Abstract
Although sensation-seeking status is associated with age of initiation and amount of drug use among adolescents, and sensitivity to the behavioral and reinforcing effects of drugs among young adults, it is unclear whether sensation-seeking status among adolescents is predictive of sensitivity to the pharmacological effects of drugs (i.e. abuse potential) as adults. This study examined the acute behavioral effects of oral diazepam and d-amphetamine in young adults, ages 18–21 years, who had consistently scored in the highest or lowest third of their grade-based cohort on a modified Sensation Seeking Scale that was completed annually between ages 10 and 14 years. Healthy participants completed 16 7.5-h test days, with test days separated by a minimum of 48 h. Each day, assessments consisting of computer task performance, verbal report of drug effects, and cardiovascular measures were completed 0, 50, 110, 170, 230, and 290 min after drug administration. Placebo and three active doses of diazepam and d-amphetamine (2.5, 5.0 and 10.0 mg/70 kg) were tested under double-blind conditions according to a randomized-block design. Typical stimulant and sedative effects were obtained with d-amphetamine and diazepam, respectively. Drug effects varied as a function of sensation-seeking status, with magnitude of effects on cardiovascular function, task performance, and report of positive drug effects being greater among high sensation seekers, and report of negative drug effects being greater among low sensation seekers. Adolescents who report high levels of sensation seeking on a consistent basis are more sensitive to pharmacological effects of stimulant and sedative drugs that are associated with abuse potential as young adults.
Keywords: abuse potential, d-amphetamine, diazepam, human, performance, personality, sensation seeking, substance abuse, verbal report of drug effect
Introduction
Sensation seeking, defined as the preference for novel, complex, ambiguous, and/or emotionally intense sensations and experiences and willingness to take risks for such experiences (Zuckerman, 1994), is associated with impulsivity, reward sensitivity, and vulnerability to drug abuse (e.g. Depue and Collins, 1999; de Wit and Richards, 2004). Adolescents and young adults characterized as high sensation or novelty seekers, using personality scales such as the Zuckerman or Cloninger inventories (Zuckerman and Link, 1968; Cloninger, 1987), are more likely to initiate drug use, begin using at an earlier age, and report greater frequency and amount of drug use compared with their low sensation seeking counterparts (Wills et al., 1994, 1995). Regular drug users and substance abusers score higher on sensation-seeking dimensions than control subjects (Kosten et al., 1994; Gelernter et al., 1997; Mitchell, 1999). High sensation seekers in drug treatment also relapse at a greater rate than low sensation seekers (Schubiner et al., 2002). These data suggest that high sensation seekers may be more vulnerable to drug abuse.
Individual differences in sensation-seeking status have been related to biological factors. Sensation seeking seems to be heritable, with twin studies suggesting that genetic factors account for as much as 58% of the variance in sensation-seeking status (Fulker et al., 1980). Sensation-seeking levels are high in children of alcoholics, who are at increased genetic risk for drug abuse (Loukas et al., 2001). High and low sensation seekers exhibit differences in physiological responses to novel stimuli (Neary and Zuckerman, 1976; Netter et al., 1996), and differences in hormone and enzyme levels and neuro-transmitter system function have been reported as a function of sensation-seeking status (Schooler et al., 1978; Balada et al., 1992; Netter et al., 1996). Although evidence is mixed, studies suggest that variation in dopamine receptor genes may also be linked to individual differences in sensation-seeking status (Ebstein et al., 1996; Ekelund et al., 1999; Suhara et al., 2001). Given a biological basis for sensation-seeking status, sensation-seeking status among adolescents should predict subsequent sensitivity to the pharmacological effects of drugs of abuse.
Several clinical studies suggest that adults reporting characteristics associated with high sensation-seeking status are more sensitive to the behavioral effects of drugs than are low sensation seekers (e.g. de Wit et al., 1987; Cheong and Nagoshi, 1998; Sax and Strakowski, 1998; Hutchison et al., 1999; Perkins et al., 2000, 2008; Alessi et al., 2003; Kelly et al., 2006; White et al., 2006; Stoops et al., 2007; Fillmore et al., 2009; cf. Carrol et al., 1982; Nagoshi et al., 1991; Chait, 1993; Corr and Kumari, 2000). As such, high sensation seekers, who are more likely to engage in high-risk behaviors, including drug use, may also be at greater risk for repeated use, given enhanced sensitivity to pharmacological effects. However, given that sensation-seeking status was assessed among adults in those studies, it remains uncertain whether sensation-seeking status assessed during adolescence would predict the relative sensitivity to the pharmacological (i.e. reinforcing) effects of drugs in later life.
The purpose of this study was to evaluate the association between sensation-seeking status among adolescents and subsequent sensitivity to the pharmacological effects of drugs as young adults. Although there are heritable factors associated with sensation-seeking status, social psychological factors can also influence expression of the trait (Stacy et al., 1991). As such, participants were recruited in a manner designed to maximize stable differences in sensation-seeking status over time, thereby increasing the likelihood that trait expression was associated with heritable factors. Three consecutive cohorts of adolescents, entering the sixth grade in Fayette County, Kentucky, completed a modified version of the Sensation Seeking Scale (Form V) for 4 consecutive years, as part of an evaluation of the efficacy of a drug prevention program (Clayton et al., 1996; Lynam et al., 1999). Individuals scoring in the top or bottom third of their grade-based cohort on each assessment were contacted as young adults and invited to participate in a follow-up study examining drug abuse potential. It was assumed that individuals identified as high or low sensation seekers at a relatively early age (i.e. 6th grade) and who remained as such over a 4-year period would be highly likely to continue to express differences in sensation-seeking status over time, independent of social psychological influences. A small subset of 17 young adults (11 high and six low sensation seekers) met these stringent eligibility criteria and completed a randomized, placebo-controlled, double-blind evaluation of the abuse potential of diazepam and d-amphetamine, to determine whether individual differences in sensitivity to drug effects were associated with their sensation-seeking status as adolescents.
Methods
Subjects
Three cohorts of middle-school students in Fayette County, Kentucky (n=5608) completed a modified version of Form V of the Sensation Seeking Scale (drug-use items were removed; Clayton et al., 1996) annually, over 4 consecutive years between sixth and tenth grade; those scoring in the upper or lower third of the distribution of scores from their cohort during each of the four annual assessments (n=679) served as potential participants. Those who could be recontacted as young adults (i.e. between ages 18 and 22 years) and agreed to be contacted about follow-up study participation, completed a brief telephone interview addressing general medical and legal status, and reported good health and occasional stimulant and sedative use (e.g. alcohol, caffeine), served as the potential pool of study participants (n=123).
From this pool, those volunteers with compatible schedules who were potentially interested in study participation were invited to complete medical screening and training activities on separate days before the start of the study (n=43). During the medical screening, all details of study participation were discussed, and volunteers completed locally developed health and personal history questionnaires, a 17-item drug use questionnaire derived from the Addiction Severity Index (McLellan et al., 1992), the 13-item version of the Michigan Alcoholism Screening Test (Selzer et al., 1975), the Eysenck Personality Inventory (Eysenck, 1986), the Addiction Research Center Maturation Scale (Martin et al., 1977), the Brief Symptom Inventory (Derogatis and Melisaratos, 1983), and the Beck Depression Inventory, short form (Beck and Beck, 1972). Volunteers also completed the Sensation Seeking Scale (Form V) and a locally developed Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-based attention-deficit/hyperactivity disorder and conduct disorder symptom checklist. Blood chemistry, liver function, and urinalysis tests were also conducted. Volunteers were excluded from participation (n=16) if they were using psychoactive medications, or had current symptoms or histories of any medical condition that would put them at increased medical risk from study participation, such as major psychiatric disorders, alcohol or drug abuse or dependence, central nervous system disorders, cardiovascular or liver/kidney disorders based on the questionnaires and follow-up discussion. During training, participants practiced the study tasks until performance was consistent and accurate across consecutive trials.
The final sample consisted of 11 high (four female) and six low (five female) sensation seekers, ages 18–21 years, who had completed 13–17 years of education. Three female low sensation seekers identified themselves as African-American, one high sensation seeker identified himself as Asian, and the remaining participants identified themselves as Caucasian.
Participants provided written consent before participation and received financial compensation (payments for medical screening, training, per diem payments, task earnings, and a bonus for completing all scheduled test days and abstaining from drug use before scheduled sessions). The University of Kentucky Medical Institutional Review Board reviewed and approved the study.
Procedure
Participants completed 16 7.5-h test days, each separated by a minimum of 48 h, under isolated conditions, in a hospital room within the General Clinical Research Center of the University of Kentucky Chandler Medical Center. Performance tasks and drug effect questionnaires were presented on a computer (PowerMac 8600/250, Apple Computer, Cupertino, California, USA) and 14-inch color monitor while participants were seated at a desk. Testing occurred at the same time each day. Participants were requested to abstain from the use of any medication, including alcohol, for 24 h and to abstain from eating for 4 h before the start of all test days. At the beginning of each test day, participants completed a Field Sobriety test and provided breath and urine samples. Samples were tested to verify the absence of drug use (i.e. alcohol, cocaine, benzodiazepines, barbiturates, marijuana, amphetamines, and opiates) or pregnancy. Participants then consumed a caffeine-free breakfast (two pieces of toast with butter, cereal, milk) during a 15-min interval.
Ten minutes after completing the meal, participants completed a baseline (i.e. predrug) assessment. The test dose was administered 50 min postmeal, and assessments were repeated 50, 110, 170, 230, and 290 min after dose administration. Each assessment was approximately 35 min in duration. After the final assessment, participants repeated the Field Sobriety tests, received per-diem payments, and were discharged from the hospital when Field Sobriety performance was consistent with performance at the start of the day before drug administration and participants reported no residual drug effects.
Assessments
Each assessment included measurement of task performance, cardiovascular function, and verbal report of drug effects.
Task performance
Tasks [Repeated Acquisition of Response Sequences (RA), Digit Symbol Substitution (DSST)] commonly used to assess the effects of drugs with abuse potential (Roache, 1991) were included to examine drug effects on psychomotor performance and learning efficiency in high and low sensation seekers. The RA task (Fischman, 1978; Kelly et al., 2005) consisted of two components, a learning component that was presented for 3 min, and a performance component, presented for 1 min. Participants were required to learn a new 10-response sequence on four buttons during the learning component, while the 10-response sequence remained unchanged during the performance component throughout the study. Participants received two cents per point during both the learning and performance components, and response rates and patterns of correct and incorrect responses throughout the learning and performance components were monitored as indices of drug effects on performance and learning ability. A 2-min computerized version of the DSST (McLeod et al., 1982; Kelly et al., 2005) was used to examine psychomotor performance. Participants received one cent per correct trial, and trial rate and accuracy were used in as an index of performance.
Cardiovascular function
Cardiovascular measures are also commonly used to assess the effects of drugs with abuse potential (Roache, 1991). In this study, cardiovascular measures (heart rate, systolic and diastolic blood pressure) were collected during a 5-min stress task during which participants completed mathematical addition problems (McCubbin et al., 1988, 1992), as well as during 5-min rest intervals occurring before and after the mathematical task. The difficulty of the addition problems (i.e. numbers of digits to be added) and the duration of time to enter the sum on the keyboard were systematically manipulated during each assessment based on participant performance in an attempt to maintain a consistent level of difficulty for all participants, regardless of mathematical ability or drug performance effects. Cardiovascular measures were collected every 60 s with an oscillometric blood pressure machine (Sentry II, NBS Medical, Costa Mesa, California, USA). As the level of task difficulty was continuously changing based on ongoing performance, drug effects on task performance were not examined.
Verbal report of drug effect measures
Verbal report measures [Visual-Analog Scales (VAS), Profile of Mood States (POMS), Addiction Research Center Inventory (ARCI)] commonly used to assess drug abuse potential (Fischman and Foltin, 1991) were included to examine subjective drug effects among high and low sensation seekers. VAS items (I feel stimulated, stressed, sedated, hungry, anxious, light-headed, thirsty, sleepy, sick to my stomach, down, high; and a drug effect, as well as: I like the drug effect) were completed by placing a mark along a computerized line containing 100 discrete units and anchored on the left by ‘Not At All’ and on the right by ‘Extremely’. An experimental version of the POMS (McNair et al., 1971) was also examined, consisting of 72 adjectives rated along a five-point scale, yielding scores on eight mood clusters: Anxiety, Depression, Anger, Vigor, Fatigue, Confusion, Friendliness, and Elation, and two derived scales [Arousal: (Anxiety+Vigor) – (Fatigue+Confusion); Total Positive: Elation − Depression]. The 49-item short form of the ARCI (Martin et al., 1971) yielded information on five dimensions: LSD scale, Amphetamine scale, Benzedrine Group (BG) scale, Morphine-Benzedrine Group (MBG) scale, and the Pentobarbital, Chlorpromazine, Alcohol Group (PCAG) scale.
Drug
Animal models have shown repeatedly that direct and indirect dopamine agonists function as more efficacious reinforcers among high novelty-seeking animals. This study was designed to examine the cross-species generality of these findings by examining the behavioral effects of an indirect dopamine agonist (d-amphetamine) among low and high sensation seekers. For comparison, the behavioral effects of diazepam, a sedative drug with known abuse potential having no direct effect on dopamine function, were examined. Previous studies have shown that the behavioral effects of therapeutic doses of d-amphetamine and diazepam, both Food and Drug Administration-approved medications, could be tested safely in healthy volunteers without extensive histories of drug-taking behavior. Multiple doses were examined within the therapeutic window to test the possibility that high and low sensation seekers would show differential sensitivity to doses associated with initial exposure to the drugs. A range of doses of each drug that are commonly used for therapeutic purposes were tested to examine sensitivity at levels associated with initial exposure to the drugs. d-Amphetamine (0, 2.5, 5, and 10 mg/70 kg) and diazepam (0, 2.5, 5, and 10 mg/70 kg) doses, adjusted based on subject body weight, were prepared in size 00 opaque capsules with lactose filler by the investigational pharmacy at the University of Kentucky. Each dose was tested on two occasions.
Statistical analysis
Group differences in demographic and other characteristics were examined with Student’s t-tests (two-tailed). Results were considered significant at P value of less than 0.05. The main study consisted of a double-blind, double-dummy placebo-controlled, randomized block design consisting of one between-subject variable (high vs. low sensation seeking status) and three within-subject variables (dose, time, and replication). Minimal interactions between replication and either dose or sensation-seeking status were observed, so for the sake of clarity, the replication factor was dropped from the model. Data from d-amphetamine and diazepam test days were analyzed as separate datasets and analyzed as a linear mixed-model analysis of covariance (ANCOVA) (SPSS, v. 13.0, Chicago, Illinois, USA), with sensation-seeking status (high vs. low), dose (0, 2.5, 5, and 10 mg/70 kg), and time (50, 110, 170, 230, and 290 min) as fixed factors, subjects as a random factor, and predose assessment data (time 0), sex, and alcohol use serving as covariates. Baseline assessment results were included as covariates to control for sensation-seeking group differences that were identified on multiple measures during the preliminary analysis. Sex and alcohol use were also included as covariates because of group differences on these variables. Ethnicity was not included as a covariate because only Caucasians were represented in both the high and low sensation seeker groups. Significant interactions were examined using simple-effects models. To minimize the number of follow-up comparisons, significant effects of time and group by time interactions were not examined, as they were tangential to the primary focus of the study, follow-up comparison testing was conducted only when main effects of the ANCOVAs or main effects of the simple-effect follow-up ANCOVAs were significant, and each dose was compared with placebo only. Drug main effects and interactions were typically dose-related, but significant dose effects were limited primarily to the high dose of both diazepam and d-amphetamine. For the sake of clarity, only the effects of placebo and the high doses (10 mg/70 kg) of both diazepam and d-amphetamine are presented graphically.
Results
Sensation-seeking group characteristics
Table 1 presents the results of the assessment questionnaires. As anticipated, given that high and low sensation seekers were recruited based on the stability of their sensation-seeking scores between grades 6 and 10, high sensation seekers scored significantly higher on the total and all four subscales of the Sensation Seeking Scale (Form V) than low sensation seekers. High sensation seekers also scored higher on the extraversion scale of the Eysenck Personality Inventory (P<0.05) and on the ARC Maturation Scale (P<0.005), and endorsed more Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-based symptoms of conduct disorder (P<0.05) than low sensation seekers, although none of the participants met diagnostic criteria for conduct disorder. No statistically significant group differences were obtained on reports of depression, attention-deficit hyperactivity symptoms, or on any of the Brief Symptom Inventory scales (data not presented).
Table 1.
Characteristics of low and high sensation seekers
| Sensation seeking status
|
||
|---|---|---|
| Low | High | |
| Age (years) | 19.3 (0.5)a | 19.4 (0.3) |
| Education (years) | 13.0 (0.4) | 12.7 (0.3) |
| Drug use | ||
| Caffeine (mg/day) | 68.4 (35.2) | 82.5 (31.6) |
| Tobacco users (%) | 66.7 | 36.4 |
| Cigarettes/day | 2.2 (1.2) | 6.1 (5.5) |
| Alcohol (drinks/week)* | 2.7 (2.7) | 8.0 (3.4) |
| Marijuana users (%) | 50.0 | 45.5 |
| Occasions/month | 5.5 (5.8) | 17.6 (4.2) |
| Cocaine (occasions/month) | 0 | 0 |
| Eysenck personality inventory | ||
| Neuroticism | 10.7 (1.5) | 9.6 (1.2) |
| Extraversion* | 10.3 (1.6) | 15.2 (1.1) |
| SMAST | 0.3 (0.4) | 0.6 (0.3) |
| Beck depression | 4.0 (1.9) | 6.5 (2.2) |
| ADHD symptoms | 4.8 (1.5) | 8.8 (1.7) |
| Conduct disorder symptoms** | 2.8 (0.7) | 9.8 (2.0) |
| Sensation seeking scale (Form V) | ||
| Thrill and adventure seeking* | 3.8 (1.4) | 8.2 (0.8) |
| Experience seeking** | 3.7 (0.5) | 6.9 (0.8) |
| Disinhibition* | 1.8 (0.7) | 4.9 (0.9) |
| Boredom susceptibility*** | 0.5 (0.4) | 3.6 (0.5) |
| Total*** | 9.8 (1.9) | 23.6 (2.3) |
ADHD, Attention Deficit Hyperactivity Disorder; SMAST, Short Michigan Alcohol Screening Test.
Mean (SE).
P <0.05.
P < 0.01.
P < 0.001.
Table 1 also presents drug use by high and low sensation seekers. Reported alcohol intake by high sensation seekers was significantly greater than low sensation seekers (8.0±3.4 vs. 2.7±3.4 drinks/week). Short Michigan Alcohol Screening Test (SMAST) scores were low and not significantly different between groups. Other drug use was minimal and not different between groups. None of the participants reported a history of regular benzodiazepine, amphetamine, methylphenidate, or cocaine use, or any drug use during the month before the study.
Diazepam effects
Task performance measures
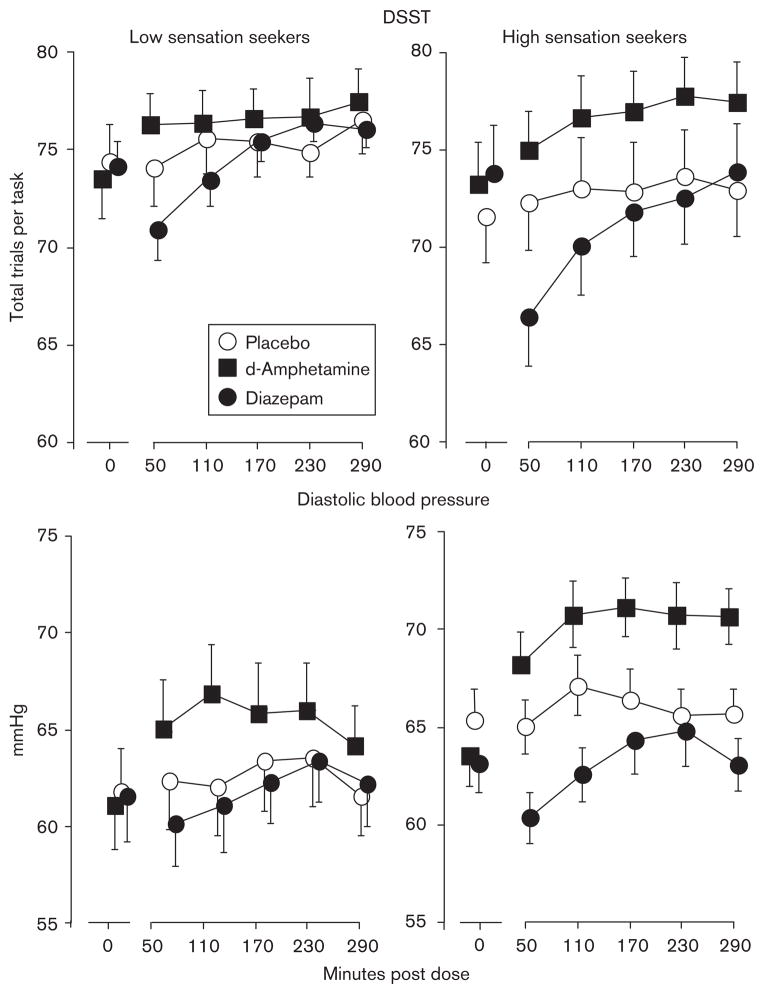
Table 2 presents the results of the linear mixed-model ANCOVA (main effects of group and drug, and group × drug interactions, only) for the diazepam dataset. Typical sedative-like impairment was observed on several task performance measures, as indicated by significant diazepam dose effects and dose by time interactions. Diazepam decreased the number of DSST trials that were completed, although the magnitude of the effect varied as a function of sensation-seeking status, with greater impairment occurring among high than low sensation seekers. Simple effects analyses indicated that significant group differences were apparent only at the 10mg/70 kg dose [F(1,15)=16.7, P<0.001], and significant diazepam effects were observed only among high sensation seekers [F(3,605)=37.8, P<0.001]. Figure 1 (upper panels) presents the effects of the 10 mg/70 kg doses of diazepam (filled circles) on DSST trial rate for low (upper left panel) and high (upper right panel) sensation seekers. Peak effects occurred 50 min postdose in both low and high sensation seekers, although the magnitude of impairment was greater among high sensation seekers.
Table 2.
Linear mixed model ANCOVA F-test results [sensation seeking group (SS), dose (D) and group × dose interactions, with baseline measures, sex and alcohol intake serving as covariates] and level of significance for diazepam and d-amphetamine datasets
| Diazepam
|
d-Amphetamine
|
|||||
|---|---|---|---|---|---|---|
| SS | Dose | SS × D | SS | Dose | SS × D | |
| DSST | ||||||
| Trial rate | 3.41 | 1.36 | 13.60*** | 2.70 | 2.67 | 3.22* |
| Percent correct | 0.29 | 0.57 | 2.27 | 0.06 | 2.33 | 0.73 |
| Repeated acquisition | ||||||
| Learning | ||||||
| Correct rate | 1.60 | 0.54 | 0.26 | 1.18 | 0.16 | 3.07* |
| Incorrect rate | 1.23 | 2.92* | 0.16 | 1.00 | 0.82 | 1.56 |
| Index of curvature | 0.36 | 0.38 | 0.96 | 0.01 | 2.44 | 1.49 |
| Performance | ||||||
| Correct rate | 0.89 | 3.46* | 1.76 | 0.14 | 7.54*** | 0.36 |
| Incorrect rate | 0.57 | 2.76* | 3.53* | 2.68 | 1.63 | 0.27 |
| Cardiovascular | ||||||
| Rest | ||||||
| Heart rate | 0.71 | 0.77 | 2.13 | 0.33 | 2.07 | 0.09 |
| Systolic | 0.40 | 3.65* | 3.06* | 0.06 | 6.75*** | 1.77 |
| Diastolic | 0.29 | 1.64 | 3.27* | 0.15 | 2.95* | 3.88** |
| Task | ||||||
| Heart rate | 0.96 | 0.99 | 0.64 | 1.09 | 3.74* | 0.24 |
| Systolic | 0.98 | 3.31* | 1.03 | 0.53 | 6.98*** | 2.18 |
| Diastolic | 0.51 | 1.85 | 3.31* | 0.09 | 0.84 | 2.58 |
| ARCI | ||||||
| PCAG | 0.70 | 6.94*** | 1.86 | 0.00 | 2.21 | 0.42 |
| BG | 8.90** | 5.32*** | 4.29** | 10.91** | 3.98** | 2.69* |
| MBG | 8.54* | 1.20 | 0.85 | 11.42** | 1.60 | 2.30 |
| LSD | 0.23 | 2.75* | 0.19 | 0.07 | 0.13 | 1.20 |
| Amphetamine | 6.95* | 3.82** | 4.24** | 11.78** | 2.67* | 1.70 |
| POMS | ||||||
| Anxiety | 1.15 | 1.21 | 2.00 | 1.25 | 7.33*** | 1.06 |
| Depression | 0.27 | 0.59 | 4.58** | 0.25 | 0.16 | 2.26 |
| Anger | 0.89 | 0.40 | 1.20 | 0.48 | 1.47 | 2.82* |
| Vigor | 6.42* | 4.42** | 3.53* | 8.00* | 9.99*** | 2.88* |
| Fatigue | 0.16 | 1.14 | 2.88* | 0.52 | 1.02 | 1.91 |
| Confusion | 0.83 | 1.40 | 4.69** | 1.24 | 1.09 | 0.35 |
| Friendly | 6.23* | 0.15 | 1.73 | 9.26** | 1.31 | 0.09 |
| Elation | 8.85** | 1.16 | 0.37 | 15.19** | 4.71** | 0.74 |
| Arousal | 1.48 | 6.25*** | 0.62 | 1.64 | 12.66*** | 1.99 |
| Total positive | 5.76* | 5.48*** | 4.07** | 9.81** | 8.09*** | 4.29** |
| VAS | ||||||
| Stimulated | 5.51* | 3.25* | 1.96 | 6.26* | 2.07 | 1.33 |
| Stressed | 0.15 | 1.43 | 3.14* | 0.19 | 2.27 | 1.30 |
| Sedated | 0.00 | 10.69*** | 1.31 | 0.00 | 0.64 | 0.05 |
| Hungry | 0.03 | 2.10 | 1.85 | 0.01 | 2.19 | 2.40 |
| Anxious | 0.02 | 2.16 | 1.09 | 0.15 | 0.33 | 0.51 |
| Light-headed | 1.13 | 4.33** | 2.17 | 0.39 | 2.05 | 2.93* |
| Thirsty | 0.56 | 0.93 | 3.11* | 0.55 | 1.56 | 8.93*** |
| Sleepy | 0.89 | 4.78** | 2.78* | 1.81 | 0.04 | 1.13 |
| Sick-to-stomach | 0.03 | 1.68 | 0.37 | 0.34 | 1.51 | 0.82 |
| Down | 0.99 | 4.86** | 0.47 | 0.45 | 3.32* | 0.72 |
| High | 1.15 | 1.27 | 0.42 | 3.75 | 1.82 | 0.50 |
| Feel drug | 2.84 | 15.46*** | 1.51 | 1.16 | 3.97** | 0.34 |
| Like drug | 4.11 | 0.17 | 0.57 | 6.38* | 1.49 | 1.56 |
ANCOVA, analysis of covariance; ARCI, Addiction Research Center Inventory; BG, Benzedrine-Group; DSST, Digit Symbol Substitution Task; LSD, Lysergic Acid Diethylamide; MBG, Morphine-Benzedrine Group; PCAG, Pentobarbital, Chlorpromazine, Alcohol Group; POMS, Profile of Mood States; VAS, Visual Analog Scale.
P < 0.05.
P < 0.01.
P < 0.001.
Fig. 1.
Time-course effects of d-amphetamine (10 mg/70 kg) and diazepam (10 mg/70 kg) on the number of trials completed per 90 s Digit Symbol Substitution Task (DSST) (upper panels) and resting diastolic blood pressure (lower panels) for low (left panels) and high (right panels) sensation seekers. Error bars represent ± 1 SEM.
Diazepam increased the number of incorrect responses (i.e. errors) during both the learning and performance components of the RA task, but decreased the number of correct responses only during the performance component. Group differences in diazepam effects were also observed on incorrect responses, but simple-effects testing only revealed a significant group difference at the 5.0mg/70 kg dose (P<0.01), resulting from a decrease in incorrect responses (i.e. improved performance) among low sensation seekers. The magnitude of diazepam-induced increases in incorrect responses on RA task performance at the 10.0 mg/70 kg dose was comparable among low and high sensation seekers.
Cardiovascular measures
As anticipated, resting heart rate and blood pressure were increased while performing mathematical tasks (data not presented). Diazepam decreased systolic and diastolic blood pressure while resting and while completing mathematical problems, and the magnitude of diazepam effects varied as a function of sensation-seeking status. While at rest, simple-effects analyses indicated that significant diazepam effects occurred only at the highest dose among high sensation seekers for both systolic [F(3,604)=112.56, P<0.001] and diastolic [F(3,605)=6.99, P<0.001] pressure. Figure 1 (lower panels) presents the effects of the 10 mg/70 kg doses of diazepam on resting diastolic blood pressure for low (lower left panel) and high (lower right panel) sensation seekers. Similar to diazepam effects on DSST performance, peak effects occurred 50min postdose in both low and high sensation seekers, and the magnitude of effect was greater among high sensation seekers. While performing mathematical problems, the highest diazepam dose (10mg/70 kg) decreased diastolic blood pressure only among high sensation seekers [F(3,604)=8.53, P<0.001].
Verbal report of drug effect measures
Sedative-like reports were observed on the verbal-report scales. On the ARCI, diazepam increased PCAG, BG, and Lysergic Acid Diethylamide Group scale and decreased Amphetamine scale ratings. Diazepam decreased the Arousal, Vigor, and Total Positive scales of the POMS. On the VAS, diazepam increased ratings of Feel Drug, Light-Headed, Down, Sedated, and Sleepy and decreased ratings of Stimulated.
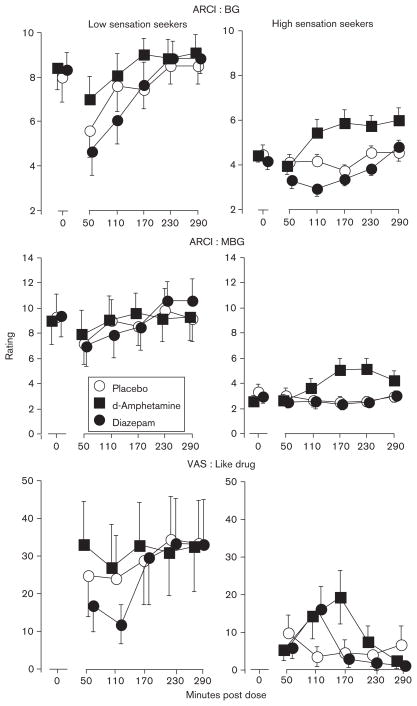
Differences in verbal reports of the sedative effects and abuse potential of diazepam were observed for low and high sensation seekers. Figure 2 presents the effects of diazepam on self-report measures associated with drug abuse potential (i.e. ARCI BG and MBG scales, VAS Like Drug). The top panels present the effects of the 0 and 10 mg/70 kg doses of diazepam on the BG scale of the ARCI for low (left panel) and high (right panel) sensation seekers. Simple-effects analyses of the sensation seeking by dose interaction indicated that diazepam decreased ratings to a greater extent among low sensation seekers [F(3,606)=6.43, P<0.001]. Diazepam decreased BG ratings occurring 50 and 110 min postdose for low sensation seekers, whereas small magnitude decreases occurred only 110 min postdrug for high sensation seekers. Although no statistically significant sensation seeking by dose interactions were observed on the ARCI MBG or VAS Like Drug scales, the main effect of sensation-seeking status was significant for MBG ratings and approached significance for Like Drug ratings. Figure 2 indicates that diazepam effects were qualitatively different for low and high sensation seekers, in that diazepam decreased ARCI MBG and VAS Like Drug ratings in low sensation seekers, whereas the drug had no effect on ARCI MBG ratings and increased VAS Like Drug ratings among high sensation seekers.
Fig. 2.
Time-course effects of d-amphetamine (10 mg/70 kg) and diazepam (10 mg/70 kg) on the Addiction Research Center Inventory (ARCI) Benzedrine-Group (BG) (upper panels) and Morphine-Benzedrine Group (MBG) (middle panels) scales, and on Visual Analog Scale (VAS) Like Drug (lower panels) ratings for low (left panels) and high (right panels) sensation seekers. Error bars represent ± 1 SEM.
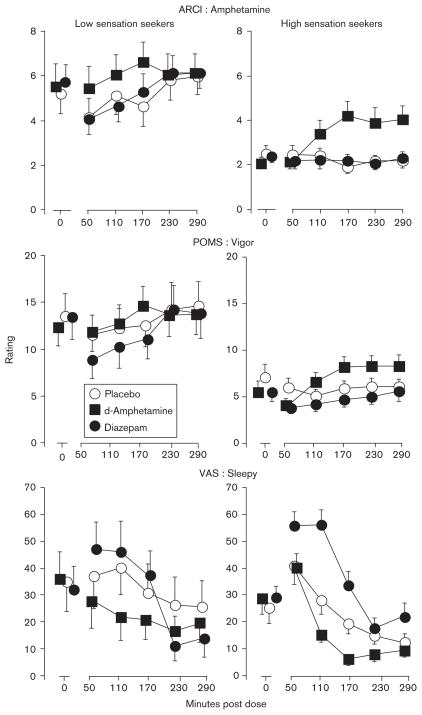
Figure 3 presents the effects of diazepam on self-report measures of stimulant and sedative drug effects (i.e. ARCI Amphetamine, POMS Vigor, VAS Sleepy). Simple-effects analyses of the sensation-seeking status by dose interaction on the Amphetamine scale of the ARCI (top panels) indicated significant diazepam effects among low sensation seekers only [F(3,605)=3.86, P<0.01], with decreased ratings occurring 50 and 110 min at both the 0 and 10 mg/70 kg dose conditions, while no changes in ratings occurred over time among high sensation seekers. Simple-effects analyses of the sensation seeking by dose interaction on the Vigor scale of the POMS (middle panels) revealed that diazepam effects were significant for low sensation seekers only (P<0.01), with decreased ratings at the 10 mg/70 kg dose condition occurring 50, 110, and 170 min, while no changes in ratings occurred over time among high sensation seekers. Simple-effects analyses of the sensation-seeking status by dose interaction on the VAS Sleepy scale (bottom panels) indicated that diazepam effects were observed only among high sensation seekers [F(3,605)=11.33, P<0.001] at the 5 mg/70 kg (P<0.001) and 10 mg/70 kg (P<0.001) doses. Peak effects occurred 50 and 110 min postdose in both low and high sensation seekers, although the magnitude of effect was greater among high sensation seekers. Similar effects were observed on the POMS Fatigue scale (data not shown), with significant diazepam effects occurring only among high sensation seekers [F(3,604)=5.65, P<0.001] at the 5 mg/70 kg (P<0.01) and 10mg/70 kg (P<0.001) doses. Results from the VAS Sleepy and POMS Fatigue scales are similar to those obtained on task performance in that diazepam engendered greater increases in ratings of sedative effects among high sensation seekers.
Fig. 3.
Time-course effects of d-amphetamine (10 mg/70 kg) and diazepam (10 mg/70 kg) on the Addiction Research Center Inventory (ARCI) Amphetamine (upper panels) and Profile of Mood States (POMS) Vigor (middle panels) scales and on Visual Analog Scale (VAS) Sleepy (lower panels) ratings for low (left panels) and high (right panels) sensation seekers. Error bars represent ± 1 SEM.
Diazepam effects on other verbal rating scales that have been associated with drug-abuse potential and/or aversive (i.e. negative) drug effects were also different for low and high sensation seekers. Simple-effects analyses of the sensation seeking status by dose interactions on the Depression and Total Positive scales of the POMS and on VAS Thirsty indicated that diazepam increased ratings on the Depression [F(3,606)=3.94, P<0.01] and Thirsty [F(3,606)=3.53, P<0.05] scales and decreased ratings on the Total Positive [F(3,607)=5.35, P<0.001] scales only in low sensation seekers. Diazepam increased ratings on the POMS Confusion scale among both low [F(3,604)=3.67, P<0.05] and high [F(3,604)=4.34, P<0.01] sensation seekers, but significant diazepam effects occurred at the 2.5 and 5mg/70 kg doses among high sensation seekers, whereas increases in Confusion ratings occurred at the 10 mg/70 kg dose in the low sensation seekers. Analyses of the sensation-seeking status by dose interaction on VAS Stressed ratings did not yield any significant simple effects, although at the 10 mg/70 kg dose, ratings were elevated among low but not high sensation seekers. These data indicate that diazepam decreased low sensation seekers ratings on several scales associated with abuse potential (POMS Total Positive) and increased ratings on scales associated with aversive drug effects (POMS Depression and Confusion, VAS Stressed).
d-Amphetamine effects
Task performance measures
Table 2 also presents the results of the linear mixed-model ANCOVA with the d-amphetamine dataset. d-Amphetamine increased the number of DSST trials completed, but the magnitude of the effect varied as a function of sensation-seeking status. The upper panels of Fig. 1 present the effect of the 0 and 10 mg/70 kg doses of d-amphetamine on DSST trial rate. d-Amphetamine increased response rate relative to placebo to a greater extent among high sensation seekers, although the simple-effects analysis of the sensation-seeking status by dose interaction did not yield any significant effects.
During the learning component of the RA task, d-amphetamine improved acquisition efficiency, based on the index of curvature, and decreased the number of incorrect responses (i.e. dose × time interactions, P<0.05, in both cases). During the performance component, d-amphetamine increased the number of correct responses but had no effect on incorrect rate. These data suggest that d-amphetamine improved both RA acquisition and performance, although by enhancing learning efficiency during the learning component, and by increasing response rate during the performance component. d-Amphetamine effects on the number of correct responses during the learning component of the RA varied as a function of sensation-seeking status. As with the DSST, analysis of the sensation-seeking status by dose interaction did not yield any significant simple effects, although d-amphetamine effects approached significance among high sensation seekers only [F(3,605)=2.48, P=0.06)], suggesting that d-amphetamine enhanced performance in high sensation seekers only.
Cardiovascular measures
d-Amphetamine increased systolic and diastolic blood pressure during the rest intervals, and heart rate and systolic pressure during mathematical task performance. d-Amphetamine effects on diastolic blood pressure varied as a function of sensation-seeking status. Figure 1 (lower panels) presents the effects of d-amphetamine on resting diastolic blood pressure; as with DSST performance, the magnitude of d-amphetamine effect was greater among high sensation seekers in that simple effects analysis indicted greater effect sizes among high [F(3,605)=9.03, P<0.001] than among low [F(3,605)=6.24, P<0.01] sensation seekers.
Verbal report of drug effect measures
d-Amphetamine increased VAS ratings of Feel Drug, stimulant-like ratings on the ARCI Amphetamine scale and the Arousal scale of the POMS, and ratings on scales that have been associated with drug abuse potential, including the BG scale of the ARCI and the Vigor, Elation, Arousal, and Total Positive scales of the POMS. d-Amphetamine also increased negative ratings on the POMS Anxiety and VAS Down scales.
d-Amphetamine effects varied as a function of sensation-seeking status on several verbal report scales associated with the abuse potential of drugs (Table 2, FFig. 2). For example, simple effects analyses indicated that significant d-amphetamine-induced increases in ARCI BG scale ratings occurred among high sensation-seekers only [(3,608)=7.18, P<0.001]. Significant main effects of sensation-seeking status were observed on the ARCI MBG and VAS Like Drug scales, with high sensation seekers reporting lower ratings on these scales under all conditions, including placebo. Figure 2 indicates that relative to placebo, d-amphetamine-induced increases in ratings on these scales occurred among high sensation seekers only.
The effects of d-amphetamine on self-report measures of stimulant and sedative drug effects are presented in Fig. 3. Significant main effects of sensation-seeking status were observed on the ARCI Amphetamine scale (top panels), with lower ratings among high sensation seekers occurring under all conditions. However, as with the MBG and VAS Like Drug scales, d-amphetamine-induced increases in ratings on the Amphetamine scale were observed among high sensation seekers only. The middle panels of Fig. 2 present the effects of d-amphetamine doses on POMS Vigor. Simple effects analyses indicated that the 10 mg/70 kg dose of d-amphetamine increased POMS Vigor ratings in high sensation seekers only. d-Amphetamine decreased VAS ratings of sleepy (bottom panels) equally in low and high sensation seekers.
d-Amphetamine effects on other verbal rating scales that have been associated with drug abuse potential and/or aversive (i.e. negative) drug effects also varied as a function of sensation-seeking status. Simple-effects analyses of sensation-seeking status by dose interactions on the POMS Anger scale indicated that d-amphetamine increased ratings only in low sensation seekers at the 2.5 mg/70 kg dose [F(3,605)=5.45, P<0.001]. Similarly, greater d-amphetamine effects among low sensation seekers were observed on the VAS Light-Headed and Thirsty scales, with simple-effects analyses indicating greater changes in Light-Headed [F(3,605)=4.45, P<0.01] and Thirsty [F(3,605)=11.95, P<0.001] scale ratings among low sensation seekers. One notable exception to the general pattern of results (i.e. greater positive d-amphetamine effects among high sensation seekers, greater negative effects among low sensation seekers) was also apparent in that simple effects analyses of the sensation seeking by dose interaction on the POMS Total Positive scale indicated increased ratings among low sensation seekers only [F(3,605)=6.53, P<0.001].
Discussion
Diazepam and d-amphetamine, examined using a double-blind repeated-measures design in which each drug was tested in every subject, engendered characteristic sedative- like and stimulant-like effects, respectively. A range of therapeutic doses of diazepam (2.5–10 mg/70 kg) impaired performance on psychomotor and cognitive tasks, decreased blood pressure, and engendered a sedative-like profile of verbal reports of drug effect, including increases on the ARCI PCAG, POMS Fatigue, and VAS Sedated and Sleepy scales, in a dose-dependent manner. In contrast, a therapeutic dose range of d-amphetamine (2.5–10 mg/70 kg) enhanced psychomotor and cognitive task performance, increased blood pressure, and engendered a stimulant-like profile of verbal reports of drug effect, including increases on the ARCI Amphetamine and BG and POMS Elation, Arousal, Vigor, and Total Positive scales, also in a dose-dependent manner.
Young adult groups of high and low sensation seekers were established, based on the consistency with which subjects scored in the upper or lower third of their age-based cohorts on the sensation-seeking personality questionnaire annually over a 4-year period of adolescence (6th through 10th grade). After controlling for sensation-seeking group differences in baseline performance, sex and alcohol use, differences in the magnitude of diazepam-induced sedative effects and d-amphetamine- induced stimulant effects were observed among the high and low sensation-seeking groups. Diazepam engendered significantly greater sedative effects (i.e. performance impairment on the DSST and RA tasks, sedative-like effects on the POMS Fatigue and VAS Sleepy scales) among high sensation seekers. In contrast, diazepam decreased verbal-report measures related to abuse potential (i.e. VAS Like Drug, POMS Total Positive, and ARCI MBG scales) and stimulant effects (i.e. POMS Vigor and ARCI A scales), and increased ratings on verbal-report measures that have been categorized as negative (i.e. increases in POMS Depression and Confusion and VAS Stressed scales) in low sensation seekers. d-Amphetamine improved performance on the DSST and RA tasks, and increased verbal-report measures associated with drug abuse potential (ARCI BG and POMS Vigor scales) only among high sensation seekers. Furthermore, the magnitudes of d-amphetamine effects on the ARCI MBG and Amphetamine scales and on VAS Like Drug were greater among high than low sensation seekers. In contrast, greater sensitivity to d-amphetamine effects among low sensation seekers was generally limited to measures associated with negative drug effects (e.g. POMS Anger, VAS Light-Headed and Thirsty scales). These results are consistent with previous studies showing that young adults who report sensation-seeking-type personality characteristics are more sensitive to the effects of drugs associated with abuse liability than low sensation seekers (e.g. de Wit et al., 1987; Cheong and Nagoshi, 1998; Sax and Strakowski, 1998; Hutchison et al., 1999; Perkins et al., 2000, 2008; White et al., 2006; Kelly et al., 2006; Stoops et al., 2007; Fillmore et al., 2009), and suggest that low sensation seekers may be more sensitive to negative drug effects. Importantly, these results also extend previous studies by showing that the association between sensation-seeking status and vulnerability to drug abuse can be determined based on the sensation-seeking status of adolescents.
Individual differences in vulnerability to the abuse potential of benzodiazepines are influenced, in part, by sedative-use history (e.g. Woods et al., 1992). Individuals with a history of heavy alcohol use, for example, show greater sensitivity to the reinforcing effects of benzodiazepines. In this study, alcohol use was greater among high sensation seekers. However, none of the subjects reported heavy or problematic alcohol use (none reported more than 14 drinks per week, and SMAST scores were low), and individual differences in alcohol use were controlled as a covariate in the statistical analyses. Diazepam decreased verbal reports associated with drug abuse potential (e.g. VAS Like Drug, POMS Total Positive, and ARCI MBG scales) among low sensation seekers, but had no effect on these measures among high sensation seekers. Furthermore, despite reporting greater alcohol use, high sensation seekers exhibited greater sensitivity to diazepam-induced sedation and performance impairment. As such, it is not likely that group differences in diazepam effects can be explained by heavier alcohol use among high sensation seekers.
High and low sensation seeker groups also differed on factors other than alcohol use, including sex, ethnicity, extraversion, conduct disorder symptoms, levels of maturation, and on baseline (i.e. predrug) performance on several of the study measures. Some of these differences would be expected based on the strict selection criteria that were used to establish group status. Only those individuals who were consistently in the upper or lower third of their grade-matched cohort on the Sensation Seeking Scale in 4 consecutive years met eligibility criteria as high and low sensation seekers. These individuals also remained high and low on their sensation-seeking status as young adults, suggesting that sensation-seeking status was stable over a 6–11-year interval (i.e. between the ages of 10 and 22 years). Extraversion and conduct disorder symptoms are positively correlated with sensation-seeking status (de Wit and Richards, 2004; Martin et al., 2004), so group differences on these factors is not surprising. Previous research has shown that drug effects vary as a function of both sex and ethnicity. Sex and ethnicity distributions varied across groups, with 36 and 86% of the high and low sensation seeking groups being female, and 91 and 50% of the high and low sensation seeking groups being Caucasian. Baseline differences in ratings on several verbal-report scales were also apparent (e.g. ARCI MGB, BG, Amphetamine; POMS Vigor, Arousal, Anger). Although it is not possible to rule out the potential influence of these factors on group differences in drug effect, other studies (e.g. Sax and Strakowski, 1998; Hutchison et al., 1999; Perkins et al., 2000, 2008; Kelly et al., 2006; White et al., 2006; Stoops et al., 2007; Fillmore et al., 2009) have shown group differences in sensitivity to drug effects similar to those reported in this study (i.e. high sensation seekers showing greater sensitivity to measures of drug abuse potential than low sensation seekers). In these studies, group differences in sensation-seeking status were not associated with sex or ethnic distribution, extraversion, conduct disorder symptoms, maturation, drug use, or baseline differences on outcome measures, suggesting that sensation-seeking group differences in sensitivity are independent of such factors.
It is also possible that pharmacokinetic variation among high and low sensation seekers could contribute to group differences in the behavioral effects of drugs observed in this study, particularly given the available evidence for biological/genetic influences on sensation-seeking status. If blood levels of d-amphetamine and/or diazepam were greater among high sensation seekers, for example, it would be anticipated that behavioral effects would also be greater in this group. In this study, the effects of both diazepam and d-amphetamine were greater among high sensation seekers than low sensation seekers on a number of variables. However, the magnitude of drug effects was not consistent across measures. In addition, qualitative differences between groups in sensitivity to drug effects were not consistent, as some measures indicated greater sensitivity to drug effects among low sensation seekers. For example, diazepam decreased ratings of POMS Vigor and Total Positive, and d-amphetamine increased Anger ratings only among low sensation seekers. Given qualitative and quantitative differences in the magnitude of drug effects across measures, group differences in drug pharmacokinetics does not seem to be the most parsimonious explanation for differential drug effects among high and low sensation seekers. However, future studies examining pharmacokinetics among high and low sensation seekers will be required to rule out pharmacokinetic influences on group differences in sensitivity to drug effects.
A number of study limitations are apparent. The participation requirements of this study were substantial (16 days, each lasting 7.5 h), and in combination with the strict eligibility criteria that limited the number of eligible participants, resulted in a relatively small sample size. The proportion of the pool of eligible participants that completed the study (17 of 123) was low, limiting the generality of the results, as well as the statistical power to detect group × dose interactions. In addition, although multiple doses within the therapeutic range were examined (0, 2.5, 5, and 10 mg/70 kg), the peak dose tested in this study was limited.
Despite the limitations in sample size and statistical power, the results of this study provide clear evidence that the behavioral effects of both diazepam and d-amphetamine vary among young adults who scored consistently in the upper or lower third of their grade-matched cohorts on the Sensation Seeking scales over 4 years of adolescence. Sensation-seeking status is associated with individual differences in impulsivity and reward sensitivity (e.g. Depue and Collins, 1999; de Wit and Richards, 2004). d-Amphetamine enhanced performance (e.g. DSST and RA response rate) and increased verbal report measures associated with drug abuse potential (e.g. ARCI BG, POMS Vigor) only among high sensation seekers, whereas diazepam impaired task performance by high sensation seekers and decreased verbal report measures associated with drug abuse potential among low sensation seekers. These results suggest that high sensation-seeking adolescents with elevated levels of impulsivity and reward sensitivity may be at increased vulnerability to abuse potential of drugs, and support a strategy of targeting high sensation seekers for drug abuse prevention efforts (Palmgreen et al., 1995; Stephenson et al., 2002).
Acknowledgments
The authors thank Cleeve Emurian, Fran Wagner, Beth Eaves, Karen McClanahan, and Oriaku Akuma-Kalu Njoku for assistance in conducting and analyzing the results of this study. Support for this study was provided by P50 05312 from the National Institute on Drug Abuse.
References
- Alessi SM, Greenwald M, Johanson CE. The prediction of individual differences in response to d-amphetamine in healthy adults. Behav Pharmacol. 2003;14:19–32. doi: 10.1097/00008877-200302000-00002. [DOI] [PubMed] [Google Scholar]
- Balada F, Torrubia R, Arque JM. Thyroid hormone correlates of sensation seeking and anxiety in healthy human females. Neuropsychobiology. 1992;25:208–213. doi: 10.1159/000118839. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beck RW. Screening depressed patients in family practice. A rapid technic. Postgrad Med. 1972;52:81–85. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- Carrol EN, Zuckerman M, Vogel WH. A test of the optimal level of arousal theory of sensation seeking. J Pers Soc Psychol. 1982;42:572–575. doi: 10.1037//0022-3514.42.3.572. [DOI] [PubMed] [Google Scholar]
- Clayton RR, Cattarello AM, Johnstone BM. The effectiveness of drug abuse resistance education (Project DARE): 5-year follow-up results. Prev Med. 1996;25:307–318. doi: 10.1006/pmed.1996.0061. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Chait LD. Factors influencing the reinforcing and subjective effects of d-amphetamine in humans. Behav Pharmacol. 1993;4:191–199. [PubMed] [Google Scholar]
- Cheong J, Nagoshi CT. Effects of sensation seeking, instruction set, and alcohol/placebo administration on aggressive behavior. Alcohol. 1999;17:81–86. doi: 10.1016/s0741-8329(98)00036-6. [DOI] [PubMed] [Google Scholar]
- Corr PJ, Kumari V. Individual differences in mood reactions to d-amphetamine: a test of three personality factors. J Psychopharmacol. 2000;14:371–377. doi: 10.1177/026988110001400406. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;45:19–55. [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res. 1987;11:52–59. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–517. doi: 10.1017/s0140525x99002046. discussion 518–569. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, et al. Dopamine D4 receptor (D4DR) exon III polymorphism associated with human personality trait of novelty seeking. Nat Gen. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Lichtermann D, Jarvelin MR, Peltonen L. Association between novelty seeking and the type 4 dopamine receptor gene in a large Finnish cohort sample. Am J Psychiatry. 1999;156:1453–1455. doi: 10.1176/ajp.156.9.1453. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The biological basis of personality. Springfield, Illinois: Charles C. Thomas; 1986. [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alcohol Depend. 2009;100:91–99. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW. Cocaine and amphetamine effects on repeated acquisition in humans. Fed Proc. 1978;37:618. [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Fulker DW, Eysenck SB, Zuckerman M. A genetic and environmental analysis of sensation seeking. J Res Pers. 1980;14:261–281. [Google Scholar]
- Gelernter J, Kranzler H, Coccaro E, Siever L, New A, Mulgrew CL. D4 dopamine-receptor (DRD4) alleles and novelty seeking in substance-dependent, personality-disorder, and control subjects. Am J Hum Genet. 1997;61:1144–1152. doi: 10.1086/301595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp Clin Psychopharmacol. 1999;7:493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Hienz RD, Zarcone TJ, Wurster RM, Brady JV. Crewmember performance before, during, and after spaceflight. J Exp Anal Behav. 2005;84:227–241. doi: 10.1901/jeab.2005.77-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology (Berl) 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ball SA, Rounsaville BJ. A sibling study of sensation seeking and opiate addiction. J Nerv Ment Dis. 1994;182:284–289. doi: 10.1097/00005053-199405000-00006. [DOI] [PubMed] [Google Scholar]
- Loukas A, Fitzgerald HE, Zucker RA, von Eye A. Parental alcoholism and co-occurring antisocial behavior: prospective relationships to externalizing behavior problems in their young sons. J Abnorm Child Psychol. 2001;29:91–106. doi: 10.1023/a:1005281011838. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Milich R, Zimmerman R, Novak SP, Logan TK, Martin C, et al. Project DARE: no effects at 10-year follow-up. J Consult Clin Psychol. 1999;67:590–593. doi: 10.1037//0022-006x.67.4.590. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli B, Himelreich K, Brenzel A, et al. Sensation seeking and symptoms of disruptive disorder: association with nicotine, alcohol, and marijuana use in early and mid-adolescence. Psychol Rep. 2004;94:1075–1082. doi: 10.2466/pr0.94.3.1075-1082. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Martin WR, Hewett BB, Baker AJ, Haertzen CA. Aspects of the psychopathology and pathophysiology of addiction. Drug Alcohol Depend. 1977;2:185–202. doi: 10.1016/0376-8716(77)90026-6. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Surwit RS, Williams RB., Jr Opioid dysfunction and risk for hypertension: naloxone and blood pressure responses during different types of stress. Psychosom Med. 1988;50:8–14. doi: 10.1097/00006842-198801000-00002. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Cheung R, Montgomery TB, Bulbulian R, Wilson JF. Aerobic fitness and opioidergic inhibition of cardiovascular stress reactivity. Psychophysiology. 1992;29:687–697. doi: 10.1111/j.1469-8986.1992.tb02047.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Method Instrum. 1982;14:463–466. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States (manual) Educational and Industrial Testing Services, Educational and Industrial Testing Services 1971 [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR, Rodriguez LA. Impulsivity, sensation seeking, and behavioral and emotional responses to alcohol. Alcohol Clin Exp Res. 1991;15:661–667. doi: 10.1111/j.1530-0277.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Neary RS, Zuckerman M. Sensation seeking, trait and state anxiety, and the electrodermal orienting response. Psychophysiology. 1976;13:205–211. doi: 10.1111/j.1469-8986.1976.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Netter P, Hennig J, Roed IS. Serotonin and dopamine as mediators of sensation seeking behavior. Neuropsychobiology. 1996;34:155–165. doi: 10.1159/000119318. [DOI] [PubMed] [Google Scholar]
- Palmgreen P, Lorch EP, Donohew L, Harrington NG, Dsilva M, Helm D. Reaching at-risk populations in a mass media drug abuse prevention campaign: sensation seeking as a targeting variable. Drugs Soc. 1995;8:29–45. [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Wilson A. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Exp Clin Psychopharmacol. 2000;8:462–471. doi: 10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology (Berl) 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Roache JD. Performance and physiological measures in abuse liability evaluation. Br J Addict. 1991;86:1595–1600. doi: 10.1111/j.1360-0443.1991.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM. Enhanced behavioral response to repeated d-amphetamine and personality traits in humans. Biol Psychiatry. 1998;44:1192–1195. doi: 10.1016/s0006-3223(98)00168-1. [DOI] [PubMed] [Google Scholar]
- Schooler C, Zahn TP, Murphy DL, Buchsbaum MS. Psychological correlates of monoamine oxidase activity in normals. J Nerv Ment Dis. 1978;166:177–186. doi: 10.1097/00005053-197803000-00003. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered short Michigan alcoholism screening test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Newcomb MD, Bentler PM. Social psychological influences on sensation seeking from adolescence to adulthood. Pers Soc Psychol Bull. 1991;17:701–708. [Google Scholar]
- Stephenson MT, Morgan SE, Lorch EP, Palmgreen P, Donohew L, Hoyle RH. Predictors of exposure from an antimarijuana media campaign: outcome research assessing sensation seeking targeting. Health Commun. 2002;14:23–43. doi: 10.1207/S15327027HC1401_2. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addict Behav. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. Neuroimage. 2001;13:891–895. doi: 10.1006/nimg.2001.0761. [DOI] [PubMed] [Google Scholar]
- White TL, Lott DC, de Wit H. Personality and the subjective effects of acute amphetamine in healthy volunteers. Neuropsychopharmacology. 2006;31:1064–1074. doi: 10.1038/sj.npp.1300939. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J Subst Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Wills TA, DuHamel K, Vaccaro D. Activity and mood temperament as predictors of adolescent substance use: test of a self-regulation mediation model. J Pers Soc Psychol. 1995;68:901–916. doi: 10.1037//0022-3514.68.5.901. [DOI] [PubMed] [Google Scholar]
- Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Zuckerman M, Link K. Construct validity for the sensation seeking scale. J Consult Clin Psychol. 1968;32:420–426. doi: 10.1037/h0026047. [DOI] [PubMed] [Google Scholar]