Abstract
Liposomes have been investigated extensively as a vaccine delivery system. Herein the adjuvant activities of liposomes with different net surface charges (neutral, positive, or negative) were evaluated when admixed with protein antigens, ovalbumin (OVA, pI = 4.7), Bacillus anthracis protective antigen protein (PA, pI = 5.6), or cationized OVA (cOVA). Mice immunized subcutaneously with OVA admixed with different liposomes generated different antibody responses. Interestingly, OVA admixed with net negatively charged liposomes prepared with DOPA was as immunogenic as OVA admixed with positively charged liposomes prepared with DOTAP. Immunization of mice with the anthrax PA protein admixed with the net negatively charged DOPA liposomes also induced a strong and functional anti-PA antibody response. When the cationized OVA was used as a model antigen, liposomes with net neutral, negative, or positive charges showed comparable adjuvant activities. Immunization of mice with the OVA admixed with DOPA liposomes also induced OVA-specific CD8+ cytotoxic T lymphocyte responses and significantly delayed the growth of OVA-expressing B16-OVA tumors in mice. However, not all net negatively charged liposomes showed a strong adjuvant activity. The adjuvant activity of the negatively charged liposomes may be related to the liposome’s ability (i) to up-regulate the expression of molecules related to the activation and maturation of antigen-presenting cells and (ii) to slightly facilitate the uptake of the antigens by antigen-presenting cells. Simply admixing certain negatively charged liposomes with certain protein antigens of interest may represent a novel platform for vaccine development.
Keywords: Protein antigen, antibody response, CTL, anti-tumor activity, antigen uptake, APC activation
1. INTRODUCTION
New approaches to vaccine development using recombinant protein antigens have significant advantages over traditional vaccines consisted of live attenuated pathogens, whole inactivated organisms, or inactivated toxins. However, recombinant protein antigens alone are often poorly immunogenic or non-immunogenic and, thus, need a potent vaccine adjuvant to enhance the resultant immune responses.1 In recent years, particulate carriers have been extensively researched as vaccine delivery systems. It is generally accepted that many particle carriers have adjuvant activity, which is likely due to the particle’s ability to facilitate the uptake of antigens by APC. Moreover, enhancement of antigen stability, presentation of multiple copies of antigens, and the ability to include immunomodulators are also the advantages of using particulates as a vaccine delivery system.2
Liposomes are a well known lipid-based particulate vaccine delivery system. The adjuvant activity of liposomes was first reported by Allison and Gregoriadis in 1974 using diphtheria toxoid as an antigen.3 Since then the adjuvant activity of liposomes has been extensively studied.4–8 Many parameters that may affect the adjuvant activity of liposomes have been investigated. It was found that a variety factors such as particle size, surface charge, lipid composition, method of antigen loading, to name a few, in a liposome-based vaccine formulation can potentially shape the resultant immune responses against the antigen of interest. There were numerous studies defining the adjuvant activity of negatively and positively charged liposomes and examining the effect of the charge of liposomes on their adjuvant activities, but the results were rather conflicting and inconclusive.3, 9–27 For example, Allison and Gregoriadis (1974) reported that diphtheria toxoid in negatively charged liposomes elicited significantly higher antibody levels than in positively charged liposomes.3 However, Nakanishi et al. reported that positively charged liposomes containing a protein antigen were more potent inducers of antigen-specific CTL responses than negatively charged and neutral liposomes.25 Similarly, Afrin et al. showed that Leishmania donovani antigens encapsulated in positively charged liposomes induced the strongest level of protection against experimental visceral leishmaniasis in mice, followed by neutral and negatively charged liposomes, respectively.26 On the contrary, in another study using the recombinant glycoprotein of Leishmania antigen,27 it was shown that antigens entrapped in neutral liposomes conferred a significantly higher protection and Th1 type immune responses than antigens entrapped in positively charged liposomes, whereas the negatively charged liposomes favored the induction of a Th2 type immune response.
In the present study, the adjuvant activities of liposomes with different net surface charges (i.e., neutral, positively charged, or negatively charged) were further investigated. Very often, protein antigens were either entrapped inside liposomes or covalently conjugated onto the surface of liposomes when liposomes were used as a vaccine delivery system.3, 28–35 Entrapment of protein antigens into liposomes has many advantages. For example, it improves the stability of the proteins by protecting it from degradation after injection.28 Using BSA as a model antigen, Shek and Sabiston (1982) showed that tryposinization of liposomes with entrapped BSA did not reduce the anti-BSA immune response induced by the liposomes, but trypsinizatoin of liposomes with surface adsorbed BSA significantly reduced the anti-BSA response induced.28 Moreover, it is thought that liposomes with antigens entrapped inside can act as a depot and allow the slow and persistent release of the antigen. Similarly, conjugation of antigens onto liposomes has its advantages as well. For example, it was shown that conjugation of lysozyme as an antigen onto neutral liposomes induced significantly stronger antibody responses than entrapment of it in the liposomes.36 However, a significantly more convenient approach is to simply mix the antigen of interest with pre-formed liposomes, similar to the mixing of protein antigens with aluminum hydroxide or aluminum phosphate in suspensions, which are used in many human vaccines. Using model antigens OVA and the PA protein of Bacillus anthracis with a pI value of 4.7 and 5.6, respectively, the adjuvant activities of net neutral, net positively charged, and net negatively charged liposomes were evaluated by simply mixing the antigens with the liposomes before injecting them into mice. It was found that net negatively charged liposomes prepared with certain negatively charged lipids have a potent adjuvant activity when admixed with protein antigens.
2. MATERIALS AND METHODS
2.1. Materials
DOTAP, DOPA, DOPC, DOPS, and DOPG were from Avanti Polar Lipids, Inc. (Alabaster, AL). BSA, horse serum, Chol, DCP, OVA (Cat. # A5503), HMD, EDC, TMB, FITC, and MTT assay kit were from Sigma-Aldrich (St. Louis, MO). B. anthracis PA protein and lethal factor were from List Biological Laboratories, Inc. (Campbell, CA). Goat anti-mouse immunoglobulin (IgG, IgG1, and IgG2a) were from Southern Biotechnology Associates, Inc. (Birmingham, AL). Class I (Kb)-restricted peptide epitope of OVA (SIINFKEL) was from Ana Spec Inc. (Fremont, CA). Phycoerythrin (PE)-labeled anti-I-A[b] and FITC-labeled anti-CD80 antibodies were from BD Biosciences (San Diego, CA). RT2 First Strand Kit, RT2 SYBR® Green/ROX™ qPCR Master Mix, RT2 Profiler™ Mouse Dendritic and Antigen Presenting Cell PCR Array were from SABioscience (Frederick, MD). CFSE and SuperScript® III First-Strand Synthesis SuperMix for qRT-PCR were from Invitrogen (Carlsbad, CA). Taq DNA polymerase was from New England Biolabs (Ipswich, MA).
2.2. Cells and cell lines
Culture medium, FBS, and antibiotics were from Invitrogen. The DC2.4 cells (a mouse DC line) were grown in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL of penicillin, and 100 µg/mL of streptomycin. The OVA-expressing B16-OVA cells were kindly provided by Dr. Edith M. Lord and Dr. John Frelinger from the University of Rochester Medical Center (Rochester, NY) 37 and grown in RPMI 1640 medium with 5% FBS and 400 µg/mL of Geneticin (G418). The J774A.1 cells (mouse macrophages) were grown in DMEM medium supplemented with 10% FBS, 100 U/mL of penicillin and 100 µg/mL of streptomycin. BMDC of C57BL/6 female mice were from Astarte Biologics (Redmond, WA) and grown in DMEM high glucose medium with 10% FBS, 100 U/mL of penicillin, and 100 µg/mL of streptomycin.
2.3. Preparation and characterization of liposomes
Liposomes were prepared using the thin film hydration method.38 Briefly, a thin film of net neutral phospholipids (DOPC) and Chol (1:1 molar ratio, 20 mg total lipid) was formed in the bottom of 7-mL glass scintillation vial by chloroform evaporation. The lipid thin film was then hydrated and dispersed in 1 mL of PBS (10 mM, pH 7.4) by vigorous mixing at room temperature. The resultant liposomes were extruded through 400 and 100 nm filters, sequentially, using the Mini-Extruder (Avanti Polar Lipids, Inc). To prepare net positively or negatively charged liposomes, DOPC was replaced by DOTAP or DOPA, respectively. For the preparation of different negatively charged liposomes, DOPS, DOPG, or DCP was used as follow: DOPS:Chol (1:1, m/m), DOPG:DOPC:Chol (1:2:1, m/m/m), and DCP:DOPC:Chol (1:2:1, m/m/m), all with 20 mg of total lipids. The endotoxin level in the liposome preparations (measured in liposomes prepared with DOTAP, DOPA, and DOPG) was estimated to be 0.11–0.35 EU/ml using a ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit from GenScript (Piscataway, NJ).
An equal volume of liposomes and protein in solution (OVA or PA in PBS, 10 mM, pH 7.4) were mixed. The mixture was then vortexed for 3–5 s and incubated at room temperature for at least 15 min prior to further use.
Cationized OVA (cOVA) was prepared as previously described.39 Briefly, 20 mg of OVA was dissolved into 1 mL of PBS. HMD (2 mL, 2 M, pH 6.8) and EDC (54 mg) were added, and the mixture was stirred at room temperature for 2 h. The reaction was stopped by adding 1 mL of glycine solution (2 M) followed by another h of stirring. The cOVA was purified using a PD-10 column (GE Healthcare Bio-Sciences Corp, Piscataway, NJ) and concentrated using ultra-centrifugation filter tubes (30 kDa cut-off) (Millipore, Billerica, MA). Protein concentration was determined using the Bradford reagent (Sigma-Aldrich). The cOVA-liposome mixtures were prepared as described above.
The particle size of liposomes and liposome-protein mixtures was determined using a Coulter N4 Plus Submicron Particle Sizer (Beckman Coulter Inc., Fullerton, CA). The turbidity was determined by measuring the absorbance at 655 nm using a BioTek Synergy™ HT Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, VT).
The percent of protein associated with the liposomes was determined by ultracentrifugation. Briefly, the antigen-liposome mixture was centrifuged at 150,000 × g for 1 h using a Beckman-Coulter Optima™ TLX Benchtop Ultracentrifuge (Brea, CA), and the protein content in the supernatant was determined using a CBQCA protein quantitation kit (Molecular Probes, Inc., Eugene, OR). The amount of proteins associated with the liposomes was derived by subtracting the amount of proteins in the supernatant from the total amount of proteins added. Sucrose gradient centrifugation was used for the protein-DOTAP liposome mixture because their mixture did not precipitate after simple centrifugation. Sucrose gradient was prepared with the following layering: 60% sucrose (0.4 mL), protein-DOTAP liposome mixture (0.1 mL), 25% sucrose (0.4 mL), and 10% sucrose (0.2 mL). After 1 h of centrifugation (200,000 × g), 0.1 mL fractions (11 total) were collected. Protein concentration in each fraction was quantified using the CBQCA kit. The liposome-associated protein peaked at fraction 2, while the protein alone peaked at fraction 8. The % of protein associated with liposomes was calculated using the trapezoidal rule. All experiments were repeated as least 3 times.
2.4. Immunization studies
All animal studies were carried out following the National Institutes of Health guidelines for animal care and use. Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin and at Oregon State University. Female BALB/c or C57BL/6 mice, 6–7 weeks of age, were from Simonsen Laboratories, Inc. (Gilroy, CA) or Charles River Laboratories (Wilmington, MA). The vaccine formulations were administrated by subcutaneous (s.c.) injection once in every two weeks, three times for OVA-liposome mixtures (10 µg OVA/mouse/injection) or two times for PA-liposome mixtures (5 µg PA/mouse/injection) and cOVA-liposome mixtures (10 µg cOVA/mouse/injection). Proteins admixed with Alum (30 µg (or 50 for PA)/mouse/injection, USP grade from Spectrum Chemicals & Laboratory Products, Cardena, CA) were used as a positive control. Mice in the negative control group were injected with sterile PBS. Two weeks after the last injection, mice were euthanized to collect blood.
2.5. Enzyme-linked immunosorbent assay (ELISA)
Antigen-specific IgG, IgG1 and IgG2a levels were determined using ELISA.40 Briefly, polystyrene, medium binding 96-well plates (BD Biosciences) were coated with 100 ng of OVA, cOVA, or PA in 100 µL of carbonate buffer (pH 9.6) overnight at 4°C. For anti-OVA or anti-cOVA antibody determination, the plates were washed with PBS/Tween 20 (10 mM, pH 7.4, 0.05% Tween 20) and blocked with blocking solution (5% horse serum in PBS/Tween 20, v/v) for 1 h at 37°C. Serum samples were diluted 100, 1,000, and 10,000 times (or two-fold serially for titers) in blocking solution and then added to the plates following the removal of the blocking solution. After 2 h of incubation at 37°C, the samples were removed, and the plates were washed five times with PBS/Tween 20. Horseradish peroxidase-conjugated goat anti-mouse immunoglobulins (IgG, IgG1, or IgG2a, 5,000-fold dilution in 1.25% horse serum in PBS/Tween 20, v/v) were added into the plates, followed by another hour of incubation at 37°C. The plates were again washed five times with PBS/Tween 20. After 15 min of incubation at room temperature with TMB solution, followed by the addition of stop solution (0.2 N sulfuric acid), the presence of bound specific antibody was detected at 450 nm. Anti-PA antibody was determined similarly, except that the 5% horse serum in PBS/Tween 20 was replaced by 4% BSA in PBS/Tween 20. Specific antibody responses were reported as the OD450 values or as antibody titers. Antibody titers were determined by considering any absorbance value higher than the 2-fold of the mean (2 × mean) of the negative control group as positive. Total IgG was measured in both BALB/c and C57BL/6 mice. IgG subtypes were measured in BALB/c mice only.
2.6. Anthrax lethal toxin neutralization assay
Lethal toxin neutralization activity was determined as previously described.41 Briefly, confluent J774A.1 cells were seeded (1 × 104 cells/well) in sterile, 96-well, clear-bottom plates and incubated overnight at 37°C, 5% CO2. Fresh medium containing PA (800 ng/mL) and lethal factor (200 ng/mL) was mixed at an equal volume with diluted serum sample and incubated for 2 h at 37°C. The cell culture medium was removed, and the serum/lethal toxin mixture was added into each well at the final concentration of 400 ng/mL of PA and 100 ng/mL of lethal factor. The cells were then incubated for 2 h at 37°C, 5% CO2. Cell viability was determined using the MTT assay with untreated and lethal toxin alone treated cells as controls.
2.7. In vivo CTL assay and mouse tumor prevention study
In vivo CTL assay was carried out as previously described.42 Female C57BL/6 mice were immunized with OVA-DOPA liposome complexes (20 µg OVA/mouse) on days 0, 7, and 14. OVA adjuvanted with IFA (Sigma-Aldrich) (25 µL/mouse) or sterile PBS were used as positive and negative controls, respectively. On day 21, the splenocytes from naïve mice were harvested and labeled with 0.2 µM of SIINFEKL followed by labeling with a high concentration of CFSE (10 µM, CFSEHigh). Same splenocytes without SIINFEKL labeling were labeled with a low concentration of CFSE (1 µM, CFSELow). Ten million cells from each population were mixed and injected intravenously via the tail vein into the immunized mice. Three h after the injection, mice were euthanized, and the relative abundance of CFSEHigh and CFSELow cells in the splenocyte preparation was determined using a flow cytometer (FC500 Beckman Coulter EPICS V Dual Laser Flow Cytometer, Fullerton, CA).
For the tumor prevention study, 28 days after the first immunization, B16-OVA cells (1 × 105) were subcutaneously injected into the flank of the mice (n = 5). The tumor size was measured using a caliper and reported based on the following equation: Tumor size (mm) = ½ [width + length]. Mice were euthanized 25 days after the tumor cell injection, and their blood was collected.
2.8. In vitro uptake of OVA admixed with liposomes by DC2.4 cells
Liposomes were mixed with FITC-labeled OVA (FITC-OVA), which was prepared following the manufacturer’s instruction (Sigma-Aldrich). DC2.4 cells (1.0 × 105 cells/well) were seeded into 48-well plates and allowed to grow overnight. The cells were then incubated with 200 µL of the FITC-OVA-liposome mixtures for 3 h at 37°C, 5% CO2. After incubation, the cells were washed three times with cold PBS, lysed with a lysis buffer (0.5% Triton X-100), and centrifuged at 14,000 × g to collect the supernatant. The fluorescence intensity in a supernatant was measured using a BioTek Synergy™ HT Multi-Mode Microplate Reader. As controls, cells were incubated with FITC-OVA alone or fresh medium alone.
2.9. CD80 and MHC II expression on DC2.4 cells after in vitro stimulation
DC2.4 cells (5.0 × 105 cells/well) were seeded into six-well plates. After overnight incubation, the cells were then incubated with 25 µL of DOTAP liposomes, DOPA liposomes, or OVA solution (5 µg OVA) at 37°C, 5% CO2. Cells were also treated with sterile PBS as a negative control or LPS from E. coli (200 ng, Sigma) as a positive control. After 16 h, cells were washed twice with staining buffer (1 % FBS and 0.1 % NaN3 in PBS), stained with FITC-labeled anti-CD80 antibody or PE-labeled anti-I-A[b] MHC II for 20 min at 4°C, washed twice, and analyzed with a FACSCalibur flow cytometer (BD Biosciences).
2.10. Mouse dendritic cell and antigen-presenting cell PCR array
DC2.4 cells (5.0 × 105 cells/well) were seeded into six-well plates. After overnight incubation, the cells were incubated with 25 µL of DOPA liposomes or sterile PBS for 24 h at 37°C, 5% CO2. Total RNA was extracted using an RNeasy Mini Kit from Qiagen (Valencia, CA) according to the manufacturer’s instruction. One µg of total RNA was used to synthesize cDNA using a RT2 First Strand Kit. Diluted first strand cDNA was used to prepare an experimental cocktail (RT2 SYBR® Green/ROX™ qPCR Master Mix, diluted cDNA, nuclease-free water). Twenty-five µL of the experimental cocktail was added to each well in the PCR array. Real-time PCR was performed using an ABI 7900HT from Applied Biosystems, Inc. (Foster City, CA). The plate was incubated at 95ºC for 10 min and cycled 40 times at 95ºC for 15 s, 60ºC for 1 min. The Ct values were obtained from SDS Software 2.3 (Applied Biosystems) and used to calculate the fold change in gene expression by the Web-Based PCR Array Data Analysis software from SABioscience.
2.11. Reverse transcription PCR
Total RNA was isolated from DC2.4 cells treated with 25 µL of DOPA liposomes or PBS for 24 h as described above. Two-step RT-PCR was performed. One µg of total RNA was used to synthesize cDNA using SuperScript® III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen). Primer pairs for mouse B2m (forward, 5’-ACCGGCCTGTATGCTATCCAGAAA-3’, reverse, 5’-AAGCATTGGGCACAGTGACAGACT-3’), IL-6 (forward, 5’-ATCCAGTTGCCTTCTTGGGACTGA-3’, reverse, 5’-AACGCACTAGGTTTGCCGAGTAGA-3’) and β-actin (forward, 5’-TGTGATGGTGGGAATGGGTCAGAA-3’, reverse, 5’-TGCCACAGGATTCCATACCCAAGA-3’) were synthesized by IDT technology (Coralville, IA). The cDNA was used for PCR reaction mixture that included the template DNA, primers, and Taq DNA polymerase. The mixture was incubated at 95ºC for 5 min and cycled 20 times for B2m (33 times for IL-6) at 95ºC for 30 s, 60ºC for 1 min, and 68ºC for 1 min using an Eppendorf Mastercycler (Hauppauge, NY). PCR products were analyzed using agrose gel electrophoresis, and the band intensity was measured using the Syngene G-box GeneSnap software (Syngene, IL).
2.12. Determination of cytokine concentration
DC2.4 cells (1 × 106 cells/well) were seeded into six-well plates. After overnight incubation, the cells were incubated with 25 µL of DOPA liposomes or DOPS liposomes at 37°C, 5% CO2. As controls, cells were also treated with sterile PBS, LPS (200 ng), DOTAP liposomes, or DOPC liposomes. After 24 h incubation, the supernatant was collected and analyzed for CCL-17 and IL-6 using a mouse CCL-17 ELISA kit from R&D Systems (Minneapolis, MN) and a mouse IL-6 ELISA kit (BD Biosciences), respectively.
BMDC (3 × 105 cells/well) were seeded in twelve-well plates and incubated overnight. Cells were stimulated with 12.5 µL of DOPA liposomes or DOPS liposomes for 24 h. PBS and LPS (100 ng) were used as controls. The CCL-17 and IL-6 production in supernatant were determined.
2.13. Statistics
Statistical analyses were completed using ANOVA followed by Fisher’s protected least significant difference procedure. A p-value of ≤ 0.05 (two-tail) was considered statistically significant.
3. RESULTS
3.1. Preparation and characterization of OVA-liposome mixtures
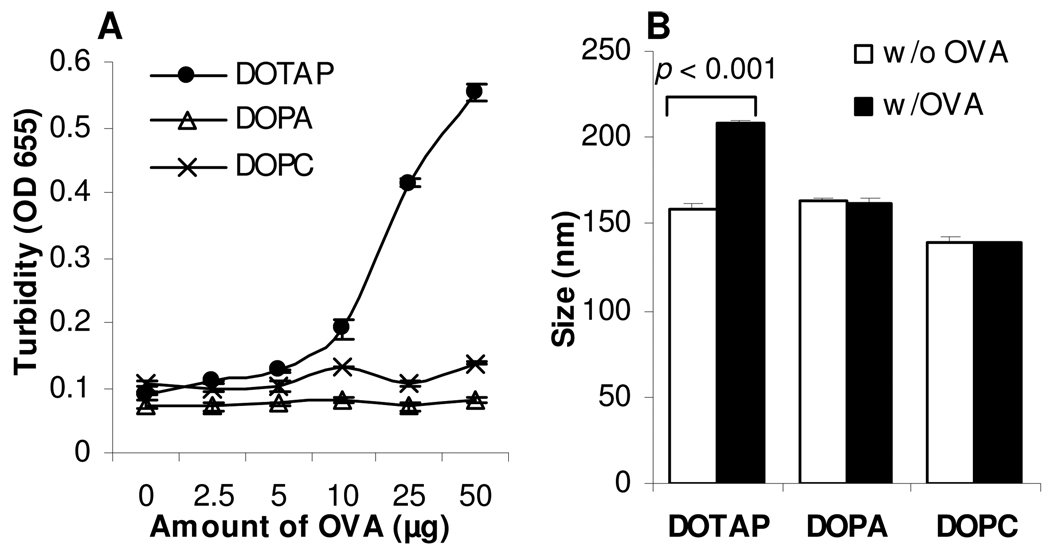
Net neutral, positively charged, and negatively charged liposomes were prepared using DOPC, DOTAP, and DOPA, respectively. The mean size for the neutral, positively charged, and negatively charged liposomes was 140 ± 4, 159 ± 3, and 163 ± 3 nm, respectively. OVA-liposome mixtures were prepared by simply mixing an equal volume of liposomes and different concentrations of OVA in solution (e.g., 2.5, 5, 10, 25, or 50 µg in 25 µL). As shown in Fig. 1A, significant aggregations were formed when the positively charged liposomes (DOTAP) were mixed with high concentrations of OVA, as indicated by the increased turbidity of the mixtures (Fig. 1A). At a concentration less than 5 µg OVA/50 µL, the turbidity of the mixture was only slightly different from that of the liposomes alone. Therefore, OVA-liposome mixtures containing 100 µg/mL of OVA were used in the immunization studies. The particle sizes of liposomes and OVA-liposome mixtures (final OVA concentration, 100 µg/mL) were not different for the neutral (DOPC) and net negatively (DOPA) charged liposomes, whereas the size of OVA-DOTAP liposome mixture was significantly larger than that of the DOTAP liposomes alone (Fig. 1B). Finally, the percentage of OVA associated with the net neutral, positively, and negatively charged liposomes was estimated to be 53 ± 1%, 91 ± 3%, and 46 ± 1%, respectively.
Figure 1. Preparation of OVA-liposome mixtures.
A. Twenty-five µL of liposomes with different net charges (neutral, DOPC; positive, DOTAP; negative, DOPA) were mixed with an equal volume (25 µL) of OVA solution containing various amount of OVA. After 15 min of incubation at room temperature, the turbidity was measured at 655 nm.
B. The size of liposomes alone or the OVA-liposome mixtures. The final concentration of the OVA was 5 µg in a final volume of 50 µL. Data shown are mean ± SD (n = 3).
3.2. OVA admixed with net negatively charged liposomes was as immunogenic as OVA admixed with net positively charged liposomes
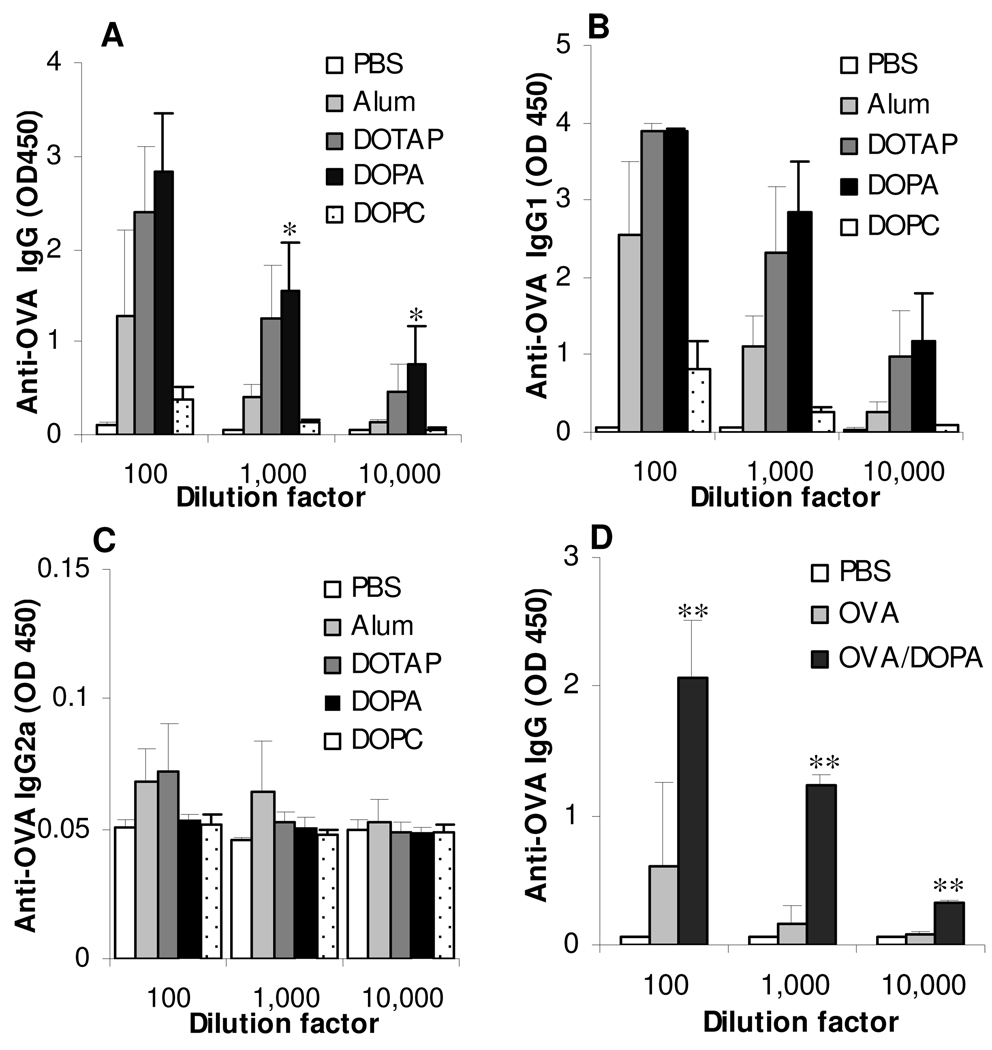
To evaluate and compare the adjuvant activities of liposomes with different net charges, mice were immunized with OVA admixed with DOPC, DOTAP, or DOPA liposomes. As expected, the OVA-DOTAP liposome mixture induced a strong anti-OVA IgG response (Fig. 2A), but the OVA-negatively charged DOPA liposome mixture induced an anti-OVA IgG response that was as strong as that induced by the OVA-DOTAP liposome mixture (Fig. 2A). The OVA-DOPC liposome mixture was only weakly immunogenic (Fig. 2A). The anti-OVA antibody response was IgG1 biased because both OVA-DOTAP liposome mixture and OVA-DOPA liposome mixture induced a strong anti-OVA IgG1 response (Fig. 2B), whereas no significant level of anti-OVA IgG2a was detected in the serum of all immunized mice (Fig. 2C). In Fig. 2D, the anti-OVA IgG response induced by OVA in PBS or OVA admixed with DOPA liposomes were compared, confirming that the strong OVA-specific antibody responses observed above was not simply due to the OVA protein alone.
Figure 2. Immunization of mice with OVA admixed with net positively or net negatively charged liposomes induced a strong OVA-specific serum IgG response.
BALB/c mice (n = 5) were dosed with OVA admixed with neutral (DOPC), net positively charged (DOTAP), or net negatively charged (DOPA) liposomes. As controls, mice (n = 4) were injected (s.c.) with OVA adjuvant with Alum or PBS alone. The IgG (A), IgG1 (B), and IgG2a (C) levels in the serum samples were determined on day 41 after the serum samples were diluted 100-, 1,000-, and 10,000-fold. D. A comparison of the anti-OVA IgG levels in mice immunized with OVA in PBS or OVA admixed with DOPA liposomes (OVA/DOPA). *, For the anti-OVA IgG level, DOPA vs. Alum, p = 0.004 after 1,000-fold dilution, p = 0.02 after 10,000-fold dilution. DOTAP vs. DOPA, p = 0.422 after 1,000-fold, p = 0.205 after 10,000-fold dilution. **, p < 0.05, OVA/DOPA vs. OVA. Data shown are mean ± SD.
3.3. Net negatively charged DOPA liposomes admixed with anthrax protective antigen protein induced a functional anti-PA antibody response
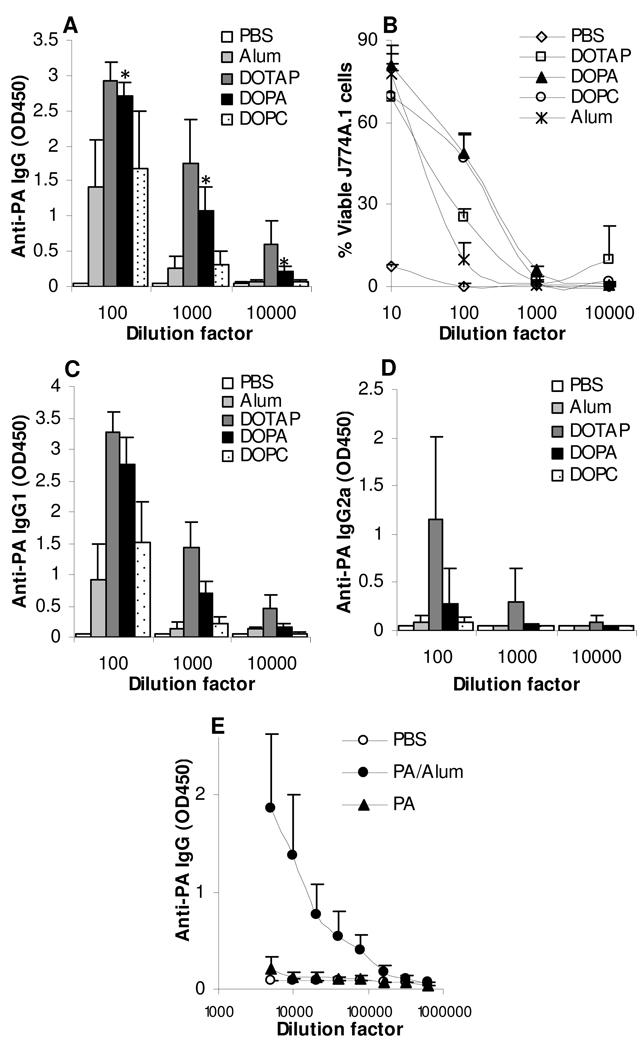
When the PA protein was used as an antigen, the PA-DOPA liposome mixture induced an anti-PA IgG response significantly stronger than that induced by PA adjuvanted with Alum (Fig. 3A). The PA-neutral DOPC liposome mixtures induced an anti-PA IgG response similar to that induced by the PA adjuvanted with Alum, and the anti-PA IgG levels in mice immunized with the PA-DOPC liposome mixture or PA adjuvanted with Alum were not different from that in mice that were injected with sterile PBS at the 10,000-fold dilution (Fig. 3A). The anti-PA antibodies induced by the PA-liposome mixtures were functional because the anti-sera were able to neutralize anthrax lethal toxin and protect mouse macrophages (J774A.1) from the lethal toxin challenge (Fig. 3B). Again, all three liposomal formulations and the PA admixed with Alum induced a strong anti-PA IgG1 response (Fig. 3C). Anti-PA IgG2a was only detected in the serum samples in mice that were treated with PA admixed with the DOTAP liposomes or the DOPA liposomes, not in mice treated with PA admixed with the DOPC liposomes or Alum (Fig. 3D). In Fig. 3E, the anti-PA IgG response induced by PA in PBS was compared to that induced by PA admixed with Alum (50 µg/mouse/injection).
Figure 3. Immunization of mice with PA admixed with liposomes with different net charges induced strong and functional PA-specific antibody responses.
BALB/c mice (n = 5) were dosed with PA admixed with liposomes (DOPC, DOTAP, or DOPA) on days 0 and 14. Control mice were injected with PA admixed with Alum or PBS alone. Mice were bled on day 26.
A. Anti-PA IgG levels in mouse serum samples.
B. The anti-PA antiserum protected mouse macrophages from anthrax lethal toxin challenge. Mouse serum samples were diluted 10-fold serially, and incubated with J774A.1 cells in the presence of anthrax lethal toxin. The neutralization activity was determined with 3 replicates.
C, D. Serum anti-PA IgG1 and IgG2a levels.
E. Serum anti-PA IgG level in mice immunized with PA in PBS or PA admixed with Alum (PA/Alum, 5 µg/50 µg). Data reported are mean ± SD (n = 5 except in E where n = 4 or 5). In A, * p < 0.003, PA/Alum vs. PA/DOPA. For PA/DOTAP vs. PA/DOPA, p = 0.18, 0.07, 0.05 at 100-, 1,000-, and 10,000-fold dilutions, respectively. At 10,000-fold dilution, PA/Alum is not different from PBS (p = 0.10). In E, the values of the PA/Alum and PBS were different (p < 0.05), except at 640,000-fold dilution.
3.4. The specific antibody responses induced by liposomes with different charges were not significantly different when cationized OVA was used as the antigen
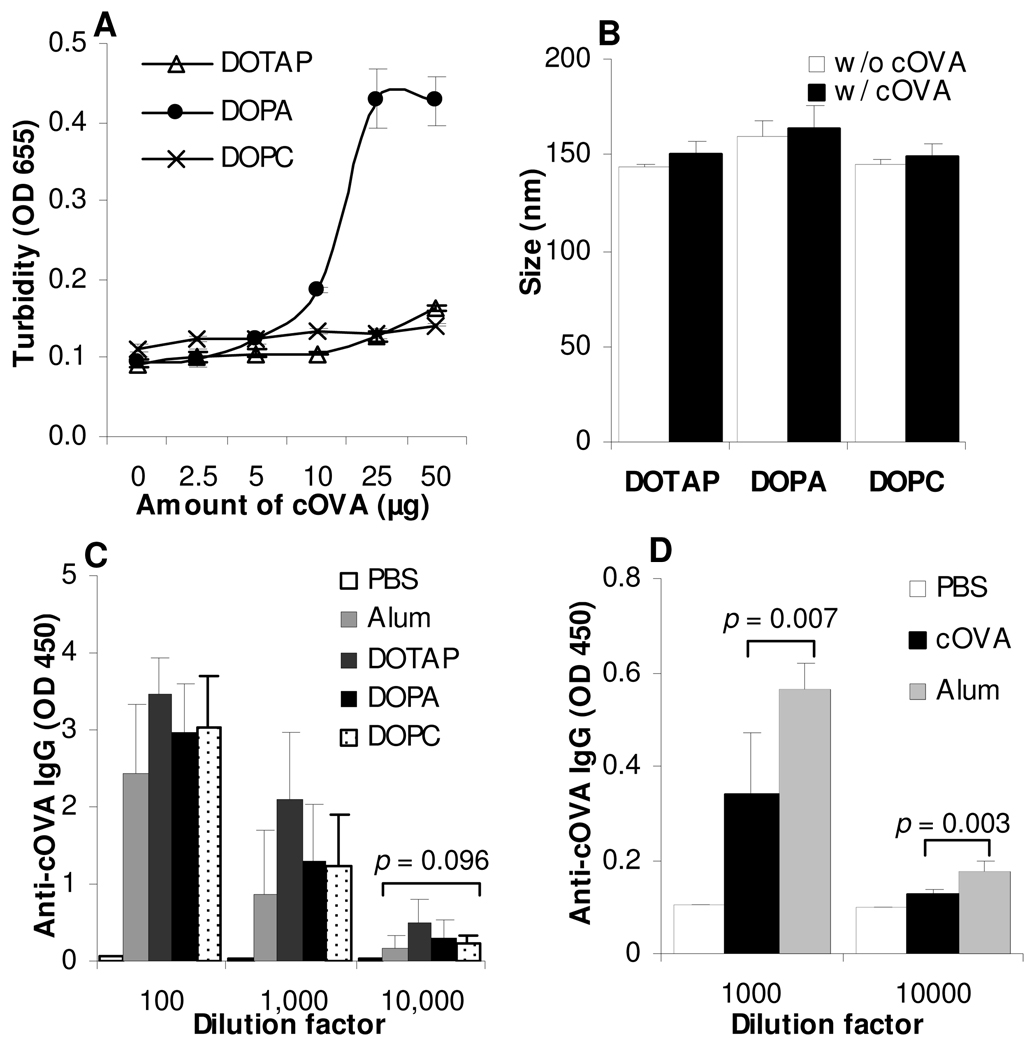
The pI of the OVA is 4.7. After cationization, the OVA and the cOVA migrated in opposite directions when applied on an agrose gel with Tris-boric acid buffer (pH 8.5) (data not shown), demonstrating the successful cationization of the OVA. As expected, when the negatively charged DOPA liposomes were mixed with high concentrations of cOVA, significant aggregations were formed as shown by the increase in turbidity (Fig. 4A), whereas no aggregation was observed when the cOVA was mixed with the positively charged DOTAP liposomes or the neutral DOPC liposomes (Fig. 4A). When 5 µg of cOVA in 25 µL of PBS was mixed with 25 µL of liposomes, the particle sizes of liposomes and cOVA-liposome mixtures were not significantly different (Fig. 4B). Therefore, the formulations containing 100 µg/mL of cOVA were used to immunize mice. Interestingly, cOVA admixed with three different liposomes induced comparable levels of anti-cOVA IgG responses (Fig. 4C). The cOVA itself was strongly immunogenic (Fig. 4D), although inclusion of Alum as an adjuvant still helped significantly.
Figure 4. When admixed with cationized OVA, liposomes with different net charges showed comparable adjuvant activities.
A. Twenty-five µL of liposomes with different net charges (neutral, DOPC; positive, DOTAP; negative, DOPA) were mixed with an equal volume of cOVA in solution containing various amount of cOVA. After 15 min of incubation at room temperature, the turbidity was measured at 655 nm.
B. The size of liposomes and the cOVA-liposome mixtures. The final concentration of the cOVA was 5 µg in 50 µL.
C. Anti-cOVA IgG responses. BALB/c mice (n = 5) were dosed with cOVA admixed with liposomes on days 0 and 14. The IgG levels were determined on day 20 after the serum samples were diluted 100-, 1,000-, and 10,000-fold. ANOVA for Alum, DOTAP, DOPA, and DOPC revealed p values of 0.181, 0.138, and 0.096 at 100-, 1,000-, and 10,000-fold dilution respectively.
D. The immunogenicity of the cOVA alone. BALB/c mice (n = 5) were dosed with cOVA alone or cOVA admixed with Alum on days 0 and 7. The IgG levels were determined on day 20 after the serum samples were diluted 1,000- or 10,000-fold. Data shown are mean ± SD (n = 3 in A, B).
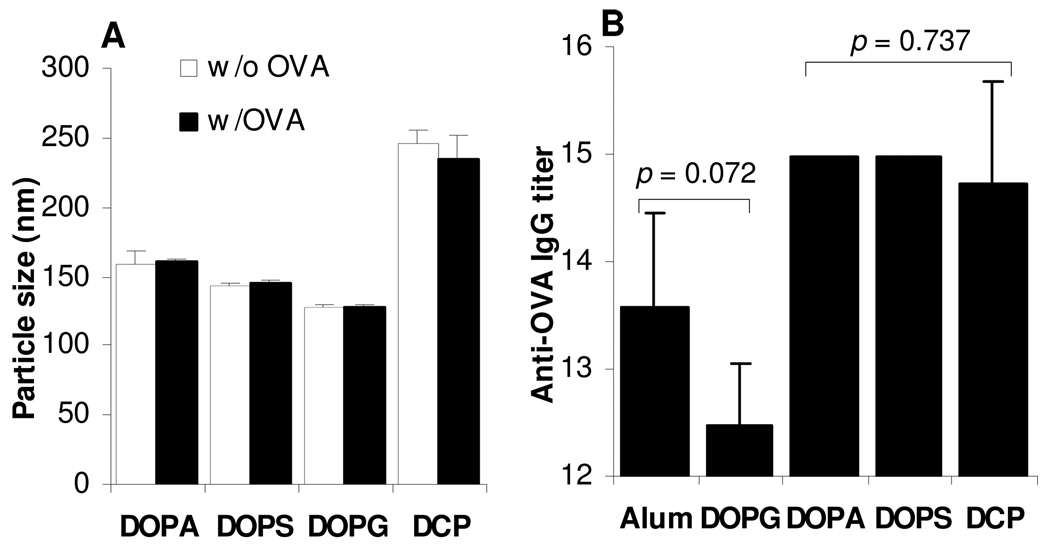
3.5. Net negatively charged liposomes prepared with different lipids had different adjuvant activities
To evaluate the adjuvant activities of different negatively charged liposomes, OVA was admixed with liposomes prepared with 3 other different negatively charged lipids (DOPS, DOPG, and DCP) and used to immunize mice (Fig. 5A). The sizes of liposomes prepared with different negatively charged lipids were different (p < 0.001, ANOVA) (Fig. 5A), but for all 4 liposomes, the sizes of the liposomes and the corresponding OVA and liposome mixture were not significantly different (Fig. 5A). The anti-OVA IgG titers in the serum samples of mice immunized with OVA admixed with DOPA liposomes, DOPS liposomes, and DCP liposomes were strong, whereas the OVA-DOPG liposome mixture seemed to be only weakly immunogenic (Fig. 5B).
Figure 5. Net negatively charged liposomes prepared with different lipids had different adjuvant activities.
A. The size of liposomes prepared with different negatively charged lipids (DOPA, DOPS, DOPG, or DCP) before (□) and after (■) admixing with OVA (5 µg/50 µL).
B. Serum anti-OVA IgG responses induced by OVA admixed with different net negatively charged liposomes. BALB/c mice (n = 4–5) were dosed with OVA admixed with liposomes (DOPA, DOPS, DOPG, or DCP) on days 0, 14 and 28. Mice were bled on day 49. ANOVA comparison of DOPA, DOPS, and DCP revealed p value of 0.737. Data reported are mean ± SD (n = 3 for A, 4–5 for B).
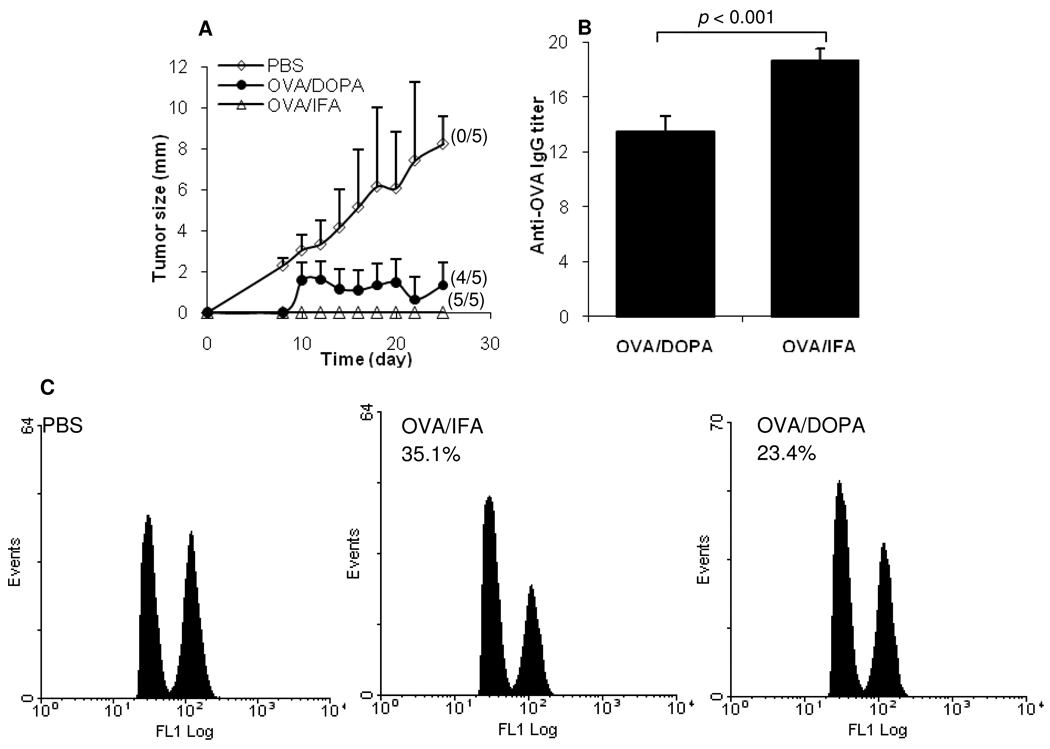
3.6. Immunization with OVA admixed with the negatively charged DOPA liposomes significantly delayed the growth of B16-OVA tumors in mice
Mice immunized with OVA admixed with DOPA liposomes or with OVA adjuvanted with IFA were subcutaneously injected with the OVA-expressing B16-OVA tumor cells, and the growth of tumors was monitored. Tumors became visible 8 days after the injection and grew continuously in the un-immunized mice (Fig. 6A). In contrast, tumors grew very slowly in mice immunized with OVA admixed with DOPA liposomes (Fig. 6A), and 4 of the 5 immunized mice were tumor-free by the end of the study. Tumors did not grow in mice immunized with OVA adjuvanted with IFA, but granulomas were visible at the injection site. The anti-OVA IgG titers shown in Fig. 6B were from the serum samples of the mice 25 days after the tumor cell injection. The anti-OVA IgG titer in mice immunized with OVA adjuvanted with IFA was significantly higher than that in mice immunized with OVA admixed with DOPA liposomes.
Figure 6. Immunization of mice with OVA admixed with DOPA liposomes significantly delayed the growth of B16-OVA tumors in mice and induced a specific CTL response.
A. Tumor growth curve. C57BL/6 mice (n = 5) were immunized with OVA admixed with DOPA liposomes, OVA adjuvanted with IFA, or PBS alone on days 0, 7, and 14. On day 28, mice were s.c. injected with B16-OVA cells. The numbers in parenthesis indicate tumor-free mice 25 days after the tumor cell injection.
B. Titers of anti-OVA IgG in mice 25 days after the tumor injection. Data shown are mean ± SD.
C. Immunization with OVA admixed with DOPA liposomes induced OVA-specific CTL responses. C57BL/6 mice were dosed with PBS, OVA/IFA, or OVA admixed with DOPA liposomes (OVA/DOPA) on days 0, 7, and 14. OVA-specific CTL responses were measured on day 21. Numbers shown are the % lytic activity. Experiment was repeated in two mice with similar results.
3.7. OVA admixed with the DOPA liposomes induced OVA-specific CTL responses
To further characterize the immune responses induced by the OVA-DOPA liposome mixture, the OVA-specific CTL response induced was measured using an in vivo CTL assay. As shown in Fig. 6C, CTL response (23.4% lytic activity) was detected in mice immunized with OVA admixed with DOPA liposomes, and 35.1% lytic activity was detected in mice immunized with OVA adjuvanted with IFA. In a separate experiment, the OVA (SIINFKEL)-specific in vivo CTL activity in mice immunized with the OVA-DOPA liposome mixture was compared to that in mice immunized with OVA admixed with Alum or OVA adjuvanted with IFA. Again, OVA adjuvanted with IFA induced the strongest CTL activity, followed by OVA admixed with DOPA liposomes. On average, only a weak CTL activity was detected in mice immunized with OVA admixed with Alum or OVA alone in sterile PBS (data not shown).
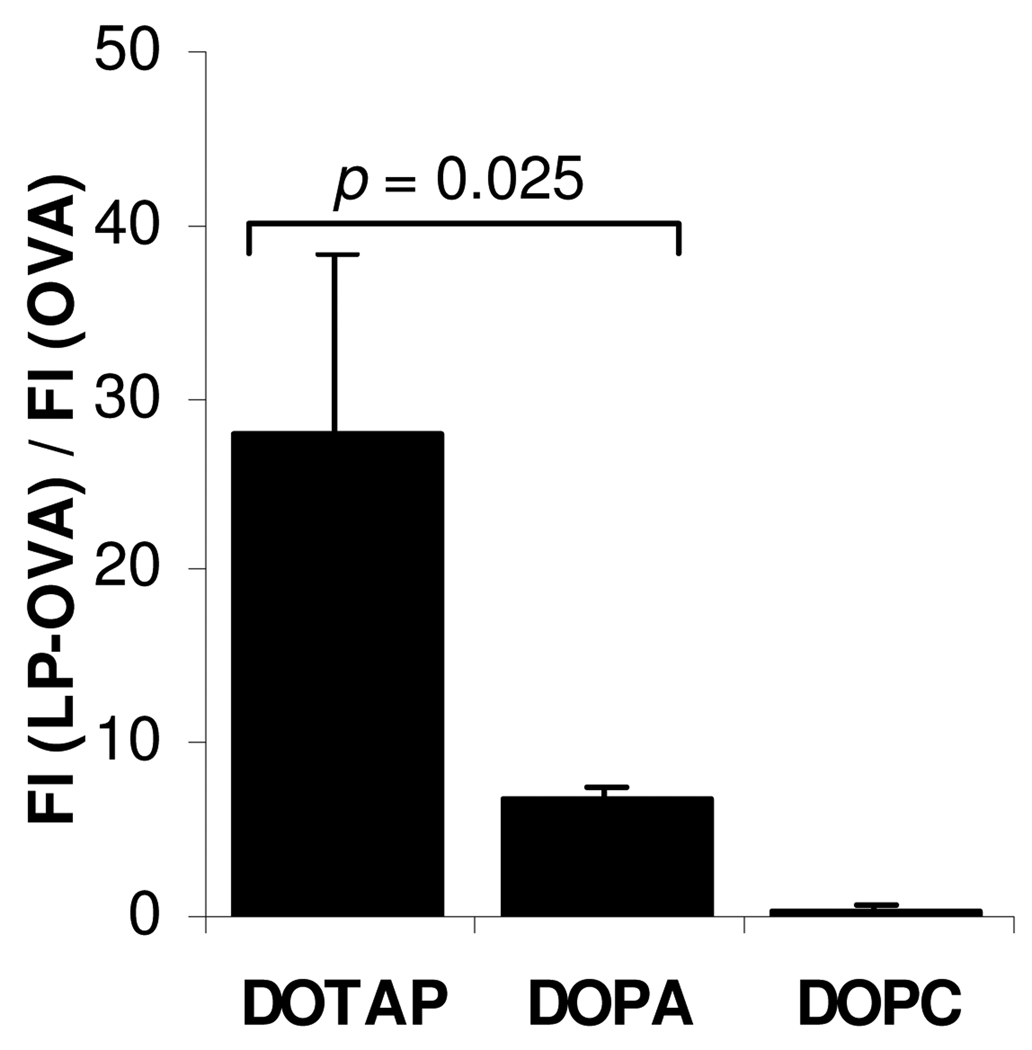
3.8. The negatively charged DOPA liposomes only slightly enhanced the uptake of the OVA by DC2.4 cells in culture
FITC-labeled OVA was admixed with liposomes with different net charges and incubated with DC2.4 cells. As shown in Fig. 7, the DOPA liposomes only slightly facilitated the uptake of the OVA by DC2.4 cells, far less than the DOTAP liposomes. The neutral DOPC liposomes did not significantly affect the uptake of OVA by DC2.4 cells (Fig. 7).
Figure 7. In vitro uptake of OVA admixed with liposomes with different charges by DC2.4 cells.
Cells (1.0 × 105) were incubated with FITC-OVA admixed with DOPC (neutral), DOTAP (positively charged), or DOPA (negatively charged) liposomes for 3 h at 37°C. Data reported are the ratio of fluorescence intensities of cells treated with FITC-OVA admixed with liposomes over that treated with FITC-OVA alone. Data shown are mean ± SD. (FI, fluorescence intensity; LP, liposomes)
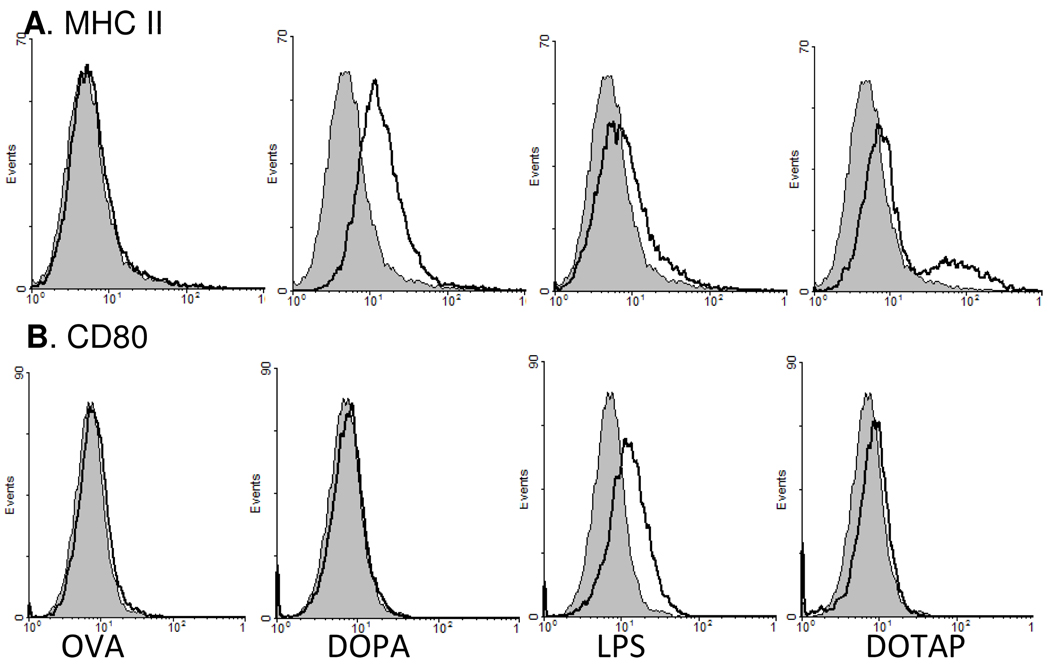
3.9. The negatively charged DOPA liposomes up-regulated the expression of genes related to DC activation and maturation
The DOPA liposomes significantly up-regulated the expression of MHC II molecules on the DC2.4 cells (Fig. 8A), but did not have any detectable effect on the expression of the CD80 (Fig. 8B), even with increased concentration of DOPA liposomes (data not shown). DOTAP liposomes, in contrast, slightly up-regulated both MHC II and CD80 on the DC2.4 cells (Fig. 8).
Figure 8. The expression of MHC II (A) and CD80 (B) on DC2.4 cells after in vitro stimulation.
Cells were incubated with DOTAP, DOPA, or OVA solution (5 µg OVA) for 16 h and stained with anti-I-A[b] MHC II or anti-CD80 antibody and analyzed using a flow cytometer. The graphs of the treatment groups (dark line) were overlaid on the graph of cells incubated with sterile PBS (gray area). Data shown were one representative from three independent experiments with similar results.
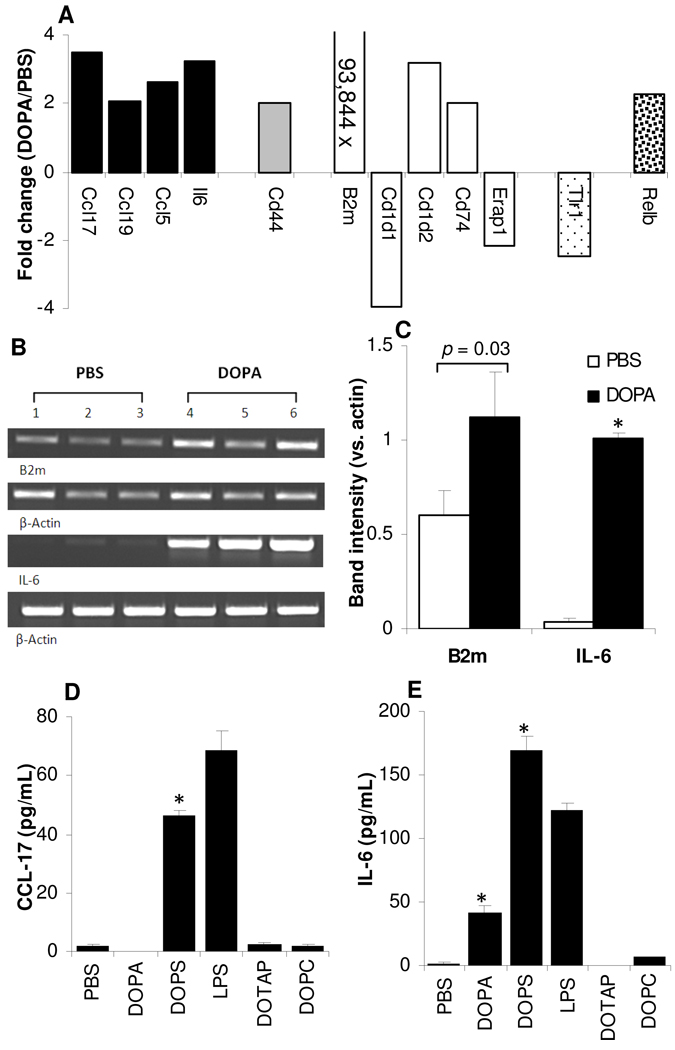
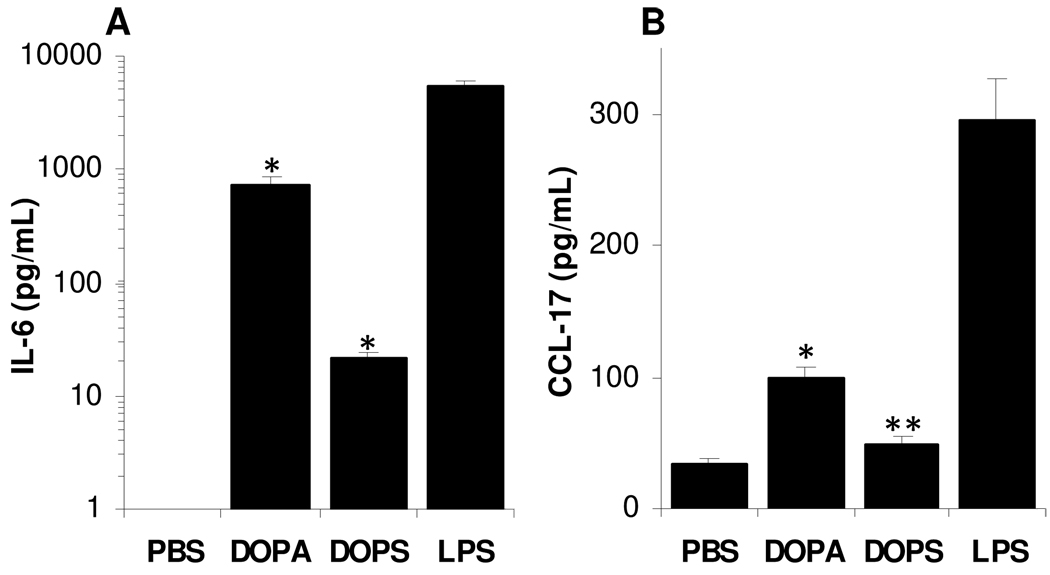
Real-time PCR revealed the differential expression of 12 genes related to DC and APC activation and maturation on the DC2.4 cells after stimulation with the DOPA liposomes: (i) cytokines, chemokines and their receptors (Ccl17, Ccl19, Ccl5, and Il6), (ii) antigen uptake (Cd44), (iii) antigen presentation (B2m, Cd1d1, Cd1d2, Cd74, and Erap1), (iv) cell surface receptors (Tlr1), and (v) signal transduction (Relb) (Fig. 9A). The over-expression of B2m and IL-6 mRNA was confirmed by semi-quantitative RT-PCR (Figs. 9B, C). ELISA assay also confirmed the up-regulated expression of the CCL-17 and IL-6 by DC2.4 cells after stimulation with the negatively charged DOPA or DOPS liposomes, although the CCL-17 level in DC2.4 cells stimulated with the DOPA liposomes was not different from that when the cells were treated with sterile PBS (Figs. 9D, E). Incubation with the positively charged DOTAP liposomes and the net neutral DOPC liposomes did not significantly up-regulate the secretion of the CCL-17 and IL-6 by the DC2.4 cells (Figs. 9D, E). Finally, the DOPA and DOPS liposomes up-regulated the expression of CCL-17 and IL-6 in mouse BMDC as well (Fig. 10), confirming the findings in the DC2.4 cells.
Figure 9. Effect of the negatively charged DOPA liposomes on DC2.4 cells.
A. The up-regulation (+) and down-regulation (−) of genes related to DC activation and maturation by DOPA liposomes detected using a PCR array. Genes with ≥ 2-fold change in mRNA expression are shown. The B2m mRNA expressed was up-regulated by 93,844-fold.
B. RT-PCR products of B2m, IL-6, and β-actin mRNA in DC2.4 cells after stimulation with DOPA liposomes (20 cycles for B2m, 33 cycles for IL-6).
C. RT-PCR data revealed the up-regulation of B2m and IL-6 mRNA by DOPA liposomes. Shown are ratios of the band intensity of the B2m or IL-6 gene mRNA over that of the β-actin in B.
D, E. DOPA and DOPS liposomes up-regulated the expression of CCL-17 (D) and IL-6 (E) in DC2.4 cells. In C, D, and E, *, p < 0.001 vs. PBS. Data shown are mean ± S.D. (n = 3).
Figure 10. DOPA and DOPS liposomes up-regulated the expression of IL-6.
(A) and CCL- 17 (B) in mouse BMDC as well. *, p < 0.001 vs. PBS, ** p = 0.04 vs. PBS. Data shown are mean ± S.D. (n = 3).
4. DISCUSSION
In the present study, it was showed that protein antigens admixed with certain net negatively charged liposomes, such as those prepared with the negatively charged DOPA lipid, induced a strong and functional antibody response when subcutaneously injected into mice. The antibody response was IgG1 biased. Immunization with an antigen admixed with negatively charged liposomes also induced antigen-specific CTL responses and prevented the growth of antigen-expressing tumor cells in mice. The adjuvanticity of the negatively charged liposomes was likely related to their ability (i) to regulate the expression of genes related to DC activation and maturation and (ii) to slightly facilitate the uptake of the antigens by APC.
It is interesting to find that when simply admixed with protein antigens, net negatively charged liposomes prepared with certain negatively charged lipids such as DOPA showed potent adjuvant activity. In cell culture, the negatively charged DOPA liposomes only slightly enhanced the uptake of the OVA protein as a model antigen by mouse DC2.4 cells, significantly less effective than the positively charged liposomes prepared using DOTAP lipid (Fig. 7), but the negatively charged DOPA liposomes and the positively charged DOTAP liposomes both showed a strong adjuvant activity (Figs. 2, 3). Therefore, mechanisms other than the ability to enhance antigen uptake have likely contributed to the adjuvant activity of the negatively charged DOPA liposomes when simply admixed with antigens. The DOPA liposomes also significantly up-regulated the expression of genes related to DC activation and maturation (Figs. 9–10). For example, the DOPA liposomes up-regulated the expression of MHC II, B2m (a component of MHC I molecule), Cd1d2, and Cd74, which are related to antigen presentation by APC.43, 44 The DOPA liposomes and the DOPS liposomes also significantly up-regulated the expression of chemokines such as CCL-17 and CCL-19 (Figs. 9, 10). It is known that immature DC can produce inflammatory chemokines including CCL-5. As they mature, DC lose the ability to produce inflammatory chemokines and switch to produce chemokines such as CCL-17 and CCL-19, which attract T and B lymphocytes.45, 46 It is speculated that the ability for certain negatively charged liposomes to induce DC activation and maturation may explain their adjuvant activity. Finally, the preliminary observation that the positively charged DOTAP liposomes and the net neutral DOPC liposomes failed to significantly up-regulate the secretion of the CCL-17 and IL-6 by DC2.4 cells suggested that the mechanisms of adjuvant activity of differently charged liposomes are likely different.
Data in Fig. 5 showed that not all negatively charged liposomes had a strong adjuvant activity. For example, negatively charged liposomes prepared with DOPA, DOPS, and DCP were shown to have comparable and strong adjuvant activities when admixed with OVA, but the net negatively charged liposomes prepared with the DOPG lipid showed only a weak adjuvant activity (Fig. 5B). It was unlikely that the size of the OVA-liposome mixtures was related to the different adjuvant activities of the four different negatively charged liposomes because the sizes of the DOPA, DOPS, and DOPG liposomes admixed with OVA were around 130–160 nm, whereas the size of the DCP liposome-OVA admixture was around 250 nm. More experiments need to be completed to understand why certain negatively charged liposomes have a strong adjuvant activity while others do not.
The OVA-DOPA liposome mixture induced a stronger OVA-specific antibody response than OVA admixed with Alum (Fig. 2A). Similarly, the PA-DOPA liposome mixture also induced a stronger PA-specific antibody response than PA admixed with Alum (Fig. 3A). The purpose of using the PA as an antigen was two-fold. Firstly, it allowed us to confirm that the adjuvant activity of the negatively charged liposomes prepared with DOPA was not limited to the OVA as an antigen. Secondly, the use of PA allowed us to evaluate the neutralizing activity of the specific antibodies induced. Anthrax is a toxin-mediated disease. B. anthracis produces two toxins, lethal toxin and edema toxin. Lethal toxin is comprised of PA protein and anthrax lethal factor; edema toxin is made of PA and edema factor. PA protein is required for the entrance of lethal factor and edema factor into cells, and only when inside cells, the lethal factor and edema factor are toxic.47–48 Therefore, neutralizing anti-PA antibodies were showed to be able to effectively protect against anthrax infection or against the anthrax toxins. As shown in Fig. 3B, the anti-PA antibodies induced by the PA-DOPA liposome mixture were functional. Finally, it seemed that the negatively charged liposomes prepared with the DOPA can help improve not only antibody responses, but also cellular responses against a protein antigen. The OVA-DOPA liposome mixture induced OVA-specific CTL responses, and immunization of mice with OVA admixed with the DOPA liposomes prevented the growth of the OVA-expressing B16-OVA tumor cells in mice (Fig. 6).
Three antigens were used in the present study. OVA is a 385 amino acid protein with a molecule weight of 45 kDa and a pI value of 4.7. PA contains 735 amino acids; its molecular weight is 83 kDa, and its pI is 5.6. Therefore, at pH 7.4, both OVA and PA should be predominately net negatively charged. In order to understand the adjuvant activity of the negatively charged DOPA liposomes when an antigen with a pI value of larger than 7.4 is used, OVA was cationized by adding hexamethylene diamine to it to generate the cOVA protein. Data in Fig. 4C showed that the cOVA-DOPA liposome mixture also induced a strong anti-cOVA IgG response. In agreement with the previous finding that a catioinized protein is more immunogenic than the original protein,49, 50 the cOVA itself was strongly immunogenic, which may explain why the cOVA-DOPA liposome mixture, the cOVA-DOTAP liposome mixture, and the cOVA-DOPC liposome mixture were all strongly immunogenic (Fig. 4D). At physiological pH (7.4), OVA and PA are expected to be mainly negatively charged, whereas the cOVA is expected to be mainly positively charged. That explains the finding that more than 90% of the OVA was found binding to the positively charged DOTAP liposomes in PBS (pH 7.4). Although to a less extent, binding of OVA with the negatively charged DOPA liposomes and the net neutral DOPC liposomes was also detected, possibly due to interactions other than electrostatic interaction. It also explains the increase in turbidity when a large amount of cOVA was admixed with the negatively charged DOPA liposomes, but not the DOTAP liposomes (Fig. 4A). As mentioned above, the adjuvant activity of the negatively charged liposomes may be related to the liposome’s ability (i) to up-regulate the expression of molecules related to the activation and maturation of APC and (ii) to facilitate the uptake of the antigens by APC. However, when antigens with a pI value larger or smaller than 7.4 were used (i.e., cOVA vs. OVA), the extent to which the antigens strongly bound to the negatively charged liposomes was expected to be different. Therefore, the extent to which the adjuvant activity of the negatively charged liposomes can be attributed to their ability to facilitate antigen uptake by APC may vary depending on antigens used. The same reasoning may be applied to the net positively charged liposomes. Finally, when the net neutral DOPC liposomes were used, only antigens such as the PA and cOVA were able to induce specific antibody responses (Figs. 3–4). Therefore, the mechanisms of adjuvanticity of the liposomes with different net charges are likely different.
As mentioned earlier, when liposomes were used as an antigen/vaccine delivery system, often the antigen of interest was entrapped in the liposomes 29–31 or covalently conjugated onto the surface of the liposomes.32–34, 51 With the exception of the complexing of plasmid DNA with positively charged liposomes, very rarely were immunizations carried out using antigens simply admixed with pre-formed liposomes. The simple admixture of antigens with pre-formed liposomes is convenient and commercially favorable because it will avoid the procedures of entrapping antigens into the liposomes or chemically conjugating antigens onto the surface of the liposomes. Based on the finding in the present study that the negatively charged DOPA liposomes were more potent than Alum in increasing the immunogenicity of OVA or PA (as an antigen), and that the OVA admixed with the negatively charged DOPA liposomes also induced specific CTL responses, it is concluded that negatively charged liposomes may have the potential to become a suitable alternative to Alum to be admixed with certain antigens in vaccine development. Other advantages of using the negatively charged liposomes, instead of Alum, may include i) the possibility of combining various antigen types such as recombinant proteins and live attenuated viruses in a single delivery system and ii) the faster clearance of the negatively charged liposomes than Alum from the injection site. However, it is premature to generalize that all protein antigens can be simply admixed with negatively charged liposomes to induce a strong immune response. Only three antigens (OVA, PA, and cOVA) were evaluated in the present study, and the negatively charged liposomes prepared with DOPG did not show a potent adjuvant activity when admixed with OVA (Fig. 5). Moreover, data from an early report showed that simply mixing BSA as a model antigen with negatively charged liposomes prepared with egg lecithin, Chol, and phosphatidic acid failed to induce any anti-BSA immune responses.52 Nonetheless, it is interesting that all three antigens tested in the present study were able to induce strong specific antibody responses when simply admixed with the net negatively charged DOPA liposomes.
5. CONCLUSIONS
In conclusion, it was showed that liposomes with different net charges had different adjuvant activities, depending on the antigens used. Certain net negatively charged liposomes showed a potent adjuvant activity when simply admixed with the antigen of interest, and the negatively charged liposomes promoted both antibody and cellular immune responses. The strong adjuvant activity of the negatively charged liposomes may be attributed to their ability to induce the activation and maturation of APC. Simply admixing certain negatively charged liposomes with certain antigens of interest may represent a novel and convenient strategy for vaccine development.
ACKNOWLEDGMENT
This work was supported in part by National Institutes of Health grants (AI070538, AI078304) (to Z. Cui). N.Y. is a graduate student at Oregon State University, and she was supported in part by a fellowship from Payap University, Chiang Mai, Thailand.
Abbreviations
- Alum
aluminum hydroxide
- APC
antigen presenting cells
- BMDC
bone marrow-derived dendritic cells
- BSA
bovine serum albumin
- CFSE
5-(and-6-)-carboxylfluorescein diacetate succinimidyl ester
- Chol
cholesterol
- CTL
cytotoxic T lymphocyte
- DC
dendritic cells
- DCP
dicetyl phosphate
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane (chloride salt)
- DOPA
1,2-dioleoyl-sn-glycero-3-phosphate (sodium salt)
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPG
1,2-dioleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt)
- DOPS
1,2-dioleoyl-sn-glycero-3-phospho-L-serine (sodium salt)
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide methiodide
- FBS
fetal bovine serum
- FITC
fluorescein-5(6)-isothiocyanate
- HMD
hexamethylene diamine
- IFA
incomplete Freund’s adjuvant
- LPS
lipopolysaccharides
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OVA
ovalbumin
- PA
protective antigen
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- pI
isoelectric point
- TMB
type 1/2 CD4+ T helper (Th1/2), 3,3’,5,5’-tetramethylbenzidine solution
References
- 1.Liu JJ, Cepica A. Current approaches to vaccine preparation. Can Vet J. 1990;31(3):181–189. [PMC free article] [PubMed] [Google Scholar]
- 2.O'Hagan DT, Singh M, Ulmer JB. Microparticle-based technologies for vaccines. Methods. 2006;40(1):10–19. doi: 10.1016/j.ymeth.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Allison AG, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252(5480):252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 4.Davis D, Davies A, Gregoriadis G. Liposomes as adjuvants with immunopurified tetanus toxoid: the immune response. Immunol Lett. 1987;14(4):341–348. doi: 10.1016/0165-2478(87)90016-2. [DOI] [PubMed] [Google Scholar]
- 5.Gregoriadis G, Panagiotidi C. Immunoadjuvant action of liposomes: comparison with other adjuvants. Immunol Lett. 1989;20(3):237–240. doi: 10.1016/0165-2478(89)90086-2. [DOI] [PubMed] [Google Scholar]
- 6.Richards RL, Rao M, Wassef NM, Glenn GM, Rothwell SW, Alving CR. Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS,S malaria antigen. Infect Immun. 1998;66(6):2859–2865. doi: 10.2307/1366431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alving CR. Liposomes as carriers of antigens and adjuvants. J Immunol Methods. 1991;140(1):1–13. doi: 10.1016/0022-1759(91)90120-5. [DOI] [PubMed] [Google Scholar]
- 8.Jaafari MR, Ghafarian A, Farrokh-Gisour A, Samiei A, Kheiri MT, Mahboudi F, Barkhordari F, Khamesipour A, McMaster WR. Immune response and protection assay of recombinant major surface glycoprotein of Leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine. 2006;24(29–30):5708–5717. doi: 10.1016/j.vaccine.2006.04.062. [DOI] [PubMed] [Google Scholar]
- 9.Galdiero F, Carratelli CR, Nuzzo I, Bentivoglio C, De Martino L, Gorga F, Folgore A, Galdiero M. Beneficial effects of myristic, stearic or oleic acid as part of liposomes on experimental infection and antitumor effect in a murine model. Life Sci. 1994;55(7):499–509. doi: 10.1016/0024-3205(94)00742-x. [DOI] [PubMed] [Google Scholar]
- 10.Aramaki Y, Suda H, Tsuchiya S. Interferon-gamma inductive effect of liposomes as an immunoadjuvant. Vaccine. 1995;13(18):1809–1814. doi: 10.1016/0264-410x(95)00117-j. [DOI] [PubMed] [Google Scholar]
- 11.Yotsumoto S, Aramaki Y, Kakiuchi T, Tsuchiya S. Induction of antigen-dependent interleukin-12 production by negatively charged liposomes encapsulating antigens. Vaccine. 2004;22(25–26):3503–3509. doi: 10.1016/j.vaccine.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 12.Yotsumoto S, Kakiuchi T, Aramaki Y. Enhancement of IFN-gamma production for Th1-cell therapy using negatively charged liposomes containing phosphatidylserine. Vaccine. 2007;25(29):5256–5262. doi: 10.1016/j.vaccine.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Walker C, Selby M, Erickson A, Cataldo D, Valensi JP, Van Nest GV. Cationic lipids direct a viral glycoprotein into the class I major histocompatibility complex antigen-presentation pathway. Proc Natl Acad Sci U S A. 1992;89(17):7915–7918. doi: 10.1073/pnas.89.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afrin F, Ali N. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect Immun. 1997;65(6):2371–2377. doi: 10.1128/iai.65.6.2371-2377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guy B, Pascal N, Francon A, Bonnin A, Gimenez S, Lafay-Vialon E, Trannoy E, Haensler J. Design, characterization and preclinical efficacy of a cationic lipid adjuvant for influenza split vaccine. Vaccine. 2001;19(13–14):1794–1805. doi: 10.1016/s0264-410x(00)00386-8. [DOI] [PubMed] [Google Scholar]
- 16.Hartikka J, Bozoukova V, Yang CK, Ye M, Rusalov D, Shlapobersky M, Vilalta A, Wei Q, Rolland A, Smith LR. Vaxfectin, a cationic lipid-based adjuvant for protein-based influenza vaccines. Vaccine. 2009;27(46):6399–6403. doi: 10.1016/j.vaccine.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, Sawdey M, Wloch MK, Tonsky K, Norman J, Manthorpe M, Wheeler CJ. Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine. 2001;19(15–16):1911–1923. doi: 10.1016/s0264-410x(00)00445-x. [DOI] [PubMed] [Google Scholar]
- 18.Jiao X, Wang RY, Feng Z, Alter HJ, Shih JW. Modulation of cellular immune response against hepatitis C virus nonstructural protein 3 by cationic liposome encapsulated DNA immunization. Hepatology. 2003;37(2):452–460. doi: 10.1053/jhep.2003.50051. [DOI] [PubMed] [Google Scholar]
- 19.Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, Jr, Huang L. Immunostimulation of dendritic cells by cationic liposomes. Mol Membr Biol. 2006;23(5):385–395. doi: 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- 20.Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J Liposome Res. 2009;19(1):2–11. doi: 10.1080/08982100902726820. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Yan W, Huang L. A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer Immunol Immunother. 2008;57(4):517–530. doi: 10.1007/s00262-007-0390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Z, Han SJ, Vangasseri DP, Huang L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol Pharm. 2005;2(1):22–28. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
- 23.Yan W, Chen W, Huang L. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol Immunol. 2007;44(15):3672–3681. doi: 10.1016/j.molimm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Yan W, Huang L. The effects of salt on the physicochemical properties and immunogenicity of protein based vaccine formulated in cationic liposome. Int J Pharm. 2009;368(1–2):56–62. doi: 10.1016/j.ijpharm.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Nakanishi M, Tanaka K, Mayumi T. Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Control Release. 1999;61(1–2):233–240. doi: 10.1016/s0168-3659(99)00097-8. [DOI] [PubMed] [Google Scholar]
- 26.Afrin F, Rajesh R, Anam K, Gopinath M, Pal S, Ali N. Characterization of Leishmania donovani antigens encapsulated in liposomes that induce protective immunity in BALB/c mice. Infect Immun. 2002;70(12):6697–6706. doi: 10.1128/IAI.70.12.6697-6706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badiee A, Jaafari MR, Khamesipour A, Samiei A, Soroush D, Kheiri MT, Barkhordari F, McMaster WR, Mahboudi F. The role of liposome charge on immune response generated in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) Exp Parasitol. 2009;121(4):362–369. doi: 10.1016/j.exppara.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Shek PN, Sabiston BH. Immune response mediated by liposome-associated protein antigens. II. Comparison of the effectiveness of vesicle-entrapped and surface-associated antigen in immunopotentiation. Immunology. 1982;47(4):627–632. [PMC free article] [PubMed] [Google Scholar]
- 29.Bhowmick S, Mazumdar T, Sinha R, Ali N. Comparison of liposome based antigen delivery systems for protection against Leishmania donovani. J Control Release. 2010;141(2):199–207. doi: 10.1016/j.jconrel.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Steers NJ, Peachman KK, McClain S, Alving CR, Rao M. Liposome-encapsulated HIV-1 Gag p24 containing lipid A induces effector CD4+ T-cells, memory CD8+ Tcells, and pro-inflammatory cytokines. Vaccine. 2009;27(49):6939–6949. doi: 10.1016/j.vaccine.2009.08.105. [DOI] [PubMed] [Google Scholar]
- 31.Antimisiaris SG, Jayasekera P, Gregoriadis G. Liposomes as vaccine carriers. Incorporation of soluble and particulate antigens in giant vesicles. J Immunol Methods. 1993;166(2):271–280. doi: 10.1016/0022-1759(93)90368-h. [DOI] [PubMed] [Google Scholar]
- 32.Snyder SL, Vannier WE. Immunologic response to protein immobilized on the surface of liposomes via covalent azo-bonding. Biochim Biophys Acta. 1984;772(3):288–294. doi: 10.1016/0005-2736(84)90145-7. [DOI] [PubMed] [Google Scholar]
- 33.Takagi A, Matsui M, Ohno S, Duan H, Moriya O, Kobayashi N, Oda H, Mori M, Kobayashi A, Taneichi M, Uchida T, Akatsuka T. Highly efficient antiviral CD8+ T-cell induction by peptides coupled to the surfaces of liposomes. Clin Vaccine Immunol. 2009;16(10):1383–1392. doi: 10.1128/CVI.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohno S, Kohyama S, Taneichi M, Moriya O, Hayashi H, Oda H, Mori M, Kobayashi A, Akatsuka T, Uchida T, Matsui M. Synthetic peptides coupled to the surface of liposomes effectively induce SARS coronavirus-specific cytotoxic T lymphocytes and viral clearance in HLA-A*0201 transgenic mice. Vaccine. 2009;27(29):3912–3920. doi: 10.1016/j.vaccine.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shek PN, Heath TD. Immune response mediated by liposome-associated protein antigens. III. Immunogenicity of bovine serum albumin covalently coupled to vesicle surface. Immunology. 1983;50(1):101–106. [PMC free article] [PubMed] [Google Scholar]
- 36.Latif N, Bachhawat BK. The effect of surface charges of liposomes in immunopotentiation. Biosci Rep. 1984;4(2):99–107. doi: 10.1007/BF01120305. [DOI] [PubMed] [Google Scholar]
- 37.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 38.Szoka F, Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 39.Cui Z, Mumper RJ. Coating of cationized protein on engineered nanoparticles results in enhanced immune responses. Int J Pharm. 2002;238(1–2):229–239. doi: 10.1016/s0378-5173(02)00079-0. [DOI] [PubMed] [Google Scholar]
- 40.Sloat BR, Sandoval MA, Hau AM, He Y, Cui Z. Strong antibody responses induced by protein antigens conjugated onto the surface of lecithin-based nanoparticles. J Control Release. 141(1):93–100. doi: 10.1016/j.jconrel.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sloat BR, Cui Z. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharm Res. 2006;23(6):1217–1226. doi: 10.1007/s11095-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 42.Qiu F, Cui Z. CD4+ T helper cell response is required for memory in CD8+ T lymphocytes induced by a poly(I:C)-adjuvanted MHC I-restricted peptide epitope. J Immunother. 2007;30(2):180–189. doi: 10.1097/01.cji.0000211330.61019.6f. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto SI, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. Identification of genes specifically expressed in human activated and mature dendritic cells through serial analysis of gene expression. Blood. 2000;96(6):2206–2214. [PubMed] [Google Scholar]
- 44.Granucci F, Castagnoli PR, Rogge L, Sinigaglia F. Gene expression profiling in immune cells using microarray. Int Arch Allergy Immunol. 2001;126((4)):257–266. doi: 10.1159/000049522. [DOI] [PubMed] [Google Scholar]
- 45.Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol. 1998;161(6):3096–3102. [PubMed] [Google Scholar]
- 46.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29(5):1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Kenney RT, Yu J, Guebre-Xabier M, Frech SA, Lambert A, Heller BA, Ellingsworth LR, Eyles JE, Williamson ED, Glenn GM. Induction of protective immunity against lethal anthrax challenge with a patch. J Infect Dis. 2004;190(4):774–782. doi: 10.1086/422694. [DOI] [PubMed] [Google Scholar]
- 48.Scobie HM, Young JA. Interactions between anthrax toxin receptors and protective antigen. Curr Opin Microbiol. 2005;8(1):106–112. doi: 10.1016/j.mib.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Pardridge WM, Bickel U, Buciak J, Yang J, Diagne A. Enhanced endocytosis and anti-human immunodeficiency virus type 1 activity of anti-rev antibodies after cationization. J Infect Dis. 1994;169(1):55–61. doi: 10.1093/infdis/169.1.55. [DOI] [PubMed] [Google Scholar]
- 50.Muckerheide A, Apple RJ, Pesce AJ, Michael JG. Cationization of protein antigens. I. Alteration of immunogenic properties. J Immunol. 1987;138(3):833–837. [PubMed] [Google Scholar]
- 51.Ernst WA, Kim HJ, Tumpey TM, Jansen AD, Tai W, Cramer DV, Adler-Moore JP, Fujii G. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24(24):5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Shek PN, Sabiston BH. Immune response mediated by liposome-associated protein antigens. I. Potentiation of the plaque-forming cell response. Immunology. 1982;45(2):349–356. [PMC free article] [PubMed] [Google Scholar]