Abstract
Corneal confocal microscopy is a growing technique for the study of the cornea at the cellular level, providing images comparable to ex vivo histochemical methods. In vivo confocal microscopy (IVCM) has an enormous potential, being a noninvasive procedure that images the living cornea, to study both its physiological and pathological states. Corneal nerves are of great interest to clinicians and scientists due to their important roles in regulating corneal sensation, epithelial integrity, proliferation, wound healing, and for their protective functions. IVCM enables the noninvasive examination of corneal nerves, allowing the study of nerve alterations in different ocular diseases, after corneal surgery, and in systemic diseases. To date, the correlation of sub-basal corneal nerves and their function has been studied in normal eyes, keratoconus, dry eye, contact lens wearers, and in neurotrophic keratopathy, among others. Further, the effect of corneal surgery on nerves has been studied, demonstrating the regenerative capacity of corneal nerves and the recovery of sensation. Moreover, IVCM has been applied in the diagnosis of peripheral diabetic neuropathy and the assessment of progression in this systemic disease. The purpose of this review is to describe the principles, applications, and clinical correlation of IVCM in the study of corneal nerves in different ocular and systemic diseases.
Keywords: cornea, nerves, innervation, esthesiometry, sensation, confocal microscopy
INTRODUCTION
The cornea is the most densely innervated tissue in the human body, supplied by the terminal branches of the ophthalmic division of the trigeminal nerve as ciliary nerves. Nerve bundles enter the peripheral mid-stroma in a radial pattern, course anteriorly, and give multiple branches innervating the anterior and mid-stroma.1 Branches from the nerves in the anterior stroma penetrate Bowman’s layer throughout the central and peripheral cornea. These branches then divide and run parallel to the superficial corneal surface, between Bowman’s layer and the basal epithelium, configuring the sub-basal nerve plexus that supplies the overlying corneal epithelium. In addition to these sensory fibers, the cornea contains autonomic sympathetic nerve fibers that may have specific physiologic roles.
The high density of sensitive neural structures within the corneal epithelium is important to allow the detection of stimuli of small magnitude. Thus, corneal sensation elicits a very sensitive defense reflex, crucial for the protection of the cornea and for the eye as a whole. However, corneal innervation provides not only corneal sensation and a protective function, but also has trophic functions, exerting an influence on the regulation of epithelial integrity, proliferation, and wound healing.1,2,3 The blinking and tearing reflexes are elicited by a reflex arch that includes the ocular surface, intact corneal innervation, interconnecting nerves from the functional unit, and lacrimal glands. Compromised function in any part of this reflex arch results in impaired ocular health.4
Studies of the cellular and molecular mechanisms underlying the pathogenesis of corneal epithelial disorders in neurotrophic keratopathy have led to the understanding of the important role the trigeminal nerve plays in maintenance of corneal health and function. Histochemical studies have revealed the presence of various neurotransmitters, including substance P, calcitonin gene-related peptide, neuropeptide Y, vasoactive intestinal peptide, galanin, methionine-enkephalin, catecholamines, and acetylcholine in the cornea.5
Human corneal nerves have also been studied extensively by both light and electron microscopy.6 Studies using these techniques may be unreliable because human corneal nerves are known to degenerate within fourteen hours of death.7 The sub-basal corneal nerve plexus is not visible by conventional slit-lamp biomicroscopy. However, sub-basal corneal nerves are clearly visible by in vivo confocal microscopy, which allows the examination of the living human cornea at the cellular level (Figure 1A). Studies of corneal nerves by IVCM have contributed to the understanding of the human cornea in health, disease, and following surgery. This method is rapid, noninvasive and precise, with good interobserver variability.8
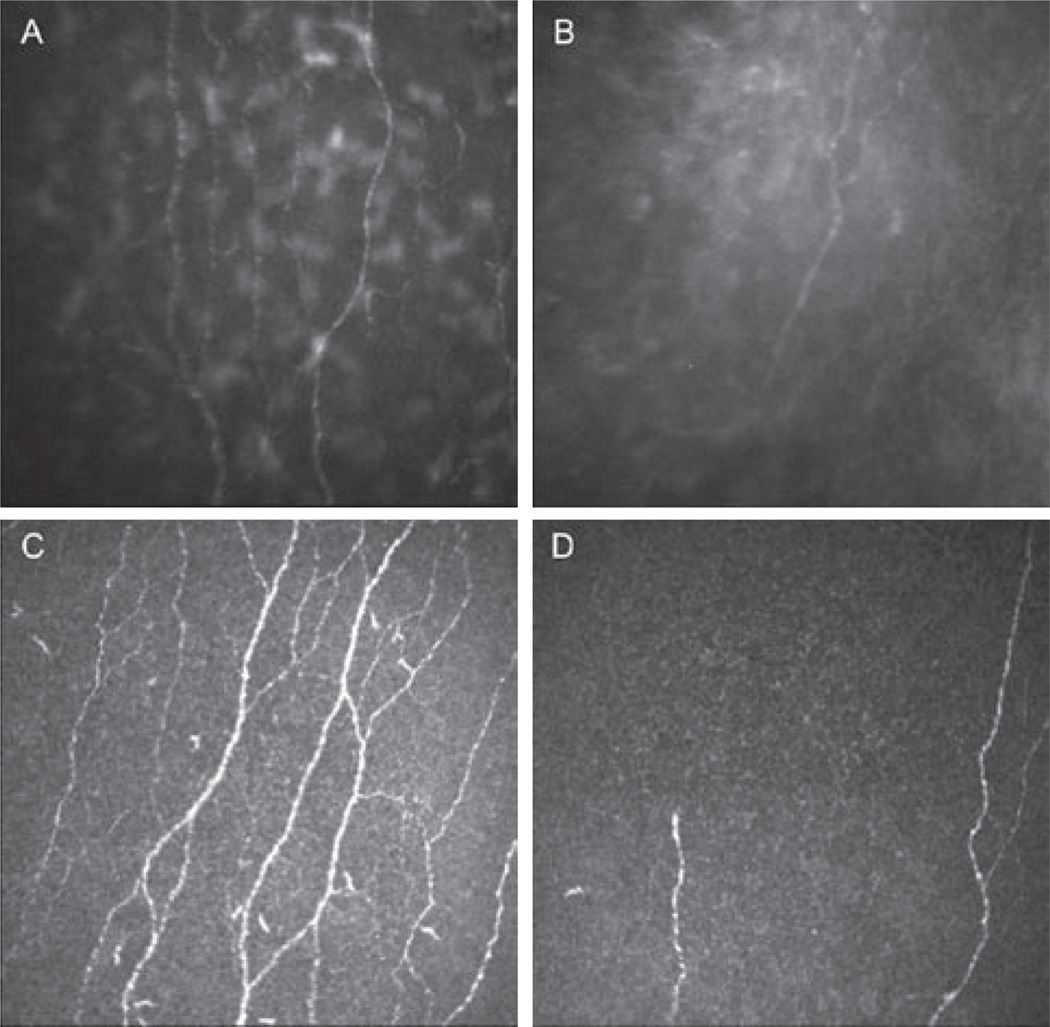
FIGURE 1.
Normal corneal sub-basal nerve plexus and after herpes simplex keratitis with two types of confocal microscopes. (A) Normal sub-basal nerve plexus. Slit scanning confocal microscopy (SSCM) Confoscan 4. (B) Herpes simplex keratitis with severe sensation loss. Note the decrease in total nerve count, length, and branching. SSCM. (C) Normal sub-basal nerve plexus. Laser scanning confocal microscopy (LSCM) HRT3/RCM. (D) Herpes simplex keratitis with severe sensation loss. Note the decrease in nerves density and branching. LSCM.
Goldman described a confocal slit system with line illumination in 1940, and demonstrated 3D imaging of the eye. However, the confocal microscope was invented and patented by Minsky in 1957.9 In 1968, the tandem scanning confocal microscope (TSCM) and later the slit scanning confocal microscope (SSCM) were developed, but it was not until 1985 that confocal microscopy was used by Lemp et al. to examine the cornea.10 A more recent improvement in this technology was the use of coherent light to produce laser scanning confocal microscopes (LSCM). All confocal microscopes use the same basic principle to obtain a high-resolution image, in the context of the confocality of the examined object with the light source and the detector plane, hence the term “confocal.”11
The exponential use of IVCM over the last years has led to significant strides in understanding the role of corneal nerves in health, ocular, and systemic diseases. The purpose of this article is to review the literature assessing the sub-basal corneal nerve plexus by IVCM in different pathological states, and their clinical correlation.
MATERIALS AND METHODS
Articles assessed for inclusion in this review were identified by an electronic search of Medline databases in November 2009 using individual and combinations of keywords “confocal,” “microscopy,” “in vivo,” “corneal,” “nerves,” “sensation” and “sensitivity,” and by review of the reference section of the identified publications. Review and synthesis of the selected literature that has studied the correlation of the subbasal corneal nerve density with corneal sensation was included.
RESULTS
Normal Subjects
Few studies have quantitatively analyzed the sub-basal nerve plexus by IVCM and its relationship with corneal sensitivity in normal human corneas.1,12–14 Studies performed in normal subjects demonstrated that corneal sensation decreases with age.15,16 However, the data regarding the correlation of aging to reduction in sub-basal nerve density are variable.12,14,17 Further, a recent study performed by Niederer et al. showed a corresponding linear decline in sub-basal nerve density of 0.9% per year.18
Contact Lens Wear
Long-term contact lens wear has been associated with a considerable reduction in corneal sensitivity,19 although it does not appear to affect corneal nerve density, distribution, or morphology.20,21 Corneal sensitivity alteration has been shown to vary between different types of contact lenses, with rigid gas permeable lenses associated with a lower corneal sensitivity than PMMA lenses.20 Further, cessation of contact lens wear is associated with a recovery of corneal sensitivity.22 The lack of correlation between nerve function and structural changes may be due to a sensory adaptation to the permanent mechanical stimulus by contact lenses.23 Another possibility favored by Murphy et al. is the attribution of the loss of sensation in soft contact lens wearers to metabolic causes.19
Keratoconus
The pathophysiology of keratoconus has yet not been completely elucidated, though there appear to be some environmental and genetically predisposing factors.24 Sub-basal nerve density has been shown to be lower in corneas with keratoconus, and qualitatively appeared more tortuous in keratoconic corneas as compared to controls, with abnormal architecture in the region of the cone.25–28 Diminishment of nerve density has been significantly correlated with loss of corneal sensation in keratoconus patients, this correlation being stronger in keratoconic patients who wore contact lenses.26 A positive correlation between nerve density with the severity of the disease has been demonstrated as well.27
Regarding the density of the basal epithelium, various studies have reported decreased epithelial cell density,26, 27, 29, 30 with a correspondent increase in cell area. Corneal sub-basal nerve alteration may lead to changes in basal epithelial cell density, and may be involved in the pathogenesis of the disease.26 Brookes et al. have shown that the destructive process in keratoconus involves the nerves, or their associated Schwann cells, which express proteolytic enzymes (cathepsin B and G) more extensively in keratoconus as compared to normal corneas.31
Dry Eye Syndrome
Decreased corneal sensation has been demonstrated in dry eye patients,32–34 although other studies have shown a hypersensitivity.35,36 Similarly, studies present conflicting results regarding the effect of dry eye on sub-basal nerve density. Several studies have observed a significantly reduced sub-basal nerve density in both Sjögren’s syndrome and non-Sjögren’s syndrome dry eye compared to controls, which correlated to corneal sensation in these patients.34,36,37 In contrast, other studies have noted no difference in sub-basal nerve density,33,38 but rather demonstrated increased corneal nerve density in a subgroup with Sjögren’s syndrome.39 The variability of results in regard to the correlation of corneal sensitivity and sub-basal nerve density may be attributed to different stages and severity of dry eye in patients enrolled in these studies. However, there is agreement among the studies that sub-basal corneal nerve tortuosity is significantly increased. An increased number of beadlike formations has been noted and interpreted as metabolically active transmitter-containing nerve fibers, which attempt to improve the abnormal epithelial trophism.38, 40 Alternatively, the beadlike formations are thought to represent nerve damage, followed by secretion of nerve growth factors due to inflammatory processes.37
Clinical correlation to slit-lamp biomicroscopy findings has been shown in several studies. Benitez et al. demonstrated that the number of sub-basal nerves and the level of corneal sensation correlates with Schirmer’s test results.34 Further, Zhang et al. have shown that corneal Rose Bengal staining correlates positively with nerve density, but is inversely related to beading of nerves.39 The results of IVCM studies in dry eye patients strongly suggest a role of corneal nerve density and morphology, as well as function, in the pathogenesis of this disease. The discrepancy between signs and symptoms, as well as the increase in patient symptoms in the face of corneal sensation loss, could be explained by injury of corneal nerve endings due to inflammatory processes, followed by altered excitability in regenerated nerves.
Neurotrophic Keratopathy and Infectious Keratitis
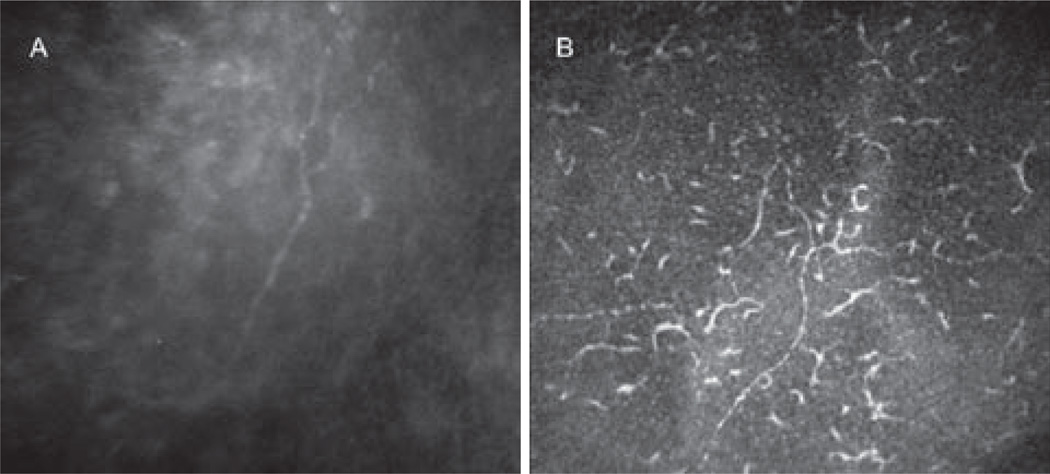
Several recent studies by our group have demonstrated the role of corneal nerves and the correlation with corneal sensation in patients with neurotrophic keratopathy, in patients with herpes simplex keratitis (HSK)41 and herpes zoster ophthalmicus (HZO).42 These studies demonstrated a significant decrease in total number of sub-basal nerve fibers, as well as nerve density in both HZO and HSK eyes, strongly correlating with the decrease in corneal sensation (Figure 1 A–D). Further, IVCM revealed that the loss of the sub-basal nerve plexus started within days in acute HSK. Interestingly, the contralateral unaffected eyes also presented with a loss of sub-basal nerve plexus as compared with normal subjects.41, 42 Moreover, profound HZO- and HSK-induced changes were observed in the superficial epithelium, which showed an increase in cell size, a decrease in cell density, and squamous metaplasia in both HSK and HZO, strongly correlating with decreased corneal sensation and nerve density.43, 44 Similarly, a profound diminishment of the sub-basal corneal nerve plexus was also observed in patients with fungal and Acanthamoeba keratitis.45 More recent studies demonstrated that the decrease in sub-basal corneal nerve density is associated with increased density and morphological changes of central epithelial dendritic cells in HSK, bacterial, fungal and Acanthamoeba keratitis, suggesting a direct interaction between the immune and nervous system in the cornea (Figure 2A–B).46
FIGURE 2.
Corneal sub-basal nerve plexus in Infectious keratitis. (A) Slit scanning confocal microscopy (Confoscan 4). Decrease in nerve density. (B) Laser scanning confocal microscopy (HRT3/RCM). Decrease in nerve density and increase in dendritic cells.
Corneal Surgery
In addition to corneal disease, IVCM enables us to morphologically and functionally correlate corneal reinnervation after surgery. It has been demonstrated that the corneal sub-basal nerve plexus is not a static, but a dynamic, structure.47 Corneal reinnervation is affected by several factors, including the time elapsed after surgery, the patient’s age, the preoperative diagnosis, and the surgical procedure.
Corneal Transplantation
Full-thickness penetrating keratoplasty involves transection of all corneal nerves in both the host and donor cornea. The nerves slowly regenerate over time, although nerve morphology remains abnormal. The study with the longest follow-up has been performed by Richter et al.,48 reporting that none of the patients after penetrating keratoplasty recovered normal nerve morphology or sensitivity during the follow-up period of up to 3 years. Reinnervation of the central cornea with sub-basal nerves occurred at 2 years, and with stromal nerves, at 7 months postoperatively.48 In another study by Darwish et al.,49 although no sub-basal nerves were detected at 12 months, corneal sensation improved over the 12-months period to near normal levels, suggesting that IVCM may not be able to detect some of the finer regenerating sub-basal nerve fibers.49 Comparison of nerve morphology between eyes with nonsurgical trauma and corneal grafts indicated that surgically induced scar formation may limit nerve regeneration in grafts. Two cross-sectional studies demonstrated reduced sub-basal nerve density and increased tortuosity, even 40 years after surgery.50,51
Corneal Refractive Surgery
Photorefractive Keratectomy (PRK)
The sub-basal corneal nerve plexus is undetectable in the treatment area of post-PRK corneas.52 These nerves have been shown to slowly regenerate, returning to normal nerve density within 2 years.53 Nevertheless, morphological alterations can still be observed up to 5 years following surgery.52, 54 Recovery of corneal sensitivity after PRK, in contrast, has been reported to start at 4–6 weeks after surgery, and appears to be completed within 6–12 months of surgery.55, 56
Laser In Situ Keratomileusis (LASIK)
The recovery of corneal sensation after LASIK has been estimated to be approximately 6 months.57,58 Within the first month following surgery, the number of subbasal and stromal nerve fiber bundles decreases by 90% as compared to the preoperative values.59 During the first year after LASIK, reinnervation occurs, with corneal nerves being detected in the central cornea by 6 months.60 However, the nerve density after LASIK remains less than a half of the pre-operative values even at 12 months.59,61 In addition, decreased sub-basal nerve density is observed at 2 and 3 years after LASIK,60 and even at 5 years following surgery, nerve regeneration appears to remain incomplete.53 A strong correlation has been observed between corneal sensation and sub-basal nerve morphology and density after LASIK.57,61 Interestingly, a study comparing corneal wound healing and nerve regeneration showed no difference between flaps created with femtosecond laser as compared to mechanical microkeratome.62
Laser-Assisted Sub-epithelial Keratectomy (LASEK)
Corneal sensation has been shown to return to preoperative levels at 3 months after LASEK surgery, although sub-basal nerve density was still at half of pre-operative values 6 months following surgery.63 However, in a separate study, no difference was observed in nerve recovery between LASIK and LASEK.64
Systemic Diseases
IVCM is an accurate non-invasive method for diagnosis and assessment of the progression of systemic diseases with peripheral neuropathy, such as diabetes. Reduced corneal sub-basal nerve density and increased nerve fiber tortuosity in diabetes have been documented utilizing IVCM, and correlated with the stage of peripheral neuropathy.65–67 Rosenberg et al. demonstrated a correlation between reduced corneal nerve bundles with loss of corneal sensation, and severity of somatic neuropathy in patients with type 1 diabetes.66 In addition, IVCM allows detection of early peripheral neuropathy, as decreased nerve density has been shown to precede impairment of corneal sensitivity. Further, corneal nerves recover with improved glycemic control or within 6 months after pancreatic transplantation in patients with type 1 diabetes.68
A reduction in neurotrophic stimuli in severe neuropathy may induce a thin epithelium, potential leading to recurrent erosions.66 This may explain the significantly higher risk of development of postoperative epithelial complications and relatively poor refractive results in diabetic patients who undergo LASIK.69 Nerve fiber damage and repair in the corneal stroma has been shown to be partially regulated by collagen, fibronectin, and proteoglycans, as well as a number of growth factors including tumor growth factor-β, fibroblast growth factor, and nerve growth factor, many of which are upregulated in diabetes.67
DISCUSSION
The main function of the cornea is transparency, for which corneal innervation is key. Corneal sensation, epithelial metabolism, cell adhesion, and wound healing all depend on adequate corneal innervation. The mechanisms governing corneal nerve integrity and their structure are potentially complex. To date, the assessment of corneal innervation has only been possible through esthesiometry techniques. The advent of IVCM, together with the improving technological advances in this field, now allow for in vivo examinations of corneal nerve morphology and density in great detail. IVCM enables a direct comparison of corneal sensory innervation and clinical findings, such as sensitivity measured by esthesiometry, epithelial indemnity and transparency. Corneal dennervation has been shown to occur in local and systemic disease, as well as after surgical and pharmacological interventions.
The relationship between corneal innervation and sensation has been studied in normal human corneas and in several diseases, such as dry eye, primary Sjögren’s syndrome, keratoconus, and diabetes. Further, the correlation between corneal reinnervation and esthesiometry has been observed after several surgical procedures, including penetrating keratoplasty and laser refractive surgery. However, conflicting results have been reported regarding the correlation of corneal sub-basal nerve density and sensation. The differences in some of the studies can be attributed to the different confocal microscopes used, which have different resolution, depth of field, and capacity to evaluate corneal nerves. A standardized method of analysis remains to be developed after rigorous validation. Furthermore, consistent image acquisition is necessary in order to use confocal microscopy in prospective and multicenter studies, allowing data to be compared between different microscopes. Additionally, some of these studies utilized different methods for the assessment of corneal sensitivity. Moreover, a consistent esthesiometry method is necessary to measure corneal sensation in a reproducible fashion.
In conclusion, the use of IVCM and esthesiometry allows the detection of corneal nerve morphology, density and function, opening the way for possible new lines of treatment for local and systemic diseases. Understanding the pathogenesis of nerve degeneration and regeneration both in health and in disease will potentially enable the development of novel strategies to prevent nerve destruction or to stimulate nerve regeneration, in order to restore corneal sensation and retain transparency.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005;24:608–613. doi: 10.1097/01.ico.0000154384.05614.8f. [DOI] [PubMed] [Google Scholar]
- 3.Beuerman RW, Schimmelpfennig B. Sensory dennervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69:196–201. doi: 10.1016/0014-4886(80)90154-5. [DOI] [PubMed] [Google Scholar]
- 4.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Nishida T. Neurotrophic mediators and corneal wound healing. Ocul Surf. 2005;3:194–202. doi: 10.1016/s1542-0124(12)70206-9. [DOI] [PubMed] [Google Scholar]
- 6.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 7.Müller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–994. [PubMed] [Google Scholar]
- 8.Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–860. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 9.Patel DV, McGhee CN. Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques: a major review. Clin Exp Ophthalmol. 2007;35:71–88. doi: 10.1111/j.1442-9071.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 10.Lemp MA, Dilly PN, Boyde A. Tandem-scanning (confocal) microscopy of the full-thickness cornea. Cornea. 1985;4:205–209. [PubMed] [Google Scholar]
- 11.Bochert R, Zhivov A, Kraak R, Stave J, Guthoff RF. Contribution to comprehension of image formation in confocal microscopy of cornea with Rostock cornea module. Br J Ophthalmol. 2005;89:1351–1355. doi: 10.1136/bjo.2004.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupcheva CN, Wong T, Riley AF, McGhee CN. Assessing the sub-basal nerve plexus of the living healthy human cornea by in vivo confocal microscopy. Clin Exp Ophthalmol. 2002;30:187–190. doi: 10.1046/j.1442-9071.2002.00507.x. [DOI] [PubMed] [Google Scholar]
- 13.Patel DV, McGhee CN. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005;46:4485–4488. doi: 10.1167/iovs.05-0794. [DOI] [PubMed] [Google Scholar]
- 14.Patel DV, Tavakoli M, Craig JP, Efron N, McGhee CN. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea. 2009;28:735–740. doi: 10.1097/ICO.0b013e318193e0e3. [DOI] [PubMed] [Google Scholar]
- 15.Millodot M. The influence of age on the sensitivity of the cornea. Invest Ophthalmol Vis Sci. 1977;16:240–242. [PubMed] [Google Scholar]
- 16.Roszkowska AM, Colosi P, Ferreri FM, Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004;218:350–355. doi: 10.1159/000079478. [DOI] [PubMed] [Google Scholar]
- 17.Erie JC, McLaren JW, Hodge DO, Bourne WM. The effect of age on the corneal subbasal nerve plexus. Cornea. 2005;24:705–709. doi: 10.1097/01.ico.0000154387.51355.39. [DOI] [PubMed] [Google Scholar]
- 18.Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007;91:1165–1169. doi: 10.1136/bjo.2006.112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy PJ, Patel S, Marshall J. The effect of long-term, daily contact lens wear on corneal sensitivity. Cornea. 2001;20:264–269. doi: 10.1097/00003226-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 20.McLaren SV, McLaren JW, Hodge DO, Bourne WM. Confocal microscopy in vivo in corneas of long-term contact lens wearers. Invest Ophthalmol Vis Sci. 2002;43:995–1003. [PubMed] [Google Scholar]
- 21.Oliveira-Soto L, Efron N. Morphology of corneal nerves in soft contact lens wear. A comparative study using confocal microscopy. Ophthalmic Physiol Optics. 2003;23:165–174. doi: 10.1046/j.1475-1313.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 22.Millodot M. Effect of long-term wear of hard contact lenses on corneal sensitivity. Arch Ophthalmol. 1978;96:1225–1227. doi: 10.1001/archopht.1978.03910060059011. [DOI] [PubMed] [Google Scholar]
- 23.Niederer R, McGhee C. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog Retin Eye Res. 2009 Nov 26; doi: 10.1016/j.preteyeres.2009.11.001. [Epub ahead of print] PMID: 19944182. [DOI] [PubMed] [Google Scholar]
- 24.Edwards M, McGhee CN, Dean S. The genetics of keratoconus. Clin Exp Ophthalmol. 2001;29:345–351. doi: 10.1046/j.1442-9071.2001.d01-16.x. [DOI] [PubMed] [Google Scholar]
- 25.Patel DV, McGhee CN. Mapping the corneal sub-basal nerve plexus in keratoconus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2006;47:1348–1351. doi: 10.1167/iovs.05-1217. [DOI] [PubMed] [Google Scholar]
- 26.Patel DV, Ku JY, Johnson R, McGhee CN. Laser scanning in vivo confocal microscopy and quantitative aesthesiometry reveal decreased corneal innervation and sensation in keratoconus. Eye. 2009;23:586–592. doi: 10.1038/eye.2008.52. [DOI] [PubMed] [Google Scholar]
- 27.Niederer RL, Perumal D, Sherwin T, McGhee CN. Laser scanning in vivo confocal microscopy reveals reduced innervation and reduction in cell density in all layers of the keratoconic cornea. Invest Ophthalmol Vis Sci. 2008;49:2964–2970. doi: 10.1167/iovs.07-0968. [DOI] [PubMed] [Google Scholar]
- 28.Simo Mannion L, Tromans C, O’Donnell C. An evaluation of corneal nerve morphology and function in moderate keratoconus. Contact Lens Anterior Eye. 2005;28:185–192. doi: 10.1016/j.clae.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Mocan MC, Yilmaz PT, Irkec M, Orhan M. In vivo confocal microscopy for the evaluation of corneal microstructure in keratoconus. Curr Eye Res. 2008;33:933–939. doi: 10.1080/02713680802439219. [DOI] [PubMed] [Google Scholar]
- 30.Hollingsworth JG, Efron N, Tullo AB. In vivo corneal confocal microscopy in keratoconus. Ophthalmic Physiol Opt. 2005;25:254–260. doi: 10.1111/j.1475-1313.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 31.Brookes NH, Loh IP, Clover GM, Poole CA, Sherwin T. Involvement of corneal nerves in the progression of keratoconus. Exp Eye Res. 2003;77:515–524. doi: 10.1016/s0014-4835(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 32.Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. 1996;15:235–239. doi: 10.1097/00003226-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Hoşal BM, Ornek N, Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye. 2005;19:1276–1279. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 34.Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007 Jan;48:173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 35.De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137:109–115. doi: 10.1016/s0002-9394(03)00897-3. [DOI] [PubMed] [Google Scholar]
- 36.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren’s syndrome. Exp Eye Res. 2008;86:879–885. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007 May;48:2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 38.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Corneal innervation and morphology in primary Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Chen J, Luo L, Xiao Q, Sun M, Liu Z. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005;24:818–824. doi: 10.1097/01.ico.0000154402.01710.95. [DOI] [PubMed] [Google Scholar]
- 40.Benítez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45:3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 41.Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit B, Bayhan HA, Dana R, Pavan-Langston D. Corneal Sensation and Subbasal Nerve Alterations in Patients with Herpes Simplex Keratitis: An In Vivo Confocal Microscopy Study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dastjerdi MH, Hamrah P, Dana MR, Pavan-Langston D. Comparison of corneal nerve and corneal sensation in herpes zoster keratopathy with in vivo confocal microscopy. [May 10, 2007];Invest Ophthalmol Vis Sci. 2007 48:3646. http://abstracts.iovs.org/cgi/content/abstract/48/5/3646. [Google Scholar]
- 43.Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit B, Bayhan HA, Dana R, Pavan-Langston D. Corneal sensation and subbasal nerve alterations in patients with Herpies Simplex Keratitis: An in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamrah P, Schrems WA, Hoesl LM, Dastjerdi MH, Dana R, Pavan-Langston D. Corneal epithelial and stromal changes in patients with herpes simplex keratitis: An in vivo confocal microscopy study. [April 11, 2008];Invest Ophthalmol Vis Sci. 2009 50:2389. http://abstracts.iovs.org/cgi/content/abstract/50/5/2389. [Google Scholar]
- 45.Kurbanyan K, Hoesl LM, Schrems WA, Hamrah P. Morphology and density of corneal nerves in acanthamoeba and fungal keratitis: An in vivo confocal microscopy study. [April 11, 2009];Invest Ophthalmol Vis Sci. 2009 50:2402. http://abstracts.iovs.org/cgi/content/abstract/50/5/2402. [Google Scholar]
- 46.Cruzat A, Mantopoulos D, Zheng L, Hamrah P. In vivo confocal microscopy study of epithelial dendritic cells and subbasal nerve plexus in infectious keratitis. 26th Biennial Cornea Conference Abstracts; Boston, MA. 2009. [Google Scholar]
- 47.Patel DV, McGhee CN. In vivo laser scanning confocal microscopy confirms that the human corneal sub-basal nerve plexus is a highly dynamic structure. Invest Ophthalmol Vis Sci. 2008;49:3409–3412. doi: 10.1167/iovs.08-1951. [DOI] [PubMed] [Google Scholar]
- 48.Richter A, Slowik C, Somodi S, Vick HP, Guthoff R. Corneal reinnervation following penetrating keratoplasty--correlation of esthesiometry and confocal microscopy. Ger J Ophthalmol. 1996 Nov;5:513–517. [PubMed] [Google Scholar]
- 49.Darwish T, Brahma A, Efron N, O’Donnell C. Subbasal nerve regeneration after penetrating keratoplasty. Cornea. 2007 Sep;26:935–940. doi: 10.1097/ICO.0b013e3180de493f. [DOI] [PubMed] [Google Scholar]
- 50.Niederer RL, Perumal D, Sherwin T, McGhee CN. Corneal innervation and cellular changes after corneal transplantation: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48:621–626. doi: 10.1167/iovs.06-0538. [DOI] [PubMed] [Google Scholar]
- 51.Patel SV, Erie JC, McLaren JW, Bourne WM. Keratocyte density and recovery of subbasal nerves after penetrating keratoplasty and in late endothelial failure. Arch Ophthalmol. 2007;125:1693–1698. doi: 10.1001/archopht.125.12.1693. [DOI] [PubMed] [Google Scholar]
- 52.Moilanen JA, Vesaluoma MH, Müller LJ, Tervo TM. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003;44:1064–1069. doi: 10.1167/iovs.02-0247. [DOI] [PubMed] [Google Scholar]
- 53.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Linna T, Tervo T. Real-time confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr Eye Res. 1997;16:640–649. doi: 10.1076/ceyr.16.7.640.5058. [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Santonja JJ, Sakla HF, Cardona C, Chipont E, Alió JL. Corneal sensitivity after photorefractive keratectomy and laser in situ keratomileusis for low myopia. Am J Ophthalmol. 1999;127:497–504. doi: 10.1016/s0002-9394(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 56.Kauffmann T, Bodanowitz S, Hesse L, Kroll P. Corneal reinnervation after photorefractive keratectomy and laser in situ keratomileusis: an in vivo study with a confocal videomicroscope. Ger J Ophthalmol. 1996;5:508–512. [PubMed] [Google Scholar]
- 57.Perez-Gomez I, Efron N. Change to corneal morphology after refractive surgery (myopic laser in situ keratomileusis) as viewed with a confocal microscope. Optom Vis Sci. 2003;80:690–697. doi: 10.1097/00006324-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Bragheeth MA, Dua HS. Corneal sensation after myopic and hyperopic LASIK: clinical and confocal microscopic study. Br J Ophthalmol. 2005;89:580–585. doi: 10.1136/bjo.2004.046888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002;43:3660–3664. [PubMed] [Google Scholar]
- 60.Calvillo MP, McLaren JW, Hodge DO, Bourne WM. Corneal reinnervation after LASIK: prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci. 2004;45:3991–3996. doi: 10.1167/iovs.04-0561. [DOI] [PubMed] [Google Scholar]
- 61.Linna TU, Vesaluoma MH, Perez-Santonja JJ, Petroll WM, Alió JL, Tervo TM. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Invest Ophthalmol Vis Sci. 2000;41:393–397. [PubMed] [Google Scholar]
- 62.Sonigo B, Iordanidou V, Chong-Sit D, et al. In vivo corneal confocal microscopy comparison of intralase femtosecond laser and mechanical microkeratome for laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2006;47:2803–2811. doi: 10.1167/iovs.05-1207. [DOI] [PubMed] [Google Scholar]
- 63.Darwish T, Brahma A, O’Donnell C, Efron N. Subbasal nerve fiber regeneration after LASIK and LASEK assessed by non-contact esthesiometry and in vivo confocal microscopy: prospective study. J Cataract Refract Surg. 2007;33:1515–1521. doi: 10.1016/j.jcrs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 64.Darwish T, Brahma A, O’Donnell C, Efron N. Subbasal nerve fiber regeneration after LASIK and LASEK assessed by noncontact esthesiometry and in vivo confocal microscopy: prospective study. J Cataract Refract Surg. 2007;33:1515–1521. doi: 10.1016/j.jcrs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 65.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–688. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 66.Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915–2921. [PubMed] [Google Scholar]
- 67.Kallinikos P, Berhanu M, O’Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–422. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 68.Mehra S, Tavakoli M, Kallinikos PA, et al. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30:2608–2612. doi: 10.2337/dc07-0870. [DOI] [PubMed] [Google Scholar]
- 69.Fraunfelder FW, Rich LF. Laser-assisted in situ keratomileusis complications in diabetes mellitus. Cornea. 2002;21:246–248. doi: 10.1097/00003226-200204000-00002. [DOI] [PubMed] [Google Scholar]