Abstract
A growing body of evidence suggests that gonadal steroids such as estradiol (E2) alter neural responses not only in brain regions associated with reproductive behavior, but also in sensory areas. Because catecholamine systems are involved in sensory processing and selective attention, and because they are sensitive to E2 in many species, they may mediate the neural effects of E2 in sensory areas. Here, we tested the effects of E2 on catecholaminergic innervation, synthesis, and activity in the auditory system of white-throated sparrows, a seasonally breeding songbird in which E2 promotes selective auditory responses to song. Non-breeding females with regressed ovaries were held on a winter-like photoperiod and implanted with silastic capsules containing either no hormone or E2. In one hemisphere of the brain, we used immunohistochemistry to quantify fibers immunoreactive for tyrosine hydroxylase or dopamine beta-hydroxylase in the auditory forebrain, thalamus, and midbrain. E2 treatment increased catecholaminergic innervation in the same areas of the auditory system in which E2 promotes selectivity for song. In the contralateral hemisphere, we quantified dopamine, norepinephrine and their metabolites in tissue punches using HPLC. Norepinephrine increased in the auditory forebrain, but not the midbrain, after E2 treatment. We found evidence of interhemispheric differences, both in immunoreactivity and catecholamine content did not depend on E2 treatment. Overall, our results show that increases in plasma E2 typical of the breeding season enhance catecholaminergic innervation and synthesis in some parts of the auditory system, raising the possibility that catecholamines play a role in E2-dependent auditory plasticity in songbirds.
Keywords: dopamine, dopamine beta-hydroxylase, norepinephrine, tyrosine hydroxylase, white-throated sparrow
INTRODUCTION
In seasonally breeding vertebrates, the timing of gonadal steroid release ensures that steroid-dependent reproductive behaviors occur at the appropriate time of year. Behavioral responses to sociosexual signals change dramatically in many species of fish, frogs, birds, and rodents when plasma estradiol (E2) rises to breeding levels (reviewed by Maney, 2010; Maney & Pinaud, 2011). To understand how E2 alters behavioral responses to sensory signals, we have studied areas of the auditory forebrain thought to participate in the encoding of behavioral relevance in songbirds. The caudomedial nidopallium (NCM) receives input from the thalamorecipient Field L and is heavily interconnected with the caudomedial mesopallium (CMM). NCM and CMM are proposed to be analogous to the supragranular layers of mammalian auditory cortex (Vates et al., 1996) or to mammalian auditory association cortex (Pinaud & Terleph, 2008; Tremere et al. 2009). These areas have received a great deal of attention largely because they show selective responses to stimuli that are particularly salient to the listener (Mello and Clayton, 1994; Jarvis et al., 1995; Gentner et al., 2001; Maney et al., 2003; Leitner et al., 2005). We previously reported that in CMM, NCM and MLd (nucleus mesencephalicus lateralis, pars dorsalis, or auditory midbrain) of female white-throated sparrows, expression of the immediate early gene product ZENK (Egr-1) was selective for conspecific song only when plasma E2 was at breeding levels (Maney et al., 2006). In non-breeding females with low plasma E2, the ZENK response to song was indistinguishable from that to frequency-matched tones. E2-dependent changes in the responsiveness of auditory processing centers may therefore promote recognition of and attention to conspecific male song during the breeding season when it is particularly relevant to the female.
E2-induced changes in auditory function, particularly changes involving stimulus salience, could be related to one or more cognitive processes that depend on catecholamines. These compounds are widely understood to shape the response properties of sensory networks involved in selective attention in mammals (see Hurley et al., 2004 and Aston-Jones and Cohen, 2005 for review) and context-dependent modulation of forebrain plasticity (see Castelino and Ball, 2005). In songbirds, catecholaminergic (CA) projections to the forebrain have been hypothesized to affect the auditory processing of behaviorally relevant social signals such as song (e.g., Appeltants et al., 2002a,b; Cardin and Schmidt, 2004; LeBlanc et al., 2007; Riters and Pawlisch, 2007). Noradrenergic denervation of the forebrain reduces behavioral and neural responses to song as well as behavioral and neural selectivity for sexually stimulating song (Appeltants et al., 2002b; Lynch and Ball, 2008; Vyas et al., 2008). Any modulation of CA systems is therefore likely to affect the auditory processing of conspecific song as well as the resulting behavioral responses. In male zebra finches, catecholamine levels and turnover in the auditory forebrain are regulated by gonadal steroids (Barclay and Harding, 1990). In this study, we investigated the effects of systemic E2 treatment on central CA activity in females of a seasonally breeding species, the white-throated sparrow. We quantified catecholamines and their metabolites using high pressure liquid chromatography (HPLC) and the density of fibers immunoreactive for the catecholamine synthetic enzymes tyrosine hydroxylase (TH) and dopamine beta-hydroxylase (DBH) using immunohistochemistry (IHC).
METHODS
Animals
All procedures in this study were approved by the Emory University Institute for Animal Care and Use Committee and adhered to NIH standards. We collected sixteen female white-throated sparrows (Zonotrichia albicollis) in mist nets in Atlanta, Georgia during the fall of 2007. We determined their sex by polymerase chain reaction (PCR) analysis of a blood sample (Griffiths et al., 1998) and confirmed sex by necropsy at the end of the study. The birds were housed at the Emory University animal care facility in indoor walk-in flight cages and supplied with food and water ad libitum. We held day length constant at 10:14h light-dark, which corresponds to the shortest day the birds would experience during the winter at the capture site. We kept the birds under these conditions for two months to ensure that photorefractoriness was broken before the start of the study (Wolfson, 1958; Shank, 1959).
Hormonal Manipulation
Because our goal was to compare the anatomical distribution of the effects of E2 on CA innervation with the previously described effects on the ZENK response (Maney et al., 2006; Sanford et al. 2010), we followed our standard procedures for housing and hormonal manipulation. We transferred the birds in pairs to small rooms where they were housed individually in two adjacent cages (38 × 38 × 42 cm) per room. We held the day length at 10:14 h light-dark throughout the experiment to prevent elevation of endogenous plasma E2 (Wolfson, 1958; Shank, 1959). On the day the birds were transferred to individual cages, we implanted each one with a subcutaneous silastic capsule (length 12 mm, ID 1.47 mm, OD 1.96 mm, Dow Corning, Midland, MI) sealed at both ends with A-100S Type A medical adhesive (Factor 2, Lakeside, AZ). In order to balance hormone treatment within each pair, one bird in each pair (n=8) received an empty implant and the other (n=8) received an implant containing 17beta-estradiol (Steraloids, Newport, RI). Capsules were implanted using topical anesthetic (lidocaine, 1%). This dose of E2 increases plasma levels to those typical of the breeding season within seven days in this species (Maney et al., 2006, 2008) and likely does so within two days (Moore, 1983). We have established that seven days of hormone treatment results in a dramatic rise in ZENK expression and selectivity in auditory areas (Sanford et al., 2010) and since our present goal was to reproduce those conditions, we treated for the same amount of time. Seven days after implantation, we rapidly decapitated the birds and quickly harvested the brains. After removing the cerebellum in order to clearly visualize the midline, we used a clean razor blade to bisect each brain into hemispheres. We fixed one hemisphere in 5% acrolein as previously described (Maney et al., 2003, 2005) and flash-froze the other in powdered dry ice for HPLC analysis at the University of North Carolina. The hemisphere that was fixed (right or left), the room (one of six identical rooms), and the position of each bird inside the room (to the right or the left of the other bird in that room) were balanced across treatments.
Immunohistochemistry
We cut the fixed hemispheres into three series of 50 μm parasagittal sections using a freezing sliding microtome. We immunolabeled one series for TH and another for DBH using standard IHC protocols (Maney et al., 2001, 2003, 2005; LeBlanc et al., 2007). Briefly, we incubated the first series of sections with an anti-TH antibody (ImmunoStar; Hudson, WI; see section on antisera below) diluted 1:2000 (LeBlanc et al., 2007) then labeled the TH with a biotinylated secondary antibody and the ABC method (Vector, Burlingame, CA). We visualized the immunolabeling with diaminobenzidine (LeBlanc et al., 2007). For the second series of sections, we pre-treated with avidin and biotin, incubated with anti-DBH antibody (ImmunoStar; see section on antisera below) diluted 1:16,000, and subsequently labeled the DBH using a biotinylated secondary antibody and the ABC method. We visualized DBH immunolabeling with nickel-enhanced diaminobenzidine (Shu et al., 1988). We processed each series of brain sections in two separate runs of IHC in which the experimental (E2-treated) and blank (empty implants) conditions were balanced across runs. Following IHC, we mounted all of the sections onto microscope slides, dehydrated them, and coverslipped in DPX (Sigma, St. Louis, MO).
Antisera
We used a mouse monoclonal antibody generated against denatured TH and purified from rat PC12 cells (ImmunoStar Cat#22941). According to the manufacturer, it recognizes a 62kD band corresponding to TH in rat and does not cross-react with dihydropterdine reductase, DBH, phenyletholamine-N-methyltransferase, phenylalanine hydroxylase or tryptophan hydroxylase using Western blot methods. It has a wide species cross-reactivity and has been validated by preadsorption studies in a range of vertebrates (Olsson et al., 2008). This antibody labels a catecholamine-typical pattern of neurons and fibers in a wide variety of birds (e.g., Bailhache and Balthazart, 1993; Moons et al., 1994; Reiner et al., 1994; Soha et al., 1996; Appeltants et al., 2001; Roberts et al., 2001) including white-throated sparrows (Balthazart and Ball, 1996; LeBlanc et al., 2007) and was used by Reiner et al. (1994) to perform an exhaustive characterization of the distribution of TH-immunoreactivity in birds. Anti-TH antibodies from other sources and anti-DA antibodies produce the same neural distribution in birds (e.g., Bottjer, 1993; Metzger et al., 1996). In our material, the antibody labels all major TH cell groups A1-A15 and fibers in a distribution typical of TH. An antibody against the phosphorylated form of TH (Genetex, Irvine, CA, Cat#16557), which we have validated in preadsorption studies in white-throated sparrow, labels an identical distribution of cells in this species.
To label DBH we used a polyclonal antibody generated in rabbit against DBH purified from bovine adrenal medulla (ImmunoStar Cat#22806). The manufacturer notes that on Western blot the antibody detects a triplet at approximately 72-74kD. It labels an NE-like distribution of cells and fibers in a variety of birds (e.g., Bailhache and Balthazart, 1993; Karle et al., 1996; Castelino and Ball, 2005; Sockman and Salvante, 2008), including white-throated sparrows (LeBlanc et al., 2007). Sockman and Salvante (2008) reported that preadsorption with antigen supplied by the manufacturer of the antibody eliminates all labeling in brain sections from European starling (Sturnus vulgaris).
For each IHC described here, omission of the primary or the secondary antibodies resulted in a complete loss of specific staining in all cases.
Regions of Interest and Data Collection
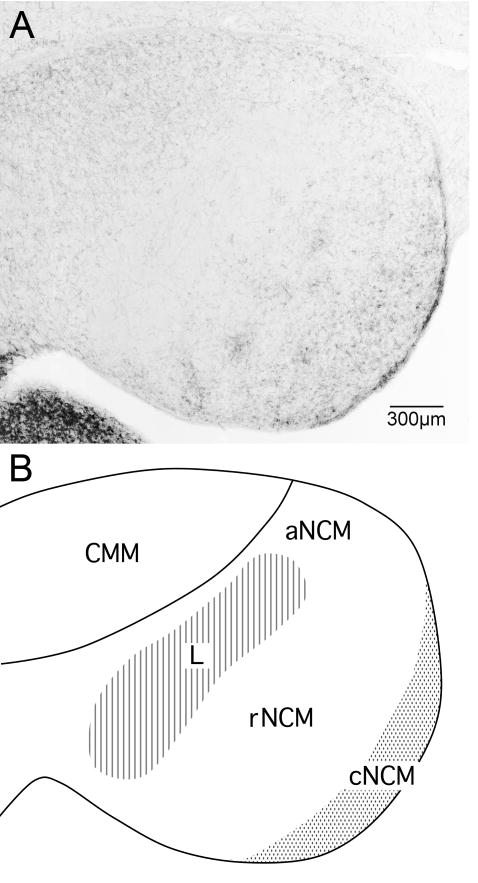
Our primary goal in this study was to map the effects of E2 on CA innervation and to determine the extent to which those effects overlap, anatomically, with the effects on the ZENK response we previously described (Sanford et al., 2010). In order to compare the anatomical distributions of the effects of E2 on CA innervation and the effects on the ZENK response, we needed to partition NCM into the same domains used in our previous study. Sanford et al. (2010) divided the region both rostro-caudally and dorso-ventrally into four primary domains: rostro-dorsal (rdNCM), rostro-ventral (rvNCM), caudo-dorsal (cdNCM) and caudo-ventral (cvNCM). Because the dorsal and ventral domains are hodologically and neurochemically similar within both rostral NCM and caudal NCM, and because the effects of E2 on ZENK expression are similar in the dorsal and ventral portions of each domain (Sanford et al., 2010), in the current study we collapsed our regions of interest into rostral and caudal domains (rNCM and cNCM) without the dorsal and ventral divisions (Fig. 1). Like Sanford et al. (2010), we also sampled from an apical domain (aNCM) located dorsal to Field L2 (Fig. 1). In the published literature, this region is usually considered part of NCM but may overlap the dorsal portion of Field L (Fortune and Margoliash, 1992).
Fig. 1.
Parasagittal view of tyrosine hydroxylase immunoreactivity (A) in the auditory forebrain of the white-throated sparrow. (B) We quantified catecholamine innervation, content, and metabolites in the following regions of the auditory forebrain (Sanford et al., 2010): the caudomedial mesopallium (CMM) and three domains of the caudomedial nidopallium (NCM): rostral NCM (rNCM), apical NCM (aNCM), and caudal NCM (cNCM). Rostral is to the left. Scale bar = 300 μm.
Using material from Sanford et al. (2010), we recently found that the ZENK response in the auditory thalamic nucleus Ovoidalis (Ov), which is homologous to the mammalian medial geniculate (Karten, 1967), is also sensitive to E2 treatment (T. Tran, unpublished; see also Maney & Pinaud, 2011). Maney et al. (2006) showed that E2 treatment increases the selectivity of the ZENK response in the auditory midbrain (MLd), which is homologous to the mammalian inferior colliculus (Karten, 1967). In order to test whether E2 treatment alters CA innervation and function in these areas, we estimated the density of immunoreactive fibers in Ov and MLd in addition to the regions of the auditory forebrain described above.
Image Acquisition and Regions of Interest
We conducted all image analyses while blind to treatment group. We used the 10x objective on a Zeiss Axioskop microscope attached to a Leica DFC480 camera and Macintosh G5 computer. For all regions we captured rectangular images corresponding to the field of view of the camera (870 X 690 μm). We held the light level constant for all photos, which were approximately 46 MB in size. We defined the regions of interest within CMM and NCM using the criteria of Sanford et al., (2010). We acquired images of CMM and NCM in four consecutive sections between ~350 and ~800 μm from the midline in all birds. The first section chosen was the medial-most in which we could discern CMM and Field L (Sanford et al., 2010). Field L was easily visible in the TH immunostained series as a region relatively poor in TH immunoreactivity (Reiner et al., 1994); we aligned the DBH immunostained sections with the alternate TH immunostained series to confirm the location of Field L.
For each series in each bird, five separate images were acquired from the same four sections. From those photos we quantified fiber density in CMM, aNCM, cNCM and two domains of rNCM (dorsal and ventral, which were later combined), as defined by Sanford et al. (2010), as follows. To acquire images of CMM, the two upper corners of each photograph were positioned along the dorsal boundary of CMM and one of the lower corners was positioned adjacent to the lamina mesopallium. The remaining four photos, of NCM, were positioned so that they would encompass the domains defined by Sanford et al. (2010; see above). When defining our regions of interest for sampling, we opened all four photos at the same time in order to avoid overlap. We first defined cNCM as a strip of tissue approximately 275 μm from the caudal boundary of NCM. We selected it by tracing the caudal boundary with the ImageJ freehand tool and using the straight line tool to define its rostral boundary. The images of cNCM captured the majority of that domain, spanning 870 μm from dorsal to ventral, but did not include the dorsal and ventral tips. The rostral domains were sampled using the ImageJ circle tool. For rNCM, we sampled from two areas, one ventral and one more dorsal (Sanford et al., 2010). We sampled the ventral region of interest using a circle about 550 μm in diameter, placed so that its ventral boundary was adjacent to the ventral edge of the telencephalon and its caudal boundary did not overlap with what we defined as cNCM. We defined the dorsal region of interest using a circle about 550 μm in diameter, placed immediately dorsal to the ventral one and not overlapping cNCM. For aNCM, we placed a circle with a diameter of about 350 μm dorsal to Field L so that its dorsal boundary lay adjacent to the ventricle. In no case did these circles overlap the region of relatively poor TH-immunoreactivity that Reiner et al. (1994) identified as Field L. Because we did not conduct tract tracing or another method that would enable us to discern absolutely the boundary between rNCM and the adjacent subregions of Field L (Vates et al., 1996), it is possible that our samples of aNCM and rNCM may have captured a bit of L1 and L3, respectively. We are confident in any case that the rostral regions we sampled correspond exactly to those exhibiting E2-dependent selectivity as described by Sanford et al. (2010).
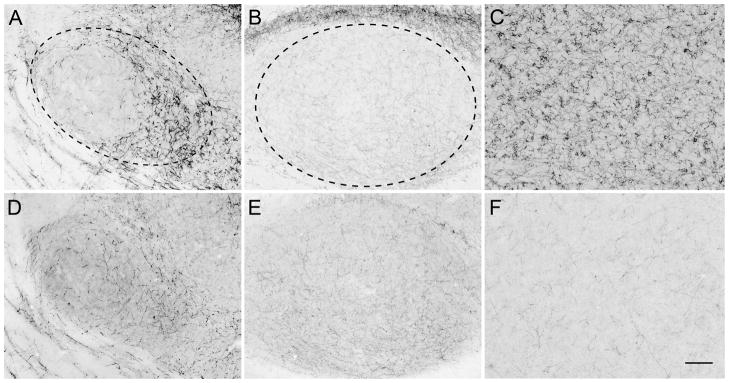
Ov was visible in two to three sections in each bird. For each series we chose the two sections in which it was the largest, spanning 150 μm from medial to lateral and traced the outline of the region, including its shell and core (Vates et al., 1996; Fig 2A, D). To acquire images of MLd, we chose the section in which it was the largest (Maney et al., 2006) and the two immediately medial and lateral to it so that we sampled from five sections spanning 600 μm. We traced the outline as depicted in Fig. 2B. We also quantified fiber density in a non-auditory region, the apical part of the hyperpallium (HA), to assess the specificity of the effects of E2 on auditory regions. Typical images of HA, which were acquired from the same sections used for Ov, are shown in Fig. 2C, F.
Fig. 2.
Fibers immunoreactive for tyrosine hydroxylase (A, B, C) or dopamine beta-hydroxylase (D, E, F) in the auditory thalamus (Ov; A, D), the auditory midbrain (MLd; B, E) and the apical part of the hyperpallium (HA; C, F), a visual area, in an estradiol-treated female white-throated sparrow. The regions of interest in which we quantified fiber density are delineated by dashed lines in A and B. The apparent cell bodies in HA are actually immunoreactive fibers forming basket-like structures (Metzger et al., 1996). Rostral is to the left. Scale bar = 100 μm.
Estimation of TH- and DBH-IR fiber density
Our hypothesis was that E2 treatment would increase the density of the CA innervation, in other words the total volume of CA fibers innervating our regions of interest (Kritzer & Kohama, 1998; LeBlanc et al., 2007). We therefore estimated immunoreactive fiber density from our acquired images in units of area (e.g., Kritzer & Kohama, 1998; Gerhardt et al., 2002; Poeggel et al., 2003; Maney et al., 2005; LeBlanc et al., 2007; de Vries et al., 2008). Using ImageJ (version 1.41o, National Institutes of Health, Bethesda, MD), we converted each image to 8-bit scale and selected TH- or DBH-immunoreactive fibers using the thresholding feature (Maney et al., 2005). The level of background staining varied somewhat from section to section, so in order to best distinguish labeled fibers from the background, we set the threshold manually for each image. In each case, we adjusted the threshold such that the pixels selected by ImageJ agreed with what the observer considered, by eye, to be labeled fibers. The same observer set the threshold for all images (LLM). We performed this procedure in 2–5 images per region (see above). We then calculated the area covered by the fibers, in square microns per square mm, for each region by summing the total area covered by fibers and dividing by the total area measured. Because Sanford et al. (2010) showed that the ZENK response is modulated similarly in the dorsal and ventral areas of rNCM, we combined our dorsal and ventral rNCM samples.
Test of interrater reliability
Like most published methods for estimating fiber density, the manual adjustment of threshold is based on the observer’s subjective opinion of which parts of the image represent immunoreactive fibers. We therefore performed a test of interrater reliability. For this analysis we used 60 images of DBH staining in CMM, which contained the lightest, least obvious fibers of all the labeling in our study (see Fig. 3B). Fifteen different animals were represented in this sample. Two different observers chose thresholds independently for these 60 images. Both observers used the same computer monitor located in the same room, and the lighting in the room was held constant.
Fig. 3.
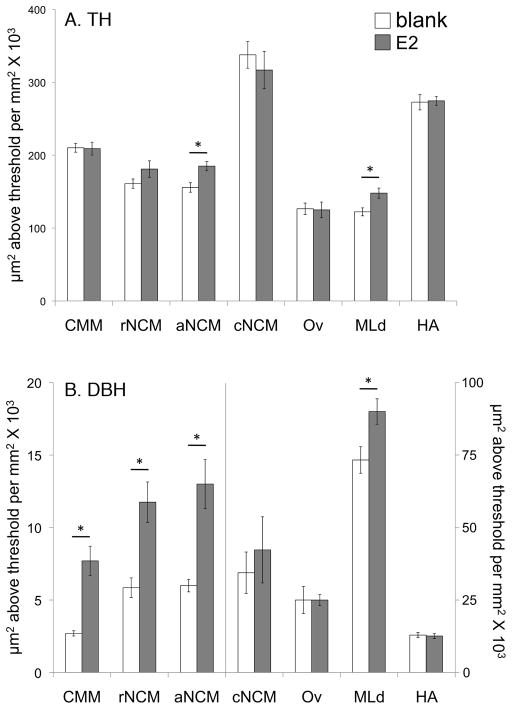
Estradiol (E2) treatment increased the estimated density of fibers immunopositive for (A) tyrosine hydroxylase (TH) in aNCM and MLd; and (B) dopamine beta-hydroxylase (DBH) in CMM, rNCM, aNCM, and MLd. MLd, auditory midbrain. HA, apical part of the hyperpallium. Ov, n. Ovoidalis. Other abbreviations, see Fig. 1. *E2 condition differs from blank condition, p < 0.02. See text for actual p levels.
Quantification of Catecholamines and Metabolites
The methods for quantification of catecholamines and metabolites are published elsewhere (Sockman and Salvante, 2008), and we reiterate the relevant portions here. We determined the concentration of catecholamines and metabolites by reversed-phase HPLC with electrochemical detection (Kilts et al., 1981). Because the number of brain regions we could sample via HPLC was limited (see below), we decided to sample regions in which we had previously shown E2-dependent selectivity of the ZENK response: NCM, CMM, and MLd (Maney et al., 2006). The chromatographic system consisted of a SM-909 isocratic HPLC pump (ANSPEC, Ann Arbor, MI), Basic+ Marathon type 816 Autosampler (Spark Holland, Netherlands), Model 400 potentiostat (EG&G Princeton Applied Research, Oak Ridge, TN) and TurboChrom software Version 4.1 (Perkin Elmer, Waltham, MA) running on a PC. We separated the compounds using a Monochrom C18 3 μm column (100 × 4.6 mm, MetaChem, Torrence, CA) with a mobile phase consisting of sodium phosphate (7.1 g), citric acid (5.76 g), disodium EDTA (50 mg), sodium octyl sulfonate (350 mg), and methanol (130 ml) topped-up to one liter total volume with double-distilled, deionized water, with pH lowered to 3.9 with hydrochloric acid. We filtered the mobile phase through a 20 μm filter (Kontes Scientific Glassware and Instruments, Vineland, NJ) before use, and flow rate was 0.8 ml/min. We maintained the electrode potential at 650 mV with respect to an Ag/AgCI reference electrode. We prepared standard solutions containing a fixed amount (30 ng) of the internal standard (isoproterenol, Sigma) and variable amounts of each of the four external standards (Sigma): dopamine (DA), norepinephrine (NE), dihydroxyphenylacetic acid (DOPAC; the principal metabolite of dopamine) and 3-methoxy-4-hydroxyphenylglycol (MHPG; the principal metabolite of norepinephrine). We included a five-point standard curve in each assay (5 × 50 μL injections), and used linear regression to fit a line through the standard curve points (r2 > 0.99 for all four components in each assay).
We sectioned the frozen, non-fixed hemispheres at 10 C in the sagittal plane at 300 μm on a cryostat, thaw mounted the sections onto glass slides, and rapidly re-froze them on pulverized dry ice. Using a chilled custom-made, thin-walled stainless steel spring-loaded punch tools, we micropunched a region of CMM (1 mm i.d.) and of NCM (1.5 mm i.d.), each for which the anatomical boundaries have been described (Sockman et al., 2002). Because the diameter of the punch tool was greater than the diameters of any of the individual domains within NCM, we did not further divide NCM into domains for the HPLC analysis. We took a micropunch (1 mm i.d.) from a region of MLd from two consecutive sections. We expelled the tissue punches into 1.9 ml polypropylene microcentrifuge tubes (one for each brain region), froze them on dry ice and stored them at −80 C until assay. Immediately before assay, we added mobile phase (225 μl) containing 30 ng isoproterenol to each tube. We sonicated the samples and then centrifuged them at 16,000 g for 15 minutes at 4 C. We aspirated the supernatant and injected 50 μl from each sample into the HPLC system. We calculated catecholamine and metabolite concentrations (pg) by first correcting the peak heights for percent recovery of the internal standard (i.e., the height of the isoproterenol peak) for each sample, and then comparing the values to those obtained for the corresponding catecholamines or metabolites in the standard curve.
Statistical Analysis
All of our dependent variables were measured in the same set of animals and are linked to catecholamine synthesis. We therefore could not assume that they were independent of each other. For this reason we began our analysis with a single repeated-measures MANOVA that included data acquired by IHC and HPLC from all birds. Because the HPLC data set included only three regions of interest (CMM, NCM as a single region, and MLd), we could not include all of the IHC data on enzymes, which were measured in seven separate regions, in this initial analysis. The IHC data were therefore analyzed separately, with all regions of interest included, in a second analysis (see below). For the initial omnibus analysis of CMM, NCM, and MLd only, because CA innervation was measured in individual domains whereas our HPLC data were acquired from a single larger sample, we needed to collapse the NCM IHC data for individual domains into a single value for NCM for each bird. To do so, we calculated an average estimated percent area covered by fibers for each enzyme by summing the total area covered by immunoreactivity in rNCM and cNCM and dividing by the total area measured for all regions summed. aNCM was not included in this calculation because it lies dorsal to Field L, outside the area of the micropunch. This calculation produced an estimate of the average area covered by fibers immunoreactive for TH or DBH throughout the part of NCM that was sampled for HPLC analysis. We then conducted a repeated-measures MANOVA with region of interest (CMM, NCM, or MLd) and catecholamine measure (TH, DBH, DA, NE, DOPAC, or MHPG) as the within-subject variables, and hormone treatment (blank or E2) as the between-subject variable. In this procedure, therefore, we tested for an effect of E2 treatment on most of the related variables together as a whole before proceeding to analyze the data for each measure or individual region separately.
In order to determine the effect of E2 treatment on estimated TH and DBH fiber innervation in our regions of interest, including Ov, and the domains of NCM, we performed a separate repeated-measures MANOVA with enzyme (TH or DBH) and region of interest (CMM, rNCM, aNCM, cNCM, Ov, and MLd) as the within-subjects factors, and hormone treatment and hemisphere as the between-subjects factors, followed by multivariate F-tests for each region of interest. To analyze the HPLC data in the other hemisphere, we did a repeated-measures MANOVA with region of interest (CMM, NCM, or MLd) and compound (DA, NE, DOPAC, or MHPG) as the within-subject variables, and hormone treatment (blank or E2) and hemisphere as the between-subject variables, followed by multivariate F-tests for each region of interest. Effect sizes were calculated using Cohen’s d. Because we hypothesized that TH- and DBH-immunoreactivity in HA would be unaffected by E2 treatment and thus wanted to minimize Type II rather than Type I error, we analyzed the data from that region in a separate multivariate F-test.
In order to better compare the effect of E2-treatment on the estimated density of CA fiber innervation among the regions, we normalized the values from the E2-treated birds to evaluate the percent change relative to controls. We then performed a repeated measures ANOVA with normalized estimated fiber density (TH or DBH) and region of interest as within-subjects variables. We followed that analysis with post-hoc pairwise student t-tests to compare the effects of E2 in each region to that in the other regions.
In order to determine whether TH- or DBH-immunoreactivity predicted the levels of catecholamines or metabolites in each region, we ran Pearson correlation tests relating the percent area covered by TH-immunoreactivity to DA and its metabolite DOPAC, and the estimated percent area covered by DBH-immunoreactivity to NE and its metabolite MHPG in each region of interest (total of 12 Pearson tests). For this analysis, NCM was treated as a single region and we used the averaged IHC data for individual domains (see above). We evaluated the correlations with the sequential Bonferroni correction for multiple comparisons (Rice, 1989).
RESULTS
When the data were analyzed together in a single repeated-measures MANOVA (see Methods), we found no reliable effect of treatment (F1, 14 = 0.758; p = 0.399). There was, however, a significant interaction between hormone treatment and region (Wilks’ Lambda F2, 13 = 7.508; p = 0.007), meaning that there was a significant effect of E2 treatment that depended on the region of interest. There was also a significant interaction between hormone treatment and compound measured (Wilks’ Lambda F5, 10 = 3.713; p = 0.037), which indicated that E2 treatment may have affected some of our measures but not others. We then proceeded to the analyses of data collected by each method (IHC or HPLC).
Effects of E2 treatment on catecholaminergic innervation of auditory areas
The effects of E2 treatment on estimated TH and DBH fiber density are plotted in Fig. 3. A repeated-measures MANOVA that considered all regions of interest, including the domains of NCM, revealed a highly significant effect of E2 treatment on immunoreactivity for CA enzymes (F1, 12 = 15.541; p = 0.002). Post-hoc pairwise comparisons showed that E2 significantly increased the estimated density of TH innervation in aNCM (F 1, 15 = 9.615; p = 0.009; d = 1.614) and MLd (F1, 15 = 7.515; p = 0.018; d = 1.455). Estimated DBH fiber density was increased in CMM (F 1, 15 = 27.809; p < 0.001; d = 2.427), rNCM (F 1, 15 = 15.372; p = 0.002; d = 1.899), aNCM (F 1, 15 = 15.133; p = 0.002; d = 2.006), and MLd (F 1, 15 = 8.485; p = 0.013; d = 1.327). We did not detect an effect of E2 on the estimated density of TH or DBH innervation of cNCM (TH: F 1, 15 = 0.447, p = 0.516, d = 0.329; DBH: F 1, 15 = 0.288, p = 0.601, d = 0.293).
Relative effects of E2-treatment on CA fiber density in regions of interest
A repeated-measures ANOVA on normalized values from the E2-treated birds showed significant effects of enzyme (F1, 7 = 32.76; p = 0.001) and region (F6, 42 = 10.05; p = 0.001) and a significant interaction between the two (F6, 42 = 8.39; p < 0.001). Post-hoc pairwise comparisons between the areas revealed that in general, the rostral areas of NCM responded more to E2-treatment than the caudal area.
Effects of E2 treatment on levels of catecholamines and their metabolites
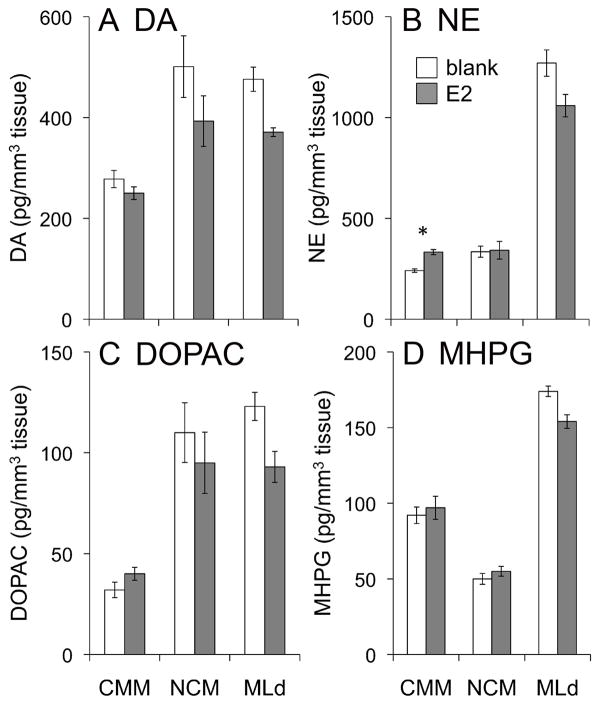
A repeated-measures MANOVA with compound and region as within-subjects measures showed no main effect of hormone treatment (F 1, 12 = 1.472; p = 0.248); however there was an interaction between treatment and region (F 2, 11 = 8.005; p = 0.007). Post-hoc pairwise comparisons revealed that the effects of E2 treatment were limited to CMM, in which norepinephrine increased (F 3, 15 = 6.876; p = 0.022; d = 1.386) (Fig. 4). We detected no other effects of hormone treatment on the concentrations of catecholamines or their metabolites in CMM, NCM, or MLd (Fig. 4).
Fig. 4.
The effects of estradiol (E2) treatment on (A) dopamine (DA) and (B) norepinephrine (NE) and their metabolites (C) dihydroxyphenylacetic acid (DOPAC) and (D) 3-methoxy-4-hydroxyphenylglycol (MHPG) in the caudomedial mesopallium (CMM), the caudomedial nidopallium (NCM), and the auditory midbrain (MLd) in female white-throated sparrows. Average protein content was 0.1 mg/mm3. * p = 0.022.
Interhemispheric differences
The repeated-measures MANOVAs conducted on the IHC data showed an overall effect of hemisphere (F1, 12 = 5.059; p = 0.044), and the parallel analysis of the HPLC data showed a significant compound by hemisphere interaction (F3, 10 = 4.564; p = 0.029). In neither analysis was there an interaction between hemisphere and treatment or between hemisphere and other variables that included treatment. Therefore, E2 treatment affected our variables of interest equally in both hemispheres in all cases. The two interhemispheric differences we found for enzyme immunoreactivity were in the same direction; our estimation of TH-immunoreactivity was higher in left than right rNCM (F1, 15 = 4.850; p = 0.048; d = 0.95), and the estimated DBH-immunoreactivity was higher in the left than the right cNCM (F1, 15 = 4.977; p = 0.046; d = 1.031). NE and its metabolite, MHPG, were both higher in the right MLd than the left (NE, F1, 15 = 5.481; p = 0.037; d = 1.725; MHPG, F1, 15 = 13.298; p = 0.003; d = 1.089).
Correlations between enzyme immunoreactivity and catecholamine levels
In order to test whether enzyme immunoreactivity predicted the levels of catecholamines and their metabolites, we looked for correlations between estimated TH fiber density and DA or DOPAC levels and between estimated DBH fiber density and NE or MHPG levels in CMM, MLd and NCM (rNCM and cNCM combined; see Methods). Pearson correlation tests revealed that estimated TH fiber density did not predict levels of DA or DOPAC as measured by HPLC. We detected no significant correlations between the percent area stained and DA or DOPAC levels in any of the regions of interest. Similarly, in most regions estimated DBH fiber density did not predict levels of NE or MHPG as measured by HPLC. The only notable correlation, between DBH-immunoreactivity and the concentration of NE in CMM (R2 = 0.334, p = 0.019) was clearly driven by the significant effect of E2 on both measures (Fig. 5). We also tried correlating the estimated fiber density of each enzyme with the summed concentrations of all of its downstream products, in other words DBH-immunoreactivity with NE and MHPG levels summed, and TH-immunoreactivity with all four products summed, but detected no significant correlations.
Fig. 5.
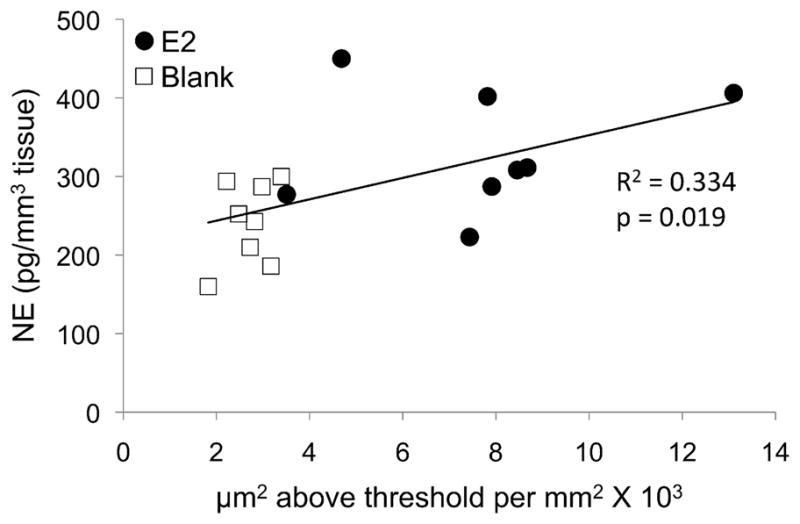
Correlation between the density of dopamine beta-hydroxylase (DBH) immunoreactive fibers and the level of norepinephrine (NE) in the caudomedial mesopallium (CMM). The relationship between DBH immunoreactivity and NE was likely driven by the significant effect of estradiol (E2) on both variables (see Figs. 3B, 4B).
Reliability of manual thresholding
Our analysis showed manual thresholding to be highly reliable. The mean thresholds and standard deviations for observers 1 and 2 were 208.36 ± 21.11 and 208.34 ± 21.31, respectively, and the correlation between them was R = 0.98. There was no difference between the thresholds set by observers 1 and 2 when compared using a paired t-test (p = 0.976) and the effect size was very low (d = 0.0009). Thresholds set by observers 1 and 2 were, on average, 1.71% different (SD = 1.28%) and never differed by more than 5%. They were within one unit of each other in 30% of cases. When the direction of the difference was taken into account, the thresholds selected by the two observers were, on average, 0.01% different.
DISCUSSION
In this study, we provide evidence that the CA innervation of the auditory system in songbirds is sensitive to E2. We found that in the auditory forebrain (CMM and NCM) and midbrain (MLd), a rise in plasma E2 from non-breeding to breeding levels causes significant increases in the estimated density of the innervating CA fibers. This finding is consistent with our previous report that E2 treatment increases the number of TH-immunoreactive cells in the ventral tegmental area, a major source of DA in the forebrain, and the locus coeruleus, the primary source of NE (LeBlanc et al., 2007). Both of these brainstem CA cell groups, which likely project to the auditory forebrain (Mello et al., 1998; Ribeiro and Mello, 2000; Appeltants et al., 2001, 2004), contain estrogen receptors (Maney et al., 2001) and therefore could be directly affected by E2 treatment.
As we have reported previously for the ZENK response, CA innervation in some areas of the auditory forebrain was not sensitive to the effects of E2. Unlike the rostral areas of NCM, the caudomedial edge (cNCM) does not respond to E2 treatment with increased ZENK expression nor does it respond with increased selectivity for song over tones (Sanford et al., 2010). Here, we showed that the CA innervation of this region is similarly insensitive to E2 treatment; whereas estimated CA fiber density increased in the rostral areas of NCM after E2 treatment, we detected no such change in cNCM (Fig. 3). The excellent anatomical match between E2-induced ZENK expression and effects on CA fiber density suggest strongly that the two may be related. This hypothesis is further supported by our observation of CA basket-like structures surrounding ZENK-immunoreactive cells in NCM (unpublished). Our results add to existing evidence on connectivity, electrophysiological responses, and neurochemical markers (Pinaud et al., 2006) that the rostral and caudal domains of NCM are perhaps functionally distinct. Future work should focus on the distinction between the rostral and caudal domains and the delineation of the rostral domains from regions of Field L proper.
How might CA input affect auditory selectivity? If the CA cells that project to auditory areas themselves fire in response to song, the ensuing catecholamine release may cause or modulate subsequent activity in a way that produces selective responses. Gale and Perkel (2010) recently demonstrated that in male zebra finches, TH-immunoreactive cells in the ventral tegmental area fire in response to sound and in fact are highly selective for conspecific song. LeBlanc et al. (2007) reported that in E2-treated female white-throated sparrows, hearing song induces ZENK expression not only in the ventral tegmental area but also in the locus coeruleus, substantia nigra, and periaqueductal gray, and that these responses are selective for song only in birds with physiological breeding levels of plasma E2. This effect was particularly striking in the locus coeruleus; in E2-treated birds, the ZENK response to song was threefold higher than the response to tones, but in blank-implanted birds the responses to song and tones were identical. Although LeBlanc et al. reported that the ZENK response in CA cell groups of the brainstem was not localized in TH positive cells, other authors have reported colocalization of immediate early gene expression and TH in these cell groups after presentation of song or other social stimuli (Bharati and Goodson, 2006; Goodson et al., 2009; Nordeen et al., 2009).
The CA cells in the brainstem need not be actively firing during song in order to play a neuromodulatory role in E2-dependent auditory selectivity. Some CA terminals may release transmitter in a nonsynaptic, or paracrine fashion, which may alter the responsivity or spontaneous firing activity in forebrain neurons (reviewed by Beaudet and Descarries, 1978). Such changes, which may be sustained over prolonged periods (Reader et al., 1979) may enable forebrain areas to respond differently to the same input depending on the behavioral state of the animal (e.g., season or social context), without changes in the selectivity of CA neurons themselves. Seasonal changes in catecholamine-based neuromodulation may enable auditory systems of female songbirds to respond selectively to male song during the breeding season and to respond less selectively outside it.
In addition to mediating changes associated with the transition from a non-breeding to breeding context, catecholamines may play a role in encoding more detailed information about social context within the breeding season. For example, in male zebra finches singing in different social contexts, NE depletion eliminates context-dependent suppression of ZENK expression in the song system (Castelino and Ball, 2005). Social context has been associated with catecholamines also in auditory structures; in female canaries, NE depletion reduces behavioral preferences for attractive male song while eliminating the selectivity of ZENK responses for those songs (Appeltants et al., 2002b; Lynch and Ball, 2008; Vyas et al., 2008). Female European starlings exposed for sustained periods to high-quality male song have higher levels of DBH and catecholamine metabolites in NCM than those exposed to sustained periods of low quality song (Sockman and Salvante, 2008). CA innervation and release in the auditory forebrain is therefore sensitive to stimulus quality and thus the quality of potential mates, and may play a role in context-dependent mate choice. Because hearing song can stimulate the secretion of reproductive hormones (Hinde and Steel, 1978; Morton et al., 1985; Bentley et al., 2000; Maney et al., 2007) it is possible that the effects of hearing song on CA systems are mediated by plasma E2. Our present results strongly support this hypothesis. Whereas Sockman and Salvante (2008) found that song quality modulated catecholamine metabolites and presumably catecholamine release, we found no evidence that release was affected by E2 treatment. This discrepancy could be due to the auditory environment; in the previous study, females heard song whereas the birds in the present study were housed with no auditory stimuli aside from a female companion. Experiments are underway to explore the effects of hearing song on CA synthesis and metabolism and the modulation of those effects by E2.
Although lateralization of CA function was not our primary focus in this study, we included hemisphere as a factor in our analysis in order to control for possible effects. We found that in some domains of NCM, TH- or DBH-immunoreactivity is higher in the left than in the right hemisphere. In MLd, the auditory midbrain, levels of NE and its metabolite MHPG are higher on the right. These findings add to a small but growing literature showing lateralization of function in the songbird auditory forebrain and midbrain as evidenced by ZENK expression (Avey et al., 2005), electrophysiological recording (George et al., 2004; Phan and Vicario, 2010), blood oxygen level dependent (BOLD) responses (Poirier et al., 2009) and behavioral assay after retrodialysis (Remage-Healey et al., 2010). This literature shows evidence of left hemisphere dominance in a variety of species and demonstrates that specific functions, such as auditory selectivity for conspecific song, can be lateralized even at the level of the midbrain (Poirier et al., 2009). In our study, interhemispheric differences did not depend on E2 treatment, meaning that both hemispheres responded equally to the hormone and that CA activity may differ between hemispheres at all times of the year.
We predicted that CA fiber density in each region would be related to catecholamine or metabolite concentrations. We did not, however, find correlations between the estimated density of enzyme-immunoreactive fibers and the concentrations of catecholamines or their metabolites. In other words, the results from our IHC measurements did not predict the results of the HPLC measurements. The most notable correlation, between estimated DBH-immunoreactivity and the concentration of NE in CMM, was driven by the effects of E2 on both variables independently. The lack of a relationship between the IHC and HPLC data raises important questions about what is actually being measured by each method and how we should expect them to relate. Because the synthetic enzymes may require posttranslational modifications such as phosphorylation, and because there are multiple downstream products of these enzymes, the relationships between the enzymes and any single product may not be linear. Even if the levels of the enzyme can be used to predict the level of monoamine and vice versa, because we have shown evidence for interhemispheric differences both in catecholamines and their synthetic enzymes, we cannot expect levels of these enzymes to relate absolutely to catecholamine levels in the contralateral hemisphere in this study. In order to determine how CA activity as measured by IHC relates to that measured by HPLC, both methods would need to be used in the same sample, for example in alternate series of sections. The fact that we detected more effects of E2 manipulation using IHC than with HPLC suggests either that the former may be a more sensitive measure than the latter or that E2 may affect the capacity to synthesize catecholamines without stimulating their synthesis directly.
In this study, we have shown that changes in plasma gonadal steroids, such as during the transition from a non-breeding to breeding state, can significantly alter the neurochemical makeup of sensory regions involved in the processing of sociosexual signals. Breeding levels of plasma E2 increased the estimated CA innervation of the auditory forebrain (NCM and CMM) and midbrain (MLd) in female sparrows that use auditory signals to choose mates. Estrogen-dependent CA innervation of sensory areas may be a conserved mechanism by which sensory systems are tuned and sensory responses modulated in order to ensure a good match between the social context of a signal and the subsequent behavioral response (reviewed by Maney & Pinaud, 2011). Future work should explore the role of CA input in auditory selectivity, and its modulation by gonadal steroids, using electrophysiological or real-time optical techniques in seasonally breeding species.
Acknowledgments
We gratefully acknowledge Allison Reid, Arundhati Murthy, Sarah Green, David Lee, and Said Saab for technical assistance in Atlanta and Dr. Richard B. Mailman and Stan B. Southerland for providing and helping with the HPLC system in Chapel Hill. We also thank Raphael Pinaud for suggestions regarding the experimental design. This research was supported by NINDS R01 NS055125 to KWS, NSF IBN-0346984 to DLM, and the Center for Behavioral Neuroscience IBN-9876754.
ABBREVIATIONS
- aNCM
apical caudomedial nidopallium
- CA
catecholaminergic
- CMM
caudomedial mesopallium
- cNCM
caudal caudomedial nidopallium
- DA
dopamine
- DBH
dopamine beta-hydroxylase
- DOPAC
dihydroxyphenylacetic acid
- E2
estradiol
- HA
apical part of the hyperpallium
- HPLC
high pressure liquid chromatography
- IHC
immunohistochemistry
- MHPG
3-methoxy-4-hydroxyphenylglycol
- MLd
dorsal lateral nucleus of the mesencephalon; auditory midbrain
- NCM
caudomedial nidopallium
- NE
norepinephrine
- Ov
ovoidalis; auditory thalamus
- rNCM
rostral caudomedial nidopallium
- TH
tyrosine hydroxylase
LITERATURE CITED
- Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: Demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tiss Res. 2001;304:237–259. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002a;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Del Negro C, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res. 2002b;133:221–235. doi: 10.1016/s0166-4328(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. Catecholaminergic inputs to aromatase cells in the canary auditory forebrain. Neuroreport. 2004;15:1727–1730. doi: 10.1097/01.wnr.0000135920.75925.1e. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Avey MT, Phillmore LS, MacDougall-Shackleton SA. Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav Brain Res. 2005;165:247–253. doi: 10.1016/j.bbr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bailhache T, Balthazart J. The catecholaminergic system of the quail brain -immunocytochemical studies of dopamine beta-hydroxylase and tyrosine-hydroxylase. J Comp Neurol. 1993;329:230–256. doi: 10.1002/cne.903290206. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Identification of catecholaminergic cell groups in the brainstem of the canary, zebra finch, white-throated sparrow and budgerigar by tyrosine-hydroxylase immunohistochemistry. Belg J Zool. 1996;126:65–78. [Google Scholar]
- Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- Beaudet A, Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3:851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Wingfield JC, Morton ML, Ball GF. Stimulatory effects on the reproductive axis in female songbirds by conspecific and heterospecific male song. Horm Behav. 2000;37:179–189. doi: 10.1006/hbeh.2000.1573. [DOI] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Comp Neurol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino CB, Ball GF. A role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata) Eur J Neurosci. 2005;21:1962–1972. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- de Vries G, Jordan M, Reza M, Rosen GJ, Immerman E, Forger NG. Sexual differentiation of vasopressin innervation of the brain: Cell death versus phenotypic differentiation. Endocrinology. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells of the field L complex in zebra finches (Taenopygia guttata) J Comp Neurol. 1992;325:388–404. doi: 10.1002/cne.903250306. [DOI] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. A basal ganglia pathway drives selective auditory responses in songbird dopaminergic neurons via disinhibition. J Neurosci. 2010;30:1027–1037. doi: 10.1523/JNEUROSCI.3585-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- George I, Vernier B, Richard JP, Hausberger M, Cousillas H. Hemispheric specialization in the primary auditory area of awake and anesthetized starlings (Sturnus vulgaris) Behav Neurosci. 2004;118:597–610. doi: 10.1037/0735-7044.118.3.597. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80:168–177. doi: 10.1046/j.0022-3042.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci. 2009;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJG. A DNA test to sex most birds. Mol Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Hinde RA, Steel E. The influence of daylength and male vocalizations on the estrogen-dependent behavior of female canaries and budgerigars, with discussion of data from other species. Adv Study Anim Behav. 1978;8:39–73. [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14 :488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Mello CV, Nottebohm F. Associative learning and stimulus novelty influence the song-induced expression of an immediate early gene in the canary forebrain. Learn Mem. 1995;2:62–80. doi: 10.1101/lm.2.2.62. [DOI] [PubMed] [Google Scholar]
- Karle EJ, Anderson KD, Medina L, Reiner A. Light and electron microscopic immunohistochemical study of dopaminergic terminals in the striatal portion of the pigeon basal ganglia using antisera against tyrosine hydroxylase and dopamine. J Comp Neurol. 1996;369:109–124. doi: 10.1002/(SICI)1096-9861(19960520)369:1<109::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Karten HJ. The organization of the ascending auditory pathway in the pigeon (Columba livia). I Diencephalic projections of the inferior colliculus (nucleus mesencephalicus lateralis, pars dorsalis) Brain Res. 1967;6:409–427. doi: 10.1016/0006-8993(67)90055-8. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Breese GR, Mailman RB. Simultaneous quantification of dopamine, 5-hydroxytryptamine and four metabolically related compounds by means of reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1981;225:347–357. doi: 10.1016/s0378-4347(00)80283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult Rhesus monkeys. J Comp Neurol. 1998;395:1–17. [PubMed] [Google Scholar]
- LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64:275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Ball GF. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain Behav Evol. 2008;72:207–214. doi: 10.1159/000157357. [DOI] [PubMed] [Google Scholar]
- Maney DL. Hormonal control of sexual behavior in female nonmammalian vertebrates. In: Breed MD, Moore J, editors. Encyclopedia of Animal Behavior. Vol. 1. Oxford: Elsevier; 2010. pp. 697–703. [Google Scholar]
- Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311:189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lake JI, Lange HS, O'Brien S. Rapid neuroendocrine responses to auditory courtship signals. Endocrinology. 2007;148:5614–5623. doi: 10.1210/en.2007-0879. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Maney DL, MacDougall-Shackleton EA, MacDougall-Shackleton SA, Ball GF, Hahn TP. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. J Comp Physiol A. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol. 2011 doi: 10.1016/j.yfrne.2010.12.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic sys- tem of the zebra finch brain: Immunocytochemical study of dopamine-b-hydroxylase. J Comp Neurol. 1998;400:207–228. [PubMed] [Google Scholar]
- Metzger M, Jiang S, Wang J, Braun K. Organization of the dopaminergic innervation of forebrain areas relevant to learning: A combined immunohistochemical/retrograde tracing study in the domestic chick. J Comp Neurol. 1996;376:1–27. doi: 10.1002/(SICI)1096-9861(19961202)376:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moons L, van Gills J, Ghijsels E, Vandensande R. Immunocytochemical localization of the L-DOPA and dopamine in the brain of the chicken (Gallus domesticus) J Comp Neurol. 1994;346:97–118. doi: 10.1002/cne.903460107. [DOI] [PubMed] [Google Scholar]
- Moore MC. Effect of female sexual displays on the endocrine physiology and behavior of male white-crowned sparrows, Zonotrichia leucophrys. J Zool. 1983;199:137–148. [Google Scholar]
- Morton ML, Pereyra ME, Baptista LF. Photoperiodically induced ovarian growth in the white-crowned sparrow (Zonotrichia leucophrys gambelii) and its augmentation by song. Com Biochem Physiol A. 1985;80:93–97. [Google Scholar]
- Nordeen EJ, Holtzman DA, Nordeen KW. Increased Fos expression among midbrain dopaminergic cell groups during birdsong tutoring. Eur J Neurosci. 2009;30:662–670. doi: 10.1111/j.1460-9568.2009.06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson C, Holmberg A, Holmgren S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. J Comp Neurol. 2008;508:756–770. doi: 10.1002/cne.21705. [DOI] [PubMed] [Google Scholar]
- Phan ML, Vicario DS. Hemispheric differences in processing of vocalizations depend on early experience. Proc Natl Acad Sci. 2010;107:2301–2306. doi: 10.1073/pnas.0900091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J Neurobiol. 2006;66:182–195. doi: 10.1002/neu.20211. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Terleph TA. A songbird forebrain area potentially involved in auditory discrimination and memory formation. J Biosci. 2008;33:145–155. doi: 10.1007/s12038-008-0030-y. [DOI] [PubMed] [Google Scholar]
- Poeggel G, Nowicki L, Braun K. Early social deprivation alters monoaminergic afferents in the orbital prefrontal cortex of Octodon degus. Neuroscience. 2003;116:617–620. doi: 10.1016/s0306-4522(02)00751-0. [DOI] [PubMed] [Google Scholar]
- Poirier C, Boumans T, Verhoye M, Balthazart J, Van der Linden A. Own-song recognition in the songbird auditory pathway: Selectivity and lateralization. J Neurosci. 2009;29 :2252–2258. doi: 10.1523/JNEUROSCI.4650-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader TA, Ferron A, Descarries L, Jasper HH. Modulatory role for biogenic amines in the cerebral cortex. Microiontophoretic studies. Brain Res. 1979;160:217–229. doi: 10.1016/0006-8993(79)90420-7. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karle EJ, Anderson KD, Medina L. Catecholaminergic perikarya and fibers in the avian nervous system. In: Smeets WFA, Reiner A, editors. Phylogeny and development of catecholamine system in the CNS of vertebrates. Cambridge University Press; Cambridge: 1994. pp. 135–181. [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Mello CV. Gene expression and synaptic plasticity in the auditory forebrain of songbirds. Learn Mem. 2000;7:235–243. doi: 10.1101/lm.34400. [DOI] [PubMed] [Google Scholar]
- Rice W. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Riters LV, Pawlisch BA. Evidence that norepinephrine influences responses to male courtship song and activity within song control regions and the ventromedial nucleus of the hypothalamus in female European starlings. Brain Res. 2007;1149:127–140. doi: 10.1016/j.brainres.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Cookson KK, Heaton KJ, Hall WS, Brauth SE. Distribution of tyrosine hydroxylase-containing neurons and fibers in the brain of the budgerigar (Melopsittacus undulatus): General patterns and labeling in vocal control nuclei. J Comp Neurol. 2001;429:436–454. doi: 10.1002/1096-9861(20010115)429:3<436::aid-cne6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sanford SE, Lange HS, Maney DL. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Dev Neurobiol. 2010;70:73–86. doi: 10.1002/dneu.20757. [DOI] [PubMed] [Google Scholar]
- Shank MC. The natural termination of the refractory period in the slate-colored junco and in the white-throated sparrow. Auk. 1959;76:44–54. [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose-oxidase DAB nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc Roy Soc Lond B. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Dev Neurobiol. 2008;68:656–668. doi: 10.1002/dneu.20611. [DOI] [PubMed] [Google Scholar]
- Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson A. Regulation of refractory period in the photoperiodic responses of the white- throated sparrow. J Exp Zool. 1958;139:349–379. doi: 10.1002/jez.1401390207. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taeniopygia guttata) J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vyas A, Harding C, McGowan J, Snare R, Bogdan D. Noradrenergic neurotoxin, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4), treatment eliminates estrogenic effects on song responsiveness in female zebra finches (Taeniopygia guttata) Behav Neurosci. 2008;122:1148–1157. doi: 10.1037/0735-7044.122.5.1148. [DOI] [PubMed] [Google Scholar]