Abstract
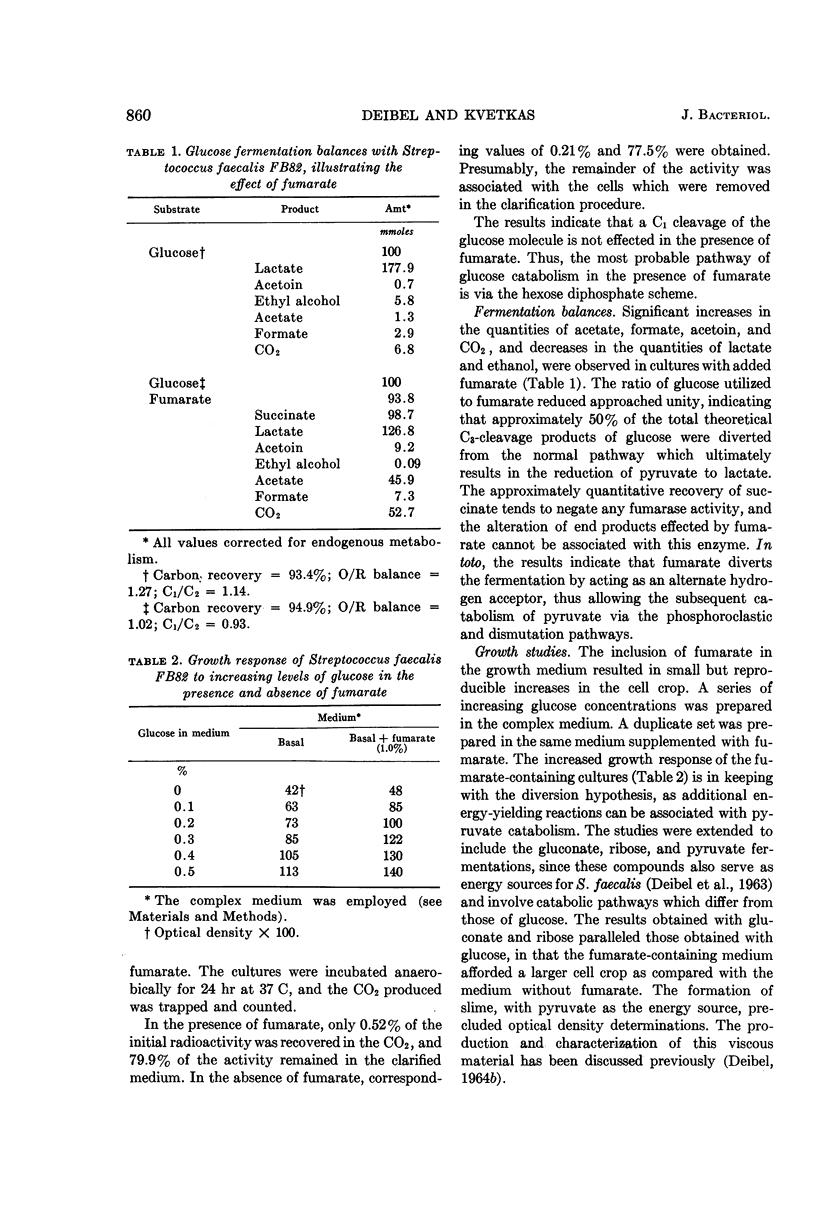
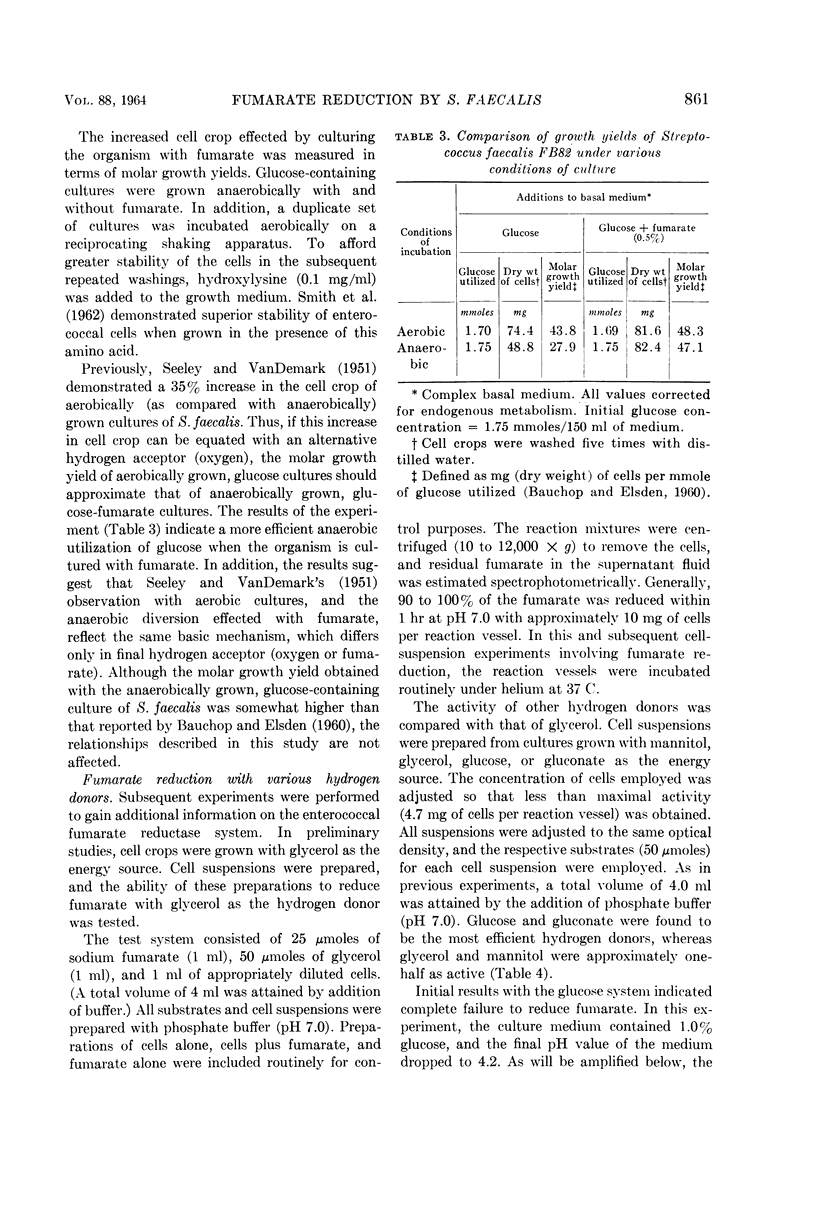
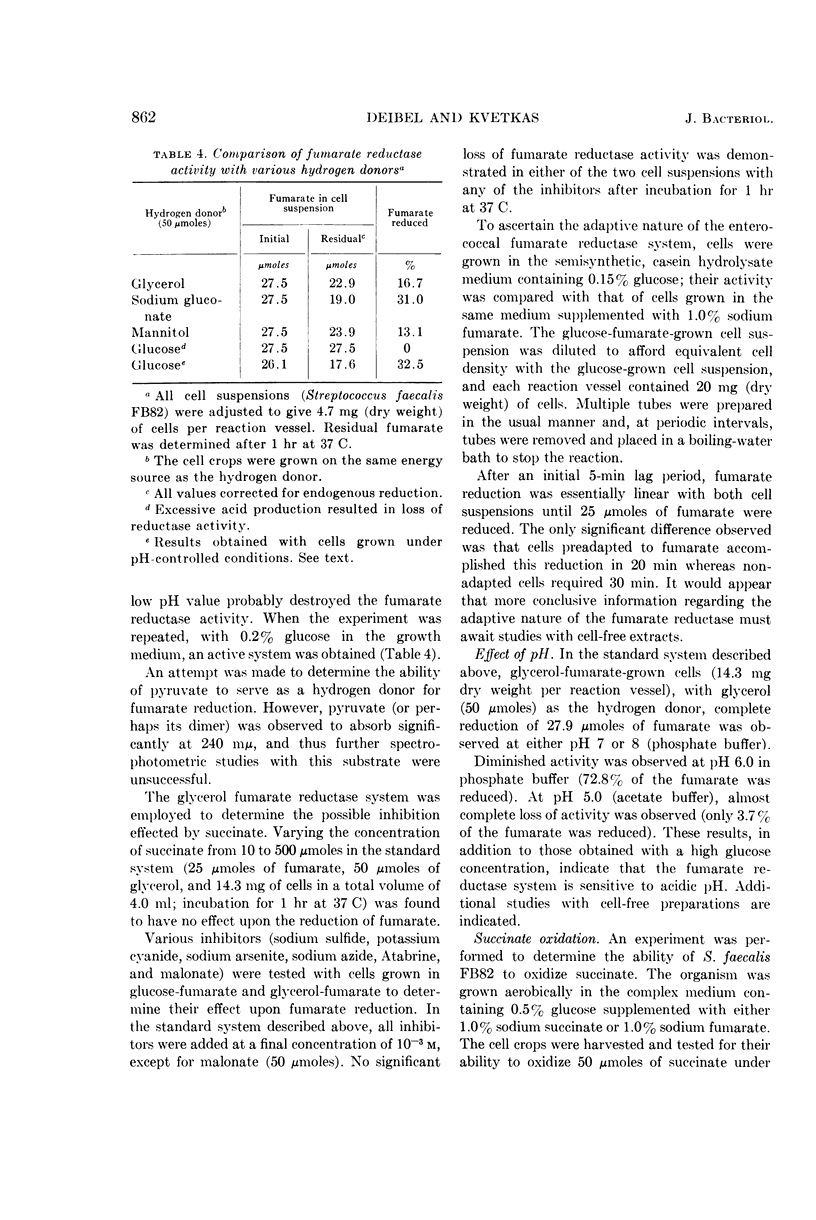
Deibel, R. H. (American Meat Institute Foundation, Chicago, Ill.), and M. J. Kvetkas. Fumarate reduction and its role in the diversion of glucose fermentation by Streptococcus faecalis. J. Bacteriol. 88:858–864. 1964.—Fumarate diverts the normal fermentation of glucose by Streptococcus faecalis FB82, as shown by the production of increased amounts of CO2, formate, acetate, and acetoin, and decreased formation of lactate and ethanol. Experiments with d-glucose-1-C14, in which low levels of labeled CO2 were recovered, indicated that C-1 cleavage of the glucose molecule was not involved. The presence of fumarate afforded consistently larger cell crops in growth studies with glucose and other energy sources. On a molar growth-yield basis, anaerobically grown, glucose-fumarate cultures were equivalent to aerobically grown, glucose cultures. The reduction of fumarate by cell suspensions indicated that glucose, gluconate, and, to a lesser extent, glycerol and mannitol could serve as hydrogen donors. Several common metabolic inhibitors had no effect upon the fumarate reductase system in cell suspensions, although some sensitivity to acidic pH was noted. Significant levels of succinate oxidation activity were not detected. Fumarate reductase activity was demonstrated in all five S. faecalis strains tested. Distribution of this ability in S. faecium strains was variable, ranging from activity comparable with that of S. faecalis to total inactivity. The observations support the conclusion that fumarate functions as an alternate hydrogen acceptor, thus allowing pyruvate to participate in the energy-yielding phosphoroclastic and dismutation pathways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- BONNICHSEN R., LUNDGREN G. Comparison of the ADH and the Widmark procedures in forensic chemistry for determinating alcohol. Acta Pharmacol Toxicol (Copenh) 1957;13(3):256–266. doi: 10.1111/j.1600-0773.1957.tb00262.x. [DOI] [PubMed] [Google Scholar]
- BRODOVSKY E. R., UTLEY M. H., PEARSON W. N. Methionine inadequacy of casein hydrolyzate as source of difficulty in vitamin assays. Science. 1958 Aug 8;128(3319):307–308. doi: 10.1126/science.128.3319.307. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H., LAKE D. E., NIVEN C. F., Jr PHYSIOLOGY OF THE ENTEROCOCCI AS RELATED TO THEIR TAXONOMY. J Bacteriol. 1963 Dec;86:1275–1282. doi: 10.1128/jb.86.6.1275-1282.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H., NIVEN C. F., Jr PYRUVATE FERMENTATION BY STREPTOCOCCUS FAECALIS. J Bacteriol. 1964 Jul;88:4–10. doi: 10.1128/jb.88.1.4-10.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H. THE GROUP D STREPTOCOCCI. Bacteriol Rev. 1964 Sep;28:330–366. doi: 10.1128/br.28.3.330-366.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel R. H. Utilization of arginine as an energy source for the growth of Streptococcus faecalis. J Bacteriol. 1964 May;87(5):988–992. doi: 10.1128/jb.87.5.988-992.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNSALUS I. C., HORECKER B. L., WOOD W. A. Pathways of carbohydrate metabolism in microorganisms. Bacteriol Rev. 1955 Jun;19(2):79–128. doi: 10.1128/br.19.2.79-128.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus I. C. Products of Anaerobic Glycerol Fermentation by Streptococci faecalis. J Bacteriol. 1947 Aug;54(2):239–244. doi: 10.1128/jb.54.2.239-244.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'KANE D. J. Influence of the pyruvate oxidation factor on the oxidative metabolism of glucose by Streptococcus faecalis. J Bacteriol. 1950 Oct;60(4):449–458. doi: 10.1128/jb.60.4.449-458.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- SEELEY H. W., VANDEMARK P. J. An adaptive peroxidation by Streptococcus faecalis. J Bacteriol. 1951 Jan;61(1):27–35. doi: 10.1128/jb.61.1.27-35.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH W. G., NEWMAN M., LEACH F. R., HENDERSON L. M. The effect of hydroxylysine on cell wall synthesis and cell stability in Streptococcus faecalis. J Biol Chem. 1962 Apr;237:1198–1202. [PubMed] [Google Scholar]
- WARRINGA M. G., SMITH O. H., GIUDITTA A., SINGER T. P. Studies on succinic dehydrogenase. VIII. Isolation of a succinic dehydrogenase-fumaric reductase from an obligate anaerobe. J Biol Chem. 1958 Jan;230(1):97–109. [PubMed] [Google Scholar]



