Summary
The cell cortex serves as a critical nexus between the extracellular environment/cell membrane and the underlying cytoskeleton and cytoplasm. In many cells, the cell cortex is organized and maintained by the Ezrin, Radixin and Moesin (ERM) proteins, which have the ability to interact with both the plasma membrane and filamentous actin. Although this membrane-cytoskeletal linkage function is critical to stability of the cell cortex, recent studies indicate that this is only a part of what ERMs do in many cells. In addition to their role in binding filamentous actin, ERMs regulate signaling pathways through their ability to bind transmembrane receptors and link them to downstream signaling components. In this review we discuss recent evidence in a variety of cells indicating that ERMs serve as scaffolds to facilitate efficient signal transduction on the cytoplasmic face of the plasma membrane.
Introduction
The cell cortex has crucial roles both in organizing the cytoskeleton and cellular morphology and in coordinating cell to cell signals. Efficient transmission of signals from the cell membrane to intracellular signaling cascades requires tight regulation of multiple signaling components in specific cellular domains. Although ERM (Ezrin-Radixin-Moesin) proteins have long been known to organize the cortical cytoskeleton by linking filamentous actin to the apical membrane of cells, recently they have also been shown to coordinate several diverse signaling events by their ability to scaffold signaling components localized to the apical domain.
The ERM proteins are structured such that intramolecular interaction between the N- and C-terminal domains masks protein-protein interaction sites and maintains the protein in an inactive state in the cytoplasm [1]. ERMs unfold and become activated in response to binding the phospholipid PIP2 and phosphorylation of a conserved threonine residue within the C-terminal domain [2]. When ERMs are activated the N-terminal domain, called the FERM (Four-point-one, Ezrin, Radixin, Moesin) domain, can bind to the cytoplasmic portion of transmembrane proteins such as CD44 and ICAMs [reviewed in 3] and a cytoplasmic membrane scaffolding protein, EBP50 [4,5]. The central region of the ERMs contains an alpha helical domain that has been shown to be important for PKA association [6], and the C-terminal domain contains a filamentous actin/FERM binding domain important for regulating F-actin and intramolecular interaction. ERMs are believed to function in a variety of cellular and developmental contexts, including organization of the apical cortex in differentiating epithelial cells, stiffening of the cell cortex during cytokinesis, epithelial integrity, and lumen morphogenesis in epithelial tubes [7].
Here we focus on the recent findings of the diverse roles of ERMs in the regulation of signaling cascades. ERMs can scaffold signaling pathway components through direct interactions or they can indirectly impinge on signaling pathways through their regulation of the actin cytoskeleton. To illustrate this point, we describe recent studies of ERMs in T cell activation, in the regulation of cAMP signaling to localize the downstream effectors PKA and Epac1, in suppression of apoptosis, and in the insulin secretory pathway.
ERMs in T cell activation
T cell activation occurs when a T cell and Antigen Presenting Cell (APC) come into contact and form an immunological synapse (IS). In response to T cell receptor (TCR) activation, dramatic changes occur at the cell membrane and in the underlying cell cortex at the IS resulting in loss of microvilli, removal of the transmembrane protein CD43, and accumulation of the TCR and associated downstream signaling components such as ZAP-70, a tyrosine kinase. Downstream of these events Interleukin-2 (IL-2), a cytokine, is produced, and is often used as an indicator of T-cell activation. Carefully controlled regulation of Ezrin and Moesin has been implicated in all of these events associated with formation of the IS, and loss of ERM protein function is associated with decreased IL-2 production in activated T lymphocytes. The role of ERMs in these processes during formation of the IS has been reviewed previously [7,8] so we will concentrate just on recent findings here.
In addition to their proposed interactions with TCR signaling effectors [7], ERM proteins have been implicated in other aspects of lymphocyte activation [8]. Several studies have shown that loss of Ezrin in T lymphocytes results in decreased IL-2 production in response to antigen binding, suggesting that Ezrin plays a positive role in T cell activation [9-11]. However, Lasserre et al. recently have shown that signaling events proximal to the TCR are actually upregulated in the absence of Ezrin [12]. These apparently paradoxical observations seem, at least in part, related to Ezrin’s ability to interact with Dlg1, which previously has been implicated in IL-2 production via NF-AT activation [13-15]. Lasserre et al. show that Ezrin colocalizes and interacts with Dlg1 in the peripheral zone of the IS. In response to TCR activation, signaling microclusters, consisting of the TCR and downstream signaling components, form at the periphery of the IS, subsequently migrate toward its center and ultimately disperse, thereby abrogating signaling. In cells depleted of Ezrin, microclusters form normally but do not migrate centripetally or disperse. Interestingly, phosphospecific antibodies for phospholipase C γ-1, linker for activation of T cells (LAT), and Erk1/2, all targets for TCR signaling, indicate greater than normal activity in activated cells depleted of Ezrin function. More detailed analysis of phospho-ERK1/2 indicated that although phosphorylation in response to TCR activation occurred with normal kinetics in Ezrin depleted cells, the progressive dephosphorylation normally observed after stimulation was delayed. Taken together, these results suggest that Ezrin is involved in a negative feedback loop that normally functions to downregulate activated TCR proximal events. The authors propose that these functions are mediated through a functional linkage with microtubules mediated by Dlg1, though the underlying mechanisms have not been elucidated.
ERMs regulate PKA signaling in lymphocytes and other cells
Cyclic AMP (cAMP), which is produced following activation of G protein coupled receptors, is used as a second messenger to activate downstream effectors, most importantly Protein Kinase-A (PKA). In its inactive form, PKA exists as a heterotetramer, with two catalytic subunits bound to two regulatory subunits. In the presence of cAMP, the catalytic and regulatory subunits separate, producing active PKA. A-Kinase Anchoring Proteins (AKAPs) bind to PKA to tightly confine it near downstream targets for efficient signaling. All three ERM proteins function as AKAPs by binding to PKA regulatory subunits [6].
In the immunological synapse, Ezrin acts as an AKAP to mediate cAMP mediated T cell repression [16]. PKA I, which binds Ezrin through its regulatory subunit, inhibits T cell activation by phosphorylating and activating C-Src kinase (Csk). Csk in turn phosphorylates and inactivates Lymphocyte-specific protein tyrosine kinase (Lck), blocking downstream T cell activation. EBP50, which also binds Ezrin, interacts with Csk-binding protein (Cbp), an adaptor for Csk [17], suggesting that it may scaffold PKA I near its effector Csk. In support of this notion, a peptide that disrupts the Ezrin/EBP50 interaction inhibits cAMP mediated IL-2 repression [18]. A similar effect has been observed in Ezrin depleted cells. This treatment also reduces levels of PKA I in T cell lipid rafts [16]. Together these results suggest that EBP50 and Ezrin scaffolding of PKA I is necessary for cAMP mediated repression of T cell activation. Whether this repression is related to that involving Ezrin, Dlg1 and microtubules described in the previous section has not, to our knowledge, been investigated.
ERMs have been shown to regulate cAMP/PKA signaling in several other tissues, including the parietal cells of the gut [19]. Unstimulated parietal cells have intracellular tubulovesicles that contain large quantities of the proton pump, H+,K+-ATPase. Upon stimulation, which increases intracellular cAMP and PKA in part through activation of histamine receptors, tubulovesicles fuse to the apical membrane to allow gastric acid secretion [20]. Ezrin is localized at the apical membrane and is required for normal acid secretion [21]. Upon histamine stimulation, activated PKA phosphorylates Ezrin within the FERM domain causing ACAP4, an ARF6 GAP, to bind Ezrin and thereby localize to the apical membrane. Ezrin depletion prevents both ACAP4 and H+,K+-ATPase from concentrating at the apical membrane, while depletion of ACAP4 results in a failure to recruit H+,K+-ATPase [19]. ARF6, a small GTPase whose activity is regulated by ACAP4, functions in regulation of membrane trafficking and requires its ability to cycle between a GTP and GDP bound state for acid secretion [22]. These data suggest that Ezrin, which might function as an AKAP to scaffold PKA in these cells (though this has not yet been demonstrated), is also an effector of PKA that functions to recruit ACAP4. ACAP4 in turn regulates ARF6 activity to control tubulovesicle fusion and proper localization of the H+,K+-ATPase for acid secretion.
In Xenopus melanocytes Moesin has been identified as an AKAP that is involved in pigment granule dispersion [23]. The movement of pigment granules found in melanophores has been an area of interest largely because it serves as a model for intracellular transport of organelles by the molecular motors dyneins, kinesins and myosins [24]. Low levels of cAMP result in aggregation of pigment granules in the center of the cell, and high levels cause dispersion of the granules toward the periphery. Moesin co-purifies with pigment granules and responds in a manner similar to pigment granules, dispersing or aggregating, when cAMP levels are modulated. Moesin likely functions to target PKA to sites of cAMP signaling because a form of Moesin that lacks the PKA binding domain but can still bind pigment granules, partially inhibited pigment granule dispersion but did not affect aggregation [23]. Moesin’s role in these cells also may require the ability to bind both pigment granules and F-actin to bring the actin cytoskeleton and associated Myosin motor proteins near to the pigment granule, but this idea remains to be formally tested.
Recent studies have found that ERMs are important for the regulation of another cAMP effector, Epac1, a guanine nucleotide exchange factor (GEF) for the Rap small GTPases. Autoinhibition of Epac proteins is released by cAMP binding, causing Epac1 to become intensely membrane associated [25-27]. This in turn leads to accumulation of activated Rap1 at the membrane [27] and increased integrin-mediated adhesion [28]. In addition, ERMs promote membrane localization of Epac1 through interactions with its N-terminal domain [27,29,30]. Both this domain and another N-terminal portion of Epac1 are necessary for full Rap1 activation and integrin mediated adhesion, and depletion of all three ERMs blocks activated Epac1 induced adhesion [29].
Thyroid cells undergo Radixin and Epac1 dependent proliferation in response to thyroid stimulating hormone (TSH) [30]. A form of Radixin that can bind Epac1 but not PKA cannot fully rescue the TSH proliferation response in Radixin knockdown cells. Additionally, the need for Radixin in TSH mediated proliferation can be bypassed by expressing a form of Rap1 that is constitutively active and has a phosphomimetic mutation at the residue phosphorylated by PKA [30]. These results suggest that both signaling components, Epac1 and PKA, are necessary for full Rap1 activation of TSH mediated proliferation, and that Radixin serves to scaffold these proteins in a cAMP-rich membrane compartment .
ERMs in apoptosis
Numerous studies have implicated ERMs in apoptotic events in a variety of cell types, including lymphocytes. Given the wide range of proposed cellular functions for ERMs, it is perhaps not surprising that they have been described to both promote and inhibit apoptosis. In T cell apoptosis, Fas ligand (FasL) binds to clusters of Fas, a cell surface receptor of the TNF family. This results in the assembly of a complex of proteins called the death inducing signaling complex (DISC). FADD, a DISC component, interacts with Fas and recruits Caspase-8, which then becomes activated and triggers apoptosis through its protease activity [31]. Ezrin was found to bind to Fas directly [32]. Studies describing a proapoptotic function have argued that Ezrin and Moesin are necessary for clustering and endocytosis of Fas, a necessary step in Fas-mediated endocytosis required for the apoptotic signal [33-36]. However, a more recent study argues that Ezrin limits the extent of cell death triggered through Fas activation [32]. In T cells stimulated with FasL, Ezrin dissociates from Fas, resulting in activation of the DISC apoptotic cascade. Knockdown of Ezrin and expression of a dominant negative Ezrin leads to an increase in Fas mediated cell death, while over expression of the full length protein slightly inhibited apoptosis [32]. Currently it is unclear whether the differences in observed roles for ERMs in Fas-mediated cell death reflect different experimental methodologies or perhaps the underlying complexity of ERM functions in these cells.
In Drosophila epithelial cells, the sole ERM protein Moesin is unambiguously required for cell survival [37-40]. Cells that are depleted of Moesin or Slik, a Sterile-20 like kinase that activates Moesin, undergo apoptosis through activation of the Jun N-Terminal (JNK) pathway [37,40]. Because one function of Moesin is to negatively regulate RhoA activity, Moesin mutant cells express abnormally high levels of active RhoA [41]. Studies in Drosophila show that RhoA binds to Slipper, a Mixed Lineage Kinase, that functions upstream of JNK [40]. Both RhoA and Slipper proteins are increased at the membrane in Moesin mutant cells, suggesting that in the absence of Moesin, RhoA recruits Slipper to the membrane, thereby triggering the JNK pathway activation and apoptosis [40].
ERMs in insulin signaling
A crucial component of insulin signaling by pancreatic ß cells is trafficking of insulin-containing secretory vesicles to the cell surface. This occurs in response to increased blood glucose, which in turn triggers increased intracellular calcium. Type 2 diabetes affects not only the ability to respond to insulin, but also insulin secretion. ERMs have recently been implicated in the release of insulin secretory granules [42]. Under low glucose conditions Ezrin and Radixin are predominantly cytoplasmic in ß cells, but in response to high glucose stimulation both become phosphorylated in a calcium-dependent manner and translocate to the plasma membrane. Cells expressing dominant negative Ezrin display mislocalized insulin secretory vesicles and reduced insulin secretion upon glucose stimulation [42]. It is currently unclear whether ERMs promote insulin secretion through their ability to organize the cortical cytoskeleton or through a scaffolding function, perhaps related to PKA signaling. However, expression of dominant negative Ezrin disrupts insulin secretion without apparent effects on filamentous actin, suggesting that the cortical cytoskeleton is not responsible for this effect.
Conclusions
The ERM proteins function in a critical cellular domain – the interface between the plasma membrane and the underlying cell cortex. In addition to their well-known role in organizing the cell cortex, recent work clearly indicates that ERMs regulate membrane-associated signaling pathways. What remains to be fully determined is to what extent the influence of ERMs on signaling results from their ability to modulate the cortical actin cytoskeleton versus an ability to directly interact with, and thereby regulate, cytoplasmic components of signal transduction pathways. Given recent evidence that ERMs act as cytoplasmic signaling scaffolds, for example by acting as AKAPs, and the abundant evidence that ERMs have multiple transmembrane binding partners, the potential for a direct role regulating signal cascades is considerable. Further work, using a combination of proteomic approaches to identify new binding partners and genetics in model systems to provide in vivo insights into functional interactions with signaling pathways, should help to unravel the role of ERMs on orchestrating the complex array of signals functioning in the cell cortex.
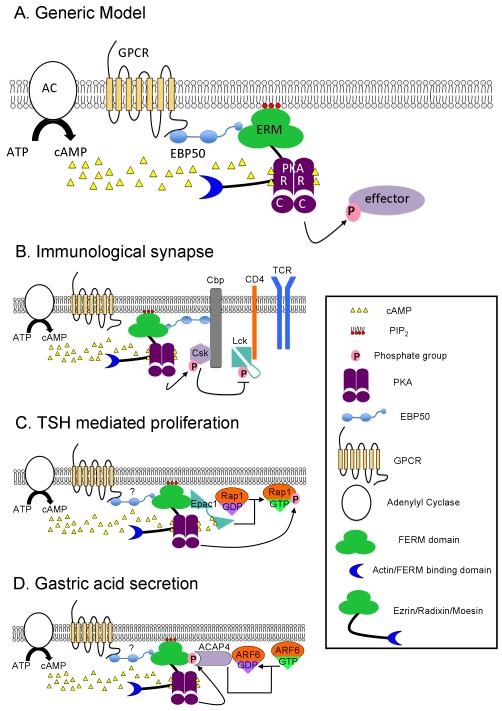
Figure 1.
ERM proteins are scaffolds for cAMP signaling pathways. A) A generic model of how ERMs act as A-kinase anchoring proteins (AKAPs). cAMP is produced following activation of GPCRs (in yellow, 7-pass transmembrane receptors), which in turn activate Adenylyl Cyclase (AC) to convert ATP to cAMP (yellow triangles). cAMP activates PKA, a heterotetramer, by binding to the regulatory (R) subunits, releasing the catalytic (C) subunits to phosphorylate nearby effector proteins. All three ERM proteins function as AKAPs, binding to the PKA regulatory subunits through their central alpha helical domain. ERM activation requires PIP2 binding (red circles) and phosphorylation of a conserved residue within the FERM or F-actin binding domain (blue crescent). EBP50 (light blue), a scaffolding protein containing two PDZ domains and a FERM binding domain, binds to ERMs via a C-terminal domain and can interact with the G-protein coupled receptors (GPCRs) through its first PDZ domain [43]. B) At the immunological synapse Ezrin mediates T cell repression by functioning as an AKAP. Cbp, a transmembrane adaptor protein, binds to both EBP50 and Csk, a Src kinase. Ezrin can bind to EBP50 and scaffold PKA to Csk. Activated PKA phosphorylates Csk, which in turn phosphorylates Lck, a kinase and downstream component of T cell activation, at a residue that renders Lck inactive. This model was adapted from [44]. C) Radixin mediates TSH mediated proliferation by scaffolding the cAMP effectors PKA and Epac1 to their effector Rap1, a small GTPase. Epac1, a GEF for Rap1, is activated by binding of cAMP which releases Epac1 auto inhibition and activate Rap1 by catalyzing the exchange of GDP for GTP. Rap1 when activated localizes to the membrane. Activated PKA phosphorylates a residue that is crucial for Rap1’s ability to mediated TSH proliferation. Although schematized here, EBP50 has not been shown to function in this pathway. D) Ezrin’s scaffolding function is required for gastric acid secretion. In parietal cells Ezrin is phosphorylated within the FERM domain by PKA, which is activated in part through activated histamine receptors. Phosphorylated Ezrin is a scaffold for ACAP4, an ARF6 GAP. ARF6 regulates membrane trafficking and requires the ability to cycle between active (GTP-bound) and inactive (GDP-bound) states for its function. Ezrin, ACAP4 and cycling ARF6 are all necessary for proper localization of the H+,K+-ATPase for gastric acid secretion. Although indicated here, EBP50 has not been shown to function in this pathway, nor has Ezrin been shown to scaffold PKA in parietal cells.
Acknowledgements
The authors would like to thank the members of the Fehon laboratory for helpful comments and discussions. This work is supported by NIH R01 GM087558.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Gary R, Bretscher A. Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins. Proc Natl Acad Sci U S A. 1993;90:10846–10850. doi: 10.1073/pnas.90.22.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 4.Reczek D, Berryman M, Bretscher A. Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci U S A. 2004;101:17705–17710. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, Freedman BD, Burkhardt JK. Ezrin and moesin function together to promote T cell activation. J Immunol. 2009;182:1021–1032. doi: 10.4049/jimmunol.182.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–750. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 11.Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, Acuto O, Dautry-Varsat A, Alcover A. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 2001;15:715–728. doi: 10.1016/s1074-7613(01)00225-4. [DOI] [PubMed] [Google Scholar]
- 12*.Lasserre R, Charrin S, Cuche C, Danckaert A, Thoulouze MI, de Chaumont F, Duong T, Perrault N, Varin-Blank N, Olivo-Marin JC, et al. Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J. 2010;29:2301–2314. doi: 10.1038/emboj.2010.127. This study shows that Ezrin is necessary for the migration and dispersion of T cell signaling microclusters and downregulation of proximal T cell signaling in response to activation by antigen. In contrast to previous studies of ERM function that examined only the endpoint of T cell activation, this study suggests that early events are actually upregulated in Ezrin-deficient cells.
- 13.Xavier R, Rabizadeh S, Ishiguro K, Andre N, Ortiz JB, Wachtel H, Morris DG, Lopez-Ilasaca M, Shaw AC, Swat W, et al. Discs large (Dlg1) complexes in lymphocyte activation. J Cell Biol. 2004;166:173–178. doi: 10.1083/jcb.200309044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Round JL, Tomassian T, Zhang M, Patel V, Schoenberger SP, Miceli MC. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J Exp Med. 2005;201:419–430. doi: 10.1084/jem.20041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Round JL, Humphries LA, Tomassian T, Mittelstadt P, Zhang M, Miceli MC. Scaffold protein Dlgh1 coordinates alternative p38 kinase activation, directing T cell receptor signals toward NFAT but not NF-kappaB transcription factors. Nat Immunol. 2007;8:154–161. doi: 10.1038/ni1422. [DOI] [PubMed] [Google Scholar]
- 16**.Ruppelt A, Mosenden R, Gronholm M, Aandahl EM, Tobin D, Carlson CR, Abrahamsen H, Herberg FW, Carpen O, Tasken K. Inhibition of T cell activation by cyclic adenosine 5′-monophosphate requires lipid raft targeting of protein kinase A type I by the A-kinase anchoring protein ezrin. J Immunol. 2007;179:5159–5168. doi: 10.4049/jimmunol.179.8.5159. This study identifies Ezrin as an AKAP for PKA I, and promotes repression of T cell activation by scaffolding downstream components necessary for cAMP mediated repression of IL-2.
- 17.Brdickova N, Brdicka T, Andera L, Spicka J, Angelisova P, Milgram SL, Horejsi V. Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton. FEBS Lett. 2001;507:133–136. doi: 10.1016/s0014-5793(01)02955-6. [DOI] [PubMed] [Google Scholar]
- 18*.Stokka AJ, Mosenden R, Ruppelt A, Lygren B, Tasken K. The adaptor protein EBP50 is important for localization of the protein kinase A-Ezrin complex in T-cells and the immunomodulating effect of cAMP. Biochem J. 2010;425:381–388. doi: 10.1042/BJ20091136. This paper shows that EBP50, an ERM binding protein, participates in the cAMP mediated repression of T cell activation described in ref. 16.
- 19*.Ding X, Deng H, Wang D, Zhou J, Huang Y, Zhao X, Yu X, Wang M, Wang F, Ward T, et al. Phospho-regulated ACAP4-Ezrin interaction is essential for histamine-stimulated parietal cell secretion. J Biol Chem. 2010;285:18769–18780. doi: 10.1074/jbc.M110.129007. This study demonstrates that Ezrin is required for gastric acid secretion in the gut. Ezrin is a target of PKA and phosphorylated Ezrin scaffolds an ARF6 GEF, ACAP4, to regulate acid secretion.
- 20.Forte JG, Zhu L. Apical recycling of the gastric parietal cell H,K-ATPase. Annu Rev Physiol. 2010;72:273–296. doi: 10.1146/annurev-physiol-021909-135744. [DOI] [PubMed] [Google Scholar]
- 21.Tamura A, Kikuchi S, Hata M, Katsuno T, Matsui T, Hayashi H, Suzuki Y, Noda T, Tsukita S. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol. 2005;169:21–28. doi: 10.1083/jcb.200410083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsukawa J, Nakayama K, Nagao T, Ichijo H, Urushidani T. Role of ADP-ribosylation factor 6 (ARF6) in gastric acid secretion. J Biol Chem. 2003;278:36470–36475. doi: 10.1074/jbc.M305444200. [DOI] [PubMed] [Google Scholar]
- 23*.Semenova I, Ikeda K, Ivanov P, Rodionov V. The protein kinase A-anchoring protein moesin is bound to pigment granules in melanophores. Traffic. 2009;10:153–160. doi: 10.1111/j.1600-0854.2008.00852.x. In this paper the authors show that Moesin functions as an AKAP in pigment granules and influences pigment granule dispersion.
- 24.Nascimento AA, Roland JT, Gelfand VI. Pigment cells: a model for the study of organelle transport. Annu Rev Cell Dev Biol. 2003;19:469–491. doi: 10.1146/annurev.cellbio.19.111401.092937. [DOI] [PubMed] [Google Scholar]
- 25.Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158:70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehmann H, Das J, Knipscheer P, Wittinghofer A, Bos JL. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature. 2006;439:625–628. doi: 10.1038/nature04468. [DOI] [PubMed] [Google Scholar]
- 27.Ponsioen B, Gloerich M, Ritsma L, Rehmann H, Bos JL, Jalink K. Direct spatial control of Epac1 by cyclic AMP. Mol Cell Biol. 2009;29:2521–2531. doi: 10.1128/MCB.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bivona TG, Wiener HH, Ahearn IM, Silletti J, Chiu VK, Philips MR. Rap1 upregulation and activation on plasma membrane regulates T cell adhesion. J Cell Biol. 2004;164:461–470. doi: 10.1083/jcb.200311093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Gloerich M, Ponsioen B, Vliem MJ, Zhang Z, Zhao J, Kooistra MR, Price LS, Ritsma L, Zwartkruis FJ, Rehmann H, et al. Spatial regulation of cyclic AMP-Epac1 signaling in cell adhesion by ERM proteins. Mol Cell Biol. 2010;30:5421–5431. doi: 10.1128/MCB.00463-10. This study shows that the N-terminus of Epac1, a cAMP effector and Rap1 GEF, binds to the ERMs at the plasma membrane. At the membrane, Epac1 promotes Rap1 activation, leading to increased integrin mediated adhesion.
- 30**.Hochbaum D, Barila G, Ribeiro-Neto F, Altschuler DL. Radixin Assembles cAMP Effectors Epac and PKA into a Functional cAMP Compartment: ROLE IN cAMP-DEPENDENT CELL PROLIFERATION. J Biol Chem. 2011;286:859–866. doi: 10.1074/jbc.M110.163816. This study demonstrates that Radixin scaffolds two cAMP effectors, Epac1 and PKA. The interaction of both Epac1 and PKA with Radixin is necessary for a TSH/Rap1 mediated proliferation response.
- 31.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Kuo WC, Yang KT, Hsieh SL, Lai MZ. Ezrin is a negative regulator of death receptor-induced apoptosis. Oncogene. 2010;29:1374–1383. doi: 10.1038/onc.2009.417. This study shows that Ezrin can limit apoptosis that is triggered through Fas activation. Ezrin binds to Fas and knockdown of Ezrin increases Fas mediated apoptosis.
- 33*.Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol. 2008;181:5963–5973. doi: 10.4049/jimmunol.181.9.5963. This paper describes a proapoptotic function of Ezrin and Moesin in which they promote Fas clustering. The authors also find that Rock phosphorylates Ezrin and Moesin and that this phosphorylation or activation of the proteins is necessary for Fas clustering.
- 34.Chakrabandhu K, Herincs Z, Huault S, Dost B, Peng L, Conchonaud F, Marguet D, He HT, Hueber AO. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 2007;26:209–220. doi: 10.1038/sj.emboj.7601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fais S, De Milito A, Lozupone F. The role of FAS to ezrin association in FAS-mediated apoptosis. Apoptosis. 2005;10:941–947. doi: 10.1007/s10495-005-0478-2. [DOI] [PubMed] [Google Scholar]
- 36.Chakrabandhu K, Huault S, Garmy N, Fantini J, Stebe E, Mailfert S, Marguet D, Hueber AO. The extracellular glycosphingolipid-binding motif of Fas defines its internalization route, mode and outcome of signals upon activation by ligand. Cell Death Differ. 2008;15:1824–1837. doi: 10.1038/cdd.2008.115. [DOI] [PubMed] [Google Scholar]
- 37.Hipfner DR, Cohen SM. The Drosophila sterile-20 kinase slik controls cell proliferation and apoptosis during imaginal disc development. PLoS Biol. 2003;1:E35. doi: 10.1371/journal.pbio.0000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hipfner DR, Keller N, Cohen SM. Slik Sterile-20 kinase regulates Moesin activity to promote epithelial integrity during tissue growth. Genes Dev. 2004;18:2243–2248. doi: 10.1101/gad.303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molnar C, de Celis JF. Independent roles of Drosophila Moesin in imaginal disc morphogenesis and hedgehog signalling. Mech Dev. 2006;123:337–351. doi: 10.1016/j.mod.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 40*.Neisch AL, Speck O, Stronach B, Fehon RG. Rho1 regulates apoptosis via activation of the JNK signaling pathway at the plasma membrane. Journal of cell biology. 2010;189:311–323. doi: 10.1083/jcb.200912010. Using Drosophila, this study demonstrates that Moesin promotes cell survival by restricting Rho1 activation, which in turn can activate JNK signaling and apoptosis.
- 41.Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–87. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- 42*.Lopez JP, Turner JR, Philipson LH. Glucose-induced ERM protein activation and translocation regulates insulin secretion. Am J Physiol Endocrinol Metab. 2010;299:E772–785. doi: 10.1152/ajpendo.00199.2010. This study demonstrates that ERMs function in insulin-containing secretory vesicle secretion.
- 43.Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, et al. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 44.Cornez I, Tasken K. Spatiotemporal control of cyclic AMP immunomodulation through the PKA-Csk inhibitory pathway is achieved by anchoring to an Ezrin-EBP50-PAG scaffold in effector T cells. FEBS Lett. 2010;584:2681–2688. doi: 10.1016/j.febslet.2010.04.056. [DOI] [PubMed] [Google Scholar]