The results of the present study suggest that ASA score ≤ 2 and use of rifampin-combination therapy are two independent factors associated with favorable outcome of patients treated for total hip or knee prosthetic infections due to S. aureus.
Abstract
Background. Variables associated with the outcome of patients treated for prosthetic joint infections (PJIs) due to Staphylococcus aureus are not well known.
Methods. The medical records of patients treated surgically for total hip or knee prosthesis infection due to S. aureus were reviewed. Remission was defined by the absence of local or systemic signs of implant-related infection assessed during the most recent contact with the patient.
Results. After a mean posttreatment follow-up period of 43.6 ± 32.1 months, 77 (78.6%) of 98 patients were in remission. Retention of the infected implants was not associated with a worse outcome than was their removal. Methicillin-resistant S. aureus (MRSA)–related PJIs were not associated with worse outcome, compared with methicillin-susceptible S. aureus (MSSA)–related PJIs. Pathogens identified during revision for failure exhibited no acquired resistance to antibiotics used as definitive therapy, in particular rifampin. In univariate analysis, parameters that differed between patients whose treatment did or did not fail were: American Society of Anesthesiologists (ASA) score, prescription of adequate empirical postsurgical antibiotic therapy, and use of rifampin combination therapy upon discharge from hospital. In multivariate analysis, ASA score ≤2 (odds ratio [OR], 6.87 [95% confidence interval {CI}, 1.45–32.45]; P = .04) and rifampin-fluoroquinolone combination therapy (OR, 0.40 [95% CI, 0.17–0.97]; P = .01) were 2 independent variables associated with remission.
Conclusions. The results of the present study suggest that the ASA score significantly affects the outcome of patients treated for total hip and knee prosthetic infections due to MSSA or MRSA and that rifampin combination therapy is associated with a better outcome for these patients when compared with other antibiotic regimens.
Prosthetic joint infections (PJIs) represent a growing public health concern in developed countries as a result of the increasing number of operations for total joint arthroplasty and a risk of postoperative infection of 1%–2% [1, 2]. Such infections are associated with substantial morbidity, increased medical costs, and reduced quality of life [3, 4]. General principles of management of PJI include a multidisciplinary approach at centers with expertise in this field. Reliable microbiological diagnosis, along with surgical procedures ranging from prosthesis removal with or without reimplantation to debridement with implant retention, and prolonged appropriate antibiotic therapy, are key elements in the management of such infections. Factors influencing the outcomes of patients with PJI have been assessed in previous studies and include retention of infected material, duration of symptoms of infection, and bacterial resistance [5–8]. However, the role of these parameters differs from one study to another and is related to study design, patients included, type of surgery, posttreatment follow-up duration, the pathogens in question, and the definition of cure. Although most PJIs are caused by Staphylococcus aureus, only a few studies on small populations, focusing on treatment of early postoperative PJIs or combining coagulase-negative staphylococci (CNS) and S. aureus PJIs, have assessed variables associated with outcome [9–16]. The purpose of the present retrospective study was therefore to identify variables associated with outcome in a large series of patients treated for S. aureus PJIs, treated according to an algorithm derived from the experience reported by Zimmerli et al [1] and several other authors [17–23].
METHODS
Study Design
This was a retrospective study of an observational cohort of patients treated for a PJI due to S. aureus. We compared characteristics of patients according to outcome. Patients were observed from initial surgical procedure for infection to most recent contact.
Study Population
All patients treated surgically for PJI due to S. aureus (mono- or polymicrobial infection) at our institution, which is currently 1 of 8 French reference centers for osteoarticular infections, were identified by searching the database of the microbiology laboratory for the items “S. aureus” and “total hip or knee prosthesis.” The study was approved by the institutional review boards of both Dron and Roger Salengro hospitals.
Definitions
PJI due to S. aureus was defined as the isolation of ≥1 strain of S. aureus from a reliable sample taken from the prosthetic site. Histological examination was not used in the present study, because this technique is not performed routinely at our institution. The term “polymicrobial infection” was used when different bacterial species were simultaneously identified from samples. “Time to infection” was defined as time from implantation of the prosthesis to clinical onset of infection and was categorized into early (≤3 months after implantation), delayed (>3 to <24 months after implantation), or late (≥24 months after implantation) infection. Acute or chronic PJI was defined as time from initiation of symptoms of infection to diagnosis lasting for, respectively, <1 and >1 month. Empiric postoperative antibiotic therapy was defined as adequate if it contained ≥1 antibiotic agent active against the pathogen(s) identified in the intraoperative samples. Antibiotic treatment based on results of intraoperative sample culture was called “definitive antibiotic therapy.” Remission was defined by the absence of local or systemic signs of infection assessed during the most recent contact with the patient and absence of the need to reoperate or to administer antibiotic therapy directed to the initial infected site from the end of treatment to the most recent contact. Failure was defined as any other outcome, including death related to the PJI. We focused the paper on infection eradication recurrence rather than on functional assessment because of the different orthopedic situations seen in our population of patients for whom assessing the functionality may have introduced imprecision for distinguishing patients in remission from those whose treatment failed.
Medical and Surgical Therapy
Medical and surgical management of each patient followed an algorithm derived from the experience reported by Zimmerli et al and several other authors [17–23]. Debridement with retention was used for patients with early postoperative or acute hematogenous infection and no implant loosening, if the duration of clinical signs and symptoms was <4 weeks and if soft tissues surrounding the prosthetic site were in good condition. In the other cases, 1-stage exchange was performed in non-immunocompromised patients having reliable preoperative microbiological information and satisfactory soft tissue. Two-stage exchange was preferred for non-immunocompromised patients whose soft tissue was damaged or for whom reliable preoperative bacterial information was unavailable. Both arthroplastic resection and arthrodesis were performed in severely immunocompromised patients or in those in whom joint replacement would not have resulted in functional benefit. For 2-stage procedures (ie, 2-stage replacement and arthrodesis), reimplantation was performed after an antibiotic treatment duration of ≥12 weeks with or without an additional antibiotic-free period of 4 weeks and if the C-reactive protein value had normalized (<10 mg/L), except when chronic inflammatory disease interfered with C-reactive protein values. A spacer was used in patients treated with 2-stage replacement of the prosthesis, even in case of methicillin-resistant S. aureus (MRSA) infection, which was the only situation that differed from the recommendations of Zimmerli et al [1]. After reimplantation of a new prosthesis or arthrodesis, the duration of antibiotic therapy depended on results of intraoperative sample cultures (ie, 2 weeks in case of negative culture results if antibiotic therapy had been stopped ≥2 weeks prior to the intervention, and 6–12 weeks in case of positive culture results). Therapeutic strategy was decided for each patient at a multidisciplinary meeting of orthopedic surgeons, infectious disease consultants, microbiologists, and anesthesiologists. In each case, the patient was aware of the different therapeutic options and took part in the final decision. All surgical procedures were performed without antibiotic prophylaxis. A combination of antimicrobial agents administered intravenously was begun intraoperatively immediately after samples were taken. It consisted of a broad-spectrum β-lactam agent (eg, cefotaxime, aztreonam, or imipenem) and a second antimicrobial agent with activity against methicillin-resistant staphylococci (vancomycin, teicoplanin, or linezolid). This treatment was continued until microbiological results of the preoperative sample culture were available and was then modified on the basis of culture results. Antibiotics were selected on the basis of patient comorbidity and prescribed at doses adapted from those proposed by Zimmerli et al [1], except for rifampin, the daily dose of which was 20 mg/kg administered in divided doses given twice a day, without exceeding daily doses of 1800 mg. After discharge from hospital, the patient was followed up by both the referring surgeon and the infectious disease consultant 1 month after discharge and at the end of antibiotic treatment. The total duration of antimicrobial therapy was 3–6 months, as proposed by Zimmerli et al [1]. Patients were then followed up by their referring surgeon once a year for a minimum of 2 years. Missing data on patient outcome after the end of antibiotic treatment were obtained by telephone contact with the patient himself/herself or the general practitioner, or when applicable, by reviewing medical records in case of rehospitalization.
Statistical Analysis
The Pearson χ2 test was used to compare qualitative variables and a 2-sample t test to compare continuous variables. A P value of <.05 was considered to reveal a significant difference. Logistic regression was used to identify independent variables associated with failure. Variables with medical or biological meaning were retained for the multivariate analysis when their effect had a P value less than .25. We constructed a receiver operating curve (ROC) to assess the validity of the model. Statistical analysis was performed using STATA, version 7.0 (StataCorp).
RESULTS
Study Population
Ninety-eight patients with PJI (71 total hip and 27 total knee prosthesis) due to S. aureus observed at our institution during the period from 2000 through 2006 were included in the study.
The main characteristics of the study population are presented in Table 1. Radiographic abnormalities consistent with diagnosis of PJI were present in 54 patients (55.1%) at the time of the first revision, including 46 patients with implant loosening.
Table 1.
Baseline Characteristics of 98 Patients With Total Hip or Knee Prosthesis Infection Due to Staphylococcus aureus According to Outcome
| Characteristic | Remission (n = 77) | Failure (n = 21) | P |
| Age, mean years ± SD | 66.3 ± 14.7 | 70.0 ± 13.4 | .29 |
| Male sex | 32 (41.6) | 11 (52.4) | .38 |
| Body mass index, mean ± SD | 28.4 ± 5.7 | 28.3 ± 7.9 | .95 |
| Diabetes mellitus | 24 (31.2) | 8 (38.1) | .54 |
| Use of steroid therapy | 10 (12.9) | 1 (4.8) | .29 |
| Ongoing cancer treatment | 4 (5.2) | 1 (4.8) | .63 |
| Concomitant Staphylococcus aureus bacteremia | 15 (19.5) | 3 (14.3) | .82 |
| Acute infection (<4 weeks duration) | 14 (19.7) | 6 (28.5) | .36 |
| Time to infection | |||
| Median months ± SD | 74.1 ± 83.4 | 63.3 ± 91.5 | .20 |
| Early (≤3 months) | 26 (33.8) | 11 (52.4) | .12 |
| Delayed (>3–24 months) | 17 (22.1) | 4 (19.1) | .76 |
| Late (≥24 months) | 34 (44.1) | 6 (28.5) | .20 |
| No. of operations since implantation, mean ± SD | 1.10 ± 1.95 | 1.71 ± 2.88 | .94 |
| Fever (temperature, >38°C) at admission | 10 (12.9) | 3 (14.3) | .87 |
| Presence of sinus tract | 27 (35.1) | 11 (52.4) | .14 |
| White blood cell count, mean ×109 cells/L ± SD | 9276.7 ± 3647.7 | 8950.5 ± 3452.1 | .71 |
| CRP level, mean mg/L ± SD | |||
| At first presentation | 98.5 ± 87.3 | 81.0 ± 66.2 | .39 |
| Prior to reimplantation | 18.8 ± 5.9 | 24.1± 37.1 | .69 |
| ASA score >2a | 23 (29.9) | 13 (61.9) | .02 |
| Methicillin-susceptible S. aureus | 65 (84.4) | 16 (76.2) | .38 |
| Methicillin-resistant S. aureus | 12 (15.6) | 5 (23.8) | .38 |
| Polymicrobial infection | 18 (25.4) | 9 (42.8) | .08 |
| Coagulase-negative staphylococci | 9 (11.7) | 5 (23.8) | .16 |
| Other bacteriab | 9 (11.7) | 4 (19.1) | .42 |
NOTE. Data are no. (%) of patients unless otherwise indicated. ASA, American Society of Anesthesiologists; CRP, C-reactive protein; SD, standard deviation.
Chronic diseases of the heart (n = 2), liver (n = 17), or kidney (n = 4), and respiratory insufficiency (n = 6) mostly related to chronic obstructive pulmonary disease.
Streptococcus viridans (n = 5), Enterococcus faecalis (n = 2), Escherichia coli (n = 3), Pseudomonas aeruginosa (n = 2), Propionibacterium acnes (n = 1).
Microbiological Findings
Microbiological results are highlighted in Table 1. S. aureus was identified as the sole bacterium isolated from samples taken during the first revision in 71 cases (72.4%) and in association with other bacterial strains in 27 cases (27.6%), including CNS in 13 cases (13.2%) and gram-negative bacilli in the other 14 cases (14.3%). MRSA was identified in 17 cases (17.3%), and all these strains were susceptible to rifampin, linezolid, teicoplanin, and vancomycin. A mean of 6.6 ± 3.6 samples were taken intraoperatively, including 4.6 ± 3.6 showing positive culture results. Of the 21 patients whose treatment failed, bacteriological data were available for 18. Sixteen patients experienced relapsing infections, which were due to S. aureus in 11 cases (methicillin-susceptible S. aureus [MSSA] = 6, MRSA = 5) and CNS in the 5 other cases. The 2 other patients had a reinfection due to Staphylococcus epidermidis. All the strains identified in these 18 patients were sensitive initially and at recurrence to rifampin and to the other antibiotics prescribed to the patients.
Surgical and Medical Treatment
Surgical procedures and antibiotic regimens are presented in Tables 2 and 3. For 34 patients who underwent 2-stage surgical procedures (ie, 2-stage replacement and arthrodesis), a gentamicin-loaded cement spacer was implanted for a mean time between initial and second intervention of 116.6 ± 14.9 days (range, 26–395 days). Patients with MRSA-related infections were treated with removal of all the infected implants in the same proportion as were patients with MSSA infections (11 [64.7%] of 17 vs 46 [56.8%] of 81; P = .74). Empiric postoperative intravenous antibiotic therapy was administered for a mean duration of 7.20 ± 4.93 days and was concordant with our algorithm in 65 patients (66.3%). Antibiotic regimens for MRSA infections were a combination of rifampin plus pristinamycin (n = 5), levofloxacin (n = 3), fusidic acid (n = 1), and teicoplanin (n = 1), and monotherapy with clindamycin (n = 3) or linezolid (n = 4). Rifampin-fluoroquinolone combinations were not prescribed because of polymicrobial infection involving resistant bacteria in 27 cases and allergic reactions or intolerance in 32 cases. A total of 6 patients were given long-term suppressive antibiotic therapy for a mean duration of 661.2 ± 157.6 days. All these patients had been treated with the debride-retain strategy and were given oral doxycycline 200 mg in 1 daily dose.
Table 2.
Characteristics of Surgical Procedures and Antibiotic Therapy in 98 Patients With Total Hip or Knee Prosthesis Infection Due to Staphylococcus aureus According to Outcome
| Characteristic | Remission (n = 77) | Treatment failure (n = 21) | P |
| Delay from onset of infection to revision, mean days ± SD | 119.4 ± 238.2 | 79 ± 111.7 | .80 |
| Removal of all infected implants | 45 (58.4) | 12 (57.1) | .99 |
| Gentamicin-loaded cement spacera | 27 (35.1) | 7 (33.3) | .84 |
| Adequate empirical postsurgical antibiotic therapyb | 73 (94.8) | 17 (80.9) | .04 |
| Rifampin-fluoroquinolone combination therapy | 37 (48.1) | 2 (9.5) | .001 |
| Rifampin combination therapy | 58 (75.3) | 10 (47.6) | .002 |
| Total duration of antibiotic therapy, mean days ± SD | 165.7 ± 108.8 | 145.1 ± 101.6 | .44 |
NOTE. Data are no. (%) of patients unless otherwise indicated. SD, standard deviation.
Including 26 patients with 2-stage replacement and 8 with arthrodesis.
At least 1 antibiotic agent active against intraoperative pathogen(s).
Table 3.
Characteristics of Treatment and Outcome of 98 Patients With Total Hip or Knee Prosthesis Infection Due to Staphylococcus aureus
| Variable | Rifampin treatment (n = 68) |
No Rifampin treatment (n = 30) |
P | ||
| Fluoroquinolone combinations (n = 39) | Other rifampin combinations (n = 29) | Linezolid monotherapy (n = 11) | Other treatment (n = 19) | ||
| Debridement-retention (n = 41) | 15/16 (93.8) | 10/15 (66.7) | 3/3 (100) | 4/7 (57.1) | .11 |
| One-stage replacement (n = 14) | 6/6 (100) | 5/5 (100) | 1/1 (100) | 2/2 (100) | … |
| Two-stage replacement (n = 26) | 12/12 (100) | 6/9 (66.7) | 4/4 (100) | 0/1 (0) | .01 |
| Arthroplastic resection (n = 9) | 1/1 (100) | 0 | 1/3 (33.3) | 2/5 (40) | … |
| Arthrodesis (n = 8) | 3/4 (75) | 0 | 0 | 2/4 (50) | … |
| Total | 37/39 (94.8) | 21/29 (72.4) | 9/11 (81.8) | 10/19 (52.6) | .002 |
NOTE. Data are proportion (%) of patients with remission.
Outcome and Prognostic Factors
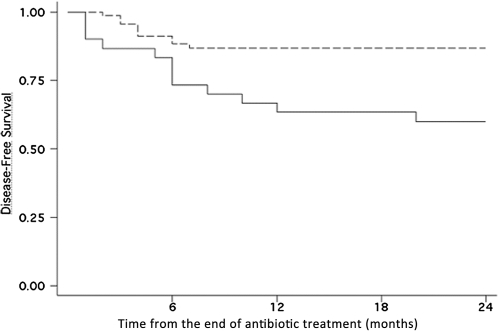
After a mean posttreatment follow-up of 43.6 ± 32.1 months, 77 patients (78.6%) were considered to be in remission. The mean delay from the end of treatment to the time of diagnosis of failure was 5.5 ± 4.4 months (range, 1–20; median, 5.5). Fourteen patients (14.3%) died from causes unrelated to PJI during a mean posttreatment follow-up of 15.8 ± 10.8 months (causes of death included malignancy in 6 patients, myocardial infarction in 4, pulmonary embolism in 2, and pneumonia in 2). Characteristics of treatment and outcome of patients are presented in Tables 2 and 3. Debridement-retention and 1- or 2-step exchange procedures were associated with similar remission rates (32 [78.0%] of 41 for debridement-retention, 14 [100%] of 14 for 1-step exchange, and 22 [84.6%] of 26 for 2-step exchange), whereas both arthroplastic resection and arthrodesis were associated with lower remission rates than the other procedures (4 [44.4%] of 9 and 5 [62.5%] of 8, respectively; P = .02]. The overall treatment failure rate for patients treated with retention, compared with removal of infected implants, was similar (Table 2). No patients had to be amputated during treatment and follow-up. The treatment failure rate was 19.7% (16 of 81) in MSSA-infected patients and 29.4% (5 of 17) in MRSA-infected patients (P = .38), whereas patients with polymicrobial infections had a 33.3% rate of treatment failure (Table 1). Patients with polymicrobial infection were treated with rifampin combinations in a significantly lower proportion than were patients with monomicrobial S. aureus infection (14 [51.8%] of 27 vs 54 [76.1%] of 71; P = .02). In univariate analysis, parameters that differed between patients whose treatment did or did not fail were American Society of Anesthesiologists (ASA) score and prescription of adequate empirical postsurgical antibiotic therapy and rifampin combination therapy upon discharge from the hospital (Table 1). Among the systemic diseases that allocated patients to ASA score >2, chronic liver disease including cirrhosis was significantly more frequent in patients with treatment failure than in those with remission (8 of 21 vs 9 of 77; P = .01). ASA score >2 was significantly less frequent in patients treated with rifampin-fluoroquinolone than in patients treated with other antibiotic regimens (Table 4). In multivariate analysis, an ASA score ≤2 (odds ratio [OR], 6.87 [95% confidence interval {CI}, 1.45–32.45]; P = .04) and the use of rifampin-fluoroquinolone combination therapy (OR, 0.40 [95% CI, 0.17–0.97]; P = .01) were 2 independent variables associated with remission. We assessed the sensitivity and specificity of the model by means of an ROC curve with an area under the curve of 0.76, which showed that the model was valid. Figure 1 shows the Kaplan-Meier plot of disease-free survival in the 98 patients according to the definitive antibotic treatment (ie, rifampin-fluoroquinolone combination versus other antibiotic regimens).
Table 4.
Characteristics and Outcome of 98 Patients With Total Hip or Knee Prosthesis Infection Due to Staphylococcus aureus According to the Antibiotic Treatment
| Variable | Fluoroquinolone combination (n = 39) | Other rifampin combinations (n = 29) | Linezolid monotherapy (n = 11) | Other treatment (n = 19) | P |
| Age, mean years ± SD | 68.8 ± 13.9 | 66.7 ± 14.2 | 64.5 ± 14.4 | 62.1 ± 17.2 | .75 |
| ASA score >2 | 8 (20.5) | 12 (41.4) | 4 (36.4) | 12 (63.1) | .02 |
| MRSA | 3 (7.7) | 7 (24.1) | 4 (36.3) | 3 (15.8) | .10 |
| 8 (20.5) | 6 (20.7) | 5 (45.4) | 8 (42.1) | .14 |
NOTE. Data are no. (%) of patients with remission unless otherwise indicated. ASA, American Society of Anesthesiologists; MRSA, methicillin-resistant S. aureus; SD, standard deviation.
Figure 1.
Kaplan–Meier estimates of the cumulative risk of failure according to the treatment group assessed at 24 months follow-up. Patients in the rifampin-fluoroquinolone treatment group had a lower risk of experiencing treatment failure than did patients treated with other antibiotic regimens (P = .003). Dotted line, rifampin-fluoroquinolone treatment group (n = 39); solid line, other regimens group (n = 59).
DISCUSSION
We report the outcome of 98 patients treated for total hip or knee PJI due to S. aureus following a defined algorithm derived from experiments reported by Zimmerli et al and several other authors [1, 17–23]. After a mean posttreatment follow-up period of >3 years, remission of infection was observed in 77 patients (78.6%), consistent with previous studies in this area, despite a higher proportion of immunocompromised patients in our study [12–16]. This characteristic of our patient population may explain the high proportion of late postoperative PJI associated with concomitant S. aureus bacteremia reported here. One-stage replacement was associated with a better outcome than that of other surgical procedures, including 2-stage replacement. The favorable conditions required for 1-stage replacement may explain the good results observed in our patients who underwent this surgical procedure. Arthroplastic resection was the surgical procedure associated with the worst outcome in our cohort of patients, with a failure rate of 55.6% (5 of 9). These results suggest that, even in case of definitive removal of all infected material in this population of patients (ie, severely immunocompromised patients or those in whom joint replacement would not have resulted in functional benefit), the infection may not be eradicated. Overall, the comparable outcome of our patients treated according to indications for retention or removal of infected implants confirms the validity of recommendations proposed by experts over the last 2 decades [1, 17–23]. The large majority of patients with chronic late postoperative infection included in the study probably explains why the delay from onset of infection to revision did not influence patient outcomes.
In the present study, the ASA score was an independent variable associated with patient outcome, consistent with results of a recent study of patients with group B streptococcal prosthetic hip infections reported by Zeller et al [24]. We also found that use of rifampin-fluoroquinolone combinations as definitive antibiotic therapy was another independent factor associated with remission of PJI due to S. aureus. The efficacy of a rifampin-containing regimen in patients with staphylococcal orthopedic implant–associated infection has also been reported in more recent observational studies [7, 25–28], confirming initial experimental model studies [29]. In our series of patients treated in the majority of cases with rifampin combinations, we found a trend toward higher failure rate only in patients with MRSA infections, compared with those with MSSA infections (29.4% and 19.7%, respectively), but this may be related to the small size of the studied population. Salgado et al described a significant poor outcome associated with MRSA infections in a series of patients treated with vancomycin without rifampin as the main treatment [30]. Nonetheless, rifampin associated with such antibiotics as fusidic acid [13, 15, 31], levofloxacin [7, 32], vancomycin or linezolid [34], and clindamycin or linezolid [31] has been associated with similar failure rates between MRSA- and MSSA-related PJIs. In the present study, patients with polymicrobial infections experienced the highest rate of treatment failure (33.3%) and also received rifampin treatment in a significantly lower proportion than did patients with monomicrobial S. aureus infection. All these data support the concept that rifampin is essential for the treatment of S. aureus–related PJIs. Use of rifampin for staphylococcal infections may be limited by the emergence of rifampin-resistant mutants, although this was not observed in our patients. According to our protocol of antibiotic therapy for PJI, rifampin was not administered as empirical therapy but exclusively as definitive antibiotic therapy consisting of a combination of 2 agents active against the pathogen(s) identified in intraoperative samples. The aim of this restriction in rifampin prescription is to prevent rifampin monotherapy for S. aureus infection, a situation likely to result in emergence of rifampin-resistant S. aureus mutants [34]. For the same reason, rifampin combinations were initially administered to our patients intravenously in order to alleviate interindividual variability in absorption of antibiotics, in particular, fluoroquinolones [35]. Despite the use of higher daily doses of rifampin in our patients than reported in most previous studies [12, 14, 23, 33] and the high proportion of our patients with chronic liver disease, rifampin had to be withdrawn in only 3 (4.3%) of 69 patients.
The present study has limitations due to its retrospective design and the fact that comparisons are made on small numbers and therefore the risk of β error leading to the absence of differences in populations compared is high. For instance, among patients with MRSA infection, those given combination therapy had a remission rate similar to that of those given monotherapy (7/11 vs 5/6; P = .60), conversely to the recent report by Ferry et al [36].
To conclude, the results of the present study suggest that patients’ ASA score significantly affects the outcome of total hip and knee prosthetic infections due to MSSA or MRSA and that rifampin combination therapy is associated with a better outcome for these patients, compared with other antibiotic regimens. Our results also suggest that inadequacy of the empirical postoperative antibiotic therapy is a risk factor for unfavorable outcome.
Acknowledgments
Potential conflicts of interest. E. S. has received travel grants from Sanofi-Aventis, participated in data monitoring boards for Merck Sharp and Dohme-Chibret, and been a speaker for Pfizer and Novartis. L. L. has received travel grants from Pfizer and been a speaker for Novartis. Y. Y. has been a consultant for Merck Sharp and Dohme-Chibret and Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern F. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 and 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Sculco TP. The economic impact of infected joint arthroplasty. Orthopedics. 1995;18:871–3. [PubMed] [Google Scholar]
- 4.McDonald DJ, Fitzgerald RH, Ilstrup DM. Two-stage reconstruction of a total hip arthroplasty because of infection. J Bone Joint Surg Am. 1989;71:834–8. [PubMed] [Google Scholar]
- 5.Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop Relat Res. 1984;182:117–26. [PubMed] [Google Scholar]
- 6.Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32:419–30. doi: 10.1086/318502. [DOI] [PubMed] [Google Scholar]
- 7.Barberán J, Aguilar L, Carroquino G. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am J Med. 2006;119:993. e7–10. doi: 10.1016/j.amjmed.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Moran E, Masters S, Berendt AR, McLardy-Smith P, Byren I, Atkins BL. Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect. 2007;55:1–7. doi: 10.1016/j.jinf.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. Arch Orthop Trauma Surg. 2000;120:570–4. doi: 10.1007/s004020000174. [DOI] [PubMed] [Google Scholar]
- 10.Brandt CM, Sistrunk WW, Duffy MC. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis. 1997;24:914–9. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- 11.Soriano A, Garcia S, Bori G. Treatment of acute post-surgical infection of joint arthroplasty. Clin Microbiol Infect. 2006;12:930–3. doi: 10.1111/j.1469-0691.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerli W, Widmer A, Blatter M, Frei R, Ochsner P. Role of rifampin for treatment of orthopaedic implant-related staphylococcal infections. JAMA. 1998;279:1537–41. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 13.Aboltins CA, Page MA, Buising KL. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clin Microbiol Infect. 2007;13:586–91. doi: 10.1111/j.1469-0691.2007.01691.x. [DOI] [PubMed] [Google Scholar]
- 14.Drancourt M, Stein A, Argenson JN, Zannier A, Curvale G, Raoult D. Oral rifampin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob Agents Chemother. 1993;37:1214–8. doi: 10.1128/aac.37.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drancourt M, Stein A, Argenson JN, Roiron R, Groulier P, Raoult D. Oral treatment of Staphylococcus spp. infected orthopaedic implants with fusidic acid or ofloxacin in combination with rifampicin. J Antimicrob Chemother. 1997;39:235–40. doi: 10.1093/jac/39.2.235. [DOI] [PubMed] [Google Scholar]
- 16.Stein A, Bataille JF, Drancourt M. Ambulatory treatment of multidrug-resistant Staphylococcus-infected orthopedic implants with high-dose oral co-trimoxazole (trimethoprim-sulfamethoxazole) Antimicrob Agents Chemother. 1998;42:3086–91. doi: 10.1128/aac.42.12.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steckelberg JM, Osmon DR. Prosthetic joint infections. In: Waldvogel FA, Bisno AL, editors. Infections associated with indwelling medical devices. 3rd ed. Washington, DC: American Society for Microbiology; 2000. pp. 173–209. [Google Scholar]
- 18.Westrich GH, Salvati EA, Brause B. Postoperative infection. In: Bono JV, McCarty JC, Thornhill TS, Bierbaum BE, Turner RH, editors. Revision total hip arthroplasty. New York: Springer-Verlag; 1999. pp. 371–90. [Google Scholar]
- 19.Ure KJ, Amstutz HC, Nasser S, Schmalzried TP. Direct-exchange arthroplasty for the treatment of infection after total hip replacement: an average ten-year follow-up. J Bone Joint Surg Am. 1998;80:961–8. doi: 10.2106/00004623-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty: a study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–23. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Schoifet SD, Morrey BF. Treatment of infection after total knee arthroplasty by debridement with retention of the components. J Bone Joint Surg Am. 1990;72:1383–90. [PubMed] [Google Scholar]
- 22.Crockarell JR, Hanssen AD, Osmon DR, Morrey BF. Treatment of infection with debridement and retention of the components following hip arthroplasty. J Bone Joint Surg Am. 1998;80:1306–13. doi: 10.2106/00004623-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin Infect Dis. 1992;14:1251–3. doi: 10.1093/clinids/14.6.1251. [DOI] [PubMed] [Google Scholar]
- 24.Zeller V, Lavigne M, Biau D, et al. Outcome of group B streptococcal prosthetic hip infections compared to that of other bacterial infections. Joint Bone Spine. 2009;76:491–6. doi: 10.1016/j.jbspin.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Laffer RR, Graber P, Ochsner PE, Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect. 2006;12:433–9. doi: 10.1111/j.1469-0691.2006.01378.x. [DOI] [PubMed] [Google Scholar]
- 26.Giulieri SG, Graber P, Ochsner PE, Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection. 2004;32:222–8. doi: 10.1007/s15010-004-4020-1. [DOI] [PubMed] [Google Scholar]
- 27.Trebse R, Pisot V, Trampuz A. Treatment of infected retained implants. J Bone Joint Surg Br. 2005;87:249–56. doi: 10.1302/0301-620x.87b2.15618. [DOI] [PubMed] [Google Scholar]
- 28.Berdal JE, Skråmm I, Mowinckel P, Gulbrandsen P, Bjørnholt JV. Use of rifampicin and ciprofloxacin combination therapy after surgical debridement in the treatment of early manifestation prosthetic joint infections. Clin Microbiol Infect. 2005;11:843–5. doi: 10.1111/j.1469-0691.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 29.Chuard C, Hermann M, Vaudax P, Waldvogel F, Lew D. Successful therapy of experimental chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob Agents Chemother. 1991;35:2611–6. doi: 10.1128/aac.35.12.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 31.Choong PF, Dowsey MM, Carr D, Daffy J, Stanley P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin based regimen. Acta Orthop. 2007;78:755–65. doi: 10.1080/17453670710014527. [DOI] [PubMed] [Google Scholar]
- 32.Vilchez F, Martínez-Pastor JC, García-Ramiro S. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17:439–44. doi: 10.1111/j.1469-0691.2010.03244.x. [DOI] [PubMed] [Google Scholar]
- 33.El Helou OC, Berbari EF, Lahr BD. Efficacy and safety of rifampin containing regimen for staphylococcal prosthetic joint infections treated with debridement and retention. Eur J Clin Microbiol Infect Dis. 2010;29:961–7. doi: 10.1007/s10096-010-0952-9. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerli W, Frei R, Widmer AF, Rajacic Z. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J Antimicrob Chemother. 1994;33:959–67. doi: 10.1093/jac/33.5.959. [DOI] [PubMed] [Google Scholar]
- 35.Dudley MN. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am J Med. 1991:45S–50S. doi: 10.1016/0002-9343(91)90311-k. [DOI] [PubMed] [Google Scholar]
- 36.Ferry T, Uçkay I, Vaudaux P, et al. Risk factors for treatment failure in orthopedic device-related methicillin-resistant Staphylococcus aureus infection. Eur J Clin Microbiol Infect Dis. 2010;29:171–80. doi: 10.1007/s10096-009-0837-y. [DOI] [PubMed] [Google Scholar]