary visual cortex via corticogeniculate pathways. Brain Res. 2000;864:95–104. doi: 10.1016/s0006-8993(00)02182-x. [DOI] [PubMed] [Google Scholar]
- Montero VM, Guillery RW, Woolsey CN. Retinotopic organization within the thalamic reticular nucleus demonstrated by a double label autoradiographic technique. Brain Res. 1977;138:407–421. doi: 10.1016/0006-8993(77)90681-3. [DOI] [PubMed] [Google Scholar]
- Mukhametov LM, Rizzolatti G, Tradardi V. Spontaneous activity of neurones of nucleus reticularis thalami in freely moving cats. J Physiol. 1970;210:651–667. doi: 10.1113/jphysiol.1970.sp009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Pedroarena C, Llinas R. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Natl Acad Sci USA. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- Pinault D, Deschenes M. Voltage-dependent 40-Hz oscillations in rat reticular thalamic neurons in vivo. Neuroscience. 1992;51:245–258. doi: 10.1016/0306-4522(92)90312-p. [DOI] [PubMed] [Google Scholar]
- Pinault D, Deschenes M. Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. Eur J Neurosci. 1998;10:3462–3469. doi: 10.1046/j.1460-9568.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- Pinault D, Smith Y, Deschenes M. Dendrodendritic and axoaxonic synapses in the thalamic reticular nucleus of the adult rat. J Neurosci. 1997;17:3215–3233. doi: 10.1523/JNEUROSCI.17-09-03215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power BD, Kolmac CI, Mitrofanis J. Evidence for a large projection from the zona incerta to the dorsal thalamus. J Comp Neurol. 1999;404:554–565. [PubMed] [Google Scholar]
- Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking interneurons during neocortical UP states. Proc Natl Acad Sci USA. 2008;105:8428–8433. doi: 10.1073/pnas.0712219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal RD, Posner MI. Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci USA. 1987;84:7349–7353. doi: 10.1073/pnas.84.20.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci USA. 2005;102:12236–12241. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun DL, Warland DK, Usrey WM. Spike timing and information transmission at retinogeniculate synapses. J Neurosci. 2010;30:13558–13566. doi: 10.1523/JNEUROSCI.0909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Petersen SE, Keys W. Saccade-related and visual activities in the pulvinar nuclei of the behaving rhesus monkey. Exp Brain Res. 1986;62:625–634. doi: 10.1007/BF00236042. [DOI] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Jayakumar J, Martin PR, Dreher B, Saalmann YB, Hu D, Vidyasagar TR. Segregation of short-wavelength-sensitive (S) cone signals in the macaque dorsal lateral geniculate nucleus. Eur J Neurosci. 2009;30:1517–1526. doi: 10.1111/j.1460-9568.2009.06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol. 2009;19:408–414. doi: 10.1016/j.conb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Lu SM, Guido W, Adams PR, Sherman SM. N-methyl-D-aspartate receptors contribute to excitatory postsynaptic potentials of cat lateral geniculate neurons recorded in thalamic slices. Proc Natl Acad Sci USA. 1990;87:4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA, Kastner S. Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J Neurosci. 2009;29:1784–1795. doi: 10.1523/JNEUROSCI.4452-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA, Richter MC, Kastner S. Retinotopic organization and functional subdivisions of the human lateral geniculate nucleus: a high-resolution functional magnetic resonance imaging study. J Neurosci. 2004;24:8975–8985. doi: 10.1523/JNEUROSCI.2413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclar G, Maunsell JH, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Res. 1990;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- Shapley R, Kaplan E, Soodak R. Spatial summation and contrast sensitivity of X and Y cells in the lateral geniculate nucleus of the macaque. Nature. 1981;292:543–545. doi: 10.1038/292543a0. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. 2. Cambridge, Mass: MIT Press; 2006. [Google Scholar]
- Sherman SM, Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res. 1986;63:1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico-pulvinar connections. Philos, Trans, R Soc Lond B Biol Sci. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumikhina S, Molotchnikoff S. Pulvinar participates in synchronizing neural assemblies in the visual cortex, in cats. Neurosci, Lett. 1999;272:135–139. doi: 10.1016/s0304-3940(99)00497-8. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol, Rev. 1977;57:386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Snow JC, Allen HA, Rafal RD, Humphreys GW. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc, Natl, Acad, Sci, U,S,A. 2009;106:4054–4059. doi: 10.1073/pnas.0810086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Kaas JH. Architectonic subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis Neurosci. 1997;14:1043–1060. doi: 10.1017/s0952523800011767. [DOI] [PubMed] [Google Scholar]
- Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. 1999;22:337–345. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- Steriade M. Acetylcholine systems and rhythmic activities during the waking--sleep cycle. Prog Brain Res. 2004;145:179–196. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Amzica F, Timofeev I. Synchronization of fast (30–40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci. 1996;16:2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986;6:68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4:402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trageser JC, Burke KA, Masri R, Li Y, Sellers L, Keller A. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol. 2006;96:1456–1463. doi: 10.1152/jn.00423.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci. 2004;24:8911–8915. doi: 10.1523/JNEUROSCI.3218-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlrich DJ, Manning KA, Feig SL. Laminar and cellular targets of individual thalamic reticular nucleus axons in the lateral geniculate nucleus in the prosimian primate Galago. J Comp Neurol. 2003;458:128–143. doi: 10.1002/cne.10568. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Christensen CA. Pulvinar lesions in monkeys produce abnormal eye movements during visual discrimination training. Brain Res. 1977;136:189–196. doi: 10.1016/0006-8993(77)90146-9. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Tootell RB, Orban GA. Attention-dependent suppression of metabolic activity in the early stages of the macaque visual system. Cereb Cortex. 2000;10:109–126. doi: 10.1093/cercor/10.2.109. [DOI] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in response to muscarinic activation between first and higher order thalamic relays. J Neurophysiol. 2007;98:3538–3547. doi: 10.1152/jn.00578.2007. [DOI] [PubMed] [Google Scholar]
- Wang HP, Spencer D, Fellous JM, Sejnowski TJ. Synchrony of thalamocortical inputs maximizes cortical reliability. Science. 2010a;328:106–109. doi: 10.1126/science.1183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hirsch JA, Sommer FT. Recoding of sensory information across the retinothalamic synapse. J Neurosci. 2010b;30:13567–13577. doi: 10.1523/JNEUROSCI.0910-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, Arend I. An object-based frame of reference within the human pulvinar. Brain. 2007;130:2462–2469. doi: 10.1093/brain/awm176. [DOI] [PubMed] [Google Scholar]
- Ward R, Danziger S, Owen V, Rafal R. Deficits in spatial coding and feature binding following damage to spatiotopic maps in the human pulvinar. Nat Neurosci. 2002;5:99–100. doi: 10.1038/nn794. [DOI] [PubMed] [Google Scholar]
- Weese GD, Phillips JM, Brown VJ. Attentional orienting is impaired by unilateral lesions of the thalamic reticular nucleus in the rat. J Neurosci. 1999;19:10135–10139. doi: 10.1523/JNEUROSCI.19-22-10135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Wilke M, Mueller KM, Leopold DA. Neural activity in the visual thalamus reflects perceptual suppression. Proc Natl Acad Sci USA. 2009;106:9465–9470. doi: 10.1073/pnas.0900714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Turchi J, Smith K, Mishkin M, Leopold DA. Pulvinar inactivation disrupts selection of movement plans. J Neurosci. 2010;30:8650–8659. doi: 10.1523/JNEUROSCI.0953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Wrobel A, Bekisz M, Kublik E, Waleszczyk W. 20 Hz bursting beta activity in the cortico-thalamic system of visually attending cats. Acta Neurobiol Exp (Wars) 1994;54:95–107. [PubMed] [Google Scholar]
- Wrobel A, Ghazaryan A, Bekisz M, Bogdan W, Kaminski J. Two streams of attention-dependent beta activity in the striate recipient zone of cat’s lateral posterior-pulvinar complex. J Neurosci. 2007;27:2230–2240. doi: 10.1523/JNEUROSCI.4004-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich K, Schneider KA, Kastner S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat Neurosci. 2005;8:1595–1602. doi: 10.1038/nn1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ichida JM, Allison JD, Boyd JD, Bonds AB, Casagrande VA. A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus) J Physiol. 2001;531:203–218. doi: 10.1111/j.1469-7793.2001.0203j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Yingling CD, Skinner JE. Selective regulation of thalamic sensory relay nuclei by nucleus reticularis thalami. Electroencephalogr Clin Neurophysiol. 1976;41:476–482. doi: 10.1016/0013-4694(76)90059-6. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Xu XX, Chen X, He S, He J. Slow recovery from excitation of thalamic reticular nucleus neurons. J Neurophysiol. 2009a;101:980–987. doi: 10.1152/jn.91130.2008. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Xu XX, He S, He J. Change detection by thalamic reticular neurons. Nat Neurosci. 2009b;12:1165–1170. doi: 10.1038/nn.2373. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]