Mild cerebral swelling on CT-scan was common in adult patients with cerebral malaria, but severity of swelling was not correlated with coma depth or survival. Mannitol as adjunctive treatment for cerebral malaria prolonged coma duration and may be harmful.
Abstract
Background. Coma is a frequent presentation of severe malaria in adults and an important cause of death. The role of cerebral swelling in its pathogenesis, and the possible benefit of intravenous mannitol therapy to treat this, is uncertain.
Methods. A computed tomographic (CT) scan of the cerebrum and lumbar puncture with measurement of cerebrospinal fluid (CSF) pressure were performed on admission for 126 consecutive adult Indian patients with cerebral malaria. Patients with brain swelling on CT scan were randomized to adjunctive treatment with intravenous mannitol (1.5 g/kg followed by 0.5 g/kg every 8 hours; n = 30) or no adjunctive therapy (n = 31).
Results. On CT scan 80 (63%) of 126 patients had cerebral swelling, of whom 36 (29%) had moderate or severe swelling. Extent of brain swelling was not related to coma depth or mortality. CSF pressures were elevated (≥200 mm H2O) in 43 (36%) of 120 patients and correlated with CT scan findings (P for trend = .001). Mortality with mannitol therapy was 9 (30%) of 30 versus 4 (13%) of 31 without adjunctive therapy (hazard ratio, 2.4 [95% confidence interval, 0.8–7.3]; P = .11). Median coma recovery time was 90 hours (range, 22–380 hours) with mannitol versus 32 hours (range, 5–168 hours) without (P = .02).
Conclusions. Brain swelling on CT scan is a common finding in adult patients with cerebral malaria but is not related to coma depth or survival. Mannitol therapy as adjunctive treatment for brain swelling in adult cerebral malaria prolongs coma duration and may be harmful.
Cerebral malaria is the most frequent severe manifestation of falciparum malaria. It presents as unrousable coma and fever, usually without focal neurological signs; seizures occur in the majority of children but only in approximately 12% of adult patients [1–4]. Depth of coma on presentation has a strong prognostic significance for a fatal outcome in both adults and children, but severe neurological sequelae are uncommon in survivors [3, 5, 6], although more frequent in children [4, 7]. The pathophysiology of cerebral malaria is incompletely understood. Autopsy studies show intense sequestration of parasitized erythrocytes in the cerebral microvasculature correlated with coma depth before death [8], suggesting that the primary pathological process is microvascular obstruction. Changes in the blood-brain barrier (BBB) have been described, but evidence for a generalized increase in BBB permeability leading to cerebral edema is conflicting [9, 10]. Defining the role of cerebral edema in cerebral malaria has important consequences for its clinical management, in particular whether adjunctive treatment with mannitol could be beneficial. At the time of the study, mannitol was used in several centers in India as adjunctive treatment in cerebral malaria. This osmotic diuretic lowers intracranial pressure but might also reduce oxygen free-radical damage [11]. In a small study, mannitol was found to reduce intracranial pressure in Kenyan children with cerebral malaria but did not prevent a poor outcome [12]. A single dose of mannitol did not reduce mortality or neurological sequelae in a larger randomized placebo-controlled trial in Ugandan children with cerebral malaria [13]. A Cochrane analysis concluded that there are insufficient data to know what the effects of osmotic diuretics are in cerebral malaria [11]. We studied the prevalence of cerebral swelling and increased intracranial pressure in adult patients with cerebral malaria. A randomized clinical trial on the efficacy of intravenous mannitol was then performed in those patients with cerebral swelling identified on computed tomographic (CT) scan.
METHODS
Study Site
The study was conducted at Ispat General Hospital in Rourkela, Orissa, India. This is a tertiary health care facility with 685 beds, an 11-bed intensive care unit, and facilities for blood banking.
Patients
Consecutive adult patients (age, ≥16 years) with coma, defined as a Glasgow Coma Score (GCS) <11, and asexual forms of Plasmodium falciparum in a peripheral blood smear sample were included into the study provided written informed consent was obtained from the relatives or accompanying attendant. Pregnancy, meningitis, or other causes of encephalopathy diagnosed by means of lumbar puncture were exclusion criteria. Ethical approval was obtained from the institutional review board of Ispat General Hospital. A medical history was taken, and a full physical examination was conducted and recorded on a standardized clinical record form. Particular attention was paid to the neurological examination, which was repeated after 1 hour in case of convulsions. A full blood count and basic biochemistry evaluation were performed for all patients. Fatal cases were reviewed by an independent data safety monitoring committee (T. K. Bose, MD, and D. Mohanty, MD).
Imaging
A CT scan (Philips, W1000, third-generation) of the brain was performed within 12 hours of hospitalization for all enrolled patients. Nonenhanced 8-mm transverse sections were obtained for the posterior fossa and 10-mm transverse sections for the rest of the brain. Additional 5-mm sections were obtained through the cerebellum and thalamus if focal abnormalities were suspected. Brain swelling was defined as loss of cerebrospinal fluid (CSF) space, small ventricles, absence of sulci, and/or compression of the perimesencephalic and chiasmatic cisterns. Mild swelling was defined as a focal or diffuse effacement of sulci or the Sylvian fissure, without compression of the supratentorial ventricular system. Moderate swelling was defined as diffuse obliteration of sulci, Sylvian fissure, and basal cisterns, including the perimesencephalic and quadrigeminal cisterns, with or without a minor mass effect in the form of a focal ventricular distortion. Severe swelling was defined as diffuse obliteration of sulci, Sylvian fissure, and basal cisterns together with an important mass effect causing near total obliteration of the supratentorial ventricular system and generalized hypodensity of brain parenchyma. Scans were read by 2 independent radiologists blinded to the clinical status of the patient. A total of 11 cases were read by a third radiologist because of incongruent interpretation, and a final decision was made by consensus, which was settled as moderate edema in 6 patients and mild edema in 5 patients.
Cerebrospinal Fluid Pressure
Lumbar puncture was performed after interpretation of the CT scan images for the potential risk of herniation. CSF pressure was measured with a spinal manometer. A small volume of 1–2 mL of CSF (the column of fluid inside the manometer) was obtained for microbiological, cytological, and biochemical analyses. Raised intracranial pressure was defined as an opening pressure ≥200 mmH2O and severe intracranial hypertension as ≥400 mmH2O.
Patient Management
Patients were closely monitored, at least once every 4 hours, for vital signs, GCS, neurological deficits, pupillary signs, and urine output. Patients were treated with a loading dose of quinine dihydrochloride 20 mg salt/kg body weight followed by 10 mg/kg body weight every 8 hours in accordance with World Health Organization guidelines [14]. The study was performed before 2006 when intravenous artesunate became first-line antimalarial treatment. When the patient was able to take foods, antimalarial therapy was changed to oral quinine sulphate (10 mg/kg every 8 hours) to complete a total course of 7 days. Seizures were treated with intravenous diazepam (10 mg) and, if convulsions persisted, with intravenous phenytoin (15 mg/kg). Whole-blood transfusion was administered if hemoglobin concentrations dropped below 5 g/dL. Patients with malaria-associated acute renal failure underwent renal replacement therapy (peritoneal dialysis or hemodialysis). Mechanical ventilator support was given in case of respiratory insufficiency. Hypoglycemia was closely monitored and treated promptly with a single dose of 50 mL dextrose 25%. Rehydration was with 0.9% NaCl or Ringer’s lactate.
Mannitol Therapy
Patients with evidence of cerebral swelling on CT scan but without renal failure (serum creatinine level, >3 mg/dL), severe anemia (hemoglobin level, <5 g/dL), or signs of pulmonary edema were randomized to receive either mannitol as adjunctive treatment or no adjunctive therapy. This was an open randomized trial. Randomization in blocks of 20 was computer generated, and the allocation codes were kept in an opaque envelope. In the adjunctive treatment group, mannitol in a dose of 1.5 g/kg body weight was infused over a period of 15 minutes, followed by 0.5 g/kg body weight every 8 hours until the patient regained consciousness or for a maximum period of 72 hours. All surviving patients were assessed neurologically at discharge; there was no follow-up beyond discharge.
Statistical Analysis
Comparisons between groups were made by means of Student t test for normally distributed continuous variables and Mann–Whitney test for nonnormally distributed continuous variables, and the χ2 test was used for comparing proportions. Odds ratios were derived where applicable. In the randomized controlled trial, survival analysis with comparison of groups by log-rank test was used. The statistical package was Stata, version 10.0 (Statacorp). The “test for trend” is included in this package and was used for determining the change in CSF pressures in relation to severity of swelling on CT scan. The intervention trial was set up as a pilot study. A total of 60 patients allowed for showing a difference in coma recovery time of 24 hours with a significance level of .05 and a power of 80%. The unexpected increase in coma duration in the mannitol-treated patients prompted the investigators in consultation with the data safety monitoring committee to refrain from extending the trial beyond the pilot phase.
RESULTS
Patients
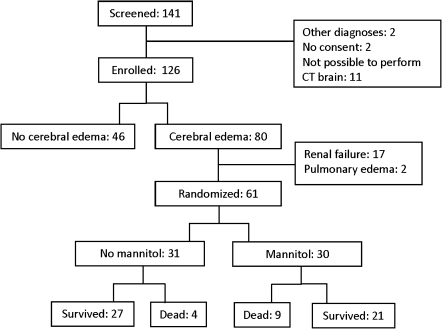
Between 2001 and 2003, a total of 141 patients with cerebral malaria were screened for eligibility, of which 126 patients were enrolled in the study (Figure 1). Clinical and laboratory values are given in Table 1. A total of 32 patients had renal failure, of which 6 received peritoneal dialysis and 2 hemodialysis. Overall, 36 (29%) of 126 patients died. None of the patients had residual neurological sequelae at the time of discharge.
Figure 1.
Trial profile.
Table 1.
Baseline Values and Disease Outcome According to the Presence of Cerebral Edema on CT Scan on Admission
| Parameter | No cerebral edema (n = 46) | Cerebral edema (n = 80) | P |
| Age, years | 37 (33-40) | 28 (25–31) | .001 |
| Female sex, no. (%) | 11 (24) | 20 (25) | >.99 |
| Fever before hospitalization, days | 5 (4–5) | 4 (4–5) | .67 |
| Altered sensorium before hospitalization, median hours (range) | 10 (2–96) | 12 (1–96) | .88 |
| Systolic blood pressure, mmHg | 116 (110–122) | 114 (109–118) | .45 |
| Glasgow Coma Scale, median (range) | 6 (3–9) | 7 (3–9) | .06 |
| Convulsions at admission, no. (%) | 6 (13) | 22 (28) | .08 |
| Cerebrospinal fluid pressure, cm H2O | 131 (113–148) | 186 (167–205) | .001 |
| Hemoglobin level, g/dL | 9.4 (8.6–10.2) | 9.9 (9.3–10.5) | .36 |
| Sodium level, mmol/L | 136 (135–138) | 136 (134–138) | .79 |
| Potassium level, mmol/L | 3.8 (3.5–4.0) | 3.8 (3.6–4.1) | .60 |
| Glucose level, mg/dL | 106 (89–123) | 107 (91–122) | .96 |
| Creatinine level,a mg/dL | 1.8 (1.4–2.2) | 1.4 (1.2–1.6) | .07 |
| Total bilirubin level,a mg/dL | 4.1 (2.9–5.9) | 4.3 (3.3–5.5) | .88 |
| Mortality,b proportion (%) | 16/44 (36) | 19/78 (24) | .21 |
NOTE. Values are mean (95% confidence interval [CI]), except where otherwise indicated.
Geometric mean (95% CI).
Excluding 4 patients with intracerebral hemorrhage or infarction.
Computed Tomographic Scans
All patients had a CT scan examination of the head within 12 hours after admission; 80 (63%) of 126 showed evidence of cerebral swelling, although in 44 patients (35%), this swelling was classified as mild. In 34 patients (27%), there was moderate swelling, and in 2 patients (2%), severe swelling. In 4 patients, other pathology was identified: intraparenchymal hemorrhage in 2 patients with concomitant moderate cerebral edema, 1 of whom died; and 2 patients with lacunar infarctions without evidence of edema. Baseline characteristics according to the presence of cerebral swelling are summarized in Table 1. The group with cerebral swelling was younger, had a higher opening pressure on lumbar puncture, and showed a trend to more frequent convulsions. There was no difference in GCS between patients with different degrees of cerebral swelling on CT scan of the brain: median GCS was 6 (range, 3–9) in the absence of swelling, 7 (range, 3–9) in patients with mild swelling, 6 (range, 3–9) in patients with moderate swelling, and 6 (range, 4–7) in patients with severe swelling. Fifteen (33%) of 46 patients without cerebral swelling had renal failure (defined as a serum creatinine level of >3 mg/dL), compared with 17 (21%) of 80 patients with cerebral swelling on CT scan (P = .20). Severe jaundice (total plasma bilirubin level, >3 mg/dL) was present in 33 (72%) of 46 patients without swelling and in 53 (68%) of 78 patients with swelling (P = .69), whereas severe anemia (hemoglobin level, <5g/dL) was present in 5 (11%) of 46 patients without swelling versus 6 (8%) of 76 patients with swelling (P = .58), and hypoglycemia (plasma glucose level, <40 mg/dL) in 4 (9%) of patients without swelling and 2 (3%) of patients with swelling (P = .19). Excluding the 4 patients with intracerebral hemorrhage or infarction identified on CT scan, mortality was 16 (36%) of 44 patients without cerebral swelling and 19 (24%) of 78 patients with cerebral swelling (P = .21). The 2 patients with severe cerebral swelling both died. At discharge, no neurological sequelae were detected in surviving patients.
CSF Pressure
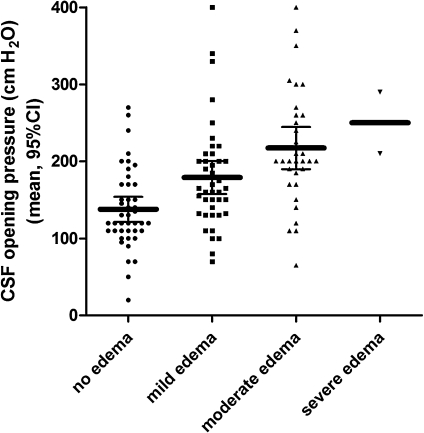
Lumbar puncture with manometry was performed for 120 of the 126 patients. No lumbar puncture was performed for 2 patients because of death shortly after the CT scan, for 3 patients because of technical difficulties in performing the puncture, and for 1 patient because of withdrawal of consent to perform the procedure by the attending relative. Median time between CT scan and lumbar puncture was 2.45 hours (range, 1.30–4.40 hours). A total of 43 (36%) of 120 patients had raised intracranial pressure (≥200 mmH2O), and 2 (2%) of 120 had severe intracranial hypertension (≥400 mmH2O). Culture of CSF samples did not grow microorganisms. Mean CSF glucose level was 112 mg/dL (95% confidence interval [CI], 103–121 mg/dL), and geometric mean CSF protein level was 69 mg/dL (95% CI, 58–79 mg/dL). An elevated (>5 × 106/L) CSF leucocyte count [range, (6–55) × 106/L] was present in 12 (10%) of 120 patients. Mean CSF pressure in these patients was 185 cmH2O (95% CI, 127–244 cmH2O). Mortality was 15 (35%) of 43 in patients with a pressure of ≥200 mmH2O and 17 (22%) of 77 in patients with lower pressures (P = .14). Mean CSF pressure was 172 mmH2O (95% CI, 157–186 mmH2O) in surviving patients and 186 mmH2O (95% CI, 155–217 mmH2O) in patients with a fatal course of the disease (P = .33). In fatal cases, median time between the lumbar puncture and the time of death was 42 hours (range, 6–384 hours). The frequency of seizures in patients with high CSF pressures (≥200 mmH2O) was 22 (51%) of 43, compared with 24 (31%) of 77 in patients with pressures <200 mmH2O (P = .03). Severity of cerebral swelling on CT scan correlated with CSF opening pressures (Figure 2; P for trend = .001). There was no correlation between CSF opening pressures at hospitalization and coma recovery times (r = –0.05; P = .63).
Figure 2.
Relation between severity of cerebral edema on computed tomographic scan and opening pressures on lumbar puncture in adult patients with slide-proven cerebral malaria; P value for trend = .001. CI, confidence interval; CSF, cerebrospinal fluid.
Mannitol Therapy
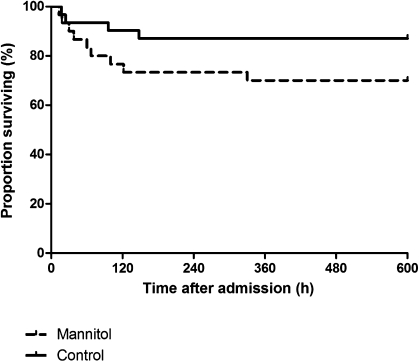
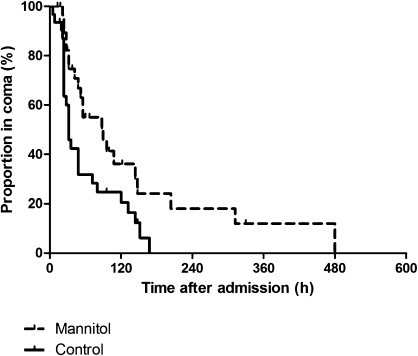
Of the 80 patients with cerebral malaria and cerebral swelling on CT scan, a total of 61 patients were randomized to receive mannitol adjunctive therapy (n = 30) or no adjunctive therapy (n = 31; Figure 1). Baseline characteristics are summarized in Table 2. The treatment groups were comparable, except for small differences in plasma sodium and potassium levels. Mortality in patients treated with mannitol was 9 (30%) of 30, compared with 4 (13%) of 31 in patients not treated with mannitol (Figure 3; log-rank test χ2 = 2.58; hazard ratio, 2.4 [95% CI, 0.8–7.3]; P = .11). When data from patients who died were censored at the moment of death, coma recovery time in surviving patients was prolonged in those treated with mannitol (Figure 4), with a median coma recovery time of 90 hours (range, 22–380 hours) with mannitol versus 32 hours (range, 5–168 hours) in patients without adjunctive treatment (Figure 4; log-rank test χ2 = 6.37; hazard ratio, 2.2 [95% CI, 1.2–4.0]; P = .01).
Table 2.
Baseline Values According to Treatment Allocation
| Parameter | No mannitol (n = 31) | Mannitol (n = 30) | P |
| Age, years | 32 (28–36) | 27 (23–31) | .08 |
| Female sex, no. (%) | 6 (19) | 10 (33) | .15 |
| Fever before hospitalization, days | 5 (4–6) | 4 (4–5) | .26 |
| Altered sensorium before hospitalization, median hours (range) | 14 (2–96) | 9 (1–37) | .13 |
| Systolic blood pressure, mmHg | 116 (108–125) | 108 (99–118) | .20 |
| Glasgow Coma Scale, median (range) | 7 (3–9) | 6 (3–8) | .12 |
| Convulsions at hospitalization, no. (%) | 9 (29) | 11 (37) | .22 |
| Cerebrospinal fluid pressure, cmH2O | 167 (138–195) | 187 (161–214) | .29 |
| Hemoglobin level, g/dL | 9.6 (8.7–10.5) | 10. 0 (9.0–11.1) | .42 |
| Sodium level, mmol/L | 134 (132–137) | 139 (136–142) | .01 |
| Potassium level, mmol/L | 3.6 (3.4–3.8) | 4.1 (3.7–4.5) | .01 |
| Glucose level, mg/dL | 95 (6) | 85 (7) | .96 |
| Creatinine level,a mg/dL | 1.2 (1.0–1.4) | 1.1 (0.9–1.2) | .36 |
| Total bilirubin level,a mg/dL | 4.6 (3.0–7.0) | 2.7 (1.9–3.9) | .06 |
NOTE. Values are mean (95% confidence interval [CI]), except where otherwise indicated.
Geometric mean (95% CI).
Figure 3.
Survival curves for patients treated with (dashed line) or without (solid line) mannitol as adjunctive treatment for cerebral malaria with cerebral edema identified on computed tomographic scan. Log-rank test χ2: 2.58; P = .11.
Figure 4.
Kaplan–Meier curves for the proportion of patients still in coma after start of treatment with (dashed line) or without (solid line) mannitol as adjunctive therapy for cerebral malaria with cerebral edema identified on computed tomographic scan. Data from patients who died were censored at the moment of death. Log-rank test χ2: 6.37; P = .01.
DISCUSSION
The most important finding of this study was that mannitol as adjunctive treatment for patients with cerebral malaria and cerebral swelling on CT scan did not reduce mortality but did prolong coma in the surviving patients. There was a trend toward increased mortality in the group treated with mannitol, but the study was not powered to show a mortality difference between treatment groups. Mannitol is an osmotic active drug that can lower intracranial pressure by absorbing extracellular fluid into the vascular compartment. Its use in cerebral malaria is common and has been advocated by several authors, but adequately powered randomized clinical trials have thus far been lacking [11]. Our results indicate that mannitol cannot be recommended as an adjunctive treatment in adult patients with cerebral malaria and may be harmful. It is possible that hyperosmolar mannitol therapy further compromises fluid exchange in the already affected microcirculation. Extensive sequestration of parasitized erythrocytes, resulting from cytoadherence to the endothelium of capillaries and postcapillary venules causing obstruction of microcirculatory flow, is a central and probably early feature in the pathophysiology of cerebral malaria [15, 16]. Adult patients who die from cerebral malaria have more prominent sequestration in the brain microvasculature, compared with severe but noncomatose fatal cases [8, 16]. These studies show that sequestration is unevenly distributed, with patent capillaries adjacent to obstructed vessels, a phenomenon recently also observed in living patients by means of video microscopy [8, 17]. This heterogeneity of microvascular obstruction allowing collateral blood supply might explain the complete recovery in the majority of patients and absence of cerebral infarction, which was also rare in the current series. The role of cerebral edema as a cause of neurological impairment is less clear [18]. Most imaging studies, using both CT and magnetic resonance imaging (MRI) scanning, find that cerebral edema is not a prominent finding in most adults with cerebral malaria but is more frequently reported in African children [19–23]. In the current study, presence of any brain swelling was recognized in 63% of adult patients with cerebral malaria but was moderate or severe in only 29% of patients. In addition, there was no correlation between depth of coma or mortality and the findings on CT scan of the brain. The results are in accordance with findings in postmortem studies in adults dying from cerebral malaria, which rarely show tentorial or brain stem herniation [24]. Patients with cerebral swelling did present more frequently with seizures, but epileptic activity itself could have contributed to the brain swelling. Other rare findings in this series included intracerebral hemorrhage and lacunar infarctions. Lacunar infarctions have been described in cerebral malaria [22] and could be related to microvascular obstruction, but we could not rule out the possibility that the infarctions predated the current disease episodes.
CSF pressures were elevated to >200 mmH2O in 43 (36%) of 120 patients, whereas 2 (2%) had severely elevated pressures of >400 mmH2O. CSF pressures correlated strongly with the severity of brain swelling identified by CT scan, which can be useful in settings where access to CT scanning is limited. CSF pressures did not correlate with coma depth or coma recovery times. Our findings are consistent with earlier reports on CSF opening pressures in adults with cerebral malaria [25]. In children, the threshold level for increased intracranial pressure is lower (100 mmH2O), and 80% of patients with pediatric cerebral malaria in Africa present with opening pressures above this threshold [26]. In the present study, lumbar puncture was performed after assessment of potential risk for herniation by CT scan. In this series, there were no complications related to the lumbar puncture and there was no relationship between the time of lumbar puncture and time of death in any patient.
Contributors to cerebral swelling in cerebral malaria include an increased blood volume caused by the sequestered biomass, cytotoxic edema, and vasogenic edema. Because parasitized erythrocytes cytoadhere to endothelium in the microcirculation and have reduced flow rates caused by increased rigidity and stickiness to other erythrocytes, the fraction of parasitized red blood cells in the cerebral microcirculation can increase to about 50 times the parasitemia in the peripheral blood [16, 27]. Because overall cerebral blood flow is uncompromised in cerebral malaria [28], this extra blood volume must be accommodated within the intracranial space and will increase intracerebral pressure. Cytotoxic edema caused by swelling of neurons and glial and other cells is the result of hypoxic, toxic, or other injury. This cannot be distinguished from vasogenic edema by CT scan but can be differentiated by diffusion-weighted MRI scanning; studies addressing this are underway. Vasogenic cerebral edema occurs if the BBB is disrupted and is characteristically reversible (albeit temporarily) by osmotic diuretics. Cytoadherence might compromise the functional integrity of the BBB, and immunohistochemical postmortem studies have shown disruption of endothelial intercellular tight junctions [9, 29]. However, studies on the functional integrity of the BBB in adults with cerebral malaria suggest that the barrier function remains grossly intact and is only modestly changed in children [29, 30]. Albumin and immunoglobulin G indices in CSF, a measure of BBB integrity, were reported mostly within the normal range in a study on Vietnamese adults with cerebral malaria [31]. The present results support the conclusion that it is unlikely that increased cerebral capillary permeability causing brain swelling is the initial cause of coma in cerebral malaria as originally suggested by Rigdon and Maegraith in the 1940s and repeated frequently since [32–34].
In conclusion, this study shows that mild to moderate cerebral swelling on CT scan and increased opening pressures on lumbar puncture are common findings in adult patients with cerebral malaria, but their presence does not correlate with coma depth or disease outcome. Mannitol as adjunctive therapy in patients with cerebral malaria and cerebral swelling is associated with prolonged coma duration and may be harmful.
Acknowledgments
The authors thank the National Vector Borne Disease Control Programme of the Government of India for support. We also express our sincere gratitude to the patients for giving consent to be the part of the study. We thank the management of Ispat General Hospital for allowing us to perform the study. We thank Prof Nicholas White and Prof Jeremy Farrar for their critical review of the manuscript.
Financial support. This work was supported by the National Vector Borne Disease Control Programme of the Government of India. A. M. D. and S. J. L. are funded by the Wellcome Trust of Great Britain.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epilepticus in childhood cerebral malaria. QJM. 1996;89:591–7. doi: 10.1093/qjmed/89.8.591. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, Lee SJ, Faiz MA, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47:151–7. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- 3.Mishra SK, Mohanty S, Satpathy SK, Mohapatra DN. Cerebral malaria in adults—a description of 526 cases admitted to Ispat General Hospital in Rourkela, India. Ann Trop Med Parasitol. 2007;101:187–93. doi: 10.1179/136485907X157004. [DOI] [PubMed] [Google Scholar]
- 4.Mohanty S, Mishra SK, Pati SS, Pattnaik J, Das BS. Complications and mortality patterns due to Plasmodium falciparum malaria in hospitalized adults and children, Rourkela, Orissa, India. Trans R Soc Trop Med Hyg. 2003;97:69–70. doi: 10.1016/s0035-9203(03)90027-7. [DOI] [PubMed] [Google Scholar]
- 5.Waller D, Krishna S, Crawley J, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21:577–87. doi: 10.1093/clinids/21.3.577. [DOI] [PubMed] [Google Scholar]
- 6.Dondorp A, Nosten F, Stepniewska K, Day N, White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 7.Idro R, Carter JA, Fegan G, Neville BG, Newton CR. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child. 2006;91:142–8. doi: 10.1136/adc.2005.077784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silamut K, Phu NH, Whitty C, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medana IM, Turner GD. Human cerebral malaria and the blood-brain barrier. Int J Parasitol. 2006;36:555–68. doi: 10.1016/j.ijpara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Patnaik JK, Das BS, Mishra SK, Mohanty S, Satpathy SK, Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994;51:642–7. [PubMed] [Google Scholar]
- 11.Okoromah CA, Afolabi BB, Wall EC. Mannitol and other osmotic diuretics as adjuncts for treating cerebral malaria. Cochrane Database Syst Rev. 2011;4:CD004615. doi: 10.1002/14651858.CD004615.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton CR, Crawley J, Sowumni A, et al. Intracranial hypertension in Africans with cerebral malaria. Arch Dis Child. 1997;76:219–26. doi: 10.1136/adc.76.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namutangula B, Ndeezi G, Byarugaba JS, Tumwine JK. Mannitol as adjunct therapy for childhood cerebral malaria in Uganda: a randomized clinical trial. Malar J. 2007;6:138. doi: 10.1186/1475-2875-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(suppl 1):S1–90. [PubMed] [Google Scholar]
- 15.Newton CR, Warrell DA. Neurological manifestations of falciparum malaria. Ann Neurol. 1998;43:695–702. doi: 10.1002/ana.410430603. [DOI] [PubMed] [Google Scholar]
- 16.Pongponratn E, Turner GD, Day NP, et al. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:345–59. [PubMed] [Google Scholar]
- 17.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 18.Gitau EN, Newton CR. Blood-brain barrier in falciparum malaria. Trop Med Int Health. 2005;10:285–92. doi: 10.1111/j.1365-3156.2004.01366.x. [DOI] [PubMed] [Google Scholar]
- 19.Cordoliani YS, Sarrazin JL, Felten D, Caumes E, Leveque C, Fisch A. MR of cerebral malaria. AJNR Am J Neuroradiol. 1998;19:871–4. [PMC free article] [PubMed] [Google Scholar]
- 20.Looareesuwan S, Warrell DA, White NJ, et al. Do patients with cerebral malaria have cerebral oedema? A computed tomography study. Lancet. 1983;1:434–7. doi: 10.1016/s0140-6736(83)91437-x. [DOI] [PubMed] [Google Scholar]
- 21.Looareesuwan S, Wilairatana P, Krishna S, et al. Magnetic resonance imaging of the brain in patients with cerebral malaria. Clin Infect Dis. 1995;21:300–9. doi: 10.1093/clinids/21.2.300. [DOI] [PubMed] [Google Scholar]
- 22.Patankar TF, Karnad DR, Shetty PG, Desai AP, Prasad SR. Adult cerebral malaria: prognostic importance of imaging findings and correlation with postmortem findings. Radiology. 2002;224:811–6. doi: 10.1148/radiol.2243010588. [DOI] [PubMed] [Google Scholar]
- 23.Yadav P, Sharma R, Kumar S, Kumar U. Magnetic resonance features of cerebral malaria. Acta Radiol. 2008;49:566–9. doi: 10.1080/02841850802020476. [DOI] [PubMed] [Google Scholar]
- 24.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria: a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 25.White NJ. Lumbar puncture in cerebral malaria. Lancet. 1991;338:640–1. doi: 10.1016/0140-6736(91)90654-8. [DOI] [PubMed] [Google Scholar]
- 26.Newton CR, Kirkham FJ, Winstanley PA, et al. Intracranial pressure in African children with cerebral malaria. Lancet. 1991;337:573–6. doi: 10.1016/0140-6736(91)91638-b. [DOI] [PubMed] [Google Scholar]
- 27.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–17. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Warrell DA, White NJ, Veall N, et al. Cerebral anaerobic glycolysis and reduced cerebral oxygen transport in human cerebral malaria. Lancet. 1988;2:534–8. doi: 10.1016/s0140-6736(88)92658-x. [DOI] [PubMed] [Google Scholar]
- 29.Brown H, Rogerson S, Taylor T, et al. Blood-brain barrier function in cerebral malaria in Malawian children. Am J Trop Med Hyg. 2001;64:207–13. doi: 10.4269/ajtmh.2001.64.207. [DOI] [PubMed] [Google Scholar]
- 30.Warrell DA, Looareesuwan S, Phillips RE, et al. Function of the blood-cerebrospinal fluid barrier in human cerebral malaria: rejection of the permeability hypothesis. Am J Trop Med Hyg. 1986;35:882–9. doi: 10.4269/ajtmh.1986.35.882. [DOI] [PubMed] [Google Scholar]
- 31.Brown HC, Chau TT, Mai NT, et al. Blood-brain barrier function in cerebral malaria and CNS infections in Vietnam. Neurology. 2000;55:104–11. doi: 10.1212/wnl.55.1.104. [DOI] [PubMed] [Google Scholar]
- 32.Maegraith B. Pathological processes in malaria. Trans R Soc Trop Med Hyg. 1948;41:687–704. doi: 10.1016/s0035-9203(48)90678-6. [DOI] [PubMed] [Google Scholar]
- 33.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–9. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 34.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22:503–8. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]