Abstract
Induction of new bone formation is frequently seen in the bone lesions from prostate cancer (PCa). However, whether osteogenesis is necessary for prostate tumor growth in bone is unknown. Recently, two xenografts, MDA-PCa-118b and MDA-PCa-133, were generated from PCa bone metastases. When implanted subcutaneously in SCID mice, MDA-PCa-118b induced strong ectopic bone formation while MDA-PCa-133 did not. To identify the factors that are involved in bone formation, we compared the expression of secreted factors (“secretome”) from MDA-PCa-118b and MDA-PCa-133 by cytokine array. We found that the osteogenic MDA-PCa-118b xenograft expressed higher levels of BMP-4 and several cytokines including IL-8, Gro, and CCL2. We demonstrated that BMP-4 secreted from MDA-PCa-118b contributed to about a third of the osteogenic differentiation seen in MDA-PCa-118b tumors. The conditioned media from MDA-PCa-118b induced a higher level of osteoblast differentiation, which was significantly reduced by treating with BMP-4 neutralizing antibody or the small molecule BMP receptor 1 inhibitor LDN-193189. BMP-4 did not elicit an autocrine effect on MDA-PCa-118b, which expressed low to undetectable levels of BMP receptors. Treatment of SCID mice bearing MDA-PCa-118b tumors with LDN-193189 significantly reduced tumor growth. Thus, these studies support a role of BMP4-mediated osteogenesis in the progression of PCa in bone.
Keywords: PCa, bone metastasis, chemokines, cytokines, osteoblast
Introduction
Prostate cancer (PCa) has a propensity to metastasize to bone. Development of bone metastases substantially shortens survival time of men with PCa and remains a major challenge in the treatment of PCa. The mechanisms that lead to the preferential growth of PCa in bone are not known. Bone metastasis from PCa typically exhibits osteoblastic lesions (1). In the histopathologic analysis of PCa bone metastases, a substantial increase in the numbers of osteoblasts and bone matrix adjacent to PCa cells are often seen. While metastases have an overall bone-forming phenotype, the clinical presentation of PCa bone metastasis suggests that it is a heterogeneous disease that also involves osteoclasts (2, 3). The complex interactions of tumor cells with osteoblasts, osteoclasts and other cells suggest that PCa cells secrete multiple factors that alter the bone microenvironment and stimulate osteoblast proliferation and/or differentiation at the sites of skeletal metastasis (4). Identification of factors that are involved in the osteoblastic bone lesions will uncover targets for therapy. However, this has been difficult due to anatomic inaccessibility, and the small amount of tumor in biopsies from bone metastasis is not sufficient for biochemical analysis.
Recently, two bone metastasis-derived xenografts, MDA-PCa-118b and MDA-PCa-133, have been generated, and they provide sufficient material for the analysis of the paracrine factors that play a role in PCa progression in bone. MDA-PCa-118b (PCa-118b) xenograft exhibits characteristics seen in patients with PCa in bone, e.g., a strong osteogenic phenotype when implanted into mouse femurs (5). Interestingly, PCa-118b induced new bone formation even when implanted subcutaneously (5). On the other hand, the MDA-PCa-133 (PCa-133) xenograft proliferates when implanted subcutaneously, but does not induce bone formation. The difference in the bone forming activity of these two xenografts reflects the heterogeneous nature of PCa bone metastases (2).
In this study, we identified the factors secreted by these two xenografts using an antibody array that detects cytokines in the conditioned medium. We found that the osteogenic PCa-118b xenograft not only expresses higher levels of osteogenic factors, e.g., BMP-4, but also osteoclastogenic factors, e.g., MCP-1 and M-CSF, compared to the PCa-133 xenograft. In addition, BMP-4 secreted from PCa-118b induced osteoblast differentiation in a paracrine fashion. Inhibition of the BMP receptor on osteoblasts by a small molecule inhibitor LDN-193189 reduces PCa-118b tumor growth. These studies provide a biochemical basis for the osteogenic phenotype of PCa-118b and support a role of osteogenesis in the progression of PCa in bone.
Materials and Methods
Materials
Recombinant human proteins were used: basic FGF (FGF-2), BMP-2, TIMP2 and IGFBP-2 (R&D, Minneapolis, MN), BMP4 (Sigma-Alrich, St. Louis, MO), Gro-α, Gro-β, Gro-γ, IL-8, SCF and BDNF (Cell Science, Canton, MA). LDN-193189 was from Axon Medchem (Netherlands). PCa xenograft tumors PCa-118b (5) and PCa-133 were derived from bone lesions of patients with castration-resistant PCa. Fingerprinting of cells isolated from PCa-118b and PCa-133 xenografts showed that their profiles are unique as expected. PC3 cell line was confirmed by fingerprinting.
Xenograft tumors and conditioned medium
Subcutaneous grafts of PCa-118b or PCa-133 were generated by implanting fragments (less than 1 mm3) of tumors subcutaneously into SCID mouse. The tumors were allowed to grow until they reached the size of 500 mm3, dissected, weighed, cut into small pieces, and suspended (250 mg wet weight per ml medium) in BGJb medium (Invitrogen) with antibiotics. The conditioned media were collected every 24 hrs for two days, spun to clarify and stored at −80°C.
Assessment of chemokines in conditioned media
Chemokines and cytokines in the conditioned media were assessed by using RayBio Human Cytokine Antibody Array C (RayBiotech, Norcross, GA) (Membranes VI and VII) according to the manufacturer’s instructions. The signal intensity of each spot was quantified by Image J. The signal intensity of the negative control was subtracted from each spot. The experiments were performed three times using conditioned medium from three different batches of xenograft tumors. BMP-4 or BMP-6 levels were measured using ELISA kits (RayBiotech, Norcross, GA).
Reverse transcription and quantitative PCR analysis
Total RNA was extracted from PCa-118b or PCa-133 using RNeasy mini kit (Qiagen). The relative mRNA level for each gene was quantified by real-time RT-PCR with SYBR Green (Applied Biosystems), using GAPDH as a control.
Measurement of luciferase and alkaline phosphatase activities in calvarial osteoblasts
The transgenic mice harboring luciferase transgene (Col-luc mice) was described previously (6). Calvarial osteoblasts from Col-luc mice or CD1 mice were incubated with conditioned medium from PCa-118b or PCa-133 with or without neutralizing antibody or LDN-193189 for three days. Cells were lysed and the luciferase activity assessed (Promega, Cat. No. E1501). Alkaline phosphatase activity was measured using p-nitrophenyl phosphate liquid substrate system (Sigma-Aldrich).
Measurement of BMP reporter activity
C2C12 cells stably expressing a BMP-responsive Id1 promoter fused to a luciferase reporter gene (C2C12/BRA) was kindly provided by Dr. Daniel B. Rifkin (7). C2C12/BRA cells were incubated with conditioned medium from PCa-118b or BMP-4 with or without 100 nM LDN-193189 for 12 hrs and the luciferase activity assessed as above.
Western blot for Smad 5 phosphorylation
Cells in 6-well plates were treated with or without 100 nM LDN-193189 and/or 0.68 ng/ml BMP4 for 16 h. LDN-193189 was added 15 min before BMP4 addition. Cell extracts were resolved by SDS-PAGE followed by Western blot analysis, using phospho-SMAD-1/5 and SMAD-5 antibodies (Cell Signaling).
Effect of LDN-193189 on tumor growth in vivo
In the first experiment, SCID mice were implanted with MDA-PCa-118b tumors. After 7 days when tumors reached measurable sizes, mice were injected with LDN-193189 (3 mg/kg) or with vehicle intraperitoneally twice a day. Tumor sizes and body weights were measured weekly. Mice were injected with calcein at three days and one day prior to sacrifice. Blood was collected and tumors were weighed. A portion of the tumors were fixed in formaldehyde for micro-computed tomography (microCT), using EVS CT (General Electric), or further decalcified for bone histomorphometric analysis, using OsteoMeasure Analysis System (Osteometrics, Inc), or flash frozen for RNA preparation. Osteocalcin in the mouse serum was determined by ELISA (Biomedical Technologies). In the second experiment, PCa-118b tumors were first digested with Accumax (eBioscience), and the isolated cells were plated overnight, digested by Accutase (eBioscience), resuspended in Matrigel in 1:1 ratio, and injected into SCID mice (1 × 106 cells/mouse) subcutaneously. Mice were treated with LDN-193189 five days post-injection.
Statistical Analysis
Data are expressed as the mean ± SD unless otherwise stated. Statistical analyses were performed using Student’s t-test (two-tailed, paired). p values less than 0.05 were considered significant.
Results
PCa-118b and PCa-133 Xenografts
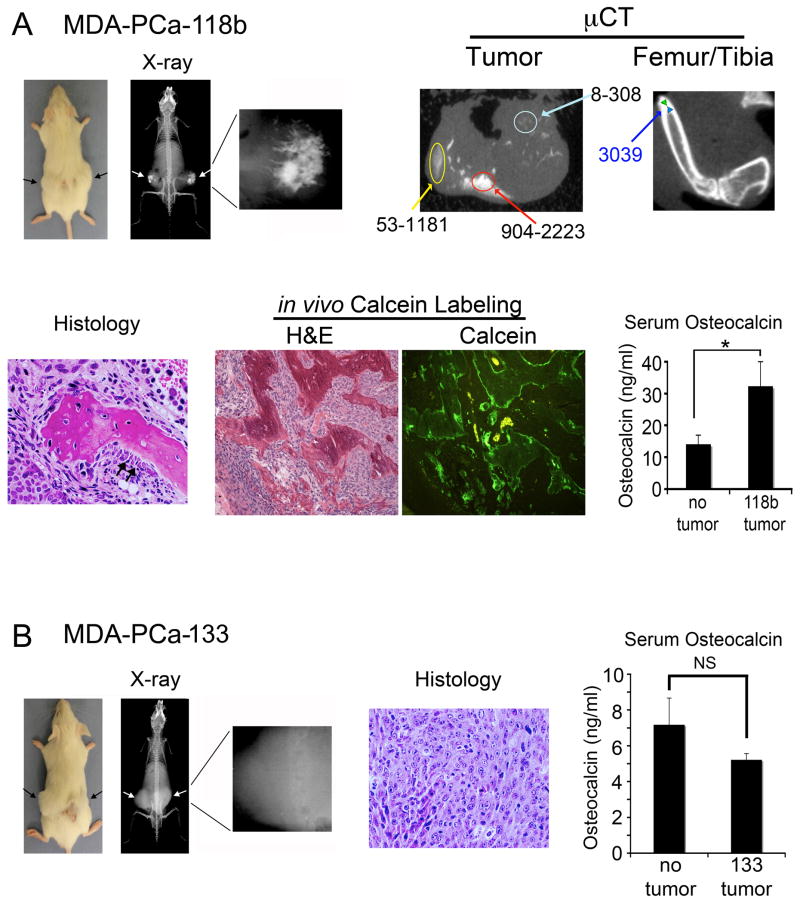
To delineate pathways that lead to osteoblastic phenotype of PCa bone metastasis, we selected PCa xenografts derived from bone metastasis with high or low osteogenic activity. PCa-118b and PCa-133 xenografts were established by implanting biopsy specimens from bone lesions into SCID mice subcutaneously. Interestingly, PCa-118b induced ectopic new bone formation as demonstrated by radiography, microCT, histology, and serum osteocalcin (Figure 1A). MicroCT analysis showed heterogeneous mineralization with various densities within the tumor (Figure 1A). In contrast, the femur/tibia bone exhibits uniform and much higher density than those in PCa-118b. Calcein labeling demonstrates that the newly formed bone is irregular. In contrast, the PCa-133 xenograft did not induce bone formation (Figure 1B).
Figure 1.
Analysis of PCa-118b and PCa-133 xenografts. (A) Subcutaneously implanted PCa-118b tumor showed mineralization (arrows) within the tumor by X-ray and microCT analyses. Three varying bone densities within the tumor are shown in comparison with the uniformed, high bone density in the femur/tibia. Histological analysis showed the presence of mineralized bone and activated osteoblasts (arrows) around the bone. Calcein labeling demonstrated the irregularity of newly formed bone. Serum osteocalcin levels are significantly higher in PCa-118b tumor bearing mice compared to those of controls. (B) Subcutaneously implanted PCa-133 tumor (arrows) did not show mineralized bone radiographically or histologically. Serum osteocalcin levels in PCa-133 tumor bearing mice were not significantly (NS) different from those in control mice. * p < 0.05.
Cytokine Array Analysis of Conditioned Medium from Xenografts
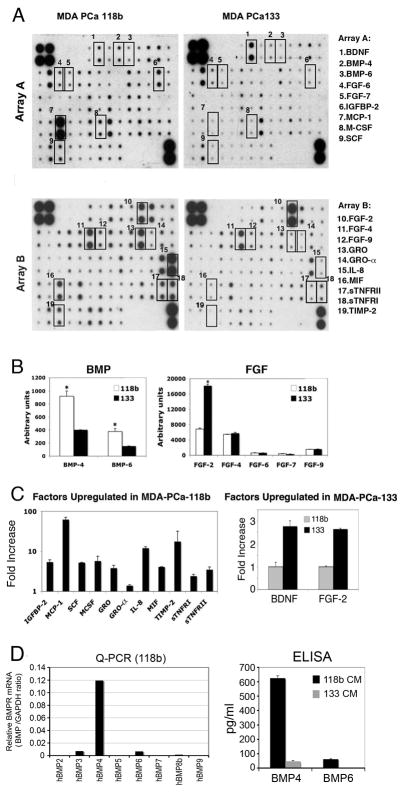
The strong bone-forming activity of PCa-118b may be due to the secretion of paracrine factors that stimulate osteoblast proliferation and/or differentiation. To identify these factors, we compared the expression of cytokines in the conditioned medium from PCa-118b and PCa-133 xenografts, using the Human Cytokine Antibody Array that detects 120 cytokines and growth factors. BMPs and FGFs are the two major families of protein factors that have effects on osteoblast proliferation and differentiation (8). The cytokine arrays indicate that PCa-118b expressed higher levels of BMP4 and BMP6 than PCa-133, while PCa-133 expressed a higher level of FGF2, but not FGF4 and FGF9, than PCa-118b (Figure 2B). Both xenografts expressed very low levels of FGF6 and FGF7 (Figure 2B).
Figure 2.
Cytokine expression profile of conditioned media from PCa-118b and PCa-133 xenografts. (A) Conditioned media from PCa-118b or PCa-133 were incubated with human antibody arrays that detected 120 cytokines. Cytokines that were differentially expressed are boxed. (B) The relative density of the array signals for BMPs and FGFs in the conditioned media of PCa-118b and PCa-133 xenografts was determined by Image J and expressed as arbitrary units. * p < 0.05. (C) Cytokines upregulated in PCa-118b versus PCa-133 conditioned media were determined by Image J and expressed as fold increase. Similar results were obtained using conditioned medium prepared from three different batches of xenograft tumors. (D) BMP RNA transcripts in PCa-118b or PCa-133 tumors were determined by qRT-PCR using primers specific to human BMPs (see primer list in Supplemental Table 1). Levels of human BMP4 and BMP6 proteins in PCa-118b and MDA-PCa-133 conditioned media were determined by ELISA.
Other factors that are highly expressed in PCa-118b compared to PCa-133 include Gro (including α, β, and γ), IL-8, TIMP-2, SCF, IGFBP-2, MIF, MCP-1, M-CSF, sTNFR1, and sTNFRII (Figure 2C). On the other hand, PCa-133 expressed a higher level of BDNF compared to PCa-118b (Figure 2C).
BMP-4 expression in PCa-118b
To confirm the cytokine array analysis, the levels of BMP RNA transcripts were determined by QPCR. Of BMPs 1–9 examined, BMP4 was the most highly expressed in PCa-118b xenograft. Low levels of BMP3 and BMP6 were also detected (Figure 2D). None of these BMP messages were detected in PCa-133 (data not shown). Further, ELISA assays of the conditioned medium showed that BMP4 in PCa-118b was 13-fold higher than that found in PCa-133 conditioned medium, with the levels at 624.8 ±17.3 pg/ml and 47.2 ±4.3 pg/ml, respectively (Figure 2D). BMP-6 in PCa-118b was at 62.4 ±2.6 pg/ml but too low to be detected in the PCa-133 conditioned medium (Figure 2D).
Effect of cytokines and conditioned medium on osteoblast proliferation and differentiation
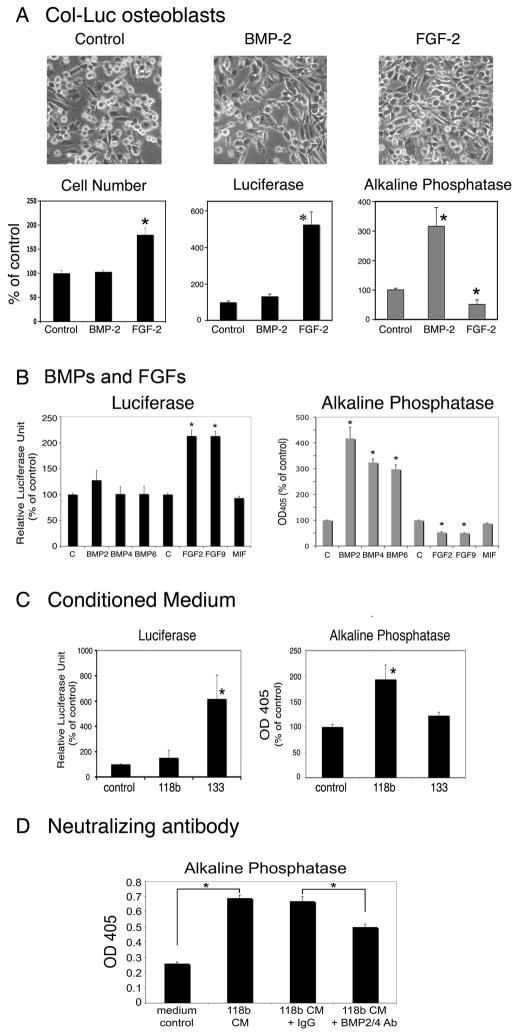
Next, we examined the effects of these cytokines on osteoblast proliferation and/or differentiation. To improve the specificity of the measurement, primary calvarial osteoblasts isolated from a 2.3 kb collagen promoter driven luciferase reporter transgenic mice (Col-luc mice) were used (6, 9). Previous studies have established that the luciferase reporter activity of the Col-luc osteoblasts correlated with osteoblast proliferation (9). Treatment with 10 ng/ml FGF-2 induces an elongated spindle shape morphology, an increase in osteoblast cell number, an increase in luciferase activity, and a small but significant inhibition of alkaline phosphatase activity (Figure 3A), demonstrating that FGF-2 stimulates Col-luc osteoblast proliferation. In contrast, treatment with 100 ng/ml BMP-2 induces a significant increase in alkaline phosphatase activity without affecting the luciferase activity (Figure 3A), suggesting that BMP-2 stimulates Col-luc osteoblast differentiation.
Figure 3.
BMPs, FGFs, and PCa-118b and PCa-133 conditioned media on osteoblast proliferation or differentiation. (A) Calvarial osteoblasts from Col-luc mice were incubated without or with BMPs (100 ng/ml) or FGF-2 (10 ng/ml) for three days. Cell morphology (x100), cell numbers, luciferase activity and alkaline phosphatase activity were measured. (B) BMP-2, BMP-4, BMP-6, FGF2, and FGF9 on Col-luc osteoblasts. (C) Conditioned media from PCa-118b or PCa-133 xenografts on Col-luc osteoblasts. (D) Neutralizing anti-BMP2/4 antibody on Col-luc osteoblasts. Each experiment was performed in triplicates and repeated at least three times. The averages of triplicates ± SD were shown. Data in A–C were expressed as a percent of control. * p < 0.05.
Next, when the Col-luc osteoblasts were treated with recombinant BMP-2, −4, or −6, a significant increase (about 300% compared to the control, n=3) of alkaline phosphatase activity was observed, with no effect on luciferase activity (Figure 3B). In contrast, treatment with 10 ng/ml of FGF-2 or FGF-9 for three days led to an increase in luciferase activity, but a decrease in alkaline phosphatase activity (Figure 3B). These observations suggest that BMP2, 4 and 6 exert similar effects on osteoblast differentiation, while FGF2 and 9 exert similar effects on osteoblast proliferation.
Cytokines, including MIF (Figure 3B), IGFBP2, BDNF, and SCF, did not have a significant effect on luciferase or alkaline phosphatase activity of Col-luc osteoblasts (Table 1). Gro (including Gro-α, β, and γ), IL-8, and TIMP-2, induced small but significant increases in alkaline phosphatase (Table 1) but not luciferase activity (data not shown). These observations suggest that these cytokines may be involved, but to a lesser extent than BMP4, in the osteogenesis of the PCa-118b xenograft.
Table 1.
Alkaline phosphatase activity in Col-luc primary mouse osteoblasts (PMOs) treated with cytokines
| Concentration (ng/ml) | Alkaline phosphatase activity (% increase over control) | |
|---|---|---|
| Control | 0 | 0±4.7 |
| GRO-α | 10 | 26.5±9.3* |
| GRO-β | 10 | 21.8±2.6* |
| GRO-γ | 10 | 20.1±5.4* |
| IL-8 | 750 | 30.2±2.0* |
| TIMP-2 | 500 | 18.5±2.9* |
| IGFBP-2 | 900 | 1.0±4.0 |
| BDNF | 500 | 0.9±5.6 |
| SCF | 50 | −3.6±7.3 |
Col-Luc PMOs were treated with cytokines for three days and alkaline phosphatase activity was measured. PMOs without treatment were used as controls and defined as 100%.
p<0.05
Osteoblast regulatory activities in the conditioned media of PCa-118b and PCa-133 xenografts
Next, the conditioned media from PCa-118b or −133 were examined for their effects on osteoblast differentiation or proliferation. Treatment of Col-luc calvarial osteoblasts with conditioned media from PCa-133 led to a 5-fold increase in luciferase activity, while PCa-118b xenograft only resulted in an average of 30% increase (Figure 3C). In contrast, conditioned medium from PCa-118b induced about a 2-fold increase in alkaline phosphatase activity compared to that from PCa-133, which induced an average of 15% increase (Figure 3C).
We further used a neutralizing antibody against BMP-4 to determine if BMP-4 might be involved in the increase in alkaline phosphatase activity seen in the CM of PCa-118b xenograft. The BMP-4 antibody inhibited about 40% of PCa-118b-induced increase in alkaline phosphatase activity (Figure 3D), while control IgG or BMP-6 antibody (data not shown) did not show an inhibitory effect. These observations suggest that BMP4 plays a significant role in PCa-118b-induced osteoblast differentiation.
BMP4 signals through BMP receptor in osteoblasts
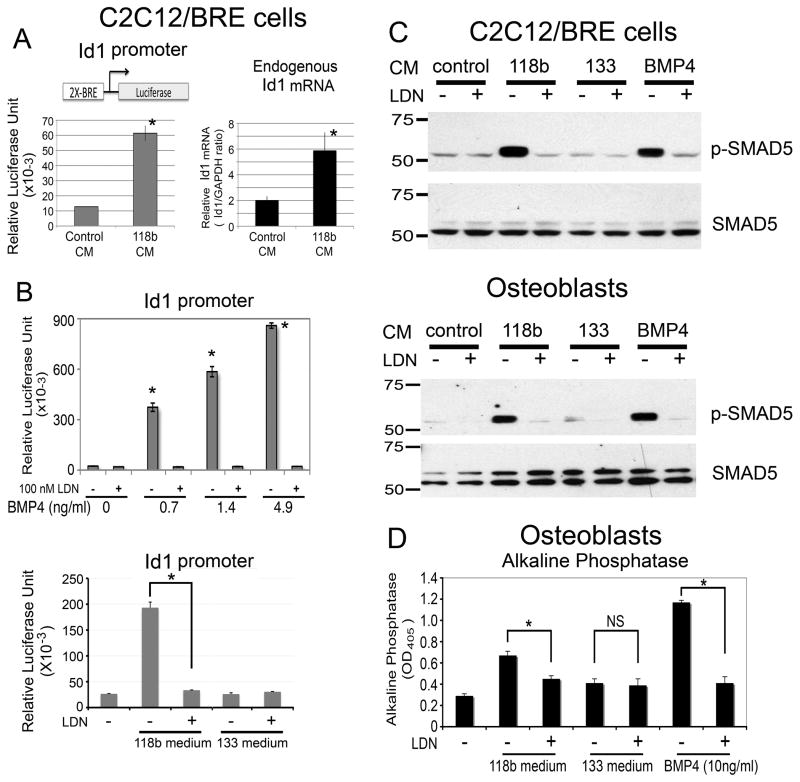
To examine whether PCa-118b conditioned medium activates BMP signaling in osteoblasts, we first examined its effect on Id1 promoter activity. Id1 is a direct target of the BMP pathway and its promoter contains multiple BMP-specific SMAD-binding elements (10). The C2C12 cell line expresses BMP receptors (7) and can be induced to become osteoblasts by BMP-2. C2C12/BRE was generated by transfecting the Id1 promoter reporter into C2C12 cells (7). When the C2C12/BRE cells were incubated with MDA-PCa-118b conditioned medium, luciferase reporter activity was increased by 5-fold, which was accompanied by a 3-fold increase in endogenous Id1 message (Figure 4A). In contrast, conditioned medium from PCa-133 did not stimulate Id1 promoter activity (Figure 4B, lower panel). LDN-193189, a small molecule inhibitor of BMP type I receptor (11), was used to specifically inhibit BMP signaling. In osteoblasts, LDN-193189 inhibited osteoblast differentiation (Fig. 4), but did not induce osteoblast cell death or inhibit osteoblast proliferation [data not shown and (11)]. Treatment of C2C12/BRE cells with 100 nM LDN-193189 led to an inhibition of BMP4-induced Id1 promoter activity at various BMP4 concentrations (Figure 4B, upper panel), suggesting that LDN-193189 blocks BMP-4-induced signaling. Further, LDN-193189 also inhibited BMP signaling to the Id1 promoter from PCa-118b conditioned medium (Figure 4B, lower panel).
Figure 4.
PCa-118b conditioned medium on BMP receptor signaling. (A) C2C12/BRE cells stably expressing a BMP-responsive Id1 promoter-luciferase reporter were incubated with PCa-118b conditioned medium for 12 hrs. Cell lysates were measured directly for luciferase activity. Endogenous Id1 and GAPDH messages were analyzed by qRT-PCR and expressed as Id1/GAPDH ratio. (B) C2C12/BRE cells were treated with increasing concentrations of either BMP4 (upper) or conditioned media from PCa-118b or PCa-133 (lower) in the presence or absence of 100 nM LDN-193189 for 12 hrs, and luciferase activities were analyzed. (C) C2C12/BRE cells (upper) or primary osteoblasts (lower) were treated as in B, and cell lysates were immunoblotted for phosphorylated Smad5 followed by reblotting for total Smad5. (D) Primary mouse calvarial osteoblasts were treated as in C except that alkaline phosphatase activity was determined after 3 days. * p < 0.05; NS, not significant.
Next, we examined the effect of PCa-118b conditioned medium on the activation of Smad5, a BMP receptor signaling molecule. In C2C12/BRE cells, conditioned medium from PCa-118b but not PCa-133 induced Smad5 phosphorylation, and this activity was inhibited by LDN-193189 (Figure 4C). Similar results were observed using calvarial mouse osteoblasts (Figure 4C). These observations suggest that PCa-118b conditioned medium activated Smad5 signaling through the BMP receptors.
We further examined the effect of LDN-193189 on conditioned medium-mediated osteoblast activity. PCa-118b conditioned medium-induced alkaline phosphatase activity was significantly reduced (about 50%) by treating the osteoblasts with 100 nM LDN-193189 (Figure 4D), consistent with the inhibition observed with BMP4 neutralizing antibody (Figure 3D). LDN-193189, at a concentration of 100 nM, had no effect on osteoblast cell number (data not shown), indicating that the inhibition of alkaline phosphatase activity by LDN-193189 is not due to toxicity. Together, these results suggest that PCa-118b conditioned medium activates BMP receptor signaling in osteoblasts, and this effect is inhibited by the BMP type I receptor inhibitor LDN-193189.
Expression of BMP receptors in PCa-118b
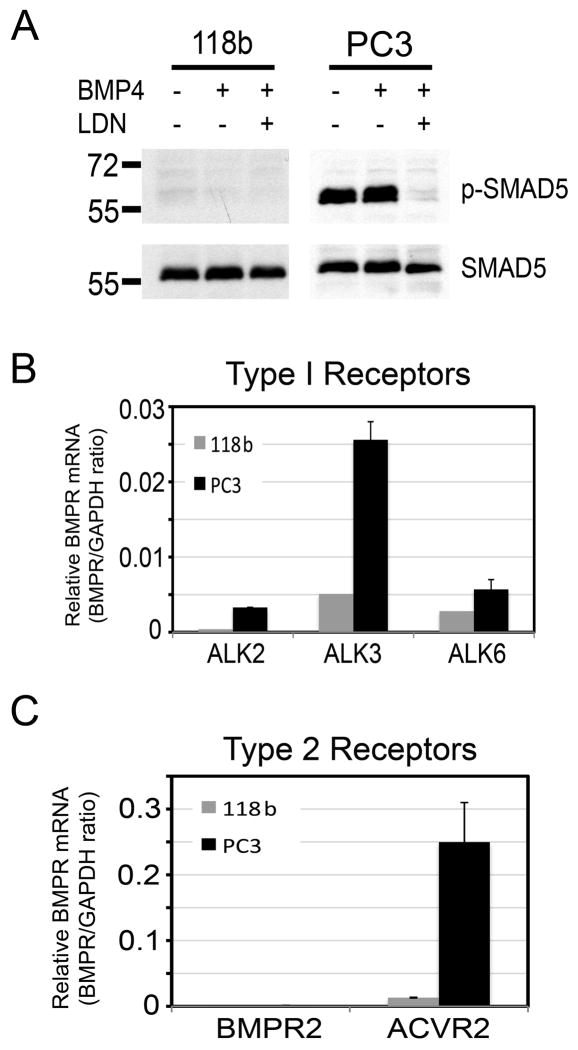
While the results showed that PCa-118b tumor secretes BMP-4 to induce osteoblast differentiation in a paracrine fashion, it is not clear whether BMP-4 may have an autocrine effect on PCa-118b tumor cells. To examine this possibility, we digested the PCa-118b tumor and cultured the isolated cells in CnT-52 medium that allows tumor cell but not osteoblast survival (data not shown). Under this condition, we did not detect phosphorylated Smad5 in BMP4-secreting PCa-118b cells, even after further BMP4 addition (Figure 5A). In addition, LDN-193189 did not affect the expression of BMP4 target gene Id2 as well as BMP4 expression in PCa-118b cells (12) (Supplemental Figure S1). Consistent with a lack of response to endogenous or exogenous BMP4, PCa-118b cells did not express significant levels of BMP type I receptors, e.g., ALK2, ALK3 and ALK6 (Figure 5B) or BMP type II receptors, e.g., BMPR2 and ACVR2 (Figure 5C). In contrast, Smad5 was activated in PC-3 cells and this activation was inhibited by LDN-193189 treatment (Figure 5A). PC-3 cells were also found to express significant levels of BMP type I and II receptors (Figure 5B and 5C). Together, these results suggest that BMP-4 secreted from PCa-118b likely has minimal autocrine effects on PCa-118b cells.
Figure 5.
Lack of BMP4 signaling on PCa-118b cells. (A) PCa-118b cells and PC3 were treated with BMP4 with or without LDN-193189 for 12 hrs, and analyzed for Smad5 phosphorylation. Levels of BMP Type I receptor (ALK2, 3, 6) (B) and Type II receptor (BMPR2, ACVRs) (C) transcripts in PCa-118b tumors and PC-3 cells were determined by qRT-PCR (see Supplemental Table 1).
Effect of LDN-193189 on PCa-118b tumor growth in vivo
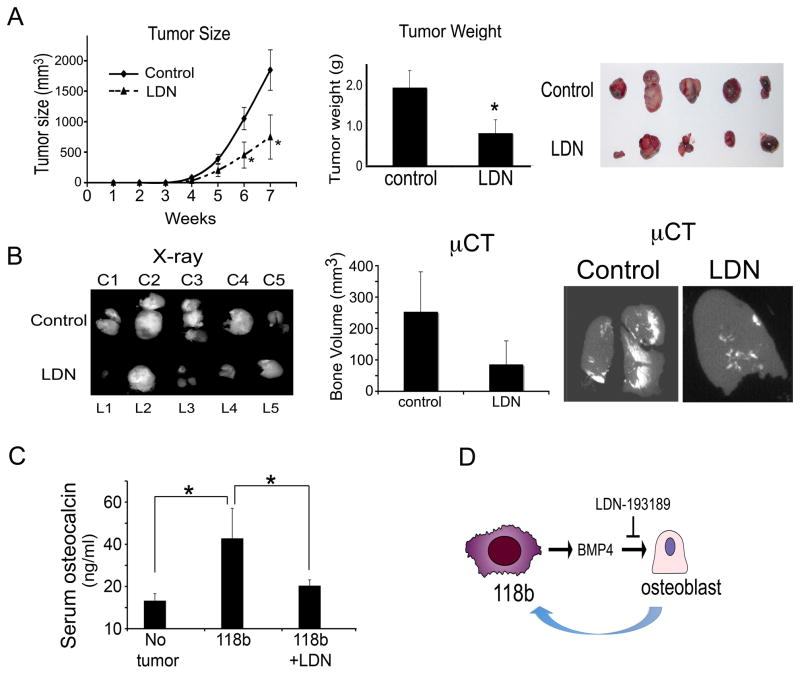
Our studies suggest that BMP4 is one of the principal paracrine factors that increase osteoblast differentiation in PCa-118b tumors. To assess the effect of BMP4-mediated osteoblast differentiation on PCa-118b tumor growth in vivo, we test the effect of LDN-193189 on PCa-118b xenograft growth in vivo. In the first experiment, LDN-193189 (3 mg/kg) was injected intraperitoneally twice a day after tumors became palpable 7 days post-implantation. The growth rates between the control vehicle- and LDN-193189-treated mice were not significantly different after the first 5 weeks, but differences in the growth rates were detected after 6 and 7 weeks post-treatment (Supplemental Figure 2). In the second experiment, cells were isolated from PCa-118b tumors and injected subcutaneously into SCID mice (1 × 106 cells per mouse). LDN-193189 or vehicle was applied to mice 5 days post-tumor cell injection before tumors were palpable. The differences in the average growth rates between these two groups, as measured by tumor size, were significant at 6 and 7 weeks post-treatment (Figure 6A). The tumor weights also showed significant differences at the termination of the study at week 7 (Figure 6A). The X-ray of the tumors showed that the ectopic bone volume and bone density were reduced in the tumors of LDN-193189-treated group compared to that of controls (Figure 6B). Quantitative determination of the bone volume by microCT, using a threshold of 300 as the cut-off for bone density measurement, showed that LDN-193189 treatment reduced the bone volume in the tumors (Figure 6B). Serum osteocalcin levels of the PCa-118b tumor bearing mice were significantly higher than those in the non-tumor bearing mice (Figure 6C). The levels of osteocalcin in the LDN-193189-treated group were reduced compared to those without the treatment (Figure 6C). Together, these observations suggest that treating PCa-118b tumor-bearing mice with LDN-193189 led to reduced bone formation and attenuated tumor growth rate. Quantitative RT-PCR for mouse osterix, Runx2, alkaline phosphatase as a measurement for osteoblast cells in the tumors showed that there were no differences in both control and LDN treated groups (Supplemental Fig. S3), suggesting that LDN-193189 reduced bone formation but does not change the proportion of mature osteoblasts in the tumor. A similar experiment was performed with PCa-133. We found that LDN-193189 has no effect on PCa-133 tumor growth. There is no evidence of bone formation in the PCa-133 tumors as measured by microCT. Further, LDN-193189 treatment did not affect serum osteocalcin levels (Supplemental Figure S4).
Figure 6.
LDN-193189 treatment attenuates PCa-118b tumor growth in vivo. (A) Cells were isolated from PCa-118b tumors, mixed 1:1 with matrigel, and injected into SCID mice subcutaneously (1 × 106 cells per mouse). LDN-193189 or vehicle treatment was started five days post-tumor cell injection. Tumor sizes were measured weekly. At week 7, tumors were removed and weighed. (B) Tumors from vehicle and LDN-193189-treated groups were X-rayed. Bone volumes were determined by microCT using a 300 value as the cut-off. (C) Serum osteocalcin levels were determined for non-tumor bearing (n=5), PCa-118b tumor bearing (n=10), and PCa-118b tumor bearing but LDN-193189-treated (n=10) mice. (D) Model of bi-directional effect between PCa-118b osteogenic tumor and osteoblasts. PCa-118b secretes BMP4 that induces osteoblast differentiation. Inhibition of the paracrine BMP4 effect on osteoblasts leads to the attenuation of PCa-118b tumor growth, suggesting that osteoblasts play a role in supporting PCa-118b tumor growth.
Discussion
Although PCa bone metastasis is frequently associated with new bone formation, it is not clear whether tumor-induced osteogenesis plays a role in PCa progression in the bone. We postulated that PCa cells secrete paracrine factors that lead to an increase in bone formation, and the newly formed bone in turn secretes factors to enhance PCa progression in bone (4). We have identified several cytokines that are differentially expressed between the osteogenic xenograft PCa-118b and non-osteogenic xenograft PCa-133. We showed that BMP4 is one of the cytokines that play a role in the osteogenesis of the PCa-118b xenograft. Because PCa-118b does not express BMP receptors, this has allowed us to explore whether blocking BMP4-induced osteogenesis affects PCa-118b tumor growth. We showed that BMP-4 induced osteoblast differentiation was reduced by the BMP receptor kinase inhibitor LDN-193189, which resulted in a decrease in PCa-118b tumor growth. These studies thus provide evidence that tumor-induced osteogenesis mediated by paracrine BMP signaling plays a role in supporting PCa progression in bone (Figure 6D), and supports our previously proposed model that osteoblast activation, resulting from PCa paracrine signaling, leads to PCa cell growth in bone (4).
Our observations raised an interesting question concerning the mechanism of decreased tumor growth upon reduction of osteogenic support by LDN-193189 treatment. We did not find significant differences in tumor cell proliferation and apoptosis, using Ki67 staining and TUNEL assays, respectively, in the control versus LDN-193189 treated PCa-118b tumors (data not shown). Our interpretation is that tumor cell growth and osteoblast differentiation are interdependent during PCa-118b tumor growth. Thus, LDN-193189 treatment resulted in a proportional decrease of both types of cells. We speculate that the interdependency of tumor cells and osteoblasts involves a wide spectrum of secreted factors mediating paracrine/autocrine signaling, and that LDN-193189 partially interrupted such an interdependency. Our studies suggest that LDN-193189 treatment led to a decrease in bone formation, as reflected in serum osteocalcin levels, and this resulted in a decrease in tumor volume due to the tumor/osteoblast interdependency.
Bone metastases of PCa predominantly result in osteoblastic lesions. However, very few cell lines or xenografts generated from human PCa bone metastasis exhibit this phenotype. MDA-PCa-118b is one of the few xenografts that elicit a strong osteogenic phenotype when implanted in SCID mice (5). How MDA-PCa-118b induces ectopic bone formation under subcutaneous site is not clear. It is likely that MDA-PCa-118b secretes several factors that are able to stimulate osteoblast proliferation or differentiation. Indeed, in a search for these secreted factors (“prostate cancer secretome”), we showed that several factors, including BMP4 secreted by MDA-PCa-118b, are able to stimulate osteoblast differentiation. In addition, previous studies by Li et al. (5) showed that FGF9, which stimulates osteoblast proliferation, also contributes to the osteoblastic phenotype of MDA-PCa-118b. Other factors yet to be identified may also be involved in the osteoblastic phenotype of MDA-PCa-118b tumor.
Many PCa-derived cell lines express BMP receptors and BMPs. Several reports showed that BMPs have autocrine effects on PCa cells. Feeley et al. (13, 14) showed that BMP-2 stimulated PC-3 and LAPC4 cell migration and invasion. Similarly, BMPs increased the in vitro invasive ability of LuCaP23.1 and C4-2B cells (15, 16). Dai et al. (16) also showed that BMPs induced VEGF protein and mRNA expression in C4-2B cells. MDA-PCa-118b is unique in that it expresses low to undetectable BMP receptors and does not respond to BMP-4. Thus, the effect of LDN-198183 on tumor growth is most likely due to the blocking of BMP-4 paracrine effects on tumor stromal cells (Fig. 6D). This property allowed us to examine, for the first time, the effect of tumor-induced osteogenesis on prostate tumor growth.
The ectopic bone formation of MDA-PCa-118b xenograft under the subcutaneous site suggests that host stromal cells/osteoblasts are recruited to the subcutaneous tumor implants. Indeed, previous study using in situ hybridization with mouse-specific probes have indicated that the osteoblasts present in the xenograft are of mouse origin (5). How the stromal cells/osteoblasts are recruited to the ectopically implanted tumor remains to be determined.
Despite the bone-forming phenotype in PCa bone metastasis, pathological analysis of the metastatic lesions in bone showed that both osteoblastic and osteolytic lesions are present in the same loci (17, 18). Histomorphometric quantification also consistently shows changes in both bone formation and resorption within metastatic foci in iliac crest biopsy samples (19). Consistent with the clinical observations, osteoclasts were also present in MDA-PCa-118b xenograft tumors (5). Thus, tumor-induced osteoblastic or osteolytic responses likely reflect the overall effects from factors that regulate osteoblast proliferation, differentiation, and osteoclast activation in vivo. Among the factors secreted from MDA-PCa-118b xenografts, MCP-1, MCSF, and IL8 have been shown to have effects on osteoclast activation (20–22). It is likely that other factors yet to be identified are also involved in the osteoclastic component in MDA-PCa-118b xenografts.
In conclusion, MDA-PCa-118b exhibits the unique properties that allow examination of the contribution of paracrine signaling in osteoblastic lesions of PCa. We demonstrate that BMP-4, an osteogenic factor, acts as one of the important paracrine factors. By modulating BMP4-mediated paracrine signaling, we found evidence that support a role of osteogenesis in the progression of PCa in bone. Further studies on the factors that induce osteogenesis in PCa bone metastasis are warranted.
Supplementary Material
Acknowledgments
We thank Dr. Nora Navone for providing MDA-PCa-133 xenograft for this study. This work was supported by grants from NIH including CA111479, P50 CA140388 and CA16672, the Prostate Cancer Foundation, the U.S. Department of Defense (PC093132), Cancer Prevention and Research Institute of Texas (CPRIT RP110327), and the Robert Wood Johnson Foundation.
References
- 1.Jacobs SC. Spread of prostatic cancer to bone. Urology. 1983;21:337–44. doi: 10.1016/0090-4295(83)90147-4. [DOI] [PubMed] [Google Scholar]
- 2.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 3.Saad F, Lipton A. Bone-marker levels in patients with prostate cancer: potential correlations with outcomes. Curr Opin Support Palliat Care. 2010;4:127–34. doi: 10.1097/SPC.0b013e32833ac6d6. [DOI] [PubMed] [Google Scholar]
- 4.Logothetis C, Lin S-H. Osteoblasts in prostate cancer metastasis to bone. Nature Reviews Cancer. 2005;5:21–8. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Mathew P, Yang J, Starbuck M-W, Zurita AJ, Liu J, et al. Androgen receptor–negative human prostate cancer cells induce osteogenesis through FGF9-mediated mechanisms. J Clin Invest. 2008;118:2697–710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossert J, Eberspaecher H, de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995;129:1421–32. doi: 10.1083/jcb.129.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zilberberg L, ten Dijke P, Sakai LY, Rifkin DB. A rapid and sensitive bioassay to measure bone morphogenetic protein activity. BMC Cell Biol. 2007;8:41. doi: 10.1186/1471-2121-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marie PJ, Debiais F, Haÿ E. Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling. Histol Histopathol. 2002;17:877–85. doi: 10.14670/HH-17.877. [DOI] [PubMed] [Google Scholar]
- 9.Lee YC, Huang CF, Murshed M, Chu K, Araujo JC, Ye X, et al. Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene. 2010;29:3196–207. doi: 10.1038/onc.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–91. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 11.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14:1363–9. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, et al. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:32941–9. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 13.Feeley BT, Krenek L, Liu N, Hsu WK, Gamradt SC, Schwarz EM, et al. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone. 2006;38:154–66. doi: 10.1016/j.bone.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Feeley BT, Gamradt SC, Hsu WK, Liu N, Krenek L, Robbins P, et al. Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J Bone Miner Res. 2005;20:2189–99. doi: 10.1359/JBMR.050802. [DOI] [PubMed] [Google Scholar]
- 15.Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005;65:8274–85. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- 16.Dai J, Kitagawa Y, Zhang J, Yao Z, Mizokami A, Cheng S, et al. Vascular endothelial growth factor contributes to the prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. Cancer Res. 2004;64:994–9. doi: 10.1158/0008-5472.can-03-1382. [DOI] [PubMed] [Google Scholar]
- 17.Charhon SA, Chapuy MC, Delvin EE, Valentin-Opran A, Edouard CM, Meunier PJ. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma with special reference to osteomalacia. Cancer. 1983;51:918–24. doi: 10.1002/1097-0142(19830301)51:5<918::aid-cncr2820510526>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88:2989–94. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Clarke NW, McClure J, George NJ. Morphometric evidence for bone resorption and replacement in prostate cancer. Br J Urol. 1991;68:74–80. doi: 10.1111/j.1464-410x.1991.tb15260.x. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–53. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 21.Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S, Skinner RA, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005;65:11001–9. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 22.Corboz VA, Cecchini MG, Felix R, Fleisch H, van der Pluijm G, Löwik CW. Effect of macrophage colony-stimulating factor on in vitro osteoclast generation and bone resorption. Endocrinology. 1992;130:437–42. doi: 10.1210/endo.130.1.1727717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.