Abstract
The human gastrointestinal tract harbors a complex and abundant microbial community reaching as high as 1013–1014 microorganisms in the colon. This endogenous microbiota forms a symbiotic relationship with their eukaryotic host and this close partnership helps maintain homeostasis by performing essential and non-redundant tasks (e.g. nutrition/energy and, immune system balance, pathogen exclusion). Although this relationship is essential and beneficial to the host, various events (e.g. infection, diet, stress, inflammation) may impact microbial composition, leading to the formation of a dysbiotic microbiota, further impacting on health and disease states. For example, Crohn’s disease and ulcerative colitis, collectively termed inflammatory bowel diseases (IBD), have been associated with the establishment of a dysbiotic microbiota. In addition, extra-intestinal disorders such as obesity and metabolic syndrome are also associated with the development of a dysbiotic microbiota. Consequently, there is an increasing interest in harnessing the power of the microbiome and modulating its composition as a means to alleviate intestinal pathologies/disorders and maintain health status. In this review we will discuss the emerging relationship between the microbiota and development of colorectal cancer as well as present evidence that microbial manipulation (probiotic, prebiotic) impacts disease development.
Keywords: microbiota, inflammation, probiotics, colorectal cancer
1. Introduction
Higher vertebrates have intimately co-existed with a myriad of microorganisms throughout evolution and this relationship has proven mutually beneficial for both entities. Although numerous external and internal surfaces are colonized by microorganisms, the human gastrointestinal (GI) tract represents the most abundant reservoir of microbes. It is estimated that the intestinal tract harbors over 100 trillion bacteria regrouped in about 1000 species. The collective prokaryote community outnumbers eukaryote cells in the human body by a factor of 10 and their collective genome is 100 times more abundant than the host genome [1]. We are now just beginning to understand the genomic composition and contribution of the microbiota to intestinal homeostasis. This vast microbial community is rapidly acquired through close contact with the mother microbiota including passage through the birth canal, breast feeding and skin contact [2]. These various modes of microbial exposures clearly impact on the intestinal microbiota composition since naturally delivered infants have a larger number of bifidobacteria species than did babies delivered by caesarean section [3]. In addition, Bifidobacterium are the prevalent genus in breast-fed infants whereas Enterococci prevail in formula-fed infants [4]. The functional implication of early differential colonization is unclear but tantalizing observations suggest a relationship with disease susceptibility [5–7]. For example, the intestinal microbiota in children from Europe and rural Africa who are exposed to a modern western diet and a rural diet respectively, have significant differences in microbial composition. The major difference was that rural Africa children showed a significant enrichment in Bacteroidetes and depletion in Firmicutes when compared to European children [7]. These differences may well explain the higher incidence of IBD in Europe than Africa [8]. In addition, microbial composition is modulated by numerous extrinsic factors such as diet, age, medication (antibiotics, NSAIDs), treatment (radiation, surgery), stress and disease [9]. However, our current lack of knowledge about microbiome core (microbial community) plasticity prevents the establishment of a health/disease microbial threshold. Nevertheless, it is clear that various biological and physical situations impact microbial composition, with some of these changes linked to disease development. It is not surprising that diseases of GI origin such as IBD and colorectal cancer have been subjected to increasingly intense investigation of the microbiota.
2. Endogenous microbiota, risk factors and development of colorectal cancer
Colorectal cancer (CRC) is among the most common worldwide cancers, accounting for over 1 million cases and about half a million deaths annually [10]. The incidence rates of CRC are higher in the Western world but are rapidly increasing in developing countries. The main two contributing factors to the occurrence of CRC are genetic (15%) and environmental factors [11]. In addition, inflammatory conditions have been shown to favor the development of CRC as patients with IBD have an annual risk increase of 1%/year (10 years post-diagnosis) [12]. Another emerging factor involved in CRC susceptibility is the intestinal microbial composition. Since the microbiota impacts numerous physiological functions related to cancer risk including control of epithelial cell proliferation/differentiation, production of essential nutrients and/or bioactive food components, prevention of overgrowth of pathogenic organisms and stimulation of intestinal immunity, recent efforts have been directed at defining the microbiota of CRC. Although few studies have tackled microbial composition in human CRC, recent reports have indicated differences between the microbiome of patients and healthy subjects. For example, the stool of CRC patients harbored increased Bacteroides-Prevotella populations compared to normal controls [13]. Another study investigated bacterial communities present at the intestinal mucosal surface of patients with adenoma. Interestingly, at the genus level, patients with adenomas showed increased abundance of Dorea spp., Faecalibacterium spp. and lower proportions of Bacteroides spp. and Coprococcus spp. than non-adenoma subjects [14]. This finding revealed that alterations in bacterial community composition is associated with CRC; although at this point, it is unclear whether these changes preceded disease onset or simply reflect a consequence of the disease.
Interestingly, factors influencing CRC development (age, NSAID, diet) were also demonstrated to impact on microbial composition. For example, Mäkivuokko et al. [15] reported that there was a significant reduction in overall numbers of microbes in elderly subjects compared with young adults. Moreover, lower numbers of Firmicutes and an increased proportion of Bacteroidetes in the elderly were observed in this study. Furthermore, the total number of microbes was higher and lower numbers of Collinsella spp. were evident in the elderly subjects who take NSAID compared both with young adults and the elderly who do not take NSAID on a regular basis. Enck et al. [16] reported that individual bacterial species consistently and significantly increased with age (E. coli, Enterococci spp.), decreased at an older age (Bacteroides spp.), or were stable throughout the life span (Lactobacilli, Bifidobacteria). These results suggest that the use of NSAIDs, combined with age, may influence the process of tumorigenesis by changing the composition of the intestinal microbiota.
High animal protein intake and a high fat diet are additional risk factors associated with colon cancer. Americans who have a high colon cancer risk typically consume a high-animal protein and fat diet, whereas Africans, who have the lowest colon cancer risk, consume a staple diet of maize meal, rich in resistant starch. O’Keefe et al. [17] analyzed the contents of colon in native Africans and African Americans and Caucasian Americans. Butyrate is produced by specific colonic bacteria, predominantly Clostridia clusters XIVa and IV of Firmicutes, from food residues such as dietary fiber or resistant starch. Their results demonstrated that the colonic content of butyrate, and all of the chief short chain fat acids (SCFA) produced by bacterial fermentation of undigested carbohydrates, were significantly higher in native Africans compared with African Americans and Caucasian Americans. In another study [18], fecal colony counts of 7-adehydroxylating bacteria were higher and Lactobacilli were lower in African Americans compared to Caucasians and colonic crypt cell proliferation rates were also higher in African Americans. These studies provide evidence that higher dietary intakes of animal products may alter the gut microbiota and therefore play a key role in carcinogenesis.
Obesity is a closely related risk factor of human CRC. Numerous studies [19–23] have verified the association between an alteration of the dominant phyla of bacteria in the gut and body weight, both in humans and animal models. The gut microbes have been shown to impact insulin resistance, inflammation, and adiposity via interactions with epithelial and endocrine cells. The intestinal epithelia cells (IECs) have a complex and mutually beneficial relationship with the gut flora. The bacteria metabolize some nutrition components in the gut, e.g. carbohydrates; in turn, the IECs metabolize the short-chain fatty acids and use them as a source of energy. In fact, the gut microbial community is essential for processing dietary polysaccharides. Bäckhed et al. [24] found that adult germ-free (GF) C57BL/6 mice given a normal microbiota harvested from the cecum of conventionally raised animals results in a 60% increase in body fat content and insulin resistance within 14 days, despite reduced food intake. Furthermore, colonization of GF mice with an “obese microbiota” results in a significantly greater increase in total body fat than colonization with a “lean microbiota”. These results identify the gut microbiota as an additional contributing factor to the pathophysiology of obesity.
The increasing understanding of GI barriers, and the adjacent GI microbiota provide evidence that these two functional entities are key to achieving and maintaining gut health [25,26]. Gut microbiota contribute to the maintenance of an intact GI barrier and disruption of this barrier can impact inflammatory and allergic diseases. The GI barrier refers to a functional entity consisting of epithelial defense and metabolic functions, the mucosal immune system and the enteric nervous system (ENS) [25]. The formation of an intestinal mucus layer is an important physical barrier protecting the intestinal epithelium against invasive micro-organisms. The mucus layer is formed by various mucin proteins which are a family of heavily glycosylated proteins including mucin1, mucin 2, mucin 3a and others. Mucins are produced mostly by Goblet cells. Mucin 2 (MUC2), the principal component of intestinal mucus, along with small amounts of related-mucin proteins, polymerizes into a gel that provides an insoluble mucous barrier that serves to protect the intestinal epithelium, Another important epithelial cell involved in bacteria-host interactions are the Paneth cells, which are critical for the maintenance of the intestinal barrier by producing zinc and antimicrobial peptides. The combined action of Goblet and Paneth cells has a significant impact on microbial composition and GI barrier function [27, 28]. Altered MUC2 expression and/or glycosylation leads to accompanying intestinal pathologies, including IBD and colon cancer [29]. The generation of a MUC2 mutant mouse model, alone or in combination with an Apc mutation, had unique pathophysiologic features useful for dissecting complex relationships between tumor development and chronic inflammation [30]. Paneth cells directly sense enteric bacteria through cell-autonomous MyD88-dependent toll-like receptor (TLR) activation, triggering expression of multiple antimicrobial factors [31]. E-cadherin is a major component of adherens junctions, which play a key role in intestinal homeostasis and barrier function. Impaired expression of E-cadherin was linked to maturation and positioning of Goblet cells and Paneth cells in the murine small intestine and colon [32].
Cheese whey protein is rich in the amino acids of threonine and cysteine and promotes synthesis of mucin. In the model of dextran sulfate sodium (DSS), which is an irritant that causes colonic inflammation (colitis) by eroding the mucosal barrier, feeding rats with a diet containing cheese whey protein reduced gene expression of inflammation markers and increased fecal mucin secretion and fecal lactobacilli and bifidobacteria counts [33]. Furthermore, the number of Goblet cells in the small intestine increased by the simultaneous incubation of Bifidobacterium bifidum IATA-ES2 with wheat gluten proteins, IFN-γ and enterobacteria using the rat intestinal loop model [34]. Thus, dysbiosis could be involved in the damage of the GI barrier and it is clear that further research is needed to fully elucidate the complex interactions between the microbiota and the GI barrier.
3. Gut microbiota, innate signaling, inflammation and colon carcinogenesis
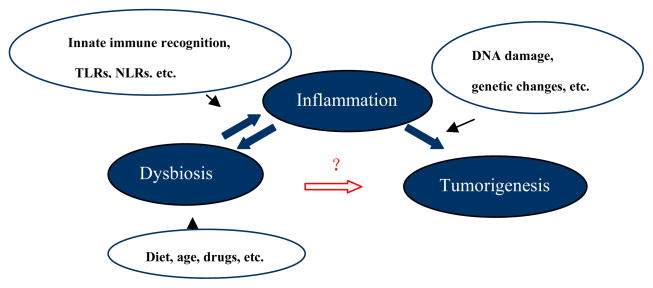
The inter-relation between bacteria and inflammation is complex as bacteria and inflammation could mutually impact upon each other. This interaction can, in turn, modulate development of CRC (see Fig. 1). As diagrammed in the figure, the types and level of activation of the various innate sensors can influence the gene expression pathways, level of inflammation and the consequences of these changes on DNA damage and chromatin alterations, which when combined with host genetic factors can lead to tumorigenesis. However, tumorigenesis is complicated and a linear association between dysbiosis-inflammation-tumorigenesis is not fully supported by other studies. Swidsinski et al [35] showed that adherent/invasive E. coli strains are found in high abundance on the colonic mucosa of patients with colorectal carcinoma and adenoma but not normal colonic mucosa, leading to hypothesis that E. coli colonization in the colon may indicate a specific microorganism can be responsible for malignant pathology. In contrast, Burn et al. [36] reported that the use of aspirin and/or resistant starch had no effect in hereditary nonpolyposis colon cancer. Moreover, while microsatellite instability (MSI) have been documented in dysplasia and development of CRC, Goel et al. [37] reported that treating ulcerative colitis patients with mesalazine or Escherichia coli Nissle for a year did not show improvement in the prevalence of MSI in the distal colon. Likewise, as suggested by other studies [13, 14], additional regulatory pathways exist between dysbiosis and tumorigenesis, other than inflammation.
Figure 1.
The evolving relationship between colon dysbiosis, inflammation and tumorigenesis. Experimental models have addressed the individual and collective role of microorganisms in the development of inflammation and CRC. Dysbiosis-inflammation-tumorigenesis provides an important model of carcinogenesis in the human colon. Please refer to the text for a more detailed discussion.
Experimental models have addressed the individual and collective role of microorganisms in the development of inflammation and CRC. For example, Enterotoxigenic Bacteroides fragilis (ETBF) secretes B. fragilis toxin (BFT) and causes human inflammatory diarrhea but also asymptomatically colonizes a proportion of the human population. In another animal study [38], GF IL-10−/− or WT mice were monoassociated with Bifidobacterium animalis subsp. The bacteria caused mild colitis in monoassociated IL-10−/− mice, whereas the intestinal tracts of WT animals remained free of inflammation. The result suggested a potential pathogenic role for this commensal bacterial species in a susceptible host. Wu et al. [39] indicated that mice chronically colonized with ETBF developed colitis and induced colonic tumors in multiple intestinal neoplasia (MIN) mice. Uronis et al. [40], showed that conventionalized IL10−/− mice exposed to the procarcinogenic compound azoxymethane (AOM) developed spontaneous colitis and colorectal carcinomas while AOM-WT mice were colitis-free and mostly developed low grade dysplasia. Interestingly, the bacterium Bacteroides vulgatus caused low level inflammation in IL10−/− mice, resulting in reduced colorectal tumors as compared to conventionalized IL10−/− mice. Moreover, GF AOM-treated IL10−/− mice showed no intestinal inflammation or CRC. The results support the concept that specific microbial entities differently impact on the development of intestinal inflammation and CRC. The molecular mechanism by which bacteria affect the development of intestinal inflammation and cancer is unclear but may be related to the triggering of a series of innate sensors responsible for microbial detection.
A number of studies have addressed the role of the host innate immune system in regulating carcinogenesis. The most studied innate sensors relating to colitis and CRC are the Nod-like receptors (NLR) and the Toll-like receptors (TLR). The interaction of gut microbiota and epithelial cells is an active process, in which NLRs and TLRs are likely directly involved. Colitis-associated cancer (CAC) is a colorectal disease where cancer arises in patients suffering from long bouts of chronic intestinal inflammation. CAC can be modeled in mice by injection of the AOM and by repeated exposure to DSS. Using a mouse model system of CAC (AOM-DSS), Chen et al. [41] demonstrated that tumors increased in Nod1-deficient mice compared to wild type mice. In addition, ApcMIN/+Nod1−/− mice, which harbor a mutation in Apc and are also deficient in Nod1, developed more tumors. This result suggests that Nod1 pathway could enhance the tumor-promoting effect of attenuated Wnt signaling. Additionally, depletion of the gut microbiota using antibiotic treatment suppressed tumor development in Nod1-deficient mice. Furthermore, NLRP3-signaling from immune cells was recently shown to play a protective role against colitis and CAC [42]. Additionally, NLRP6 has recently been shown to prevent development of CAC [43]. All together, these data support a role of the host innate immune signaling pathways in the regulation of inflammation-mediated colon cancer development.
Toll-like receptors (TLRs) belong to the Toll-like receptor/interleukin-1 receptor (TLR/IL-1R) superfamily which is defined by a common cytoplasmic Toll/interleukin-1 receptor (TIR) domain. The single immunoglobulin IL-1 receptor-related molecule (SIGIRR), a negative regulator for Toll-IL-1R signaling, plays a critical role in gut homeostasis, intestinal inflammation, and colitis-associated tumorigenesis by maintaining the microbial tolerance of the colonic epithelium [44–47]. Cells from SIGIRR-deficient mice showed enhanced activation in response to either IL-1 or certain Toll ligands [44]. In human colonic samples, SIGIRR was expressed mainly in IECs at levels significantly higher in inactive compared to active mucosa. In mice, colonic SIGIRR expression decreased rapidly after colitis development and returned gradually to basal levels [46]. SIGIRR may exert its inhibitory effect through blocking the molecular interface of TLR4, TLR7 and the MyD88 adaptor mainly via its BB-loop region [47]. Moreover, the innate adapter protein MyD88 prevents the development of CAC by transmitting IL-18 receptor signaling [48]. In contrast, TLR4 promotes the development of CRC in the AOM/DSS model [49]. TLR4 is expressed on CD4+ T cells and TLR4 triggering in CD4+ T cells affects their phenotype and their ability to provoke intestinal inflammation. In a model of spontaneous colitis [50], IL10−/−TLR4−/− mice displayed accelerated development of disease, with signs of overt colitis as early as 8 week of age, when compared with IL10−/− and IL10−/−TLR9−/− mice which did not develop colitis by 8 months. Mechanistically, Lipopolysaccharide (LPS) stimulation of TLR4-bearing CD4+ T cells inhibited ERK1/2 activation upon subsequent cell stimulation. These data highlighted the complex relationship between the microbiota and the status of inflammation and CRC in the host and supported the model where susceptibility to develop CRC is modulated by the microbiota and by the repertoire of host innate sensors. Consequently, modulation of the intestinal microbiota using probiotics or prebiotics could influence the development of tumors.
4. Probiotics and colon cancer
The term “probiotics” has been in use for several decades and the Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation defined the term in 2001 as “live micro-organisms which confer a health benefit on the host when administered in adequate amounts”[51]. Lactic acid bacteria and Bifidobacteria are the most common types of microbes used as probiotics, while certain yeasts and bacilli may also be beneficial to the host. The immunomodulatory effect of probiotic bacteria has been postulated by Metchnikoff over 100 years ago [52]. There has been an increased interest in the scientific community on the protective roles of probiotics on intestinal diseases, especially IBD and colon carcinogenesis. A meta study [53] in 2006 showed that most of the studies (epidemiological and interventional) evaluating the effect of probiotics administration on the incidence of CRC and/or of precursor lesions had positive results although the authors of this publication argue that “There are no positive data from interventional studies so far.”
4.1. In vitro studies
The precise mechanisms by which probiotics inhibit colon cancer may involve multiple pathways, including cell cycle, reactive oxygen species (ROS), apoptosis, production of specific bacterial enzymes and effects on the host metabolome. Polyamine synthesis is an early event during the G1 phase of the cell cycle and is necessary for cells to initiate their proliferative processes. Orlando et al. [54] found that Lactobacillus GG administration induced a significant reduction in polyamine biosynthesis in both the HGC-27 and DLD-1 cancer cell lines. The antiproliferative effect of the probiotic microorganisms was observed after 24 hours of the treatment. The anti-proliferative capabilities of probiotics may also relate to their ability to adhere to cells. Lee et al. [55] found that Bacillus polyfermenticus SCD was strongly adherent to Caco-2 cells and inhibited the growth of colon cancer cells in a dose dependent manner. Kim et al. [56] assessed the anticancer activity and bacterial enzyme inhibition of Bifidobacterium adolescentis SPM0212. The strain inhibited the proliferation of three human colon cancer cell lines: HT-29, SW 480, and Caco-2 and also dose-dependently inhibited TNF-α production and changes in cellular morphology. This specific bacterial strain could inhibit harmful fecal enzymes, including β-glucuronidase, β-glucosidase, tryptophanase, and urease.
The interaction of gut microbiota and epithelial cells is an active process, in which some proinflammatory cytokines are induced by probiotics from epithelial cells. TLRs are likely directly involved in this process. Paolillo et al. [57] reported Caco-2 cells exposed to L. plantarum bacteria significantly induced human beta-defensin 2 (HBD-2) mRNA expression and HBD-2 secretion in a time dependent manner compared to controls. The L. plantarum-induced increase in HBD-2 expression was inhibited by anti-TLR-2 neutralizing antibodies. Some strains of Lactobacilli can stimulate macrophages and dendritic cells to secrete IL-12, a cytokine that plays a key role in activating innate immunity. Shida et al [58] examined the IL-12-inducing ability in mouse peritoneal macrophages of 47 Lactobacillus strains belonging to 10 species. Almost all strains belonging to the Lactobacillus casei group or to Lactobacillus fermentum induced high levels of IL-12 and phagocytosis of the Lactobacilli was necessary for the IL-12 induction. Their results also demonstrated that Lactobacillus strains having a rigid cell wall resistant to intracellular digestion effectively stimulate macrophages to induce IL-12.
4.2. In vivo experiments
Probiotics contribute to the development of the mucosal immune system by influencing the innate inflammatory response and reducing mucosal inflammation. Probiotics also act through effects on dendritic, epithelial cells, native T cells in the lamina propria of the gut and can thus influence adaptive immunity. Previous studies have demonstrated the anti-inflammatory effect of probiotics on intestinal inflammation. Lactic acid bacteria are present in many foods, such as yogurt, and are frequently used as probiotics to improve some biological functions of the host. Perdigon et al. [59] found that yogurt induced a great reduction in the inflammatory immune response and inhibited tumor growth in 1,2-dimethylhydrazine(DMH) treated BALB/c mice. In addition, they observed an increase in the IgA-secreting cells and in CD4+ T lymphocytes. A yogurt based formulation containing microencapsulated live probiotic bacterial cells has been used in colon cancer prevention and therapy studies. Urbanska et al. [60] studied the properties of microencapsulated probiotic bacterial cells in a yogurt formulation in MIN mice carrying a germline APC mutation. Daily oral administration of the microencapsulated Lactobacillus acidophilus resulted in significant suppression of colon tumor incidence, tumor multiplicity, and reduced tumor size. Furthermore, treated animals exhibited fewer GI intra-epithelial neoplasia with a lower grade of dysplasia in tumors. The immunomodulating and immunostimulating properties of yogurt and fermented milks have also been well documented. Yogurt feeding itself was correlated with increased or decreased levels of some cytokines, such as TNFα, IFN-γ and interleukins [59–60]. Interestingly, Pagnini et al. [61] reported that probiotics may promote gut health through stimulation, rather than suppression, of the innate immune system. They reported that the multiple probiotic formulation VSL#3 (Streptococcus thermophilus, Bifidobacterium infantis, and Lactobacillus acidophilus) could prevent the onset of ileitis in the SAMP1/YitFc murine model by local stimulation of epithelial innate immune responses, i.e. increased production of epithelial-derived TNF-α and restoration of the epithelial barrier function in vivo. Leblanc et al. [62] reported that a yogurt diet given to BALB/c mice before and after the carcinogen 1, 2-dimethylhydrazine (DMH) treatment, inhibited tumor formation. In the DMH-yogurt group, cellular apoptosis increased during the treatment. Yogurt feeding induced TNF-α and IFN-γ expression in cells isolated from large intestine nodules and the expression of these cytokines also increased in cells obtained from Peyer’s Patches of the yogurt control group.
Another key player in colon inflammation is the presence of reactive oxygen species (ROS) and the antioxidant properties of probiotics may contribute to their broad effects [63]. Likewise, polyphenols from tea and other beverages such as red wine can play a role as possible chemopreventive agents by reducing oxidative damage, and result in an increase in gut colonization by some probiotics strains [64]. Park et al. [65] assessed the effects of Bacillus polyfermenticus on the antioxidant system and the process of colon carcinogenesis in male F344 rats. The rats fed with Bacillus polyfermenticus exhibited significantly lower numbers of aberrant crypt foci than were observed in the DMH treated group. Leukocytic DNA damage and plasma lipid peroxidation levels, as well as a lower plasma total antioxidant potential, recovered in response to supplementation with Bacillus polyfermenticus. These results indicated that Bacillus polyfermenticus could exert a protective effect on the antioxidant system and the process of colon carcinogenesis.
An underlying mechanism that might be involved in the activity of probiotics may be the induction of apoptosis. Apoptosis induction by commensal bacteria could possibly represent a physiologic “oncologic surveillance” mechanism for colonic proliferative disease prevention [66], but this hypothesis awaits further testing for confirmation.
5. Prebiotic and synbiotics
Prebiotics are non-digestible food ingredients that stimulate the growth and/or activity of bacteria in the digestive system in ways claimed to be beneficial to health. Synbiotics refer to nutritional supplements combining probiotics and prebiotics that are thought to act together; i.e. synergism. It has been suggested that a combination of a probiotic and a prebiotic, i.e. synbiotics, might be more active than either a probiotic or prebiotic alone in preventing CRC. A high olive oil-containing diet supplemented with a freeze-dried fruit and vegetable extract (OFV), increased the family Lachnospiraceae in Apc (MIN) mice and reduced the development of intestinal adenomas in this model [67]. As another example, Le Leu et al. [68] evaluated the effect of bacteria Bifidobacterium lactis and “resistant starch” (RS) and their combination (synbiotic) on their ability to protect against CRC. Bifidobacterium lactis utilizes the carbohydrate “resistant starch” (RS) as a substrate and up-regulates the acute apoptotic response to a carcinogen in the colon. Rats fed RS in combination with Bifidobacterium lactis showed a significantly lowered incidence and multiplicity of colonic neoplasms compared with the control group. No protection against cancer was seen in the group supplemented with only Bifidobacterium lactis. Interestingly, a fermented milk product containing Bifidobacterium animalis subsp. lactis DN-173 010 strain protects against development of colitis in the T-bet−/−; Rag2−/− mice through reduction of colitogenic microorganisms, microorganisms that favorthe development of colitis [69]. In addition, mice exposed to sialyl (alpha2,3) lactose-deficient milk were more resistant to DSS-induced colitis, which again is associated with the reduction of potentially colitogenic microorganisms[70]. In another diet based study, Lara-Villoslada et al. [71] reported short-chain fructooligosaccharides(SC-FOS) increased cecal Lactobacilli and Bifidobacteria counts as well as short-chain fatty acid (SCFA) production in healthy rats. In colitic rats, SC-FOS feeding caused a decrease of MPO activity, leukotriene B4 (LTB4) production and iNOS expression. This anti-inflammatory effect was evidenced by a significant reduction in the extent of colonic damage. Thus there is a growing body of data indicating that specific bacterial species, in combination with dietary changes/additions, can modulate overall gut inflammation. Long term studies are now needed to assess the impact of these mechanisms of reducing gut inflammation on CRC.
6. Clinical trials
Despite the experimental evidence in rodent models showing a beneficial effect of probiotics that inhibits the development of CRC, a systemic approach for the evaluation of probiotics leading to the substantiation of health claims in humans is very limited. A FAO/WHO Working Group has generated guidelines for the criteria and methodology for evaluation of probiotics in clinical trials [72]. Since then, several studies have been reported, generally in the form of randomized, double- blinded, measurement of efficacy and side effect compared with placebo. In one study the administration of Lactobacillus casei was tested as a method to prevent the occurrence of colorectal tumors [73]; the occurrence of tumors with a grade of moderate atypia or higher was significantly lower in the patient group after 2–4 years treated with Lactobacillus casei as compared to the control group. In a 12-week clinical trial [74] completed in 2007, polypectomized patients were treated with Lactobacillus rhamnosus GG (LGG) and Bifidobacterium lactis Bb12 (BB12) and oligofructose-enriched inulin. The treatment resulted significant changes in fecal flora of the patients as Bifidobacterium and Lactobacillus increased and Clostridium perfringens decreased. The intervention also significantly reduced colorectal proliferation in the patients. A two-period crossover study on 38 healthy men was reported with treatment periods of 4 weeks of Lactobacillus rhamnosus LC705 (LC705) together with Propionibacterium freudenreichii ssp shermanii JS (PJS) [75]. The administration of LC705 and PJS was followed by an increase in the fecal counts of Lactobacilli and Propionibacteria and a decrease in the activity of β-glucosidase with increasing counts of Propionibacteria. Recently, Gianotti et., al [76], reported in a prospective trial that in 31 CRC patients, Lactobacilli johnsonii (La1), but not Bifidobacterium longum (BB536), affects intestinal microbiota by reducing the concentration of pathogen and modulating the intestinal immune response. Thus, in clinical trials, probiotics are suggested to play a protection role in the initial process of carcinogenesis. However, it remains to be determined if long term administration of probiotics can result in significant changes in the incidence of CRC in humans. Recently, a cohot study with 12 years of follow-up on 45,241 volunteers determined that high yogurt intake was significantly associated with decreased CRC. risk, suggesting the long term administration of probiotics or probiotics formulations can reduce the incidence of CRC [77]. Some of the challenges faced in these studies will be the selection and standardization of the appropriate microorganisms, control for dietary intake, time and frequency of microorganism dosing and development of biomarkers that can be followed over the long term course of any such clinical trials.
7. Conclusions
There is a growing body of evidence that the gut microbiota contributes to colon tumorigenesis. Animal experiments have revealed that microorganisms can directly impact gut immunity and inflammation but statistically and biologically significant evidence of such effects is still needed in human trials. Probiotics can inhibit the inflammatory process by enhancing host immune responses, altering the bacterial phylotypes in the colon and impacting the gut metabolome. They may also have anti-tumor properties through direct anti-proliferative activity on tumor cells. However, further experimental models are needed to better understand the exact mechanisms involved in the effects of probiotics on the host. The results of these studies will hopefully lead to more rational and standardized approaches for the use of probiotics in the prevention and treatment of human colorectal cancer.
Research highlights.
Endogenous microbiota, risk factors and development of colorectal cancer
Gut microbiota, innate signaling, inflammation and colon carcinogenesis
Probiotics and synbiotics and their effects on initiation and development of colon cancer
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has been funded with federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E.
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitman William B, Coleman David C, Wiebe William J. Prokaryotes: The unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orrhage K, Nord CE. Factors controlling the bacterial colonization of the intestine in breast fed infants. Acta Paediatr Suppl. 1999;88:47–57. doi: 10.1111/j.1651-2227.1999.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 3.Biasucci G, Rubini M, Riboni S, Retetangos C, Morelli L, Bessi E. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86(Suppl 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Rubaltelli FF, Biadaioli R, Pecile P, Nicoletti P. Intestinal flora in breast- and bottle-fed infants. J Perinat Med. 1998;26:186–191. doi: 10.1515/jpme.1998.26.3.186. [DOI] [PubMed] [Google Scholar]
- 5.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 6.Ouwehand AC, Isolauri E, He F, Hashimoto H, Benno Y, Salminen S. Differences in Bifidobacterium flora composition in allergic and healthy infants. J Allergy Clin Immunol. 2001;108:144–145. doi: 10.1067/mai.2001.115754. [DOI] [PubMed] [Google Scholar]
- 7.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrokhyar F, Swarbrick ET, Irvine EJ. A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:2–15. doi: 10.1080/00365520150218002. [DOI] [PubMed] [Google Scholar]
- 9.Tennyson CA, Friedman G. Microecology, obesity, and probiotics. Curr Opin Endocrinol Diabetes Obes. 2008;15:422–427. doi: 10.1097/MED.0b013e328308dbfb. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 11.Ferlay J, Bray F, Pisani P, Parkin DM. IARC Cancer Base No.5 Version 2.0. Lyon, France: IARC Press; 2004. Globocan 2002: cancer incidence, mortality and prevalence worldwide. [Google Scholar]
- 12.Chambers WM, Warren BF, Jewell DP, Mortensen NJ. Cancer surveillance in ulcerative colitis. Br J Surg. 2005;92:928–936. doi: 10.1002/bjs.5106. [DOI] [PubMed] [Google Scholar]
- 13.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Van Nhieu JT, Furet JP. Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLoS One. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen XJ, Rawls JF, Randall T, Burcal L, Mpande CN, Jenkins N, Jovov B, Abdo Z, Sandler RS, Keku TO. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–147. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2010;103:227–234. doi: 10.1017/S0007114509991553. [DOI] [PubMed] [Google Scholar]
- 16.Enck P, Zimmermann K, Rusch K, Schwiertz A, Klosterhalfen S, Frick JS. The effects of ageing on the colonic bacterial microflora in adults. Z Gastroenterol. 2009;47:653–658. doi: 10.1055/s-0028-1109055. [DOI] [PubMed] [Google Scholar]
- 17.O’Keefe SJ, Ou J, Aufreiter S, O’Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139:2044–2048. doi: 10.3945/jn.109.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Keefe SJ, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J, Adada H, van der Merwe T. Why Do African Americans Get More Colon Cancer than Native Africans? J Nutr. 2007;137(1 Suppl):S175–182. doi: 10.1093/jn/137.1.175S. [DOI] [PubMed] [Google Scholar]
- 19.Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr. 2010;92:1023–1030. doi: 10.3945/ajcn.2010.29877. [DOI] [PubMed] [Google Scholar]
- 20.Shida K, Kiyoshima-Shibata J, Nagaoka M, Watanabe K, Nanno M. Induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J Dairy Sci. 2006;89:3306–3317. doi: 10.3168/jds.S0022-0302(06)72367-0. [DOI] [PubMed] [Google Scholar]
- 21.Roller M, Pietro Femia A, Caderni G, Rechkemmer G, Watzl B. Intestinal immunity of rats with colon cancer is modulated by oligofructose-enriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis. Br J Nutr. 2004;92:931–938. doi: 10.1079/bjn20041289. [DOI] [PubMed] [Google Scholar]
- 22.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 23.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 24.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bischoff SC. ‘Gut health’: a new objective in medicine? BMC Med. 2011;9:24. doi: 10.1186/1741-7015-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Wehkamp J, Fellermann K, Herrlinger KR, Bevins CL, Stange EF. Mechanisms of disease: defensins in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:406–415. doi: 10.1038/ncpgasthep0265. [DOI] [PubMed] [Google Scholar]
- 29.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006 Apr;1765(2):189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Yang K, Popova NV, Yang WC, Lozonschi I, Tadesse S, Kent S, Bancroft L, Matise I, Cormier RT, Scherer SJ, Edelmann W, Lipkin M, Augenlicht L, Velcich A. Interaction of Muc2 and Apc on Wnt signaling and in intestinal tumorigenesis: potential role of chronic inflammation. Cancer Res. 2008 Sep 15;68(18):7313–22. doi: 10.1158/0008-5472.CAN-08-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trülzsch K, Müller-Höcker J, Vogelmann R, Allgäuer M, Gerhard M, Steininger S, Wolf E, Kolligs FT. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One. 2010;5:e14325. doi: 10.1371/journal.pone.0014325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprong RC, Schonewille AJ, van der Meer R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: role of mucin and microbiota. J Dairy Sci. 2010;93:1364–1371. doi: 10.3168/jds.2009-2397. [DOI] [PubMed] [Google Scholar]
- 34.Cinova J, De Palma G, Stepankova R, Kofronova O, Kverka M, Sanz Y, Tuckova L. Role of intestinal bacteria in gliadin-induced changes in intestinal mucosa: study in germ-free rats. PLoS One. 2011;6:e16169. doi: 10.1371/journal.pone.0016169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swidsinski A, Khilkin M, Kerjaschki D, Schreiber S, Ortner M, Weber J, Lochs H. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998;115:281–286. doi: 10.1016/s0016-5085(98)70194-5. [DOI] [PubMed] [Google Scholar]
- 36.Burn J, Bishop DT, Mecklin JP, Macrae F, Möslein G, Olschwang S, Bisgaard ML, Ramesar R, Eccles D, Maher ER, Bertario L, Jarvinen HJ, Lindblom A, Evans DG, Lubinski J, Morrison PJ, Ho JW, Vasen HF, Side L, Thomas HJ, Scott RJ, Dunlop M, Barker G, Elliott F, Jass JR, Fodde R, Lynch HT, Mathers JC CAPP2 Investigators. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359:2567–2578. doi: 10.1056/NEJMoa0801297. [DOI] [PubMed] [Google Scholar]
- 37.Goel A, Mittal A, Evstatiev R, Nemeth M, Kruis W, Stolte M, Boland CR, Gasche C. In vivo effects of mesalazine or E. coli Nissle 1917 on microsatellite instability in ulcerative colitis. Aliment Pharmacol Ther. 2009;30:634–642. doi: 10.1111/j.1365-2036.2009.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moran JP, Walter J, Tannock GW, Tonkonogy SL, Sartor RB. Bifidobacterium animalis causes extensive duodenitis and mild colonic inflammation in monoassociated interleukin-10-deficient mice. Inflamm Bowel Dis. 2009;15:1022–1031. doi: 10.1002/ibd.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshua M, Uronis JM, Mühlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen GY, Shaw MH, Redondo G, Núñez G. The Innate Immune Receptor Nod1 Protects the Intestine from Inflammation-Induced Tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci U S A. 2011 May 18; doi: 10.1073/pnas.1100981108. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, A negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 45.Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, Tuohy VK, Fairchild RL, de la Motte C, Cua D, Vallance BA, Li X. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, Clin Exp Immunol. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Kadota C, Ishihara S, Aziz MM, Rumi MA, Oshima N, Mishima Y, Moriyama I, Yuki T, Amano Y, Kinoshita Y. Down-regulation of single immunoglobulin interleukin-1R-related molecule (SIGIRR)/TIR8 expression in intestinal epithelial cells during inflammation. inflammation, and tumorigenesis. Clin Exp Immunol. 2010;162:348–361. doi: 10.1111/j.1365-2249.2010.04254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong J, Wei T, Stark RW, Jamitzky F, Heckl WM, Anders HJ, Lech M, Rössle SC. Inhibition of Toll-like receptors TLR4 and 7 signaling pathways by SIGIRR: a computational approach. J Struct Biol. 2010;169:323–330. doi: 10.1016/j.jsb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’huigin C, Marincola FM, Trinchieri G. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, Abreu MT. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.González-Navajas JM, Fine S, Law J, Datta SK, Nguyen KP, Yu M, Corr M, Katakura K, Eckman L, Lee J, Raz E. TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J Clin Invest. 2010;120:570–581. doi: 10.1172/JCI40055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria (October 2001). [www.who.int/entity/foodsafety/publications/fs_management/en/probiotics.pdf “Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria”].
- 52.Anukam KC, Reid G. Probiotics: 100 years (1907–2007) after Elie Metchnikoff’s Observations. In: Mendez-Vilas A, editor. Communicating Current Research and Educational Topics and Trends in Applied Microbiology. Formatex. org; Spain: 2007. pp. 466–474. [Google Scholar]
- 53.Capurso G, Marignani M, Delle Fave G. Probiotics and the incidence of colorectal cancer: when evidence is not evident. Dig Liver Dis. 2006;38(Suppl 2):S277–282. doi: 10.1016/S1590-8658(07)60010-3. [DOI] [PubMed] [Google Scholar]
- 54.Orlando A, Messa C, Linsalata M, Cavallini A, Russo F. Effects of Lactobacillus rhamnosus GG on proliferation and polyamine metabolism in HGC-27 human gastric and DLD-1 colonic cancer cell lines. Immunopharmacol Immunotoxicol. 2009;31:108–116. doi: 10.1080/08923970802443631. [DOI] [PubMed] [Google Scholar]
- 55.Lee NK, Park JS, Park E, Paik HD. Adherence and anticarcinogenic effects of Bacillus polyfermenticus SCD in the large intestine. Lett Appl Microbiol. 2007;44:274–278. doi: 10.1111/j.1472-765X.2006.02078.x. [DOI] [PubMed] [Google Scholar]
- 56.Kim Y, Lee D, Kim D, Cho J, Yang J, Chung M, Kim K, Ha N. Inhibition of Proliferation in Colon Cancer Cell Lines and Harmful Enzyme Activity of Colon Bacteria by Bifidobacterium adolescentis SPM0212. Arch Pharm Res. 2008;31:468–467. doi: 10.1007/s12272-001-1180-y. [DOI] [PubMed] [Google Scholar]
- 57.Paolillo R, Romano Carratelli C, Sorrentino S, Mazzola N, Rizzo A. Immunomodulatory effects of Lactobacillus plantarum on human colon cancer cells. Int Immunopharmacol. 2009;9:1265–1271. doi: 10.1016/j.intimp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Shida K, Kiyoshima-Shibata J, Nagaoka M, Watanabe K, Nanno M. Induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J Dairy Sci. 2006;89:3306–3317. doi: 10.3168/jds.S0022-0302(06)72367-0. [DOI] [PubMed] [Google Scholar]
- 59.Perdigón G, Valdez JC, Rachid M. Antitumour activity of yogurt: study of possible immune mechanisms. J Dairy Res. 1998;65:129–138. doi: 10.1017/s0022029997002604. [DOI] [PubMed] [Google Scholar]
- 60.Urbanska AM, Bhathena J, Martoni C, Prakash S. Estimation of the Potential Antitumor Activity of Microencapsulated Lactobacillus acidophilus Yogurt Formulation in the Attenuation of Tumorigenesis in Apc(Min/+) Mice. Dig Dis Sci. 2009;54:264–273. doi: 10.1007/s10620-008-0363-2. [DOI] [PubMed] [Google Scholar]
- 61.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci USA. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Moreno de Leblanc A, Perdigón G. Yogurt feeding inhibits promotion and progression of experimental colorectal cancer. Med Sci Monit. 2004;10:BR96–104. [PubMed] [Google Scholar]
- 63.Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, Kundu K, Murthy N, Hansen JM, Neish AS. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med. 2009 Oct 15;47(8):1205–11. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dolara P, Luceri C, De Filippo C, Femia AP, Giovannelli L, Caderni G, Cecchini C, Silvi S, Orpianesi C, Cresci A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat Res. 2005;591:237–246. doi: 10.1016/j.mrfmmm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 65.Park E, Jeon GI, Park JS, Paik HD. A Probiotic Strain of Bacillus polyfermenticus Reduces DMH Induced Precancerous Lesions in F344 Male Rat. Biol Pharm Bull. 2007;30:569–574. doi: 10.1248/bpb.30.569. [DOI] [PubMed] [Google Scholar]
- 66.Pagnini C, Corleto VD, Hoang SB, Saeed R, Cominelli F, Delle Fave G. Commensal Bacteria and “Oncologic Surveillance” Suggestions From an Experimental Model. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S193–196. doi: 10.1097/MCG.0b013e31817f1284. [DOI] [PubMed] [Google Scholar]
- 67.Mai V, Colbert LH, Perkins SN, Schatzkin A, Hursting SD. Intestinal microbiota: a potential diet-responsive prevention target in ApcMin mice. Mol Carcinog. 2007;46:42–48. doi: 10.1002/mc.20233. [DOI] [PubMed] [Google Scholar]
- 68.Le Leu RK, Hu Y, Brown IL, Woodman RJ, Young GP. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis. 2010;31:246–251. doi: 10.1093/carcin/bgp197. [DOI] [PubMed] [Google Scholar]
- 69.Veiga P, Gallini CA, Beal C, Michaud M, Delaney ML, DuBois A, Khlebnikov A, van Hylckama Vlieg JE, Punit S, Glickman JN, Onderdonk A, Glimcher LH, Garrett WS. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18132–18137. doi: 10.1073/pnas.1011737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, Hennet T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010 Dec 20;207(13):2843–2854. doi: 10.1084/jem.20101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lara-Villoslada F, de Haro O, Camuesco D, Comalada M, Velasco J, Zarzuelo A, Xaus J, Galvez J. Short-chain fructooligosaccharides, in spite of being fermented in the upper part of the large intestine, have anti-inflammatory activity in the TNBS model of colitis. Eur J Nutr. 2006;45:418–425. doi: 10.1007/s00394-006-0610-2. [DOI] [PubMed] [Google Scholar]
- 72.Guidelines for the Evaluation of Probiotics in Food: Joint FAO/WHO Working Group meeting; London Ontario, Canada. 30 April–1 May 2002. [Google Scholar]
- 73.Ishikawa H, Akedo I, Otani T, Suzuki T, Nakamura T, Takeyama I, Ishiguro S, Miyaoka E, Sobue T, Kakizoe T. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer. 2005;116:762–767. doi: 10.1002/ijc.21115. [DOI] [PubMed] [Google Scholar]
- 74.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O’Riordan M, O’Sullivan GC, Pool-Zobel B, Rechkemmer G, Roller M, Rowland I, Salvadori M, Thijs H, Van Loo J, Watzl B, Collins JK. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–496. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 75.Hatakka K, Holma R, El-Nezami H, Suomalainen T, Kuisma M, Saxelin M, Poussa T, Mykkänen H, Korpela R. The influence of Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp. shermanii JS on potentially carcinogenic bacterial activity in human colon. Int J Food Microbiol. 2008;128:406–410. doi: 10.1016/j.ijfoodmicro.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Gianotti Luca, Morelli Lorenzo, Galbiati Francesca, Rocchetti Simona, Coppola Sara, Beneduce Aldo, Gilardini Cristina, Zonenschain Daniela, Nespoli Angelo, Braga Marco. A randomized double-blinded trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol. 2010;16:167–175. doi: 10.3748/wjg.v16.i2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pala V, Sieri S, Berrino F, Vineis P, Sacerdote C, Palli D, Masala G, Panico S, Mattiello A, Tumino R, Giurdanella MC, Agnoli C, Grioni S, Krogh V. Yogurt consumption and risk of colorectal cancer in the italian EPIC cohort. Int J Cancer. 2011 May 23; doi: 10.1002/ijc.26193. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]