Abstract
We present a new physical biology approach to understanding the relationship between the organization and segregation of bacterial chromosomes. We posit that replicated Escherichia coli daughter strands will spontaneously demix as a result of entropic forces, despite their strong confinement within the cell; in other words, we propose that entropy can act as a primordial physical force which drives chromosome segregation under the right physical conditions. Furthermore, proteins implicated in the regulation of chromosome structure and segregation may in fact function primarily in supporting such an entropy-driven segregation mechanism by regulating the physical state of chromosomes. We conclude that bacterial chromosome segregation is best understood in terms of spontaneous demixing of daughter strands. Our concept may also have important implications for chromosome segregation in eukaryotes, in which spindle-dependent chromosome movement follows an extended period of sister chromatid demixing and compaction.
In bacteria, the two defining processes of the cell cycle, chromosome replication and segregation, progress hand in hand. The roles of the enzymes and proteins involved in chromosome replication have been well studied in Escherichia coli1. Chromosome segregation, on the other hand, was described by Murray and Hunt in 1993 (REF. 2) as “among the most mysterious” events during the bacterial cell cycle, and its basic mechanism remains elusive.
At the heart of the problem is the fact that the stretched length of a chromosome is at least 1,000 times as long as the cell, thus chromosomes — be they eukaryotic or bacterial — experience a strong confinement inside a cell, and individual proteins can probe only a fraction of the chromosome owing to their own small sizes. It is this immense difference in scale between proteins and chromosomes that makes it difficult to understand how the local actions of specific proteins can result in the global changes that take place during the organization, decatenation and segregation of the bulk chromosomes. This is particularly pertinent to proteins like type II topoisomerases or the SMC (structural maintenance of chromosomes) proteins. Type II topoisomerases are known to be crucial for chromosome segregation in both bacteria and eukaryotes3, but how do they know whether two strands of DNA belong to the same or different chromosomes? Similarly, it has been proposed that DNA condensation by SMC proteins contributes to chromosome segregation4,5, but it is not clear why mixed polymers will not become even more intermingled when condensed by such proteins. These examples stress the importance of directionality of the guiding forces for the action of proteins in the organization and segregation of chromosomes.
Here, we propose a physical biology framework for understanding not only how the global physical properties of the chromosome provide directionality for the action of proteins such as topoisomerases, but also which of these physical properties should be influenced by proteins in order to establish global organization and segregation of the chromosome. Specifically, we put forward the theory that conformational entropy is a major guiding force for segregation of chromosome bulk, and that the proteins previously identified as segregation factors in fact function to create the right physical conditions for entropy-driven chromosome segregation.
In the first part of this Opinion article, we consider when entropy will drive segregation and then present a physical model of the bacterial chromosome to explain chromosome segregation in E. coli. A corollary of our model is that accurate segregation of smaller DNA circles such as plasmids cannot rely on entropic force alone, unlike chromosomes, but require active partitioning systems. Fully consistent with our proposal, cytoskeleton-based mechanisms have been described in detail for the segregation of low-copy-number plasmids6, whereas evidence is lacking for a chromosome segregation mechanism that is purely cytoskeleton based. In the second part of this Opinion article, we critically examine the protein-based DNA segregation models and explain how they can be understood in the context of the entropy model. Finally, we compare chromosome segregation in bacteria and eukaryotes, emphasizing the common physical principles.
Entropy can drive segregation
When will conformational entropy drive segregation?
As we illustrate in BOX 1 and in Supplementary information S1 (movie) and Supplementary information S2 (movie), polymers confined in a box can actively segregate, whereas disconnected but otherwise physically identical particles always mix. This can be understood in a two-dimensional example by imagining a long polymer chain as a random walk that cannot cross its own path (a so-called ‘self-avoiding random walk’). More specifically, consider a long chain on a paper surface that cannot cross itself, surrounded by a tightly confining box. Now, draw a second chain in the same box but without allowing the chains to cross. Finding overlapping (intermixed) but self-avoiding conformations is a challenge. The reason is that — using the terminology of polymer physics — the overlapping chains have fewer conformational degrees of freedom, or less conformational entropy, than the ones that are completely separated. Thus, entropic forces can actively segregate mixed polymers from one another.
Box 1 Entropy measures the degrees of freedom, not disorder, of the system.
In his influential book, What is Life?50, Schrödinger asserted that a living being reaches thermodynamic equilibrium only on its death. Defining entropy as a measure of ‘disorder’ in the last chapter, he struggled to explain how order (life) can be achieved from disorder. However, this association of life with minimal entropy can be misleading. Molecular dynamics simulations (see the figure, part a, and Supplementary information S1 (movie)) can be carried out for a long rectangular box containing two species of particles in equilibrium (blue and red) that are initially separated by a wall. As the wall is removed from the system, the two species start to mix. The driving force of this process is the well-known ‘entropy of mixing’.
Entropy, however, is more subtle than a simple measure of disorder. To see this, let us start with the mixed state of the particles and connect those of the same species, creating two long linear chains, one blue and one red (see the figure, part a). Another important condition is the excluded-volume interaction between the particles. The reader is encouraged to perform this simple computer simulation, and he or she will see that the two chains demix; that is, ‘order’ emerges out of disorder (see Supplementary information S2 (movie)).
Another example of order arising out of disorder is the phase separation of a long polymeric chain (for example, double-stranded DNA) embedded in a solution of small molecules (for example, globular proteins), in which the small molecules are depleted from the long chain because of their excluded volume (see the figure, part b). The result of this entropic interaction is the compaction of the long-chain molecule.
These are some of the many examples of entropy leading to order that we have come across since Schrödinger’s time*. They clarify what entropy really measures — namely, the degrees of freedom of the system (rather than the ‘disorder’ of the system). These two examples also point out the importance of chain connectivity, a keyword in polymer physics. This emergence of order from disorder owing to entropy arising from chain connectivity is our starting point for understanding chromosome organization and segregation in bacteria.
*Note that polymer physics was still in its infant stage during Schrödinger’s time. For example, Flory’s magnum opus, Principles of Polymer Chemistry51, appeared almost 10 years after Schrödinger’s What is Life?50.
Conformational entropy can be measured quantitatively using the concept of a ‘blob’ (REF. 7). A blob is the largest unit of a polymer, under a particular set of conditions, that shows the characteristics of an unperturbed self-avoiding chain, even in the presence of external forces such as compression or pulling. If the chromosome has internal structures within which DNA motions or dynamics influence one another, these structures can be measured experimentally and considered to be a blob as well (Supplementary information S3 (box)). A blob is also the basic unit of free energy stored in a strongly deformed polymer. Compressing or stretching an unperturbed chain requires that the polymer’s tendency to go back to its natural size — which is (contour length)3/5, so much smaller than its fully stretched length — must be overcome. Thus, a stretched chain stores free energy like an ‘entropic spring’ (REF. 8), and this entropic spring consists of a string of blobs (BOX 2). For the same reason, strongly confined chains behave like a loaded entropic spring. Indeed, as we describe below, we view the bacterial chromosome as a loaded entropic spring of blobs, in which each blob is a structural unit of the chromosome and consists of supercoiled DNA stabilized by DNA-binding proteins9, thus storing the free energy produced by the DNA–protein interactions (such as depletion interactions, as illustrated in BOX 1, DNA–gyrase interactions that generate torsion, and binding of SMC proteins for DNA condensation).
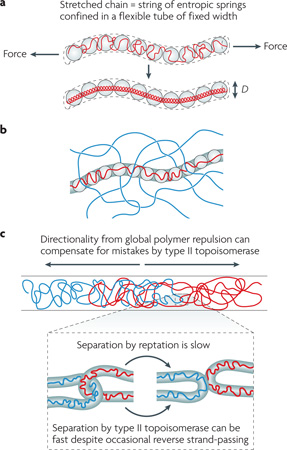
Box 2 Chain molecules in strong confinement or at high concentrations.
The effect of a cylindrical confinement on a chain is equivalent to applying a tension, which pulls and stretches the long molecule (see the figure, part a). In polymer physics, the stretched chain is described as a series of entropic springs (or ‘blobs’), the size of which is determined by the width of the tube. There is a linear relationship between the total contour length of the chain and the total number of blobs, and the free energy stored in the chain is directly proportional to the number of blobs. In other words, a blob is also a basic unit of free energy stored in a polymer and has a thermal energy of kBT (where T is the room temperature (∼300 Kelvin) and kB is the Boltzmann constant (2 cal per mol K) regardless of its physical size52,53 (for comparison, ATP hydrolysis releases ∼12 kBT of energy, and a hydrogen bond is several kBT). In other words, blobs can have different sizes but the same free energy, as long as the self-avoidance condition is met.
When the concentration of the chain is very high, transverse motions of a chain segment embedded in the meshwork of polymers is hindered. In this case, the polymer motion is best understood as sliding in a conceptual tube embedded in a polymer meshwork, also known as ‘reptation’ (see the figure, part b). Reptation is slow; it takes much longer to travel the same absolute distance by reptation in a meshwork than by diffusion in the absence of the meshwork, and the difference between the rates increases in proportion to the length of the chain itself.
Chain connectivity and excluded-volume interactions provide directionality for the segregation of intermingled chains54 (see the figure, part c). Type II topoisomerases do not need to provide or know the directionality. By occasional random strand-passing, these enzymes can accelerate the kinetics of chromosome segregation because reptation is a much slower process (see the figure, part c). However, too high a concentration of topoisomerase means that the two chains will not know their directionality, because the excluded-volume interaction between the chains and the enzyme effectively vanishes (by virtue of the high concentration of the enzyme) and the forward and reverse topoisomerase reactions will cancel each other out. We thus predict that there will be an optimal range of type II topoisomerase concentrations at which chromosome segregation is fastest, because the entropy-driven directionality and topoisomerase-dependent ‘free pass’ are in balance.
The size of the blob simultaneously influences the organization of two chains (segregation versus mixing) and their conformation (ordered versus random) in a closed confinement. To understand this, consider two chains trapped in a cylindrical pore closed by two pistons, as illustrated in FIG. 1a. When the chain concentration is low, the two chains are well separated, as their mixing is entropically costly (because blobs repel one another). As the pistons decrease the volume of the cylinder and the pressure builds up, the two chains become more compressed and, thus, their free energy increases — imagine an ideal gas being compressed. Free energy is directly proportional to the number of blobs, so compressed chains have more blobs and, accordingly, these are smaller (see Supplementary information S4 (box)).
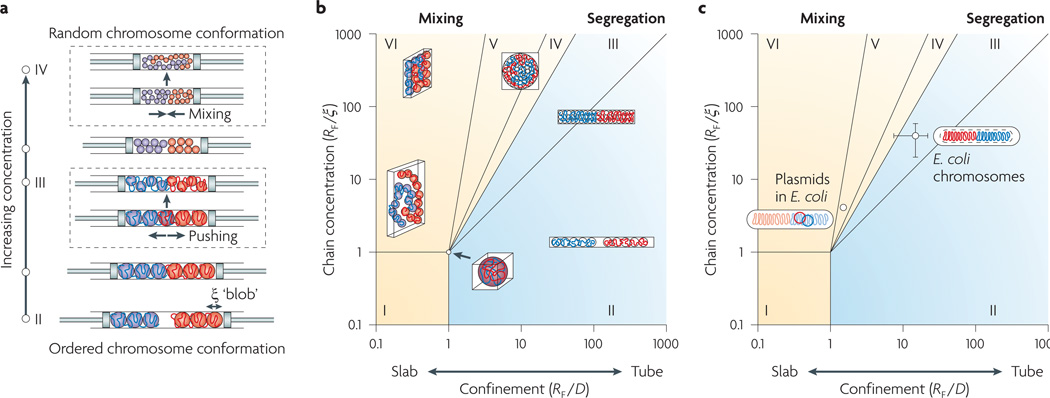
Figure 1. Predicting chromosome segregation using physical parameters of the nucleoid.
a | The piston analogy, illustrating the effect of the degree of confinement on the organization of two chains. Two long chains are confined in a cylinder of fixed width that is capped by two pistons. As the volume of the cylinder is decreased by the pistons, chains go through two simultaneous transitions: a change in the principal ordering of each chain from linear to random, and from segregation to mixing of the two chains. b | A phase diagram explaining how two long chains will segregate or mix depending on the degree of confinement and the concentration of the chains in a box. This phase diagram can be used to predict the entropy-driven segregatability of other organisms (see main text and Supplementary information S4 (box) for details). c | Escherichia coli chromosomes are in the segregation regime, whereas plasmids are in the mixing regime because they are too small. ξ, size of the structural unit (also called the correlation length); D, width of the nucleoid inside the cell; RF, Flory radius of gyration of the isolated nucleoid (the diameter of the fully expanded nucleoid released from the lysed cell).
At the beginning of the compression process, the two chains will repel each other — because the large blobs forming the chains repel one another — while maintaining their linearly ordered conformation inside the pore. However, this segregated state cannot last indefinitely. Once the blobs become substantially smaller than the width of the cylinder, the chain gains more conformational freedom by giving up its linear organization and taking on a random conformation. In other words, two transitions occur simultaneously as compression continues: from segregation to mixing, and from linear organizations to random conformations.
What will happen if we change the shape of the confinement, keeping its volume constant? (Because there is a constant chain concentration, owing to the constant volume, there will be no change in confinement free energy and therefore no change in the number and size of blobs.) A general rule is: the longer the box, the better the segregation and the stronger the linear ordering of the confined chains (see Supplementary information S4 (box); see below for segregation in a round cell).
We can translate the above qualitative descriptions into a rigorous phase diagram that can tell us the ‘segregatability’ of two confined chains in a three-dimensional box of a given size and shape. In other words, it tells us how well two chains will segregate in a particular environment owing to entropic forces (FIG. 1b). Briefly, regime I in the phase diagram describes polymers in dilute solution without any confinement, such as an isolated, free-floating DNA in dilute solution. Regime II describes a long cylindrical confinement in which the confined linearly elongated chains are well separated from one another. Regimes III, IV and V describe a closed box in which polymers are under moderate to strong confinement of varying geometries, from rod shapes to flat spheres via round shapes that are relevant to most bacteria. Regime VI describes a slab-like geometry. See Supplementary information S4 (box) for the full quantitative descriptions.
Application to Escherichia coli chromosomes and plasmids
To determine where E. coli fits on this phase diagram, we need a coarse-grained, physical model of a bacterial chromosome (summarized in FIG. 2a), which is a string of structural units (blobs) that are closely packed in the nucleoid volume. Three parameters characterize our physical model of the E. coli nucleoid: the size of the structural unit (also called the correlation length; ξ); the Flory radius of gyration (RF) of the isolated nucleoid (namely, the diameter of the fully expanded nucleoid released from the lysed cell); and the length and width (L and D) of the nucleoid inside the cell. The most important parameter is ξ, which can be measured as described in Supplementary information S3 (box).
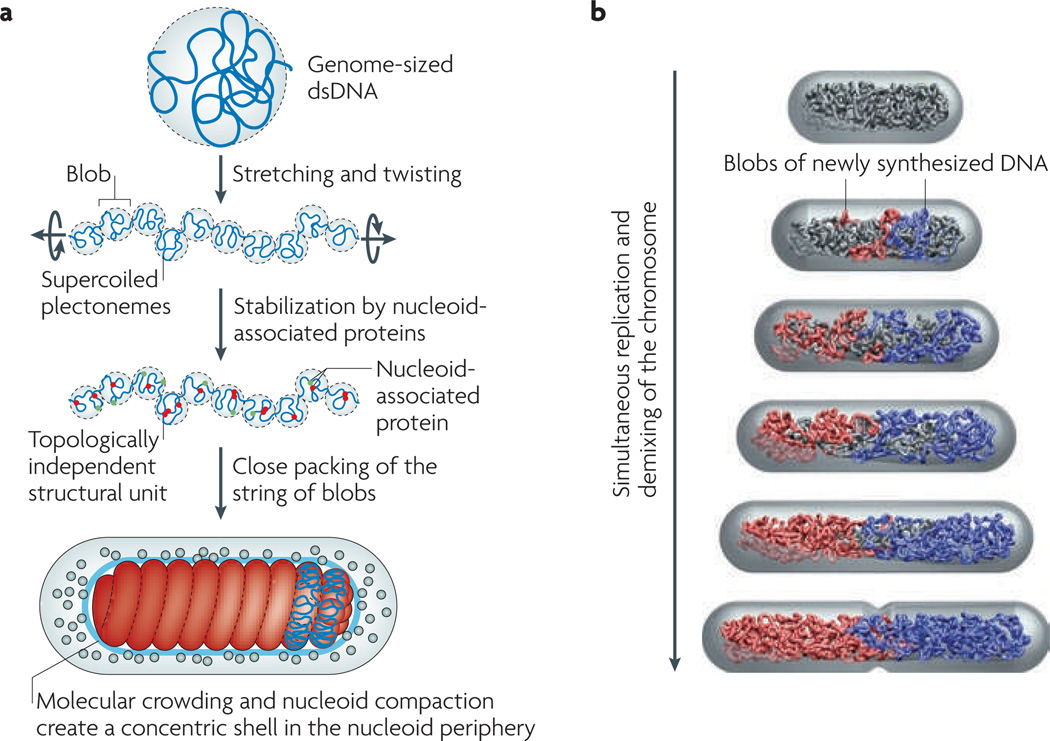
Figure 2. Physical model of a bacterial chromosome and its segregation.
a | A reductionist model of the Escherichia coli chromosome. First, we stretch a bacterial-genome-sized naked double-stranded DNA (dsDNA). This breaks the DNA into a series of blobs, the total volume of which gradually decreases as pulling continues. In parallel, we also twist the DNA to match the supercoil density of a bacterial chromosome. As a result, the DNA blobs will consist of supercoiled plectonemes (a shape of the DNA in which the two strands are intertwined). We stop the simultaneous pulling and twisting processes when the total volume of the blobs equals the target volume of the nucleoid inside the cell. Next, we ‘sprinkle’ the chromosome with nucleoid-associated proteins. These stabilize the supercoiled DNA blobs as topologically independent structural units of the chromosome. Finally, we connect the two ends of the chromosome to make it circular, and then pack it tightly in the cell. For the simpler case of chains without supercoiling, the phase diagram in FIG. 1 provides a model for the close-packed organization and segregatability of the chains inside the cell. In general, supercoiling will only increase the tendency for chromosome demixing because of the branched structure that it induces. b | The concentric shell model predicts extrusion of the newly synthesized DNA (blue and red) to the periphery of the nucleoid15. The newly replicated DNA is extruded to the periphery of the unreplicated nucleoid (grey) and forms a string of DNA blobs in the order of replication, promoted by SMC (structural maintenance of chromosomes) proteins and other nucleoid-associated proteins. In our model, the two strings of blobs repel each other and drift apart owing to the excluded-volume interaction and conformational entropy.
Experimentally, E. coli str. B/r H266 is the only organism for which these parameters have been measured10,11 (see BOX 2 and Supplementary information S3 (box)). They are: D = 0.24 µm, L = 1.39 µm, RF = 3.3 µm; and ξ = 87 nm. Thus, E. coli str. B/r H266 belongs to regime III, in which the strongly compressed individual chromosomes gain maximum conformational entropy when they remain segregated and linearly ordered (FIG. 1c; see Supplementary information S4 (box)). Similarly, our phase diagram can be used to make a prediction for other bacteria such as Vibrio cholerae (which has two chromosomes), Bacillus subtilis and Caulobacter crescentus. At present, neither the size of the isolated nucleoid nor the size of the structural unit have been measured for these organisms, although we expect B. subtilis and C. crescentus to belong to regime III, considering that both organisms have similar cell dimensions and genome sizes to E. coli. Future studies are needed to measure ξ for the nucleoids in these organisms, perhaps using fluorescence correlation spectroscopy techniques similar to those used in REF. 11(explained in Supplementary information S3 (box)) in order to fully test our predictions.
The next question concerns plasmid segregation in bacteria. Note that plasmids make little contribution to the amount of DNA in the cell because of their small sizes. Indeed, RF for plasmids is typically one-tenth of the RF value for the chromosome. This separates the plasmids from the chromosomes by one order of magnitude along the diagonal line between the two axes in the phase diagram (FIG. 1c; see Supplementary information S4 (box)). As a result, plasmids belong to the lower part of regime V in the phase diagram, in which polymers are not confined and the entropic repulsion between them is weak. In the absence of any specific interactions with the chromosome (such as ‘hitchhiking’), or without dedicated segregation machinery, plasmids will distribute randomly inside the cell because of their small sizes. Indeed, recent experimental results from studies of the high mobility and random distribution of the RK2 plasmid, which lacks a partitioning system, are fully consistent with our prediction12.
Segregation in round cells
Perfect symmetry of cell shape means that the confined chains do not have any preferred conformations between mixing and segregation, although their global reorganization is readily achieved13. Few data are available about chromosome organization in spherical bacteria, so we can only speculate about possible contributing factors to segregation. We think that supercoiling is the most important factor, as it gives rise to the branched structure of the bacterial chromosome14. We did not take into account the topological complexity of the polymer in our phase diagram, as it would add a third dimension to the diagram for which quantitative results are currently unknown. Nevertheless, polymer physics makes the general prediction that an increased topological complexity of polymers (and membranes), such as branching, will only increase their tendency for segregation15,16, and this will be especially strong in a confined space17. To see this intuitively, imagine two crumpled pieces of paper or nets with fine meshwork inside the cylinder in FIG. 1a. Unlike linear chains, they will never mix17. Another important factor at the cellular level is the symmetry break and invagination of the cell shape during division, which could help to resolve partially intermingled chromosomes. Indeed, real cells are never perfectly spherical. It has been shown numerically that pole-to-pole Min protein oscillations could be achieved along the long axis of a nearly round cell, in which the equatorial radii differ by as little as 5%18. This might explain the observed division of round cells at alternating perpendicular planes19.
Relationship with protein-based models
The phase diagram provides a quantitative framework for understanding the relationship between the physical state of the chromosome and its confinement and segregation, but under our reductionist approach lie biological mechanisms orchestrated by proteins. Our hypothesis is that the role of these proteins is to change the physical state of the chromosome and, thus, create the right conditions for entropic segregation (TABLE 1). We describe below how our physical model is connected to other protein-based segregation models, making experimentally testable predictions where possible.
Table 1.
Comparisons of the proposed segregation factors
| Segregation factors | Remarks | Our interpretation |
|---|---|---|
| Chromosome tethering to the membrane and growth55 |
|
|
| MreB56 (an actin homologue) |
|
|
| ParM6 (an actin homologue) |
|
|
| ParA27 |
|
|
|
migS and the bacterial ‘centromere’ (REF. 59) |
|
|
| MukB60 and SMC proteins |
|
|
| Extrusion–capture30 |
|
|
| RNA polymerase34 |
|
|
| Coupling of transcription and translation, and membrane transertion35 |
|
|
| Mechanical pushing (can be induced by cohesion)47,48 |
|
|
| Excluded-volume interactions, chain-connectivity and conformational entropy15 |
|
|
NA, not applicable; ori, origin; Par, plasmid partition protein; SMC, structural maintenance of chromosomes.
To this end, we first recapitulate the concentric-shell model for a replicating nucleoid15. This model was inspired by the observation that the fluorescently labelled E. coli nucleoid occupies a much smaller volume than the cell (BOX 1). In other words, the density of the chromosomal DNA should be much lower in the periphery of the E. coli nucleoid than at its core. The main prediction of the concentric-shell model is that newly synthesized DNA will be extruded to and will move in the periphery (the ‘outer shell’) of the nucleoid, in particular during the early stages of DNA replication (FIG. 2b). There are several advantages of this model. First, polymer repulsion is much stronger in the thin slab of the outer shell than in a space without confinement (see Supplementary information S4 (box)), so the replicating chromosome arms tend not to mix in the first place. Second, the replicating DNA can move much faster in the outer shell than inside the nucleoid bulk (BOX 2). Third, moving in the outer shell allows the principal organization of the unreplicated nucleoid to be preserved and also facilitates the sequential deposition of newly synthesized DNA in the order of replication.
Our concentric-shell model can be tested experimentally. Berlatzsky et al.20 have developed a method for labelling replicating chromosomes in slow-growing B. subtilis cells using fluorescent nucleotide derivatives. Although their results are only qualitative, the technique shows great potential for tracking DNA duplicated before and after a chosen time point using two colours. Future experiments using this method for the careful quantitative analysis of cells in balanced growth may allow newly replicated DNA strands to be tracked throughout the cell cycle.
Active transport of plasmids and ori sites in some organisms
Low-copy-number plasmids segregate21 and position themselves inside the cell (for example, plasmid R1 at the cell poles, and plasmids P1 and F at the quarter positions)22 through dedicated mechanisms. There is also increasing experimental evidence that chromosomal origin (ori) loci are localized at specific intracellular positions in E. coli (at mid-cell)23, C. crescentus (at old cell poles)24 and V. cholerae25,26. Consistent with these findings, it has been suggested that bacterial cytoskeletal proteins such as plasmid partition protein M (ParM) and ParA are the machinery for active transport of low-copy-number plasmids and ori loci, respectively. The system consisting of ParA, ParB and parS (the binding site for ParB) is widely conserved in many bacteria and contributes to ori segregation in C. crescentus and oriII segregation in V. cholerae.
However, although ParA plays an important part in chromosome segregation, its role is likely to be limited, for the following reasons. In C. crescentus, ParA is not required once ori loci are separated at the beginning of the cell cycle27. The absence of ParA alone has little effect on chromosome segregation in B. subtilis4, and there is no ParA homologue in E. coli. Also, as we discuss below, eukaryotic sister chromatids demix and move ∼0.5 µm apart before their separation by spindles, without any active pushing or pulling of the replicated DNA28. A potential problem with active transport of chromosomal ori loci for organisms that undergo multifork replication is that simple transport of multiple copies of ori may be harmful to the cell unless it also solves the ‘hierarchy dilemma’ of positioning an ori depending on its identity. An obvious experimental test of this idea would be to insert an active plasmid segregation system, such as ParM or the ParAB–parS system, into the E. coli chromosome. We predict that chromosome segregation during multifork replication in such an organism will be defective. We propose that organisms encoding ParA have adopted this protein from plasmids for a more efficient segregation of the ori domain, which is comparable in size to plasmids, rather than for segregation of the bulk chromosomes.
C. crescentus is smaller than E. coli and has a smaller cytoplasmic space surrounding its nucleoid, so the outer concentric shell of this species may not be large enough for fast diffusion of newly replicated DNA. In this case, ParA might be needed to ensure rapid segregation of the duplicated ori loci in the early stage of the replication cycle, when newly synthesized DNA would otherwise be kinetically trapped in the cell (see Supplementary information S4 (box)). The advantage of having an outer shell leads us to predict that, even in C. crescentus, duplicated ori loci will move in the periphery of the nucleoid. Finally, as the cell grows and replication continues, reducing the volume of the dense, unreplicated-DNA core, an outer shell is not needed for entropy-driven segregation15. The C. crescentus cell cycle is longer than those of E. coli and B. subtilis, which will further help segregation by entropy.
Several proteins are involved in active transport of DNA inside a bacterium. Examples include SpoIIIE, which moves DNA into the forespore during sporulation of B. subtilis, and FtsK, which resolves dimers and carries out other ‘rescue’ tasks in E. coli29. However, it is important to realize that these proteins translocate DNA at the last step of segregation by taking advantage of the directionality that is provided by the septum, which is an entirely different process from segregation of replicating chromosomes.
Segregation models based on replication, transcription, translation and tethering
In addition to the active DNA transport models, both the force of DNA ejection by DNA polymerase and the tethering of DNA polymerase have been proposed to move DNA in the cell. The finding of a fixed replication factory in B. subtilis led to the proposal of an ‘extrusion–capture’ model30, which assumed that the energy released during replication could contribute to chromosome partitioning. Recent work, however, has shown that replication forks and their associated replisomes are both independent and highly dynamic in the cell, making their role in segregation unlikely31–33. It was suggested that the negative effects of streptolydigin on chromosome segregation implicate RNA polymerases in chromosome segregation34. In addition, it has been proposed that tran-sertion (the insertion of polypeptides into membranes during translation), instead of polymerases themselves, could segregate the replicating chromosomes35. Definitive experimental support for these proposals is lacking and, in fact, the transertion model is inconsistent with the speed of separation of chromosomal loci, especially those near the ori. But even if these factors do play a part in segregation, they would not be mutually exclusive with the entropy model or with other mechanical models. For example, they could support the concentric-shell model, as large macromolecular complexes such as replisomes would be extruded to the outer shell of the nucleoid. The outer shell is also a site of active transcription and is juxtaposed to the cell membrane, facilitating the transertion process. Transertion might effectively pull the newly synthesized DNA into the outer shell of the nucleoid and then anchor it there. From our view, a more important consequence of transertion might be that completely duplicated nucleoids would be physically separated as a result of nucleoid association with the slowly growing cytoplasmic membrane.
Physical state of the chromosome
Perhaps the most important feature of our physical model of chromosome segregation is its robustness. In other words, the model does not depend on the microscopic organization of the chromosome in the structural unit (the blob) or on details of how individual proteins interact with the DNA. What is important is the size of the structural unit — that is, the effective correlation length (ξ). As discussed above (and illustrated in FIG. 1a,b), a larger blob size always results in better segregation and more ordered chain conformations. (see also REF. 36 for computer simulation results; the authors observed segregation failure only when the structural units are small). In view of this, the most obvious candidates for increasing the size of the structural unit are the chromosome condensation proteins (the SMC proteins4,5 and MukB23) and the small DNA-binding proteins (such as HU, H-NS and integration host factor (IHF))9. DNA gyrases are also important because they cause branched supercoils, promoting repulsive interactions between the chromosomes. In this case, the torsional energy generated by DNA gyrase activity is used for topological repulsion between the supercoiled chromosomes.
Our theoretical argument is supported by studies showing that the disruption of chromosome segregation caused by a deficit in MukB is substantially (but not completely) alleviated by alterations in DNA gyrase activity, leading to increased levels of negative supercoiling14,36–38. Although these data might suggest that redundant mechanisms help to drive chromosome segregation, we propose that neither class of protein forms the dedicated segregation machinery. Instead, we posit that their role is to maintain the chromosome in a specific physical condition that allows for entropy-driven segregation.
Cell growth, shape and division
As we illustrate in FIG. 1, a larger cell size means a lower concentration of chromosomal DNA (assuming that the amount of DNA remains constant), and this results in a larger ξ and better physical conditions for segregation. Similarly, a longer cell means a stronger tendency for segregation. Consequently, proteins that regulate cell growth and division are also important for ensuring that the cell reaches the appropriate size for proper segregation. Recent studies showed that B. subtilis possesses a metabolic sensor that couples nutrition availability to division, ensuring that cells reach the appropriate mass and complete chromosome segregation before cytokinesis39. Similarly, other proteins can help segregation indirectly through secondary effects. The cytoskeletal protein MreB, for example, helps the cell maintain a higher aspect ratio and possibly also promotes chromosome segregation in the final stages by regulating the activity of topoisomerase IV at mid-cell40.
Symmetry of the chromosome arms
Recently, an interesting observation on the distribution of chromosome arms (replichores) in E. coli was reported41,42. Under conditions in which cell cycles do not overlap, the E. coli nucleoid is linearly ordered, each replichore occupies one half of the cell volume and the chromosome arms segregate progressively in the order of replication43. Measuring the cell-to-cell positional variations of chromosomal markers allowed the effective spring constant of the in vivo nucleoid to be measured44. A more recent striking observation comes from an elegant study showing that leading and lagging strands go to different cellular destinations45. These findings are consistent with our model, in principle. For example, we think that the physical constraints exerted by the core of the nucleoid itself and the required movement in the outer concentric shell, together with demixing and the physical difference between leading and lagging strands, may result in the symmetry seen. Indeed, data show that the organization of the E. coli chromosome throughout the cell cycle is determined by its evolving intrinsic mechanical and physical properties, which alter under the combined effects of repulsion and radial confinement (A. Bourniquel, M. Prentiss and N. Kleckner, personal communication). However, it will be necessary to add organism-specific instructions to our general physical model to explain the detailed differences between organisms such as C. crescentus24,27 (in which the ori is at the pole and is possibly tethered) and E. coli (in which leading and lagging strands display differential motions in the outer shell)45,46. Future studies need to address the biological importance of these findings as well as their applicability to fast-growing cells and round cells.
Concluding remarks
One of the most striking events during the eukaryotic cell cycle is the separation of compact sister chromatids, aligned across the central plane of the cell, by the mitotic spindles. This hallmark of mitosis has inspired numerous proposals aimed at connecting bacterial chromosome segregation with the mitotic process. However, analogies between the two domains of life, such as the presence of a bacterial ‘centromere’ or ‘mitotic machinery’, have contributed little to our understanding of bulk chromosome segregation in bacteria. In this Opinion article, we argue that the active segregation mechanisms are present for the segregation of low-copy-number plasmids and can also be used for the positioning of ori sites in some bacterial organisms, perhaps as a product of evolution, but are not used for the chromosome bulk.
As previously noted by several research-ers4,28,47–49, it is important to realize that eukaryotic chromosomes also go through a period of intermingling during replication and that they demix without the help of mitotic spindles or any of the analogous protein-based mechanisms that have been proposed for bacterial chromosome segregation. It is known that topoisomerase II, condensins and histones are needed to build mitotic chromosomes and segregate sister DNA strands in eukaryotes, but the driving force behind the formation of sister chromatids with largely separated DNA has been unclear. The key question here is: what guiding force causes topoisomerase to remove catenations rather than add them? We propose that entropic demixing provides the guiding force and directionality for sister strand separation during mitotic chromosome assembly, just as it does for bacterial chromosome segregation (BOX 2).
Further, the presence of multiple chromosomes poses an additional challenge for chromosome segregation in eukaryotic cells, because each daughter cell must receive not only the correct number but also the correct set of chromosomes. From our point of view, this strongly supports the argument that mitosis requires a more sophisticated, active segregation machinery and checkpoint, whereas entropy alone might be sufficient for chromosome segregation in most bacteria.
Finally, we would like to remind the reader that segregation by entropic forces, whether it is for bacterial chromosomes or eukaryotic sister chromatids, represents a robust mechanism. Although the diversity of life is the consequence of evolution, the robust nature of the physical principles in general, such as the example in chromosome segregation described here, may provide deeper insight into the basic processes shared by all life forms.
Supplementary Material
Acknowledgements
We are deeply grateful to T. Mitchison for his penetrating insight and numerous invaluable suggestions. We thank N. Kleckner and C. Woldringh for critical reading of the manuscript. We also thank J.-Y. Bouet, A. Brouniquel, A. Danchin, E. Garner, B.-Y. Ha, R. Losick, K. Maeshima, B. Mulder, A. Murray, P. Wiggins and many other colleagues for helpful discussions over the years. This work was supported by Harvard University, USA, and the US National Institutes of Health (grant P50 GM068763 to S.J.).
Glossary
- Contour length
The length of the polymer at maximum extension
- Ideal gas
A theoretical gas consisting of randomly-moving, non-interacting point particles.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
Entrez Genome Project: http://www.ncbi.nlm.nih.gov/genomeprj
Bacillus subtilis | Caulobacter crescentus | Escherichiacoli | Vibrio cholerae
UniProtKB: http://www.uniprot.org
FURTHER INFORMATION
Suckjoon Jun’s homepage: http://www.sysbio.harvard.edu/csb/jun/
Andrew Wright’s homepage: http://sackler.tufts.edu/Academics/Degree-Programs/PhD-Programs/Faculty-Research-Pages/Andrew-Wright.aspx
SUPPLEMENTARY INFORMATION
See online article: S1 (movie) | S2 (movie) | S3 (box) | S4 (box)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Suckjoon Jun, Email: sjun@cgr.harvard.edu, FAS Center for Systems Biology, Harvard University, Cambridge, Massachusetts 02138, USA.
Andrew Wright, Email: andrew.wright@tufts.edu, Department of Molecular Biology and Microbiology, Tufts University School of Medicine, 136 Harrison Avenue, Boston, Massachusetts 02111, USA.
References
- 1.Kornberg A, Baker TA. DNA Replication. 2nd edn. New York: Freeman & Company; 1992. [Google Scholar]
- 2.Murray A, Hunt T, editors. The Cell Cycle: an Introduction. New York: Oxford Univ. Press; 1993. [Google Scholar]
- 3.Wang JC. DNA topoisomerases. Annu. Rev. Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan NL, Marquis KA, Rudner DZ. Recruitment of SMC to the origin by ParB-parS organizes the origin and promotes efficient chromosome segregation. Cell. 2009;137:697–707. doi: 10.1016/j.cell.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruber S, Errington J. Recruitment of condensin to replication origin regions by ParB/SpoOJ promotes chromosome segregation in B. subtilis. Cell. 2009;137:685–696. doi: 10.1016/j.cell.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Salje J, Zuber B, Löwe J. Electron cryomicroscopy of E. coli reveals ParM filament bundles involved in plasmid segregation. Science. 2009;323:509–512. doi: 10.1126/science.1164346. [DOI] [PubMed] [Google Scholar]
- 7.deGennes P-G. Scaling Concepts in Polymer Physics. Ithaca, New York: Cornell Univ. Press; 1979. [Google Scholar]
- 8.Bloom K, Joglekar A. Towards building a chromosome segregation machine. Nature. 2010;463:446–456. doi: 10.1038/nature08912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stavans J, Oppenheim A. DNA-protein interactions and bacterial chromosome architecture. Phys. Biol. 2006;3:R1–R10. doi: 10.1088/1478-3975/3/4/R01. [DOI] [PubMed] [Google Scholar]
- 10.Woldringh CL, Odijk Tin. In: Organization of the Prokaryotic Genome. Charlebois RL, editor. Washington DC: American Society for Microbiology; 1999. pp. 171–187. [Google Scholar]
- 11.Romantsov T, Fishov I, Krichevsky O. Internal structure and dynamics of isolated Escherichia coli nucleoids assessed by fluorescence correlation spectroscopy. Biophys. J. 2007;92:2875–2884. doi: 10.1529/biophysj.106.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derman AI, Lim-Fong G, Pogliano J. Intracellular mobility of plasmid DNA is limited by the ParA family of partitioning systems. Mol. Microbiol. 2008;67:935–946. doi: 10.1111/j.1365-2958.2007.06066.x. [DOI] [PubMed] [Google Scholar]
- 13.Jun S, Arnold A, Ha B-Y. Confined space and effective interactions of multiple self-avoiding chains. Phys. Rev. Lett. 2007;98:128303. doi: 10.1103/PhysRevLett.98.128303. [DOI] [PubMed] [Google Scholar]
- 14.Sawitzke JA, Austin S. Suppression of chromosome segregation defects of Escherichia coli muk mutants by mutations in topoisomerase I. Proc. Natl Acad. Sci. USA. 2000;97:1671–1676. doi: 10.1073/pnas.030528397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun S, Mulder B. Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc. Natl Acad. Sci. USA. 2006;103:12388–12393. doi: 10.1073/pnas.0605305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trun NJ, Marko JF. Architecture of a bacterial chromosome. ASM News. 1998;64:276–283. [Google Scholar]
- 17.Vilgis TA. Polymer theory: path integrals and scaling. Phys. Rep. 2000;336:167–254. [Google Scholar]
- 18.Huang JC, Wingreen N. Min-protein oscillations in round bacteria. Phys. Biol. 2004;1:229–235. doi: 10.1088/1478-3967/1/4/005. [DOI] [PubMed] [Google Scholar]
- 19.Corbin BD, Yu X-C, Margolin W. Exploring intracellular space: function of the Min system in round-shaped Escherichia coli. EMBO J. 2002;21:1998–2008. doi: 10.1093/emboj/21.8.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berlatzky IA, Rouvinski A, Ben-Yehuda S. Spatial organization of a replicating bacterial chromosome. Proc. Natl Acad. Sci. USA. 2008;105:14136–14140. doi: 10.1073/pnas.0804982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordstrom K, Austin SJ. Mechanisms that contribute to the stable segregation of plasmids. Annu. Rev. Genet. 1989;23:37–69. doi: 10.1146/annurev.ge.23.120189.000345. [DOI] [PubMed] [Google Scholar]
- 22.Gordon GS, et al. Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell. 1997;90:1113–1121. doi: 10.1016/s0092-8674(00)80377-3. [DOI] [PubMed] [Google Scholar]
- 23.Danilova O, Reyes-Lamothe R, Pinskaya M, Sherratt D, Possoz C. MukB colocalizes with the oriC region and is required for organization of the two Escherichia coli chromosome arms into separate cell halves. Mol. Microbiol. 2007;65:1485–1492. doi: 10.1111/j.1365-2958.2007.05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viollier PH, Thanbichle M, McGrath PT, West L, Meewan M. Rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc. Natl Acad. Sci. USA. 2004;101:9257–9262. doi: 10.1073/pnas.0402606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fogel MA, Waldor MK. A dynamic, mitotic-like mechanism for bacterial chromosome segregation. Genes Dev. 2006;20:3269–3282. doi: 10.1101/gad.1496506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiebig A, Keren K, Theriot JA. Fine-scale time-lapse analysis of the biphasic, dynamic behaviour of the two Vibrio cholerae chromosomes. Mol. Microbiol. 2006;60:1164–1178. doi: 10.1111/j.1365-2958.2006.05175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toro E, Hong S-H, McAdams HH, Shapiro L. Caulobacter requires a dedicated mechanism to initiate chromosome segregation. Proc. Natl Acad. Sci. USA. 2008;105:15435–15440. doi: 10.1073/pnas.0807448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297:559–565. doi: 10.1126/science.1074757. [DOI] [PubMed] [Google Scholar]
- 29.Barre F-X. FtsK and SpoIIIE: the tale of the conserved tails. Mol. Microbiol. 2007;66:1051–1055. doi: 10.1111/j.1365-2958.2007.05981.x. [DOI] [PubMed] [Google Scholar]
- 30.Lemon KP, Grossman AD. The extrusion capture model for chromosome partitioning in bacteria. Genes Dev. 2001;15:2031–2041. doi: 10.1101/gad.913301. [DOI] [PubMed] [Google Scholar]
- 31.Migocki MD, Lewis PJ, Wake RG, Harry EJ. The midcell replication factory in Bacillus subtilis is highly mobile: implications for coordinating chromosome replication with other cell cycle events. Mol. Microbiol. 2004;54:452–463. doi: 10.1111/j.1365-2958.2004.04267.x. [DOI] [PubMed] [Google Scholar]
- 32.Bates D. The bacterial replisome: back on track? Mol. Microbiol. 2008;69:1341–1348. doi: 10.1111/j.1365-2958.2008.06378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes-Lamothe R, Possoz C, Danilova O, Sherratt DJ. Independent positioning and action of Escherichia coli replisomes in live cells. Cell. 2008;133:90–102. doi: 10.1016/j.cell.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dworkin J, Losick R. Does RNA polymerase help drive chromosome segregation in bacteria? Proc. Natl Acad. Sci. USA. 2002;99:14089–14094. doi: 10.1073/pnas.182539899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woldringh CL. The role of co-transcriptional translation and protein translocation (transertion) in bacterial chromosome segregation. Mol. Microbiol. 2002;45:17–29. doi: 10.1046/j.1365-2958.2002.02993.x. [DOI] [PubMed] [Google Scholar]
- 36.Fan J, Tuncay K, Ortoleva PJ. Chromosome segregation in Escherichia coli division: a free energy-driven string model. Comput. Biol. Chem. 2007;31:257–264. doi: 10.1016/j.compbiolchem.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Holmes VF, Cozzarelli NR. Closing the ring: links between SMC proteins and chromosome partitioning, condensation, and supercoiling. Proc. Natl Acad. Sci. USA. 2000;97:1322–1324. doi: 10.1073/pnas.040576797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dasgupta S, Maisnier-Patin S, Nordstrom K. New genes with old modus operandi: the connection between supercoiling and partitioning of DNA in Escherichia coli. EMBO Rep. 2000;1:323–327. doi: 10.1093/embo-reports/kvd077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weart RB, et al. A metabolic sensor governing cell size in bacteria. Cell. 2007;130:335–347. doi: 10.1016/j.cell.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madabhushi R, Marians KJ. Active homolog MreB affects chromosome segregation by regulating topoisomerase IV in Escherichia coli. Mol. Cell. 2009;33:171–180. doi: 10.1016/j.molcel.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Liu X, Possoz C, Sherratt DJ. The two Escherichia coli chromosome arms locate to separate cell halves. Genes Dev. 2006;20:1727–1731. doi: 10.1101/gad.388406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen HJ, Ottensen JR, Youngren B, Austin SJ, Hansen FG. The Escherichia coli chromosome is organized with the left and right chromosome arms in separate cell halves. Mol. Microbiol. 2006;62:331–338. doi: 10.1111/j.1365-2958.2006.05346.x. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen HJ, Li Y, Youngren B, Hansen FG, Austin SJ. Progressive segregation of the Escherichia coli chromosome. Mol. Microbiol. 2006;61:383–393. doi: 10.1111/j.1365-2958.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 44.Wiggins PA, Cheveralls K, Martin JS, Lintner R, Kondev J. Strong intranucleoid interactions organize the Escherichia coli chromosome into a nucleoid filament. Proc. Natl Acad. Sci. USA. 2010;107:4991–4995. doi: 10.1073/pnas.0912062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White MA, Eykelenboom JK, Lopez-Vernaza MA, Wilson E, Leach DRF. Non-random segregation of sister chromosomes in Escherichia coli. Nature. 2008;455:1248–1250. doi: 10.1038/nature07282. [DOI] [PubMed] [Google Scholar]
- 46.Reyes-Lamothe R, Wang X, Sherratt D. Escherichia coli and its chromosome. Trends Microbiol. 2008;16:238–245. doi: 10.1016/j.tim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Kleckner N, et al. A mechanical basis for chromosome function. Proc. Natl Acad. Sci. USA. 2004;101:12592–12597. doi: 10.1073/pnas.0402724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bates D, Kleckner N. Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell. 2005;121:899–911. doi: 10.1016/j.cell.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woldringh CL, van Driel R. In: Organization of the Prokaryotic Genome. Charlebois RL, editor. Washington DC: American Society for Microbiology; 1999. pp. 77–90. Ch. 5. [Google Scholar]
- 50.Schrödinger E. What is life? Cambridge, UK: Cambridge Univ. Press; 1944. [Google Scholar]
- 51.Flory P. Principles of Polymer Chemistry. Ithaca, New York: Cornell Univ. Press; 1953. [Google Scholar]
- 52.Grosberg AY, Khalatur PG, Khokhloo AR. Polymeric coils with excluded volume in dilute solution: the invalidity of the model of impenetrable spheres and the influence of excluded volume on the rates of diffusion-controlled intermacromolecular reactions. Makromol. Chem. Rapid Commun. 1982;3:709–713. [Google Scholar]
- 53.Pincus P. Excluded volume effects and stretched polymer chains. Macromolecules. 1976;9:386–388. [Google Scholar]
- 54.Liu Z, Zechiedrich EL, Chan HS. Inferring global topology from local juxtaposition geometry: interlinking polymer rings and ramifications for topoisomerase action. Biophys. J. 2006;90:2344–2355. doi: 10.1529/biophysj.105.076778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacob F, Brenner S, Cuzin F. On the regulation of DNA replication in bacteria. Cold Spring Harb. Symp. Quant. Biol. 1963;28:329–348. [Google Scholar]
- 56.Kruse T, Gerdes K. Bacterial DNA segregationby the actin-like MreB protein. Trends. Cell Biol. 2005;15:343–345. doi: 10.1016/j.tcb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Hu B, Yang G, Zhao W, Zhang Y, Zhao J. MreB is important for cell shape but not for chromosome segregation of the filamentous cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 2007;63:1640–1652. doi: 10.1111/j.1365-2958.2007.05618.x. [DOI] [PubMed] [Google Scholar]
- 58.Karczmarek A, et al. DNA and origin region segregation are not affected by the transition from rod to sphere after inhibition of Escherichia coli MreB by A22. Mol. Microbiol. 2007;65:51–63. doi: 10.1111/j.1365-2958.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- 59.Yamaichi Y, Niki H. migS, a cis-acting site that affects bipolar positioning of oriC on the Escherichia coli chromosome. EMBO J. 2004;23:221–233. doi: 10.1038/sj.emboj.7600028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niki H, Jaffe A, Imamura R, Ogura T, Hiraga S. The new gene mukB codes for a 177 kD protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 1991;10:183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.