Summary
STAT3, an essential transcription factor with pleiotropic functions, plays critical roles in the pathogenesis of autoimmunity. Despite recent data linking STAT3 with inflammatory bowel disease, exactly how it contributes to chronic intestinal inflammation is not known. Using a T cell transfer model of colitis, we found that STAT3 expression in T cells was essential for the induction of both colitis and systemic inflammation. STAT3 was critical in modulating the balance of T helper 17 (Th17) and regulatory T (Treg) cells, as well as in promoting CD4+ T cell proliferation. We used chromatin immunoprecipitation and massive parallel sequencing (ChIP-Seq) to define the genome-wide targets of STAT3 in CD4+ T cells. We found that STAT3 bound to multiple genes involved in Th17 cell differentiation, cell activation, proliferation, and survival, regulating both expression and epigenetic modifications. Thus, STAT3 orchestrates multiple critical aspects of T cell function in inflammation and homeostasis.
Introduction
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that serves critical functions in development, cell growth, and homeostasis in a variety of tissues ([Hirano et al., 2000] and [Levy and Lee, 2002]). Germline deletion in mice leads to embryonic lethality most likely due to STAT3's essential functions in mediating leukemia inhibitory factor (LIF) signaling as well as other gp130 family cytokines (Takeda et al., 1997). Conversely, inappropriate activation of STAT3 is associated with malignant transformation and the pathogenesis of various types of cancers (Haura et al., 2005). In hematopoietic cells, STAT3 is an important negative regulator of the granulocyte colony-stimulating factor (G-CSF) pathway and granulopoiesis and is essential for the actions of interleukin-10 (IL-10) (Murray, 2006).
Surprisingly, despite its critical roles in other tissues, conditional deletion of STAT3 in T cells revealed no overt developmental deficits (Takeda et al., 1998). Even though Stat3−/− T cells were reported to have reduced IL-6-dependent proliferation in vitro, mice lacking Stat3 in their T cells had normal thymic development and peripheral T cell compartments (Akaishi et al., 1998). After activation, CD4+ T cells can differentiate into specialized subsets in the periphery, which are required for proper immune regulation and host defense. In addition to previously recognized T helper 1 (Th1) and Th2 fates, naive CD4+ T cells can also differentiate into Th17 and regulatory T (Treg) cells; the balance of Th17 and Treg cells is now considered to be critical for host immunity and the preservation of tolerance ([Bettelli et al., 2008], [Stockinger and Veldhoen, 2007], [Weaver et al., 2007] and [Yang et al., 2008a]). Cytokines that activate STAT3, including IL-6, IL-21 and IL-23, are important drivers of Th17 cell differentiation and promote immunity against extracellular bacteria and fungi ([Aggarwal et al., 2003], [Bettelli et al., 2006], [Nishihara et al., 2007] and [Nurieva et al., 2007]). Accordingly, we and others have shown that STAT3 plays a critical role in the differentiation of CD4+ T cells into Th17 cells ([Chen et al., 2006], [Laurence et al., 2007], [Mathur et al., 2007], [Wei et al., 2007] and [Yang et al., 2007]). Patients with Job's or Hyper IgE Syndrome (HIES) have dominant-negative mutations of STAT3 and are deficient in Th17 cells, underscoring the close link between STAT3 and Th17 cells ([Holland et al., 2007], [Ma et al., 2008], [Milner et al., 2008] and [Minegishi et al., 2007]).
The human inflammatory bowel diseases (IBDs) comprise a wide spectrum of disorders, characterized by chronic inflammation of the intestine, and several lines of evidence point to the potential involvement of STAT3 and Th17 cells in disease pathogenesis. Genome-wide association studies have identified polymorphisms in the IL23R, JAK2, and STAT3 genes associated with increased susceptibility to IBDs ([Barrett et al., 2008] and [Duerr et al., 2006]). Furthermore, phosphorylated STAT3 and IL-17 are features of the inflammatory response in IBDs and colitis models, but the requirement for STAT3 in models of colitis, particularly with respect to T cell-dependent pathology, has not been explored ([Fujino et al., 2003] and [Suzuki et al., 2001]). Moreover, despite the link between STAT3 and models of Th17 cell-mediated diseases, our understanding of how STAT3 contributes to immunoregulation in terms of its gene targets is remarkably limited ([Harris et al., 2007] and [Liu et al., 2008]).
Using a T cell-transfer model of colitis, we found that STAT3 is essential for driving both colitis and systemic inflammation not only by modulating Th17 and Treg cell differentiation but also by allowing T cell homeostatic expansion. To comprehensively delineate STAT3 targets in T cells, we employed chromatin immunoprecipitation followed by massive parallel sequencing (ChIP-Seq). We found that STAT3 binds most of the genes shown to promote Th17 cell fate determination, as well as many genes involved in T cell survival and proliferation. Thus, STAT3 has diverse, yet critical functions in the differentiation, proliferation, and survival of T cells in the setting of chronic inflammatory disease.
Results
STAT3 Is Required for T Cell-Dependent Colitis
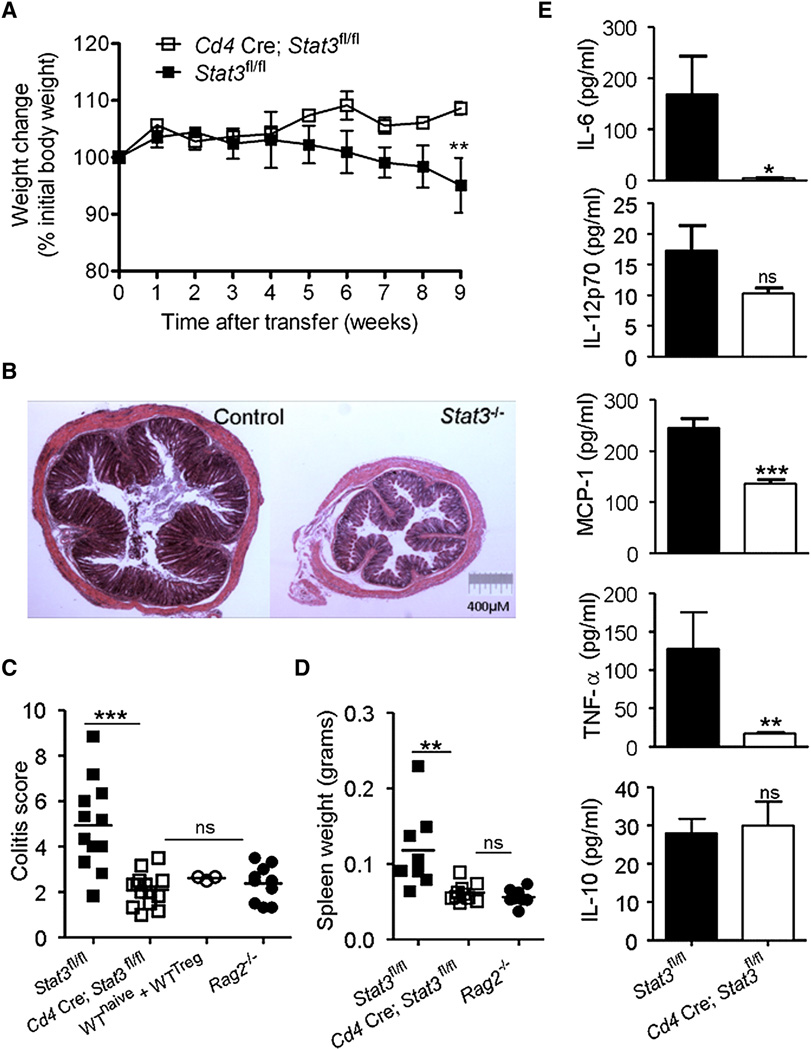
To dissect the involvement of STAT3 in chronic intestinal inflammation, we used a T cell-transfer colitis model in which lymphopenic Rag2−/− mice were reconstituted with CD4+CD45RBhiCD25− (RBhi) naive T cells from control (Stat3fl/fl) or Stat3−/− (Cd4 Cre; Stat3fl/fl) mice and monitored for development of inflammatory disease. As expected, mice reconstituted with control T cells began to lose weight at 5 weeks, whereas mice that received Stat3−/− T cells continued to gain weight (Figure 1A). We confirmed clinical signs of colitis by evaluating colonic inflammation histologically. Mice that received control T cells developed marked inflammation in the colon at 9 weeks (Figures 1B and 1C). In contrast, mice that received Stat3−/− T cells had no colonic inflammation and were indistinguishable from unreconstituted Rag2−/− controls or Rag2−/− mice that received both control naive and Treg cells.
Figure 1. Intrinsic Requirement for STAT3 in T Cell-Dependent Colitis.
(A–E) CD4+CD45RBhiCD25− naive T cells from control (Stat3fl/fl) or Stat3−/− (Cd4 Cre; Stat3fl/fl) mice were transferred into Rag2−/− mice, which were monitored for evidence of colitis.
(A) Weight loss (percentage of initial weight at day 0) was calculated for each mouse over 9 weeks. Data show mean (±SEM) weight changes for each group (n = 4–5 mice) and are representative of four independent experiments.
(B and C) Colitis was assessed histologically at 9 weeks and severity was scored by markers of inflammation (Izcue et al., 2008). Each point represents an individual mouse and the data are pooled from three independent experiments. Unreconstituted Rag2−/− mice and mice that received naive T cells with CD4+CD25+ Treg cells were included as controls.
(D and E) Systemic inflammation was assessed by measuring (D) spleen weights and (E) mean (±SEM) concentrations of inflammatory cytokines and chemokines in the serum. Data are representative of two independent experiments. *p < 0.05, **p < 0.005, ***p < 0.0005.
In addition to localized colonic pathology, transfer of naive T cells into lymphopenic hosts induces systemic inflammation manifested by splenic enlargement and elevated serum levels of inflammatory cytokines and chemokines. Accordingly, we found that Rag2−/− mice reconstituted with control T cells developed splenomegaly, associated with the presence of IL-6, IL-12p70, MCP-1, and TNF-α in the serum (Figures 1D and 1E). In contrast, mice reconstituted with Stat3−/− T cells had normal-sized spleens and significantly lower serum concentrations of inflammatory cytokines, whereas concentrations of IL-10 were similar to controls (Figure 1E). STAT3 was therefore critical for driving not only the tissue-specific pathology in the colon but also systemic inflammatory responses.
STAT3 Is Required for Th17 Cell Differentiation in Colitis
STAT3 is necessary for Th17 cell differentiation in vitro and has been implicated in Th17 cell-mediated pathology in other models of autoimmunity. To test whether STAT3 is critical for Th17 cell generation in T cell-transfer colitis, we harvested cells from the colon lamina propria (LP), mesenteric lymph nodes (MLNs), and spleens of Rag2−/− mice at 9 weeks after reconstitution with control or Stat3−/− naive T cells and measured intracellular IL-17A protein in CD4+ T cells by flow cytometry. Although IL-17A-producing T cells were present in the colon LP, MLNs, and spleen of Rag2−/− mice that received control T cells, the proportion and absolute number of IL-17A+ T cells was significantly reduced in all tissues of Rag2−/− mice reconstituted with Stat3−/− T cells (Figures 2A and 2B and Figure S1A available online). Interferon-gamma (IFN-γ) is another cytokine commonly induced during colitis and implicated in IBD pathogenesis ([O'Connor et al., 2009] and [Powrie et al., 1994]). We did not observe a reduction in the proportion of IFN-γ-producing CD4+ T cells in any of the tissues of mice reconstituted with Stat3−/− naive T cells compared to controls (Figure 2C). On the contrary, a significantly higher proportion of IFN-γ+ CD4+ T cells were present in the spleens of mice that received Stat3−/− T cells; however, the absolute number of IFN-γ+ T cells in the tissues was not significantly different between the two groups (Figure S1B). Thus, our data support the conclusion that STAT3 is necessary for IL-17A but not IFN-γ production by CD4+ T cells in this model of colitis.
Figure 2. Loss of STAT3 Abrogates Th17 but not Th1 Cell Responses.
(A–C) Rag2−/− mice were injected with naive T cells from control (Stat3fl/fl) or Stat3−/− (Cd4 Cre; Stat3fl/fl) mice.
(A) IL-17A production by CD4+ T cells in the colon LP, MLNs, and spleen was measured.
(B) The overall proportion of IL-17A+ T cells represents data pooled from two independent experiments.
(C) The proportions of IFN-γ+ CD4+ T cells in the tissues of Rag2−/− mice were enumerated and pooled from two independent experiments. See also Figure S1. *p < 0.05, ***p < 0.0001.
STAT3 Inhibits Regulatory T Cell Conversion
Treg cells are a specialized population of CD4+ T cells that express the forkhead/winged-helix protein Foxp3, and they dampen inflammatory responses and prevent autoimmunity (Josefowicz and Rudensky, 2009). In addition to eliciting inflammation, both IL-23 and IL-6 inhibit Treg cell differentiation and can thereby contribute to immune pathology ([Izcue et al., 2008] and [Korn et al., 2008]). Because IL-6-mediated inhibition of Foxp3 expression in vitro is STAT3-dependent (Yao et al., 2007), we assessed the impact of STAT3 on Treg cell homeostasis in the intestine in vivo, both at the steady state and in the context of inflammatory disease. We first examined the frequency of Foxp3+ T cells in unperturbed Cd4 Cre; Stat3fl/fl mice and littermate control Stat3fl/fl mice but found that both had equivalent proportions and absolute numbers of Foxp3+ T cells in their tissues (Figures 3A and 3B). These findings argue that STAT3 has little effect on Foxp3 expression in the absence of inflammation.
Figure 3. STAT3 Inhibits Regulatory T Cell Conversion in Colitis.
(A) The proportions and (B) absolute numbers (mean ± SEM) of Foxp3-expressing CD4+ T cells in the tissues of control (Stat3fl/fl) or Stat3−/− (Cd4 Cre; Stat3fl/fl) mice are shown. Data are representative of three independent experiments.
(C and D) Colitis was induced in Rag2−/− mice as described in Figure 1. Intracellular Foxp3 expression in the tissues is shown in (C), and in (D), proportions of Foxp3+ cells among CD4+ T cells represent data pooled from four independent experiments. See also Figure S2. *p < 0.05, **p < 0.01.
To investigate the importance of STAT3 in Treg cell differentiation in the setting of inflammation, we examined the conversion of Stat3−/− naive T cells into Foxp3+ Treg cells in the colitis model. In Rag2−/− mice reconstituted with control naive T cells, a small proportion of CD4+ T cells expressed Foxp3 in all of the tissues examined (Figures 3C and 3D). Conversely, we found a significantly greater proportion of Foxp3+ T cells in colons and spleens of Rag2−/− mice that received Stat3−/− T cells. The absolute numbers of Foxp3+ T cells were not different; however, we attribute this to poor expansion of Stat3−/− T cells (discussed below, Figure S2A). Similar proportions of IL-10+ T cells were also present in both groups of mice (Figure S2B). Thus, STAT3 appeared to inhibit the conversion of naive T cells into Foxp3+ cells in vivo in the inflammatory setting. In addition to the paucity of IL-17-producing cells, these data provide another explanation for why mice reconstituted with Stat3−/− T cells did not develop colitis or systemic disease.
STAT3 Directly Regulates Most Genes Involved in Th17 Cell Differentiation
Our data point to nonredundant, essential roles for STAT3 in regulating the balance between Th17 and Treg cell differentiation in vivo. To better define how STAT3 contributes to this decision, we identified STAT3 targets genome-wide using transcriptional profiling and chromatin immunoprecipitation coupled with massive parallel sequencing (ChIP-Seq). We found that STAT3 bound to more than 3000 genes and among its targets, we identified many genes implicated in Th17 cell differentiation (Table S1).
As previously reported, we found that STAT3 bound to the Il17a and Il21 promoters (Figure 4A) (Chen et al., 2006 Z. Chen, A. Laurence, Y. Kanno, M. Pacher-Zavisin, B.M. Zhu, C. Tato, A. Yoshimura, L. Hennighausen and J.J. O'Shea, Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells, Proc. Natl. Acad. Sci. USA 103 (2006), pp. 8137–8142. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (161)
Figure 4. STAT3 Is Directly Involved in Multiple Aspects of Th17 Cell Differentiation.
(A) ChIP-Seq was performed on Th17 cells with antibodies against phospho-STAT3 (second panel) and histone 3 lysine 4 trimethylation (H3K4me3) (lower panels). The locations of conserved noncoding sequences (CNSs) are shown in the top panel. The arrows indicate transcription directionality. Scales are constant for all genes and islands. See also Figure S3 and Table S1.
(B and C) The effect of STAT3 on gene expression in control or Stat3−/− Th17 cells was determined by (B) microarray analysis and (C) quantitative PCR.
[Chen et al., 2006], [Nurieva et al., 2007] and [Wei et al., 2007]). Additionally, STAT3 bound to the Il17f promoter and the intergenic region of the Il17a and Il17f locus. In fact, intergenic STAT3 binding sites resided within conserved noncoding sequences (CNSs) and contained STAT consensus motifs (Figure 4A and Figure S3A). We also found STAT3 bound at multiple sites in the Il6ra gene (Figure 4A) and at sites in the Il23r and the Ccr6 genes (Table S1).
Gene expression is regulated by transcription factor binding and epigenetic modifications that control accessibility of the chromatin to transcriptional machinery (Kouzarides, 2007). Epigenetic changes also affect cell lineage commitment and phenotypic stability by controlling transcription of essential genes (Goldberg et al., 2007). To assess the role of STAT3 in regulating epigenetic modifications, we cultured WT and Stat3−/− naive T cells for 3 days under Th17 cell-polarizing conditions (Figure S3B) and measured histone 3 lysine 4 trimethylation (H3K4me3), a mark found at active gene loci, by ChIP-Seq (Figure 4A and Table S1). We found that in WT T cells, STAT3-bound genes Il17a, Il17f, Il21, and Il6ra all contained H3K4me3 marks; however, these marks were absent or reduced in Stat3−/− T cells. Therefore, STAT3 regulates positive epigenetic modifications of its target genes, and likely contributes to stability of the Th17 phenotype.
To assess whether STAT3 binding and H3K4me3 correlated with modulation of gene expression, we performed transcriptional profiling using microarray and quantitative PCR. Expression of IL17a, IL21, and Il6ra was markedly impaired in Stat3−/− T cells compared to controls, particularly when the cells were differentiated with IL-6 and TGF-β (Figure 4B). We also found that expression of IL17f (not present on Affymetrix chips) was decreased in Stat3−/− T cells compared to control T cells (Figure 4C). In contrast, expression of Ccr6 was not changed (Figure 4B). Thus, both STAT3 binding and increased H3K4me3 to genes were associated with STAT3-dependent expression.
Th17 cell differentiation is associated with expression of several transcription factors, including RORγt, RORα, RUNX1, BATF, IRF-4, AHR, and c-Maf ([Bauquet et al., 2009], [Brüstle et al., 2007], [Ivanov et al., 2006], [Schraml et al., 2009], [Veldhoen et al., 2008], [Yang et al., 2008b] and [Zhang et al., 2008]). We assessed STAT3 binding to these transcription factor genes to determine whether it directly contributes to their regulation. RORγt, encoded by Rorc, is the master regulator of Th17 cell differentiation and STAT3 bound to sites within the first intron (Figure 4A). STAT3 also bound the related retinoid receptor gene, Rora, as well as the Batf, Irf4, Ahr, and Maf genes (Figure 4A). We also found that STAT3 regulated permissive H3K4me3 marks on Rorc, Rora, and Batf but not on Irf4, Ahr, or Maf. This correlated well with the magnitude of their differential expression determined by microarray (Figure 4B). Overall, these findings support a direct function for STAT3 in regulating not only gene expression but also epigenetic modifications of key Th17 cell-related genes.
Lymphopenia-Induced Expansion of CD4+ T Cells Requires STAT3
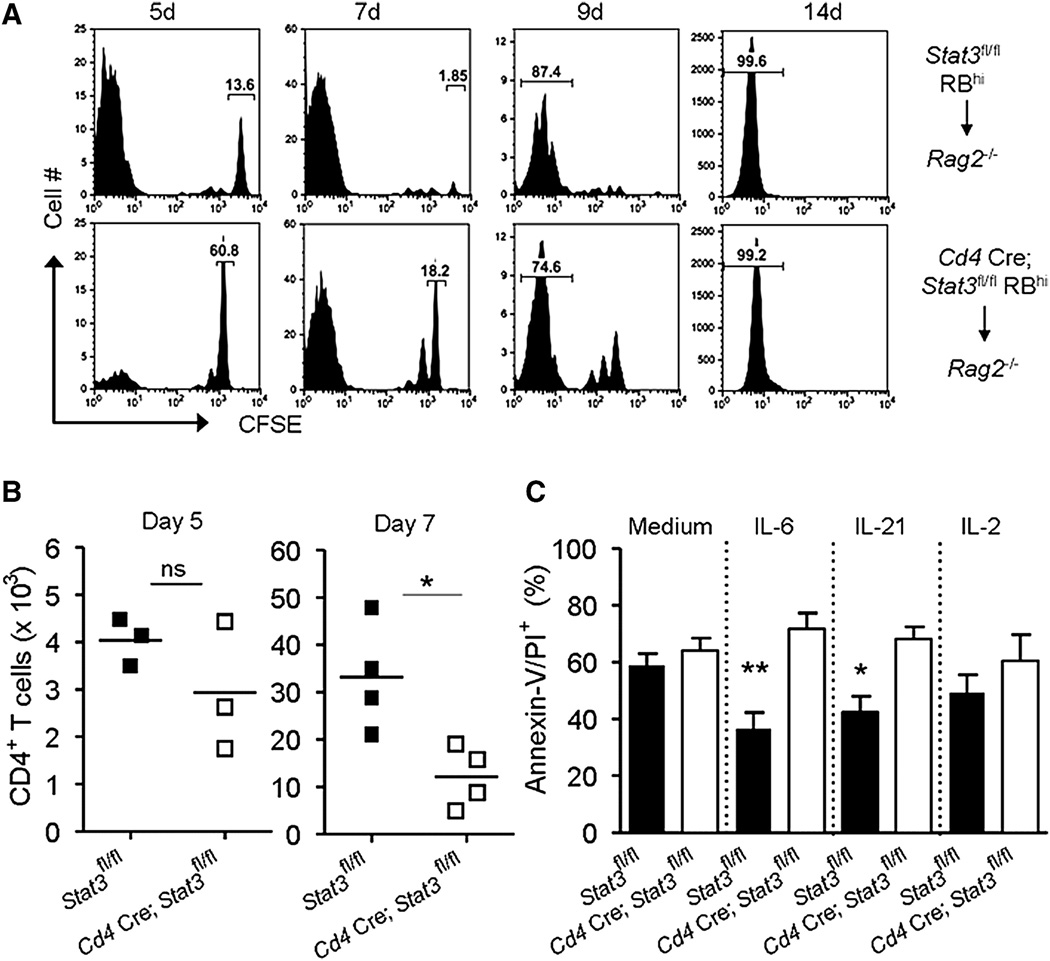
An important hallmark of disease in the colitis model is the accumulation of activated CD4+ T cells (Powrie et al., 1994). As expected, transfer of control (Stat3fl/fl) naive T cells into Rag2−/− mice resulted in the accumulation of CD4+ T cells in the colon LP, MLNs, and spleen after 9 weeks (Figure 5A). However, there were significantly fewer Stat3−/− CD4+ T cells in tissues of Rag2−/− recipient mice. Although this correlated well with a lack of inflammation (Figure 1), we were interested in determining why Stat3−/− T cells failed to accumulate in the lymphocyte-deficient environment.
Figure 5. STAT3 Is Required in Lymphopenia-Induced Expansion of CD4+ T Cells.
(A) Rag2−/− mice were reconstituted with naive (RBhi) T cells from control (Stat3fl/fl) or Stat3−/− (Cd4 Cre; Stat3fl/fl) mice. The absolute numbers of CD4+ T cells in the tissues were quantified. Data are pooled from two independent experiments.
(B) The absolute numbers of CD4+ T cells in the tissues of Rag2−/− mice cotransferred with a 1:1 ratio of wild-type (CD45.1) and Stat3−/− naive T cells. See also Figure S4.
(C) The absolute numbers of CD4+ T cells in the spleen and LN of Rag2−/− mice 2 weeks after reconstitution with wild-type (CD45.1) or Stat3−/− T cells are shown. Data are representative of two independent experiments.
(D and E) Bone marrow (BM) cells from wild-type (CD45.1) or Stat3−/− mice were transferred into irradiated Rag2−/− mice. The proportions of CD4+ and CD8+ T cells in the thymus and spleen are shown in (D), and as shown in (E), absolute numbers (mean ± SEM) of CD4+ cells in the spleen and LN were determined.
(F) BM cells from wild-type (CD45.1) and Stat3−/− mice were cotransferred into irradiated Rag2−/− mice in a 1:1 ratio and the absolute numbers of CD4+ T cells in the tissues were calculated. *p < 0.05, **p < 0.01, ***p < 0.0005.
Because Stat3−/− T cells did not elicit inflammation in the Rag2−/− mice, we could not exclude that the lack of inflammatory cytokines in the system contributed to their poor accumulation within the tissues. To address this possibility, we induced inflammation in Rag2−/− mice by concurrently transferring equal numbers of wild-type (CD45.1) and Stat3−/− naive T cells (Figures S4A and S4B). Substantial numbers of wild-type T cells accumulated in tissues of reconstituted mice, but significantly fewer Stat3−/− CD4+ T cells were present (Figure 5B). This indicates that STAT3 has an important, cell-intrinsic role in driving T cell expansion in the context of inflammation and its importance is particularly evident in a competitive setting.
In a chronically lymphopenic Rag2−/− mouse, commensal bacteria and self-antigens along with cytokines drive the initial rapid expansion of naive T cells (Surh and Sprent, 2008). Therefore, the inability of Stat3−/− T cells to accumulate could indicate a role for STAT3 in early expansion, prior to the onset of inflammatory disease. To test this, we transferred wild-type (CD45.1) or Stat3−/− naive T cells into Rag2−/− mice. At 2 weeks, we found significantly fewer Stat3−/− CD4+ T cells in the spleen and lymph nodes of the Rag2−/− recipients than wild-type T cells (Figure 5C). Importantly, neither group of recipients had signs of inflammation, supporting an essential function for STAT3 in early lymphopenia-induced T cell expansion.
Proliferation of T cells also occurs as part of normal lymphoid tissue development and in this context does not elicit inflammation. To examine the importance of STAT3 in homeostatic CD4+ T cell expansion in the setting of stem cell transplantation, we reconstituted irradiated Rag2−/− mice with bone marrow (BM) from wild-type (CD45.1) or Cd4 Cre; Stat3fl/fl mice and examined lymphoid compartments after 8 weeks. Although there were comparable proportions of CD4+ T cells in the thymus, there were significantly fewer CD4+ T cells in the spleens and lymph nodes of mice reconstituted with BM from Cd4 Cre; Stat3fl/fl mice (Figures 5D and 5E). We next used a model of competitive bone marrow reconstitution to examine the ability of Stat3−/− T cells to accumulate in the presence of normal T cells. Specifically, we irradiated Rag2−/− mice and cotransferred bone marrow cells from wild-type (CD45.1) and Cd4 Cre; Stat3fl/fl mice at a 1:1 ratio (Figure 5F). We found that the absolute number of Stat3−/− CD4+ T cells was significantly less than wild-type T cells in the peripheral lymphoid organs and in the thymus. These findings support an essential function for STAT3 in regulating CD4+ T cell proliferation not only in pathologic settings but also during their development from precursor cells; in the presence of wild-type T cells, Stat3−/− T cells are at a clear competitive disadvantage.
STAT3 Plays a Nonredundant Role in CD4+ T Cell Survival and Proliferation
The inability of Stat3−/−T cells to accumulate under both lymphopenic and homeostatic conditions could be due to defects in proliferation, increased apoptosis, or both. To address these possibilities, we first assessed the ability of carboxyfluorescein succinimidyl ester (CFSE)-labeled naive T cells to proliferate after transfer into Rag2−/− mice. We examined the CD4+ T cells in the spleen and found that the majority of control (Stat3fl/fl) T cells (86.4%) underwent multiple cell divisions after 5 days (Figure 6A). In contrast, fewer than half (39.2%) of Stat3−/− T cells had divided, as indicated by CFSE dilution. By 7 days, essentially all of the control T cells had divided (98.11%), whereas 18% of Stat3−/− T cells remained quiescent. However, by 14 days, both the Stat3−/− and control T cells had undergone multiple rounds of division; similar levels of T cell proliferation occurred in the MLNs (Figure S5A). Thus, we can conclude that STAT3 influences the rate of CD4+ T cell proliferation and loss of Stat3 delays but does not abrogate T cell proliferation. As a result, fewer Stat3−/− T cells accumulate in tissues, especially at early time points (Figure 6B and Figure S5B).
Figure 6. STAT3 Has a Nonredundant Role in T Cell Survival and Proliferation.
(A and B) Control (Stat3fl/fl) or Stat3−/− (Cd4 Cre; Stat3fl/fl) naive T cells were transferred into Rag2−/− mice and cell division was assessed by CFSE dilution.
(B) The absolute numbers of CD4+ TCR-β+ cells in the spleen at day 5 and 7 are shown; data are representative of two independent experiments.
(C) Control and Stat3−/− CD4+ T cells were cultured for 3 days in medium or with the indicated cytokines and the proportion of apoptotic (Annexin-V/PI positive) cells was determined by flow cytometry. Means (±SEM) of apoptotic cells from three independent experiments are shown. See also Figure S5. *p < 0.05; **p < 0.005.
Apoptotic cells are rapidly cleared in vivo, making it difficult to quantify cell death of adoptively transferred cells. Therefore, we further assessed cell viability in vitro by culturing naive T cells with anti-CD3 and anti-CD28 with or without STAT3-activating cytokines, IL-6 and IL-21, measuring incorporation of propidium iodide (PI) and surface expression of Annexin-V. In the absence of exogenous cytokines (medium), the proportion of apoptotic control and Stat3−/− T cells was roughly equivalent (Figure 6C and Figure S5C). Addition of IL-6 or IL-21 to cultures of control T cells significantly reduced the proportion of apoptotic cells. In contrast, Stat3−/− T cells were not responsive to IL-6 or IL-21 and had poor viability in all conditions. Activation of T cells with anti-CD3 and anti-CD28 results in endogenous IL-2 production. Consequently, addition of excess exogenous IL-2, which preferentially activates STAT5, did not enhance the viability of either the Stat3−/− or control T cells. Taken together, these findings support the contention that STAT3 regulates T cell survival and proliferation, which in turn affect their ability to expand and elicit inflammation in a lymphopenic host.
Direct Involvement of STAT3 in Regulating Cell Survival and Proliferation Genes in CD4+ T Cells
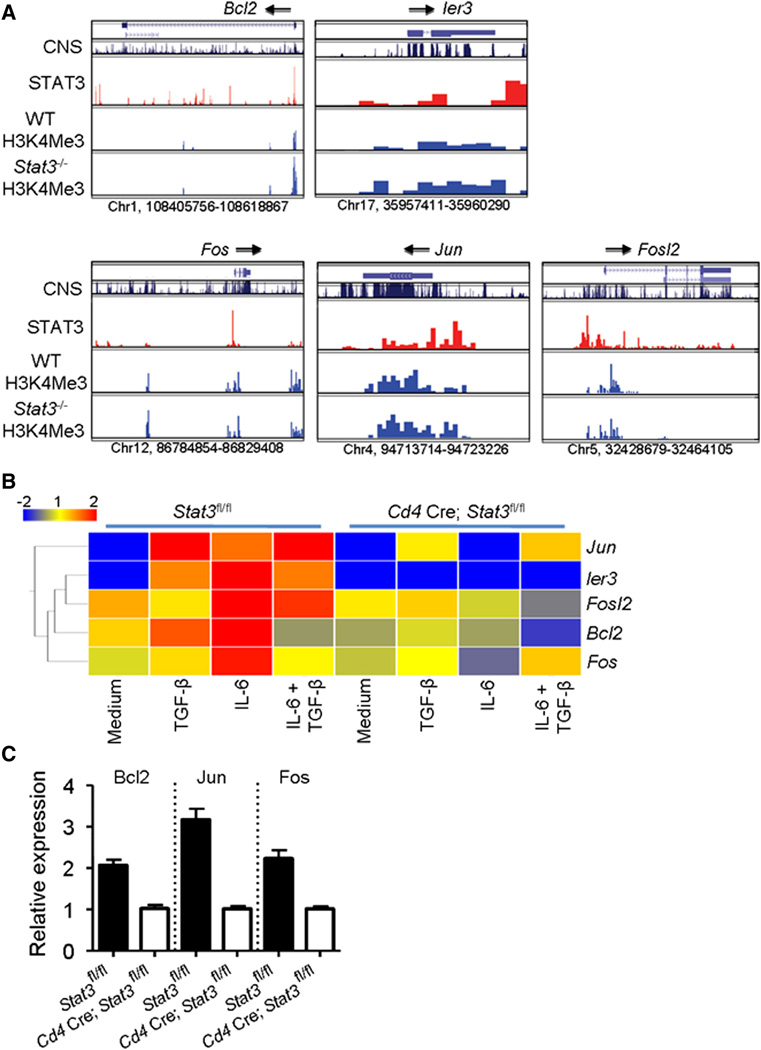
STAT3 has been previously implicated in the regulation of genes involved in cell survival and proliferation ([Bourillot et al., 2009] and [Hirano et al., 2000]). However, there is a paucity of data showing that STAT3 directly regulates expression of cell survival and antiapoptotic genes in CD4+ T cells. From our ChIP-Seq analysis, we found direct STAT3 binding to multiple survival genes, including Bcl2, Ier3, Fos, Jun, and Fosl2 (Figure 7A). Additionally, transcriptional profiling revealed that these genes were inducible in T cells by IL-6, but their induction was abrogated in Stat3−/− T cells (Figures 7B and 7C). We also noted that H3K4me3, permissive marks of active transcription, were present on all genes but were not absent in Stat3−/− T cells. These findings indicate that although expression of these genes is decreased in a STAT3-dependent manner, they remain poised for transcription. This fits with our conclusion that STAT3 is important but not the only factor involved in regulating transcription of survival genes.
Figure 7. STAT3 Directly Regulates Genes Involved in CD4+ T Cell Survival and Proliferation.
(A) STAT3 binding (second panel) and H3K4me3 modifications (lower panels) were determined by ChIP-Seq. Conserved noncoding sequences are shown in the top panel. Arrows indicate directionality of transcription. See also Table S1.
(B and C) Control or Stat3−/− T cells were cultured for 3 days with the indicated cytokines and the effect of STAT3 on gene expression was assessed by (B) microarray and (C) quantitative PCR (mean ± SEM; n = 2 samples).
Taken together, the present data establish that STAT3 directly regulates genes that control survival and proliferation of CD4+ T cells. This important function of STAT3 is manifested most dramatically when T cells undergo expansion both in pathologic settings like the colitis model and in noninflammatory lymphopenic environments. However, in contrast to its roles in Th17 cell differentiation, STAT3 is not absolutely required for T cell survival and proliferation; other factors can evidently contribute to these processes.
Discussion
STAT3 has now been genetically and functionally linked to the pathogenesis of human IBD as well as other autoimmune diseases, but our understanding of STAT3's function in T cells has been surprisingly limited. Here, we have shown that Stat3 expression by T cells is essential for their colitogenic activity in a well-described model of colitis and systemic inflammation. STAT3 contributes to T cell-mediated colitis in a number of ways. First, it is essential for the differentiation of Th17 cells in vivo; in its absence T cells are nonpathogenic and tend to divert toward a Treg cell fate. Second, STAT3 mediates signals that are crucial for CD4+ T cell proliferation and survival in vivo. Using ChIP-Seq and transcriptional profiling, we identified multiple genes that are direct targets of STAT3 in T cells. These included nearly all of the genes known to regulate Th17 cell differentiation as well as genes that promote survival and proliferation. Thus, STAT3 has diverse and nonredundant functions in T cells, both in pathogenic and homeostatic settings.
The critical function for STAT3 in Th17 cell differentiation has been studied both in vitro and in animal models of autoimmunity. Here, we provide direct evidence that STAT3 regulates the expression of multiple genes essential for Th17 cell development. We previously showed that STAT3 binds to the Il17a promoter, but the present data show that STAT3 also binds the Il17f promoter and the intergenic region of these genes (Akimzhanov et al., 2007). In the future it will be important to further examine how STAT3 binding to intergenic sites contributes to its regulation of Il17a and Il17f gene transcription. We also provide evidence that STAT3 controls chromatin accessibility at the Il17a, Il17f, and Il21 genes by regulating histone modifications. This adds another layer of complexity to STAT3's role as a transcriptional regulator.
Equally importantly, we have shown that STAT3 binds to and regulates the expression of several major transcription factor genes that drive Th17 cell differentiation. These include the genes encoding RORγt, RORα, BATF, IRF4, AHR, and c-Maf: factors important for promoting the program that leads to selective IL-17 production. Therefore, our findings support a broad role for STAT3 as a critical regulator of the Th17 cell transcriptional program. It will be useful to compare and contrast the array of targets for each of these transcription factors, including STAT3, to better define the hierarchy of interactions.
Previous in vitro studies have suggested that STAT3 inhibits Treg cell differentiation. In the present study, we found that STAT3 did not affect Treg cells in unperturbed mice. By contrast, STAT3 did inhibit naive T cell conversion into Foxp3-expressing cells in the context of inflammation, which is consistent with previous work showing that both IL-6 and IL-23 inhibit regulatory T cell differentiation in autoimmune disease ([Izcue et al., 2008] and [Korn et al., 2008]). However, it remains uncertain how this negative regulation occurs. STAT3 could regulate Foxp3 indirectly by enhancing RORγt expression, the latter being a factor that binds to and suppresses Foxp3 function. Nonetheless, Foxp3 levels are reduced in vitro by exposure to IL-6, supporting a specific inhibitory role for STAT3 in Treg cells. The mechanism underlying this effect is unclear. We also found proportionally more IFN-γ+ T cells in the absence of STAT3, but again, STAT3 did not bind the Ifng gene. However, these findings might be explained by reduced IL-17 production, which has recently been reported to inhibit IFN-γ production in the setting of colitis (O'Connor et al., 2009).
In addition to effects on T cell differentiation, we also found that STAT3 controls T cell proliferation and survival. We were surprised by the extent to which STAT3 regulates this aspect of T cell function given that the original description of mice lacking Stat3 in T cells reported no effects on thymic or peripheral T cell development. We found that Stat3−/− T cells exhibited reduced proliferation and failed to accumulate in the spleen or colon after transfer to Rag2−/− mice. Strikingly, even the presence of wild-type T cells and development of severe colitis did not allow Stat3−/− T cells to expand. These results reveal a nonredundant and cell-intrinsic requirement for STAT3 in T cell expansion during an inflammatory response. Previous reports have suggested that IL-6 is important for T cell survival in the context of inflammation (Atreya et al., 2000). Additionally, IL-23 is suggested to promote expansion of Th17 cells in peripheral tissues, and specifically in the colon (data not shown) (McGeachy et al., 2009). Therefore, at least in pathologic settings, IL-6 and IL-23 are likely candidates for driving STAT3-dependent expansion of CD4+ T cells.
The requirement for STAT3 in CD4+ T cell expansion was also clearly evident in the absence of any overt inflammatory response and particularly in the setting of competitive bone marrow reconstitution. This is unexpected because IL-7 and IL-15, which predominantly activate STAT5, have been proposed to be the principal drivers of lymphopenia-induced proliferation (Surh and Sprent, 2008). Thus, the STAT3-activating cytokine responsible for this aspect of T cell homeostasis remains to be identified.
Regardless of the cytokines responsible, it is clear from our data that STAT3 directly regulates many antiapoptotic and pro-proliferative genes in T cells. It has been previously suggested that Bcl-2 is activated by STAT3, but we provide the first evidence that STAT3 directly regulates Bcl2 expression. Fos and Jun dimerize to form the activator protein 1 (AP-1) transcription factor complex, which plays an important role in T cell activation. Interestingly, previous work has indicated that STAT3 cooperates with both Jun and Fos to enhance transcription of IL-6-responsive genes (Schuringa et al., 2001). It is intriguing therefore that STAT3 can directly regulate expression of Jun and Fos and so potentially function in a positive feedback loop to regulate gene expression. It will be important to examine these pathways in more detail to gain insights into how they work together to promote T cell expansion. Equally, the finding that proliferation is not abrogated in the absence of STAT3 and that epigenetic modifications of prosurvival genes are regulated independently of STAT3 argues that this factor acts in concert with other transcription factors to regulate proliferation and cell survival.
In conclusion, our data highlight many functions of STAT3 in CD4+ T cells not previously appreciated and emphasize the pleiotropic nature of this transcription factor. In addition to multiple roles in regulating Th17 cell differentiation, STAT3 contributes to T cell homeostasis, particularly in the context of inflammation. Coupled with STAT3's known immunosuppressive function in innate and epithelial cells, our findings support a complex role for STAT3 in the immune system ([Pickert et al., 2009] and [Takeda et al., 1999]). Given the recent genetic data implicating STAT3 in a number of human autoimmune diseases, it is clear that more careful dissection of the many functions of this key gene is warranted.
Experimental Procedures
Mice
Cd4 Cre; Stat3fl/fl mice were generated as previously described ([Lee et al., 2002] and [Wei et al., 2007]). B6.129 Rag2−/− mice and B6.SJL-CD45.1 mice were from Taconic Farms. All mice were handled and housed in specific pathogen-free facilities in accordance with the guidelines of the NIH Animal Care and Use Committee.
Adoptive Transfer Model of Colitis
CD4+ T cells were enriched from the spleen and lymph nodes of control (Stat3fl/fl) and Stat3−/− (Cd4 Cre; Stat3fl/fl) mice with an AutoMACS cell separator (Miltenyi Biotec), stained with PerCP Cy5.5 anti-CD4, FITC anti-CD25, and PE anti-CD45RB (all obtained from BD Biosciences), and naive CD4+ CD25− CD45RBhi T cells were purified (>99%) by cell sorting (Moflo,Dako Cytomation or FACSAria, BD Biosciences). CD4+CD45RBhiCD25− naive T cells (4 × 105) were injected intravenously (i.v.) into age- and sex-matched Rag2−/− mice and intestinal inflammation was monitored. Control mice were coinjected with 4 × 105 naive T cells and 1 × 105 CD4+CD45RBloCD25+ Treg cells or left unreconstituted. Mice were sacrificed at 8–10 weeks when significant weight loss occurred in the control groups. Sections of the proximal, mid-, and distal colon were fixed in buffered 10% formalin and stained with hematoxylin and eosin (H&E) (Histoserv). Inflammation was assessed histologically by methods previously described ([Izcue et al., 2008] and [Uhlig et al., 2006]). Serum was collected and protein concentrations of IL-6, IL-12p70, MCP-1, TNF-α and IL-10 were measured by cytometric bead assay (BD Biosciences).
Isolation of Leukocyte Subpopulations and Flow Cytometry
Cell suspensions were prepared from the thymus, spleen, mesenteric lymph nodes (MLNs), and colon lamina propria (LP) by methods previously described (Uhlig et al., 2006). PerCP Cy5.5- or FITC- conjugated anti-CD4, PE-conjugated anti-TCR-β, FITC-conjugated anti-CD45.1, and APC-conjugated anti-CD8 were from BD Biosciences. PE or FITC anti-Foxp3 staining set was from eBioscience. For intracellular cytokine staining, cells were cultured for 4 hr with PMA (50 ng/ml), ionomycin (1 mg/ml) and BFA (GolgiPlug; BD Biosciences), stained for CD4, fixed, and permeabilized in buffers from BD Biosciences. Cells were stained with PE or APC anti-IFN-γ, PE anti-IL-17A (all from BD Biosciences) or Alexa Fluor-647 anti-IL-17A (eBioscience), or appropriate isotype controls (BD Biosciences).
For in vitro activation, naive T cells were cultured for 3 days with 5 µg/ml each of platebound anti-CD3 and anti-CD28 (BD Biosciences) alone or with 100 U/ml IL-2 (Peprotech), 10 ng/ml IL-6 (eBioscience) and 10 ng/ml IL-21(R&D). FITC or APC anti-Annexin-V antibody and propidium iodide (BD Biosciences) were used for apoptosis assessment. Cells were acquired on the FACSCaliber (BD Biosciences) and analyzed with FlowJo 8.7.3 software (Tree Star).
Homeostatic Proliferation and Stem Cell Transplantation
For assessing early in vivo T cell expansion, Rag2−/− mice were injected i.v. with 1 × 106 naive T cells from WT (CD45.1), Stat3fl/fl, or Cd4 Cre; Stat3fl/fl mice. Spleen and lymph nodes were harvested at 2 weeks and CD4+ T cell populations were assessed by flow cytometry with CD4, CD45.1, and TCR-β antibodies. Alternatively, naive CD4+ T cells (1× 106) were purified by cell sorting, labeled with carboxyfluorescein succinimidyl ester (CFSE, 1 mM, Molecular Probes) and injected i.v. into Rag2−/− mice. After 5, 7, 9 or 14 days, spleen and MLN cells were harvested, labeled with CD4 and TCR-β antibodies and CFSE dilution was quantified by flow cytometry. In competitive studies, Rag2−/− mice were injected i.v. with 4 × 105 naive T cells from Cd4 Cre; Stat3fl/fl and WT (CD45.1) mice, mixed at a 1:1 ratio. At 10 weeks, CD4+ T cell populations were assessed by flow cytometry as above.
For stem cell transplantation, bone marrow was isolated from Cd4 Cre; Stat3fl/fl and WT (CD45.1) mice and 3 × 106 cells were injected i.v. into gamma-irradiated Rag2−/− mice (900 rads). For the competitive reconstitution experiments, Cd4 Cre; Stat3fl/fl and WT (CD45.1) bone marrow cells were mixed at a 1:1 ratio (3 × 106 cells total) and injected i.v. into irradiated Rag2−/− mice. Eight weeks later, spleen, thymus, and lymph nodes were harvested and T cell populations were assessed by flow cytometry with CD4, CD8, and CD45.1 antibodies.
ChIP-Seq and Transcriptional Profiling
Control (Stat3fl/fl) and Stat3−/− (Cd4 Cre; Stat3fl/fl) naive CD4+CD44−CD62L+ T cells were isolated and sorted on the FACSAria flow cytometer. Cells were cultured for 72 hr with CD3 (5 µg/ml) and CD28 (5 µg/ml), IL-6 (10 ng/ml), and TGF-β (2.5 ng/ml) with blocking antibodies to 10 µg/ml IL-2, 10 µg/ml IL-4, and 10 µg/ml IFN-γ. Cells were restimulated for 1 hr with IL-6 (10 ng/ml) and then processed for ChIP-Seq as previously described (Wei et al., 2009). Antibodies against histone H3K4me3 (ab8580, Abcam) and phosphoserine (PS)-STAT3 (Cat. #9134, Cell Signaling) were used. The ChIP DNA fragments were blunt-ended, ligated to the illumina adaptors, and sequenced with the Illumina Genome Analyzer II. Details of how the level of STAT3 binding and histone modifications were calculated for all genes can be found in Table S1.
For microarray analysis, control or Stat3−/− naive T cells were activated for 3 days with anti-CD3, anti-CD28 alone, or with IL-6 (10 ng/ml) and TGF-β (2.5 ng/ml). RNA was purified (MirVana miRNA isolation kit, Ambion), reverse transcribed, and biotinylated (MessageAmp II-Biotin Enhanced Kit, Ambion). Labeled aRNA (10 µg) was hybridized to GeneChip Mouse Genome 430 2.0 arrays (Affimetrix) in accordance with the manufacturer's protocols. Expression was determined with GeneChip Operating Software (GCOS; v1.1.1) and analysis was performed with GeneSpring software GX 10.0.1 (Agilent Technologies).
For quantitative PCR, RNA was purified as above and reverse transcribed (Applied Biosystems). Taqman Master Mix and primers from Applied Biosystems were used for qPCR reactions. Each assay was performed in triplicate with an ABI Fast detection system and gene expression levels were normalized to 18 s rRNA and internal controls (medium alone) with ΔΔCt calculations.
Statistics
GraphPad Prism 4.0 was used for statistical analysis (unpaired, two-tailed, t test with a confidence interval of 95%). Differences were considered statistically significant when p < 0.05.
Supplementary Material
Acknowledgments
We thank J. Simone, J.Lay, K. Zaal, and the NIAMS LACU Staff for their assistance. We thank P. Ahern, M. Asquith, and A. Izcue for advice and critical reading of the manuscript. This research was funded by the Intramural Research Program at NIAMS. W.T.W. is supported by National Institutes of Health grant #1 K22 AR53953-01. F.P. is supported by funding from the Wellcome Trust.
References
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (618) [DOI] [PubMed] [Google Scholar]
- Akaishi H, Takeda K, Kaisho T, Shineha R, Satomi S, Takeda J, Akira S. Defective IL-2-mediated IL-2 receptor alpha chain expression in Stat3-deficient T lymphocytes. Int. Immunol. 1998;10:1747–1751. doi: 10.1093/intimm/10.11.1747. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (41) [DOI] [PubMed] [Google Scholar]
- Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. View Record in Scopus | Cited By in Scopus (63) [DOI] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in crohn disease and experimental colitis in vivo. Nat. Med. 2000;6:583–588. doi: 10.1038/75068. View Record in Scopus | Cited By in Scopus (433) [DOI] [PubMed] [Google Scholar]
- Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, et al. NIDDK IBD Genetics Consortium and Belgian-French IBD Consortium and Wellcome Trust Case Control Consortium, Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat. Genet. 2008;40:955–962. doi: 10.1038/NG.175. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (1474) [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourillot PY, Aksoy I, Schreiber V, Wianny F, Schulz H, Hummel O, Hubner N, Savatier P. Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells. 2009;27:1760–1771. doi: 10.1002/stem.110. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (11) [DOI] [PubMed] [Google Scholar]
- Brüstle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 2007;8:958–966. doi: 10.1038/ni1500. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (126) [DOI] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: A landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. Article | PDF (452 K) | View Record in Scopus | Cited By in Scopus (179) [DOI] [PubMed] [Google Scholar]
- Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. View Record in Scopus | Cited By in Scopus (71) [DOI] [PubMed] [Google Scholar]
- Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pract. Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. View Record in Scopus | Cited By in Scopus (116) [DOI] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (409) [DOI] [PubMed] [Google Scholar]
- Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, et al. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (143) [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. Article | PDF (1004 K) | View Record in Scopus | Cited By in Scopus (852) [DOI] [PubMed] [Google Scholar]
- Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. Article | PDF (1306 K) | View Record in Scopus | Cited By in Scopus (83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. Article | PDF (573 K) | View Record in Scopus | Cited By in Scopus (72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. Article | PDF (510 K) | View Record in Scopus | Cited By in Scopus (1296) [DOI] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. Article | PDF (1336 K) | View Record in Scopus | Cited By in Scopus (309) [DOI] [PubMed] [Google Scholar]
- Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. Article | PDF (425 K) | Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (20) [DOI] [PubMed] [Google Scholar]
- Levy DE, Lee CK. What does Stat3 do? J. Clin. Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 2008;180:6070–6076. doi: 10.4049/jimmunol.180.9.6070. View Record in Scopus | Cited By in Scopus (31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. View Record in Scopus | Cited By in Scopus (133) [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (134) [DOI] [PubMed] [Google Scholar]
- Murray PJ. STAT3-mediated anti-inflammatory signalling. Biochem. Soc. Trans. 2006;34:1028–1031. doi: 10.1042/BST0341028. View Record in Scopus | Cited By in Scopus (24) [DOI] [PubMed] [Google Scholar]
- Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, et al. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int. Immunol. 2007;19:695–702. doi: 10.1093/intimm/dxm045. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (42) [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. View Record in Scopus | Cited By in Scopus (412) [DOI] [PubMed] [Google Scholar]
- O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. View Record in Scopus | Cited By in Scopus (65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. Article | PDF (4234 K) | View Record in Scopus | Cited By in Scopus (602) [DOI] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. View Record in Scopus | Cited By in Scopus (25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuringa JJ, Timmer H, Luttickhuizen D, Vellenga E, Kruijer W. c-Jun and c-Fos cooperate with STAT3 in IL-6-induced transactivation of the IL-6 respone element (IRE) Cytokine. 2001;14:78–87. doi: 10.1006/cyto.2001.0856. Abstract | PDF (444 K) | View Record in Scopus | Cited By in Scopus (31) [DOI] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr. Opin. Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. Article | PDF (264 K) | View Record in Scopus | Cited By in Scopus (207) [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. Article | PDF (1878 K) | View Record in Scopus | Cited By in Scopus (96) [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J. Exp. Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. View Record in Scopus | Cited By in Scopus (215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: Generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 1998;161:4652–4660. View Record in Scopus | Cited By in Scopus (283) [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. Article | PDF (469 K) | View Record in Scopus | Cited By in Scopus (504) [DOI] [PubMed] [Google Scholar]
- Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. Article | PDF (758 K) | View Record in Scopus | Cited By in Scopus (191) [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (184) [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (496) [DOI] [PubMed] [Google Scholar]
- Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. Article | PDF (1733 K) | View Record in Scopus | Cited By in Scopus (116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (224) [DOI] [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. Article | PDF (1742 K) | View Record in Scopus | Cited By in Scopus (159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. Article | PDF (1595 K) | View Record in Scopus | Cited By in Scopus (250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (70) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.