Anticoagulation, the standard treatment for deep vein thrombosis (DVT), is quite effective in preventing pulmonary emboli, but thrombolytic therapy is more effective in restoring venous patency and antegrade flow in acutely thrombosed veins. Studies have suggested that the post-phlebitic syndrome can be prevented if the obstructive thrombus is removed quickly by thrombolytic therapy rather than waiting for natural thrombolysis to occur.1–3 Before development of pharmacomechanical treatments for DVT, thrombolytic therapy was implemented by continuous infusion of thrombolytic agents directly into the thrombus often for many hours until the desired degree of clot lysis was achieved.4–7 Although effective, continuous infusion of many thrombolytic agents including tPA have been associated with major bleeding complications despite being administered directly into the clot by catheter directed therapy.8–12 In one study where pharmacokinetic measurements were conducted during continuous infusion thrombolytic therapy, elevated systemic levels of tPA were shown to persist as long as the infusion was maintained.13 Recent clinical studies have shown that thrombolysis can be achieved by instilling the thrombus with alteplase (recombinant tissue plasminogen activator, r-tPA) without continuous infusion.14–16 Because of its affinity for fibrin, intra-clot alteplase remains bound in the thrombus and continues to lyse the clot for hours after its application ceases.
Earlier studies showed that thrombi in the upper and lower extremities could be lysed with daily intra-clot injections of 20–50mg alteplase.14,15 However, pharmacokinetic measurements during these trials suggested that five- to ten- fold reductions in the dose of alteplase might also be effective.15,16 In this paper we report the outcome of clinical trials using a maximum daily dose of 4 mg alteplase for subclavian, jugular, and central vein thrombosis (SJ-CVT) and 10 mg for the larger clot burden in DVT of the lower extremity (DVT-LE).
Materials and Methods
Preparation of Thrombolytic Agent
Alteplase (Activase; Genentech, South San Francisco, CA) was diluted with sterile water (manufacturer’s instructions) to 1mg/ml and then further diluted with normal saline to a concentration of 0.1 mg/ml to increase the volume of thrombolytic agent available for intraclot injection.
Treatment Protocols
Two protocols, compliant with the Health Insurance Portability and Accountability (HIPPA) Privacy Rules, were reviewed and approved by Institutional Review Boards of the National Cancer Institute (NCI) and National Heart Lung Blood Institute (NHLBI) to prospectively evaluate the safety and efficacy of low doses of alteplase in treatment of adult patients (18 years and older) with SJ-CVT and DVT-LE respectively. Exclusion criteria for both protocols included pregnancy, macroscopic gastrointestinal or genitourinary tract bleeding, deep organ biopsy or major surgery or trauma or lumbar puncture or epidural catheters within previous 2 weeks, recent onset (<10 days) atrial fibrillation without anticoagulation, stroke or neurosurgical procedure within previous 2 months, allergy to heparin or radiographic contrast media, renal insufficiency (creatinine >2 mg/dl), thrombocytopenia <50,000/dl, other bleeding diathesis not attributable to anticoagulation, and inability to complete protocol required anticoagulation regimens or follow up evaluations.
SJ-CVT protocol
The SJ-CVT protocol was developed to address the problem of venous thrombosis caused by use venous access devices (VADs) in patients who were already enrolled in protocols at our institution for treatment of various disorders, primarily cancer. Indications for thrombolytic treatment were relief of pain or swelling, preservation of veins for future venous access, and prophylaxis against progression to superior vena cava syndrome. Patients with SJ-CVT symptomatic for less than 1 month were allowed up to 4 days of treatment receiving up to 4mg alteplase per day delivered by intraclot injection through 4 french pulse spray catheters (2cm spray segment, Angiodynamics Corp., Queensbury, New York).
For indwelling peripherally inserted central catheter (PICC) related thrombosis, the PICC was exchanged for the pulse spray catheter. Otherwise, a basilic or brachial vein was independently catheterized for introduction of the pulse spray catheter. To treat jugular vein thrombosis, a 4 french glidecatheter (Terumo Corp., Somerset, New Jersey) introduced into an ipsilateral arm vein was directed into the affected jugular vein directly or, in 14% of cases, guided by an adjunctive catheter placed directly into the thrombosed jugular vein, before exchanging for a pulse spray catheter. After each thrombolytic treatment, the pulse spray catheter was exchanged for a 4 french micropuncture sheath (Cook Inc, Bloomington, In) or catheters (Arrow International Inc, Reading, Pa ; Bard Access Systems Inc, Salt lake City, Ut) to maintain access, allow follow up venography, and/or continue anticoagulation.
Outpatient thrombolytic therapy with anticoagulation using low molecular weight heparin (LMWH, enoxaparin 1mg/kg/q12h) subcutaneous injections was offered to patients. These patients were discharged after a 2 hour recovery period following tPA injections. Most inpatients received continuous infusions of unfractionated heparin into the access sheath or catheter with dose adjusted to prolong the APTT to 1.5 –2.5 times the mean normal value. Strictures encountered during thrombolytic treatments were treated by balloon catheter angioplasty but stents were not used. Because of rapid reversibility, intravenous unfractionated heparin was the anticoagulation of choice whenever balloon dilatation was performed. If patency was restored, it was recommended that all central venous access devices on the affected side be removed and that side be avoided for future access.14 If venous patency could not be restored, the venous access device was often left in place in asymptomatic patients to allow completion of medical therapies. When sufficient lysis was achieved, continued anticoagulation with either LMWH or warfarin sodium (target INR 2.0–3.0) was prescribed until the follow up venogram 5–7 weeks later.
DVT-LE protocol
Patients were required to have first-time lower extremity DVT (DVT-LE) with thrombosis of popliteal or larger deep veins, less than 14 days old, and be able to receive at least 6 months of anticoagulation. The goals of treatment were restoration of venous function, relief of pain and swelling, and prevention of post thrombotic syndromes. The DVT-LE protocol allowed for a maximum of 4 treatments (1 treatment/day) of up to 10mg of alteplase per treatment per leg affected by DVT. Two or three access routes were used depending on distribution of thrombosis including retrograde femoral vein and antegrade posterior tibial vein access for calf vein thrombosis; right internal jugular vein access for internal iliac vein and profunda femoral vein thrombosis; and antegrade common femoral vein access for ipsilateral common iliac thrombosis.
Alteplase was delivered by rapid hand injection through pulse spray catheters (4french, 2 cm spray pattern) into thrombi in the large deep veins (popliteal vein and above) while thrombosed calf veins were catheterized via ipsilateral retrograde femoral vein access using 4 french glide catheters coaxially introduced 3 french microcatheters, or 4 french pulse spray catheters with more gentle injections of alteplase to avoid extravasation from these smaller capacity vessels.17 Ultrasound guidance was essential for catheterization of thrombosed veins in all cases. Venous stenoses were treated with balloon catheter angioplasty but self expanding uncovered stents (SMART stents, Cordis Corp, Bridgewater, NJ) were only deployed in May Thurner or common iliac vein stenoses.
After administration of alteplase, pulse spray catheters were exchanged for 4 french catheters, which were used for unfractionated heparin infusions and for contrast venography the following day to determine whether additional doses of alteplase were needed. Retrograde femoral catheters, used for the intraclot alteplase injections in femoral-popliteal DVT-LE, were exchanged for 4 or 5 french PICC lines after each thrombolytic session to preserve access to the popliteal and infrapopliteal venous segments and to infuse unfractionated heparin directly into the treated veins to prevent re-thrombosis. For both protocols, the endpoint of thrombolytic treatment was restoration of antegrade flow in the deep veins. Marder scores (described below) were used to assess extent of thrombosis before and immediately after completion of thrombolytic therapy.18
During thrombolytic therapy, all DVT-LE protocol patients received anticoagulation with unfractionated heparin (dose adjusted to maintain APTT to between 1.5 to 2.5 times the mean normal value). After each thrombolytic treatment, patients on both protocols were kept at bedrest for 2 hours to allow systemic tPA levels to normalize, and then encouraged to ambulate. Sequential compression devices were used in patients with DVT-LE whenever they were at bedrest. After thrombolytic therapy, systemic anticoagulation was continued for 6 months, - initially, with LMWH (enoxaparin 1mg/kg/q12h) followed by warfarin (INR between 2 to 3). Patients underwent repeat venogram and duplex ultrasound examination 4–8 weeks later and to assess durability, another duplex ultrasound examination, supplemented with contrast venography in all iliofemoral DVT patients at 6 months. Patients underwent quantitation of pain, swelling, lower extremity mobility prior to, at the end of thrombolytic therapy, and on the two follow-up visits in the Department of Rehabilitation Medicine. All patients were fitted for compression stockings (Jobst stockings, BSN Medical, Charlotte, NC) at the end of thrombolytic therapy and during follow up visits.
Assessment of extent of DVT-LE (Marder Scores)
Diagnosis of venous thrombosis was established by contrast venography and/or duplex ultrasonography. For DVT-LE the extent of thrombosis was quantitated by Marder scores.18 In this system deep vein segments are assigned weights (iliac = 6, common femoral = 4, superficial femoral =10, popliteal = 4, anterior tibial = 4, posterior tibial = 6, peroneal = 6). The weights of the segments containing occlusive thrombus are added to give a measure of the total pretreatment clot volume. After completion of thrombolytic treatment the weight of each segment that was originally occluded is multiplied by 1 if no thrombolysis occurred, by 0.5 if there was obvious thrombolysis but persistent complete occlusion, by 0.25 if there is flow in the vein but persistent thrombus, and by 0 if there is no evidence of residual thrombus. The post-treatment weights are added to give a measure of the total clot remaining.
Pharmacokinetic studies
For SJ-CVT protocol patients, systemic venous tPA activity was measured (immunoabsorbent assay, TriniLIZE tPA, supplied by Trinity Biotech USA, Jamestown, NY) immediately before, and immediately after completion of tPA administration, and then hourly for the next 2 hours. The same schedule was used for DVT-LE patients except that tPA measurements were extended for 4 hours after treatment because of the larger dose of tPA.
Non Protocol patients
Some patients with SJ-CVT who could not meet all anticoagulation and follow up schedules or presented following retirement of the protocol were treated off protocol with the same alteplase regimen. However, short term results (1 to 3month outcomes) of treatment were available in all patients to allow us to include these patients in determining the ability of low dose alteplase to restore antegrade flow.
Results
Treatment of SJ-CVT (Tables 1&2)
Table 1.
SJ-CVT patient demographics, ancillary diagnoses, and VAD history:
| Patient # |
Age/sex | Primary Diagnosis | Ipsilateral VAD History # : type × duration |
|---|---|---|---|
| 1P | 58WM | Lymphoma | 1: R PICC × 4 weeks |
| 2P | 49WF | Diabetes type I | 2: 9Fr LS SG×5d; 7F LIJ ×2 wks |
| 3P | 57WM | Renal cell ca | 1: LS Portacath ×2yrs |
| 4P | 24WF | CGD | 4: SG× 4d; ltPICCs:× 6wks;×1wk; ×15d |
| 5P | 57WF | Breast ca | 1: LS portacath ×22 days |
| 6P | 47WF | CLL | 1: LCV PICC ×1 week |
| 7P | 58WF | Lymphoma | 1: LSV Portacath × 8mo |
| 8P | 67WM | Lymphoma | 1: LBsV PICC × 9days |
| 9P | 25BM | Lymphoma | 1: LBsV PICC × 1week |
| 10P | 34BM | T-cell lymphoma | 2: L PICC × 5 wk;PICC × 1wk |
| 11P | 48WF | Breast ca | 1: RIJ Portacath ×3 wks |
| 12P | 64BM | Multiple myeloma | 1 :6F LIJ C ×7wks+ line infection |
| 13P | 59WM | Lymphoma | 1: L PICC × 5d |
| 14 | 60WM | Prostate ca | 1: LtSV Portacath × 9wks |
| 15 | 53WF | Aplastic anemia | 1: LIJ dialysis C-15F× 3 wks |
| 16 | 49BM | SS; CRF | 2: SG × 5d; LIJ CC 7f × 5wks |
| 17 | 22HF | Ewing's Sarcoma | 1: RIJ Portacath ×1 mo |
| 18 | 24WF | Lymphoma | 2: L PICC (1):3.5 wk; PICC(2):4d |
| 19 | 15HF | Hyper IgE (Job's) | 1: R 7 fr Hickman 2-L × 3wks |
| 20 | 47WM | Melanoma | 1: 13.5F apheresisC × 8wks |
| 21 | 15HF | Hyper IgE (Job's) | 1::Lt IJ Hickman 7fr 2L × 2 wks |
| 22 | 61WF | melanoma | 1: L: Portacath >1yr |
| 23 | 47WM | Lymphoma | 1: LCV PICC ×1 week |
| 24 | 64WF | T-cell Lymphoma | 1: R:BsV PICC ×1 week |
| 25 | 31BF | SLE | 0: Idiopathic; Sxs 4d |
| 26 | 37WM | lymphoma | 2: Port infected 6wk; Rt PICC 6wk |
| 27 | 58BF | Multiple Myeloma | 1: Lt PICC 5fr × 17d |
| 28 | 63WM | lymphoma | 2: R PICC ×2 over 2months |
| 29 | 58WF | Mesothelioma L Chest | 0: Trousseau’s syndrome; Sxs 5d |
| 30 | 51WM | Lymphoma | 1: Portacath × 3.5 yrs |
| 31 | 57BM | HIV, Lymphoma | 1: R PICC× 2mos |
| 32 | 73WM | CLL | 1: L PICC × 5d |
| 33 | 33WM | Hodgkins Lymphoma | 4: L PICCs 4 over 6 mo |
Legend:
Column 1 : P= protocol patient Column 3: ca= cancer; CGD= chronic granulomatous disease; CLL=chronic lymphocytic leukemia; SS= sickle cell anemia; CRF=chronic renal failure; SLE= systemic lupus erythematosus Column 4: R=right; L=left; S=subclavian; V=vein; SG=Swan Ganz catheter; d=day (s); IJ = internal jugular vein; wk(s)=week(s) ; PICC=peripherally inserted central catheter; C= catheter; CV=cephalic vein; BsV=basilica vein; Sxs= symptoms
Table 2.
SJ-CVT patient treatment and outcomes:
| Patient # |
Extent DVT | tPA doses* | ancillary tx | Anticoag | Patency restored? |
|---|---|---|---|---|---|
| 1P | R: SV, AV, BV | 4mg/ 4mg | 0 | LMWH-I | No |
| 2P | L: InV, SV, IJ | 4mg/4mg | 10mm TLA LInV? | UH | Yes |
| 3P | SVC, LInV | 4mg | 12mm TLA& Port removal | LMWH-I | Yes |
| 4P | L: InV, | 4mg/ 2mg | 9mm TLA LInV | LMWH-O | Yes |
| 5P | L: SV, AV, BV | 4mg/4mg/4mg | 16mg/10mg tPA | LMWH-I | No |
| 6P | L: SV, AV, CV | 4mg | 0 | UH | Yes |
| 7P | L: InV, SV | 4mg/4mg | 8mm TLA | UH | Yes |
| 8P | L: SV, AV, BV, BsV | 4mg/4mg/2mg | 0 | UH | Yes |
| 9P | L: InV, SV, AV. BV, | 3mg/4mg | 0 | LMWH-I | Yes |
| 10P | L: InV, SV.AV | 4mg/4mg | 0 | LMWH-I | No |
| 11P | R: IJ, InV | 4mg/2mg | 0 | LMWH-I | Yes |
| 12P | L: InV, LIJ, SV, AV, BV, | 4mg/4mg/4mg | 0 | UH | No |
| 13P | L: SV, AV, BV | 3mg | 0 | LMWH-O | Yes |
| 14 | SVC | 4mg/ 2mg | 0 | UH | Yes |
| 15 | L: InV, SV, AV | 4mg/4mg | removal dialysis Catheter | UH | Yes |
| 16 | L: IJ | 4mg/ 4mg | 6mm TLA LIJ stricture | UH | No |
| 17 | R: IJ, SV, INV | 4mg/ 2mg | 0 | LMWH-O | Yes |
| 18 | L: SV, AV, BV | 4mg/2mg | 0 | LMWH-I | Yes |
| 19 | R: IJ, InV | 4mg/2mg | 5mm TLA | LMWH-I | Yes |
| 20 | R: IJ, SV, InV | 2mg/4mg/4mg | 0 | UH | Yes |
| 21 | L: IJ | 4mg | 5mmTLA LIJ stricture | F-PentaS | Yes |
| 22 | L: InV, IJ, SV, BsV | 4mg/4mg/4mg | 0 | UH | No |
| 23 | L: SV, AV, CV | 4mg/2mg | 0 | LMWH-I | Yes |
| 24 | R: SV, AV, BsV | 4 mg | 0 | UH | Yes |
| 25 | R: SV | 4mg | 0 | Argatroban | Yes |
| 26 | R: AV, SV, InV, IJ | 4mg/4mg | 8mm TLA | LMWH-O | Yes |
| 27 | Lt: BV, SV | 4mg/3mg | 8mm TLA | LMWH-O | No |
| 28 | R: AV; SV | 4mg/ 4mg | 8mm TLA | UH | yes |
| 29 | L: IJ;SV; AV | 4mg/ 4mg | 0 | UH | yes |
| 30 | R: IJ; SV; InV | 4mg/4mg/4mg | 8mm TLA | UH | No |
| 31 | R: IJ, SV, AV, BV | 4mg | 0 | UH | yes |
| 32 | L: SV, AV, BV | 4mg | 0 | UH | yes |
| 33 | L: IJ, InV, SV, AV | 4mg/ 4mg | 8mm TLA | UH | yes |
Legend:
Column 1: P=protocol patient
Column 2: R=right; L=left; S=subclavian; V=vein; A=axillary; B=brachial; IJ+internal jugular; In=innominate or brachiocephalic; Bs=basilica; B=brachial.
Column 3: *only 1 dose or treatment given per day; Hence, # of doses equals # of days of thrombolytic therapy.
Column 4: 0=no other treatment; TLA= transluminal angioplasty or balloon dilatation; pt 5P received additional high tPA doses to no avail; Column 5 = LMWH-I= Lovenox as inpatient; LMWH-O= lovenox as outpatient; UH = intravenous unfractionated heparin; F–PentaS = fondaparinaux (pentasaccharide)
Thirty three consecutive patients were treated for SJ-CVT. In all but 2 patients (Table 1: patients # 25 and 29), thrombosis was associated with use of 1 or more central catheters. Restoration of patency (Table 2) was achieved in 15 (75%) of the initial 20 patients (13 “on protocol” and 7 “off protocol”). Because this success rate was statistically not different than the higher dose study previously reported (P<0.05), the protocol was closed.14 Inclusion of the 13 subsequent patients brings the overall patency restored to 26 of the 33 patients (79%). Among these 33 patients the average total dose of r-tPA was 7.1 mg (range, 3mg to 12mg) administered over an average of 2.1 days or treatments (range, 1 to 3 days or treatments). The extent of thrombosis and the doses used for each patient are tabulated in Table 2.
The duration of thrombosis or obstruction was often difficult to ascertain. Symptoms of swelling and discomfort provided a lower estimate of age of thrombus, while the history of central catheter placements provided an upper limit on the duration of venous injury. Acute thrombosis was present in the 9 patients (patients # 6P, 8P, 9P, 13P, 23, 24, 25, 29, 32) who were treated within 9 days of their first central catheter placement or developed thrombosis with no prior history of central catheterization. In this subset, venous patency was restored in all patients with an average total dose of 6mg of tPA, administered over an average of 1.6 days/treatments, and no stenoses were encountered (Fig 1). Among the remaining 24 patients with either longer duration (indwelling for more than 2 weeks) catheters or history of multiple central catheters on the affected side, balloon dilatation was required in half (12 of 24) and patency was restored in 17 (71%). In the 7 patients where patency could not be restored, venograms revealed either minimal thrombolysis (patient 12P with associated stentotrophomonas maltophilia line infection), or stenoses refractory to balloon dilatation.
Fig 1.
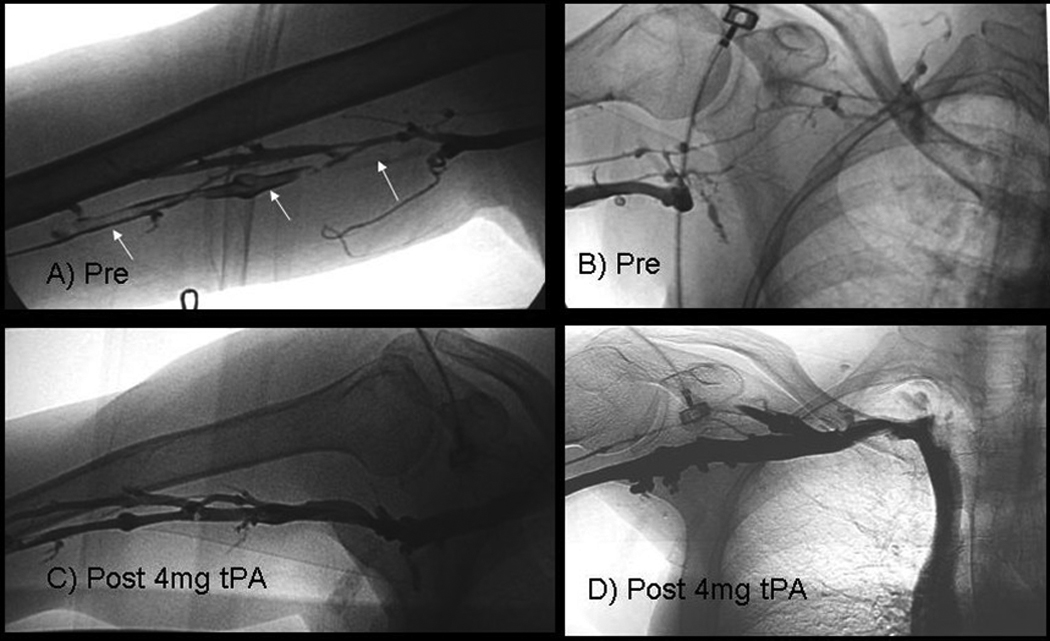
64 WF with lymphoma (patient # 24 in Tables 1,2), developed right basilic, axillary, and subclavian thrombosis, only 1week after placement first right arm (basilic vein) PICC line:
1A: Pre-treatment venogram shows thrombosed (filling defects, white arrows) basilica vein.
1B: Pre-treatment venogram shows diversion of contrast into collateral veins because of occluded axillary and subclavian veins.
1C, D: post treatment venogram obtained 1day after pulse spray injection of 4 mg of r-tPA, shows clearance of thrombi and no evidence of strictures.
Five patients (#s 4P, 13P, 17, 26, and 27) elected to have thrombolytic therapy as outpatients. After thrombolytic therapy one outpatient was hospitalized overnight after balloon dilatation of an underlying stenosis. None of the 33 patients experienced bleeding complications or required blood transfusions.
DVT-LE (Tables 3&4)
Table 3.
DVT-LE demographics, etiology, ancillary diagnoses, est. age of thrombus
| Patient # |
Age/sex | Predisposing Factors | Est. Age DVT |
|---|---|---|---|
| 1 | 45WF | Obesity | 12d |
| 2 | 57WM | Het Factor V Leiden: GCSF; sxs up to 18d | 11d |
| 3 | 55WM | Idiopathic | 10d |
| 4 | 37WF | Anterior Cruciate Ligament repair + tibial fracture | 10d |
| 5 | 38WF | AntiThrombin deficiency / obesity | 13d |
| 6 | 62WM | Airplane flight | 9d |
| 7 | 54WM | Idiopathic | 7d |
| 8 | 60WM | Lt Great toe fracture with ORIF | 12d |
| 9 | 41WM | Liposuction, Het factor V-Leiden | 9d |
| 10 | 57WM | Idiopathic | 11d |
| 11 | 53WM | Varicose veins R>L leg; 11 days sx | 14d |
| 12 | 42BF | Idiopathic | 9d |
| 13 | 47WF | Idiopathic | 14d |
| 14 | 53WM | Het factor V- Leiden; Incompetent perforator vein | 8d |
| 15 | 61WM | Idiopathic; commercial airplane pilot | 13d |
| 16 | 52WM | Prothrombin 20210 | 11d |
| 17 | 36WM | Colorectal cancer | 14d |
| 18 | 47WM | Het factor V-Leiden | 5d |
| 19 | 55WF | Idiopathic | 9d |
| 20 | 19WM | Het factor V-Leiden | 12d |
| 21 | 54WM | Het factor V-Leiden | 11d |
| 22 | 36WM | Prothrombin 20210 | 6d |
| 23 | 50BF | May Thurner | 9d |
| 24 | 38WM | Anterior Cruciate Ligament repair with previous DVT 1Pop V division | 8d |
| 25 | 18WF | Het factor V-Leiden; BCPs; May Thurner | 14d |
| 26 | 55WM | Bilateral TKR, lymphoma+ L. groin RT, trauma+ lumbar fusion, AT def | 8d |
| 27 | 58WF | Het factor V-Leiden, Premarin, varicose veins | 10d |
| 28 | 49WF | May Thurner | 8d |
| 29 | 28WF | Het factorV-Leiden, BCPs | 10d |
| 30 | 36WF | Idiopathic | 11d |
Legend:
Column 1: All patients were protocol patients..
Column 3: Het. = heterozygous; ORIF = open reduction internal fixation; BCPs= birth control pills; TKR = total knee replacement; ATdef-antithrombin deficiency; RT= radiation therapy; Column 4: d = days
Table 4.
DVT-LE Extent of DVT, Treatment, and Outcomes
| Patient # |
Extent of DVT (Thrombosed segments) | Marder scores pre/post- Tx |
Doses of tPA mg)* |
Ancillary Tx | Patency restored? |
|---|---|---|---|---|---|
| 1 | L::SFV, PV, PerV, PTV | 26 / 6 | 7.5 / 10 / 4 | None | Yes |
| 2 | L: PV, PerV, PTV | 16 / 7 | 8 / 8 / 4 | TLA (8mm):PV, Per –PTV tk | No |
| 3 | R: EIV, CFV, SFV, PFV, PV(2) PerV, PTV | 36 / 9 | 10 / 10 / 6 | TLA (6mm): PV, PerV | Yes |
| 4 | L: SFV, PV, PerV, PTV | 26 / 4 | 10 / 6 | None | Yes |
| 5 | R: SFV, PV, PerV, PTV | 26 / 4 | 8 / 10 | None | Yes |
| 6 | L: SFV, PV, PerV, PTV | 26 / 6 | 8 / 8 / 4 | None | Yes |
| 7 | R: PV(2), PerV, PTV | 16 / 4 | 8 | None | Yes |
| 8 | L: EIV, CFV, SFV, PFV, PV, PerV, PTV | 36 / 9 | 10 / 10 / 10 / 8 | TLA (8mm): SFV-PV | Yes |
| 9 | L: CIV, EIV, CFV, PFV, SFV | 20 / 4 | 8 / 5 / 4 | TLA (10mm): L CIV. | Yes |
| 10 | L: PV, PerV, PTV | 16 / 4 | 7 / 7 / 4 | TLA (8mm): PV | Yes |
| 11 | R: SFV, PV, PerV, PTV, LSV | 26 / 4 | 8 / 6 | 2mg tPA into LSV clot | Yes |
| 12 | L: SFV, PV, PerV, PTV | 26 / 4 | 8 / 6 | None | Yes |
| 13 | L: SFV, PV, PerV, PTV. | 26 / 6 | 6 / 8 | None | Yes |
| 14 | R: SFV, PV | 14 / 3 | 5 / 4 | None | Yes |
| 15 | R: SFV, PV(2), PerV, PTV | 26 / 5 | 8 / 10 / 6 | TLA (8mm): both dup. PV | Yes |
| 16 | R: SFV, PV, PerV, PTV | 26 / 7 | 7 / 10 | TLA (8mm) : PV | Yes |
| 17 | L: SFV; PV, PerV, PTV, ATV, plantar v | 30 / 7.5 | 10 / 10 / 6 | None | Yes |
| 18 | L: SFV, PV, PerV, PTV, ATV, plantar v | 30 / 7.5 | 10 / 10 / 10 | TLA: 8 mm PopV/ 6mm PerV | Yes |
| 19 | R: PV; PerV, PTV | 16 / 4 | 8 / 8 / 5 | None | Yes |
| 20 | L: IVC, CIV to PerV, PTV, IIV, PFV; R: CIV | 40+/ 10 | 10 / 10 / 8 | TLA (16mm):IVC/8mm: R CIV | Yes |
| 21 | R: SFV, PV, PerV, ATV, PTV, +plantar v | 30 / 7.5 | 10 / 8 / 8 | None | Yes |
| 22 | R: SFV; PV, PTV | 20 / 5 | 8 / 6 | TLA(8mm):SFV;(6mm}: PV | Yes |
| 23 | L: CIV, IIV, EIV | 6 / 1.5 | 6 / 4 | 12mm Stent: CIV | Yes |
| 24 | R: EIV, CFV, PFV.SFV | 20 / 5 | 10 / 8 | None | Yes |
| 25 | L: CIV, IIV, EIV, CFV | 10 / 1.5 | 6 / 6 / 4 | 14mm Stents (2): CIV | Yes |
| 26 | L: CIV, EIV, IIV, CFV, PFV, SFV, PV | 24 / 6 | 8 / 10 / 5 / 2 | TLA (8mm +10mm): L CIV | Yes |
| 27 | L: SFV, PV(2); Per V, PTV | 26 / 4 | 6 / 6 / 6 | TLA (8mm): PV; (5mm): PerV | Yes |
| 28 | L: CIV, EIV, CFV | 10 / 1 | 6 / 6 / 4 | TLA (10mm);12mm CIV stent | Yes |
| 29 | L: EIV, CFV, PFV, SFV, PV, PerV, PTV | 36 / 11 | 10 / 8 / 4 | TLA (6mm) PerV | Yes |
| 30 | L: CIV, IIV. EIV, CFV, proximal SFV | 20 / 2.5 | 8 / 6 / 6 / 6 | TLA (10 mm): CIV & EIV | Yes |
Legend:
Column 1: All patients were protocol patients.
Column 2 & 5: (vein segments): R= right; L= left; SFV= femoral vein of thigh (aka superficial femoral vein); PV(2) = popliteal vein (duplicated); PFV= profunda femoral vein; PerV=peroneal vein; PTV=posterior tibial vein; ATV= anterior tibial vein; CIV = common iliac vein; EIV = external iliac vein; IIV = internal iliac vein; CFV= common femoral vein; LSV=lesser saphenous vein.
Column 4: Patient receives only 1 tPA dose or treatment per day; Hence, # of tPA doses equals # of days of thrombolytic therapy.
Column 5: tk = trunk; TLA = transluminal angioplasty or balloon dilatation (inflated balloon diameter): followed by site of TLA
Demographics, Thrombophilia, and Distribution of Thrombosis
The average age of the 30 DVT-LE patients was 47 years (range 18 to 62 years) with estimated average age of DVT of 10 days (range 5–14 days, Table 3). Inherited thrombophilic traits were identified in 13 (43%) patients (heterozygous factor V-leiden in 9, prothrombin G20210A in 2, antithrombin deficiency in 2). Seven patients (23%) presented with ilio-femoral DVT : 3 with May Thurner syndrome (patients 23, 25,28) and 1 related to previous pelvic surgery and inguinal radiation therapy patients (26); 3 associated with factor V-Leiden (patients 9, 20, 25); and 1 idiopathic (patient 30).
The remaining 23 (77%) patients had femoral popliteal DVT. Occlusive calf vein thrombosis was present in 22 (73%)and in 3 patients (#s 17,18, and 21 or 10% of patients) thrombosis extended from the posterior tibial veins into the plantar vein of the foot (Table 4).
One patient with factor V-Leiden (patient #20) was referred to us with diagnosis of first time acute DVT of left leg based on ultrasound exam but upon catheterization was found to have previously unrecognized occlusion of the inferior vena cava and asymptomatic chronic thrombosis of the right common iliac vein with well developed collateral drainage via the right internal iliac vein to paravertebral and epidural collateral veins (Fig 2). Thrombolytic therapy was given to treat acute DVT of the left leg as well as to recanalize the inferior vena cava. A month later (after verification of continued patency of the deep veins of the left leg and inferior vena cava), the chronic stenotic right iliac vein underwent balloon angioplasty and thrombolytic therapy for 2 treatments (6mg tPA/d for 2d). By the sixth month follow up examination, venograms and clinical evaluation indicated stents were unnecessary. In other patients (patients 23,25, and 28), bare metallic stents (12–14 mm diameter) were placed in common iliac veins after thrombolytic therapy to relieve persistent obstruction from left common iliac vein stenosis or May Thurner syndrome.
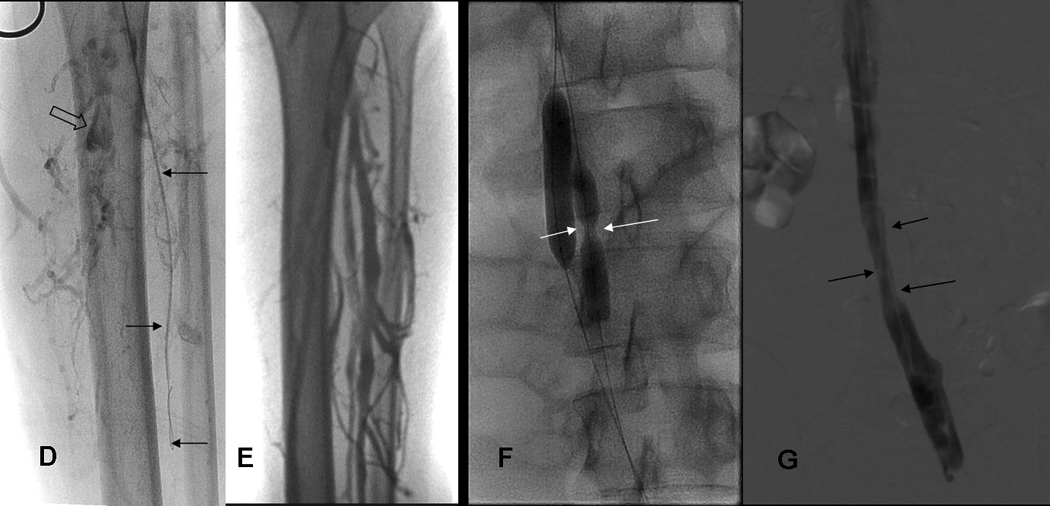
Fig 2.
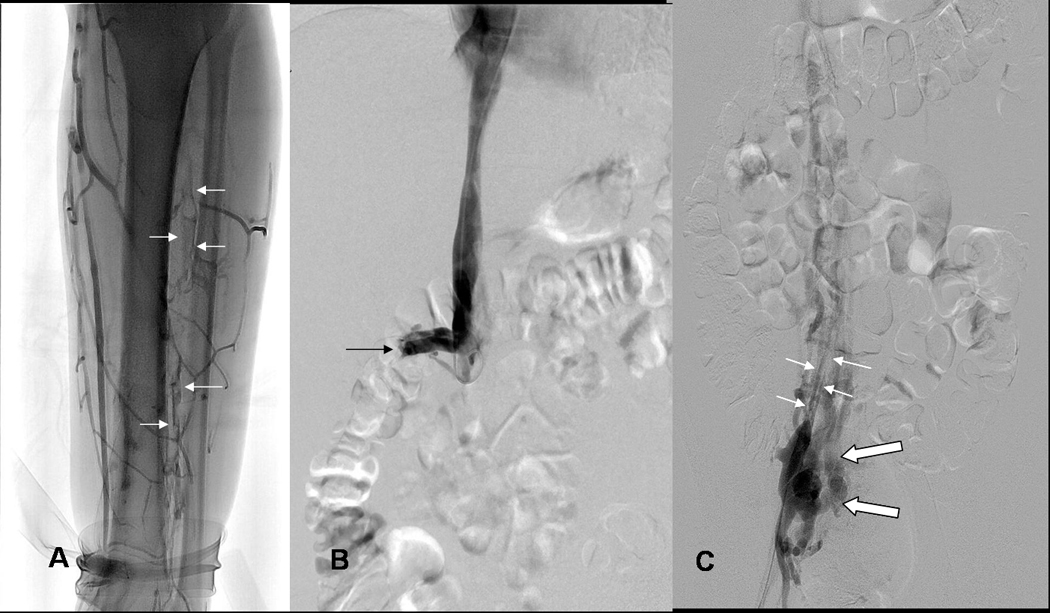
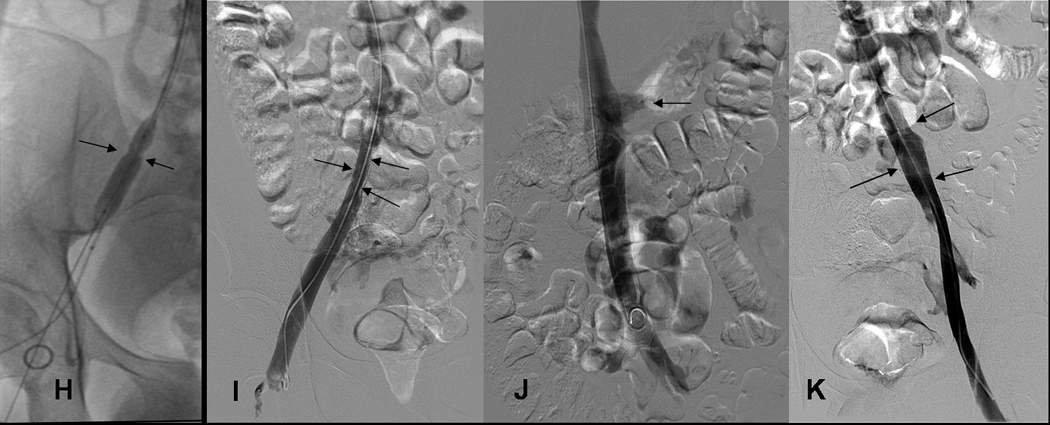
ABC: 19yo WM (patient 20) with Factor V-Leiden (FV-L) referred for acute DVT left leg.
A) Lower end of thrombosis: Left leg venogram shows thrombosis of peroneal veins (arrows) down to ankle; posterior tibial veins do not opacify due to occlusive thrombosis at mid calf level.
B) Upper end of thrombosis: IVCgram shows IVC occluded by thrombosis below renal veins (arrows, right renal vein), unsuspected because initial diagnosis was made by ultrasound examination only.
C) Asymptomatic right leg: Chronic thrombosis of right common iliac vein demonstrated by right femoral venogram shows narrow recanalized right common iliac vein (small white arrows) and collateral venous drainage from internal iliac vein to paravertebral collateral veins bypassing obstructed IVC (large open white arrows).
DEFG: 19yo WM with FV-L: Acute DVT left leg/IVC thrombosis received 3 treatments (10mg/10mg/8mg tPA).
D) Using retrograde femoral vein access, guidewire and 4 french glidecatheter (arrows) was passed down into clotted peroneal veins to allow intraclot injection of tPA. Note, thrombosis of proximal posterior tibial vein (open arrow).
E) Follow up leg venogram at 1 month shows patency of calf veins; patency also recovered in left popliteal, femoral and iliac veins (not shown).
F) IVC occlusion treated with pulse spray injections of tPA and double balloon dilatation (10 mm balloon introduced via right jugular vein, and 8 mm balloon from left femoral vein) of partially organized thrombus. Note presence of stricture in IVC (white arrows, 8mm balloon catheter).
G) Brisk flow restored in left external, left common iliac vein (mildly narrowed, arrows), and small IVC at 1 month.
HIJK: 19 yo WM with FV-L: treatment chronic DVT right iliac vein and 6 month venograms.
H) Narrowed right common iliac vein (arrows) was dilated with 10 mm balloon 1 month after treatment of acute DVT left leg and received 2 (6mg/ 6mg) treatments with tPA.
I) At 6 month follow up, despite mild narrowing (arrows), right common iliac vein is again the dominant pathway for venous return.
J) IVC patent (arrows, transient reflux into left renal vein) at 6 months.
K) Left femoral, external iliac and common iliac vein at 6 months. Left Common iliac vein (arrows) caliber has improved since 1 month study (see Fig. 2G).
Degree of Lysis and Marder Scores achieved with Thrombolysis
Venograms performed the day following last thrombolytic treatment showed that antegrade venous flow was restored in 29 of 30 patients (97%) using an average total dose of 19.7 mg (range 8 mg to 38mg) of alteplase over an average of 2.7 treatments (range 1 to 4 treatments or days) for an average dose of 7.3 mg tPA/day. Initial results of thrombolytic therapy were also evaluated using the modified Marder scores described above. For the 30 patients the mean pre-treatment Marder score was 23.5, which was reduced to 5.3 after thrombolytic treatment, implying a 77% reduction in thrombus burden. The one failure (patient #2) had a duplicated popliteal system with a patent lateral popliteal divison draining patent anterior tibial veins, but the medial popliteal division, the primary drainage pathway of the posterior tibial and peroneal veins, remained obstructed despite adequate doses of tPA and balloon dilatation. At 6 months the patient was asymptomatic in regard to pain and swelling but the medial popliteal division remained obstructed indicating continued impairment of the calf muscle pump.
Effect of Thrombolytic Therapy on Symptoms
Pain and edema were the most common symptom and sign of DVT in our patients. On presentation, three patients (#s 17, 20, and 21) required crutches for ambulation because of severe pain with weight bearing. Pain was the first symptom to improve so that after the second thrombolytic treatment all patients were able to ambulate unassisted and were pain free by end of thrombolytic treatments. Edema improved more slowly so that patients were often discharged with some residual edema that resolved by the time of their first follow up visit 4–8 weeks later.
Continued Patency Studies
Follow up evaluations (duplex ultrasound studies supplemented with venography for ilio-femoral DVT patients) were completed at 6 months in all but 2 patients : one patient (#6) although asymptomatic, cited travel costs as a financial hardship for not returning for imaging studies and the other patient (#17) who was diagnosed with advanced metastatic colorectal cancer shortly after successful thrombolytic treatment died within 2 months. In the remaining 28 patients results were durable (27/28 or 96%) in that there was no loss in patency in the 27 patients where patency was restored, while the one patient where patency was not restored did not regain patency despite 6 months of anticoagulation.
Complications
All SJ-CVT had uneventful courses of treatment and two DVT-LE patients had small hematomas related to oozing at catheter introduction sites. There were no cases of intracranial or remote bleeding, or bleeding that required blood transfusion.
Pharmacokinetic studies
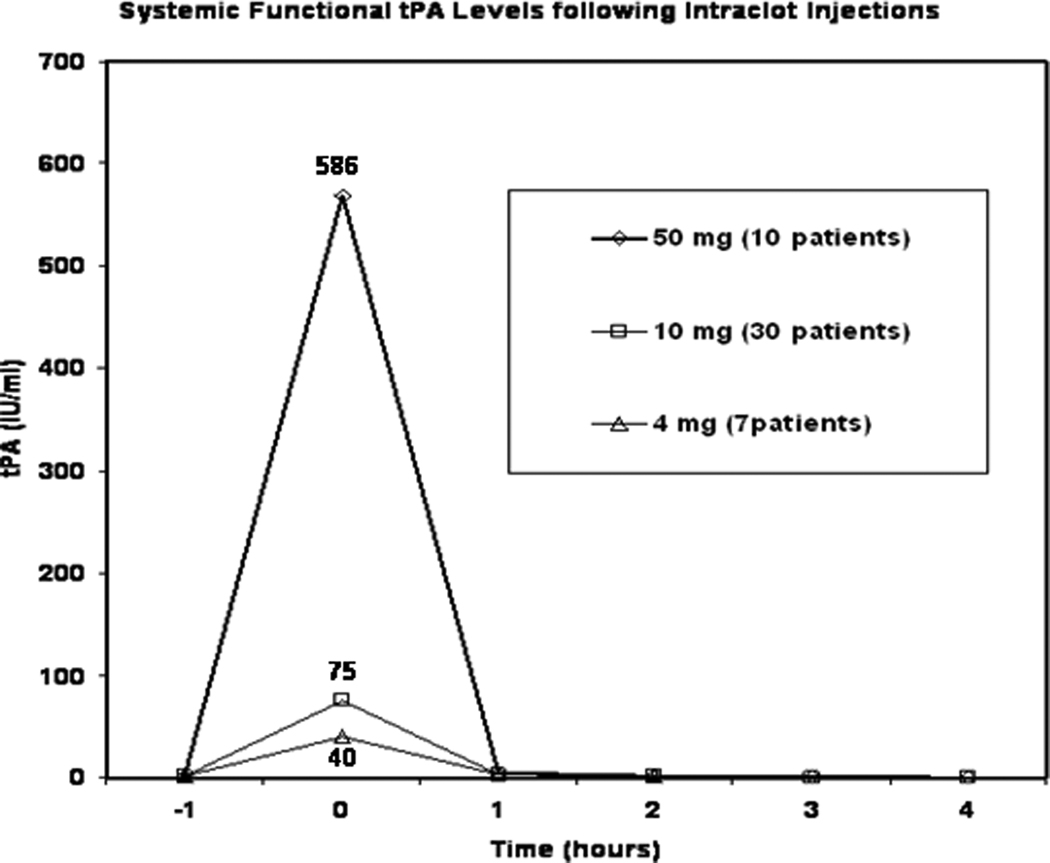
Pharmacokinetic data from SJ-CVT and DVT-LE showed that following completion of intra-clot alteplase injections, elevations in systemic tPA levels are reduced in rough proportion to reductions in administered dose but rapidly return to normal levels within 1 –2 hours after cessation of the intraclot injections. (Fig. 3).
Fig 3.
Pharmacokinetics of intraclot tPA injection. Systemic tPA levels measured immediately after cessation of intraclot injection (Time point “0 Hours”) are elevated over pre-treatment baseline (Time point “-1 hour”) level but cessation of injection allows rapid clearance of circulating tPA (within 1–2 hours) at all dose levels due to its short half life (T1/2 ~ 5 minutes). Reduction in dose from 50mg down to 10mg and 4 mg results in a roughly proportional reduction (average “0 hour” or immediate post injection systemic levels of tPA : fall from 568, 75, to 40 IU/mlrespectively) in systemic exposure to tPA. Reduction in overall dose and avoiding prolonged tPA infusions allows reduction in amount and duration of systemic exposure to circulating tPA.
Discussion
Although prolonged continuous intraclot infusion of a thrombolytic agent has been the mainstay of catheter directed thrombolysis since Dr. Charles Dotter first successfully cleared arterial thrombus with streptokinase in 1974, the introduction of recombinant tPA or alteplase, with its substantial fibrin binding, allowed effective thrombolysis without need for prolonged infusions.19 This was first anticipated from animal experiments by Giancarlo Agnelli who showed that not only did the thrombolytic action of tPA continue well beyond the termination of its infusion, but the bleeding risks of the treatment were reduced by shortening the duration of the tPA infusions.20–21
Once daily intraclot injection of tPA differs from continuous infusion catheter-directed thrombolytic therapy in a number of ways. Our selection of dose of tPA was guided by pharmacokinetic data acquired from the earlier studies using 20 to 50 mg/day doses of tPA that suggested substantially lower doses of tPA would also be effective and the current study demonstrates that 10 mg doses of the tPA produced the same benefit (patency in 97% of cases) in treating DVT-LE as 20 – 50 mg in earlier studies (80% patency), and as little as 4 mg was effective in SJ-CVT with its smaller clot burden without increasing number of treatments or days.14,15 These results for once daily intraclot injection of tPA for DVT mirror the published reports showing that progressively lower of doses of tPA have been found to be effective for treatment of dialysis graft thrombosis.22–25
Pharmacokinetic studies with the current study confirm that the lower doses reduce the amount of tPA that reaches the systemic circulation while the elimination of prolonged continuous infusions permits rapid clearance of any tPA that reached the circulation because of its short half life (T1/2 ~ 5 minutes). This reduction of the amount and duration of tPA in the systemic circulation afforded by the use of intraclot injection of low doses of tPA without continuous infusion may translate into reduction of duration and overall risks of thrombolytic therapy for treatment of DVT.
A second advantage of eliminating prolonged continuous infusions for effective thrombolysis is related to the complex presentations of DVT, particularly in the lower extremity. It is not uncommon for additional venous segments to be thrombosed such as internal iliac vein and deep femoral vein in iliofemoral DVT and multiple calf veins to be thrombosed in femoropopliteal DVT. Continuous infusion regimens require the infusion catheter to be dedicated to treatment of one set of vein segments for many hours, making it difficult to give a complete or comprehensive treatment when DVT is extensive enough to involve multiple segments. Elimination of the need for continuous infusion of tPA allows treatment of many more divisions affected by DVT more efficiently, albeit requiring additional catheterization skills and procedure time of the interventional radiologist.
As enzymatic clearance of clot is inherently a slow process compared to pharmacomechanical approaches to clot clearance, restoration of venous patency with thrombolytic therapy often requires multiple days of therapy, whether implemented by continuous infusion or by once daily intraclot injection. A disadvantage of once daily intraclot injection as opposed to continuous infusion of thrombolytic agents is a potential higher risk of re-thrombosis. The continuous elevation of local levels of thrombolytic agents in the thrombosed veins during catheter-directed continuous infusion thrombolytic therapy regimens is a powerful deterrent against local re-thrombosis whereas with once daily intraclot injection, tPA levels decrease rapidly after termination of injection before maximum fibrinolysis of the original thrombus is achieved. As stasis is a major factor in re-thrombosis we feel it is critical to try to restore flow in all deep vein segments as soon as possible, treating all important segments right from the first day on, rather than approach thrombolysis in “piecemeal” fashion, -treating one or only some segments each treatment session. In addition, lapses or interruptions of anticoagulation must be avoided as re-thrombosis can occur very quickly in the absence of the “immunity” afforded by continuous thrombolytic infusions. On the other hand, because venograms to evaluate once daily intraclot injections of tPA are performed 24 hours after the last injection of tPA, at a time when systemic and local tPA levels have long cleared, the degree of patency achieved is more likely to be representative of the patency that can be sustained by anticoagulation alone than with continuous infusion regimens where venograms are obtained by simply switching the catheter from infusion of thrombolytic agent to contrast injection. We saw no fall off in patency in the 6 month follow up studies in our DVT-LE patients compared to the historical data from the urokinase registry.4
Our studies indicate that while low doses of alteplase given as intraclot injection once daily are just as effective as published studies of catheter directed thrombolytic therapy using continuous infusions of thrombolytic agents, the potential for safer thrombolytic therapy, for outpatient therapy, and for improved treatment of complex cases of DVT are features that warrant further investigation of this approach to thrombolytic therapy.
Acknowledgements
The authors would like to acknowledge Richard O. Cannon MD for his support in the clinical studies; Ann Cullinane and Paula Merryman for their contribution to the pharmacokinetic studies; and Dr Robert Wesley for assistance in statistical analysis needed for design of the protocols.
Grant Support: These studies were supported by intramural research grants of the National Institutes of Health, and preformed under supervision of Institutional Review Boards of the National Cancer Institute and National Heart Lung Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Oral and electronic presentations:
1) This manuscript was first presented as Abstract #5 at the 33rdAnnual Scientific meeting SIR 2008 in Washington, DC in the scientific session “Venous Thromboembolic Disease, Lysis” on March 16, 2008 moderated by Dr John Kaufman
2) As an electronic poster presentation at ISET 2009.
Conflicts of Interest:
The contributors have no conflicts of interest to report.
References
- 1.Watz R, Savidge GF. Rapid Thrombolysis and preservation of venous function in high deep vein thrombosis. Acta Med Scand. 1979;205:203–298. doi: 10.1111/j.0954-6820.1979.tb06050.x. [DOI] [PubMed] [Google Scholar]
- 2.Meissner MH, Manzo RA, Bergelin RO, Markel A, Strandness DE. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surg. 1993;18:596–608. [PubMed] [Google Scholar]
- 3.Rhodes JM, Cho JS, Gloviczki P, Mozes G, Rolle R, Miller VM. Thrombolysis for experimental deep venous thrombosis maintains valvular competence and vasoreactivity. J Vasc Surg. 2000;31:1193–1205. doi: 10.1067/mva.2000.104421. [DOI] [PubMed] [Google Scholar]
- 4.Mewissen MW, Seabrook GR, Meissner M, Cynamon, et al. Catheter-directed thrombolysis for lower extremity deep vein thrombosis: Report of a national multicenter registry. Radiology. 1999;211:39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- 5.Semba C, Dake MD. Iliofemoral deep venous thrombosis: Aggressive therapy with catheter-directed thrombolysis. Radiology. 1994;191:487–494. doi: 10.1148/radiology.191.2.8153327. [DOI] [PubMed] [Google Scholar]
- 6.Bjarnason H, Kruse JR, Asinger DA, et al. Iliofemoral deep venous thrombosis: safety and efficacy outcome during 5 years of catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 1997;8:405–418. doi: 10.1016/s1051-0443(97)70581-5. [DOI] [PubMed] [Google Scholar]
- 7.Sillesen H, Just S, Jorgensen M, Baekgaard N. Catheter directed thrombolysis for treatment of ilio-femoral deep venous thrombosis is durable, preserves venous valve function and may prevent chronic venous insufficiency. Eur J Vasc Endovasc Surg. 2005;30:556–562. doi: 10.1016/j.ejvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Grunwald MR, Hoffman LV. Comparison of urokinase, alteplase, and reteplase for catheter-directed thrombolysis of deep vein thrombosis. J Vasc Interv Radiol. 2004;15:347–352. doi: 10.1097/01.rvi.0000121407.46920.15. [DOI] [PubMed] [Google Scholar]
- 9.Ouriel K, Gray B, Clair DG, Olin J. Complications associated with the use of urokinase and recombinant tissue plasminogen activator for catheter-directed peripheral arterial and venous thrombolysis. J Vasc Interv Radiol. 2000;11:295–298. doi: 10.1016/s1051-0443(07)61420-1. [DOI] [PubMed] [Google Scholar]
- 10.Goldhaber SZ, Meyerovitz MF, Green D, Vogelzang RL, et al. Randomized controlled trial of tissue plasminogen activator in proximal venous thrombosis. Amer J Med. 1990;88:235–240. doi: 10.1016/0002-9343(90)90148-7. [DOI] [PubMed] [Google Scholar]
- 11.Baekgaard N, Broholm R, Just S, Jorgensen M, Jensen LP. Long-term results using catheter-directed thrombolysis in 103 lower limbs with acute iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39:112–117. doi: 10.1016/j.ejvs.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Semba CP, Bakal C, Calis KA, Grubbs GE, et al. Alteplase as an alternative to urokinase. J Vasc Interv Radiol. 2000;11:279–287. doi: 10.1016/s1051-0443(07)61418-3. [DOI] [PubMed] [Google Scholar]
- 13.Marder VJ, Brenner B, Totterman S, Francis CW, et al. Comparison of dosage schedules of rt-PA in the treatment of proximal deep vein thrombosis. J Lab Clin Med. 1992;119:485–495. [PubMed] [Google Scholar]
- 14.Chang R, Horne MK, III, Mayo DJ, Doppman JL. Pulse –Spray Treatment of subclavian and jugular venous thrombi with recombinant tissue plasminogen activator. J Vasc Interv Radiol. 1996;7:845–851. doi: 10.1016/s1051-0443(96)70858-8. [DOI] [PubMed] [Google Scholar]
- 15.Chang R, Chen C, Kam A, Mao E, Shawker T, Horne MK., III Deep vein thrombosis of the lower extremity: direct intraclot injection of Alteplase once daily with systemic anticoagulation-results of a pilot study. Radiology. 2008;246:619–629. doi: 10.1148/radiol.2461062076. [DOI] [PubMed] [Google Scholar]
- 16.Horne MK, III, Chang R. Pharmacokinetics of pulse-sprayed recombinant tissue plasminogen activator for deep vein thrombosis. Thromb Res. 2003;111:111–114. doi: 10.1016/j.thromres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Chang R, Cannon RO, III, Chen CC, Doppman JL, Shawker TH, Mayo DJ, Wood B, Horne MK., III Daily catheter-directed single dosing of t-PA in treatment of acute deep venous thrombosis of the lower extremity. J Vasc Interv Radiol. 2001;12:247–252. doi: 10.1016/s1051-0443(07)61832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marder VJ, Soulen RL, Atichartakarn V, et al. Quantitative venographic assessment of deep vein thrombosis in the evaluation of streptokinase and heparin therapy. J. Lab Clin Med. 1877;89:1018–1029. [PubMed] [Google Scholar]
- 19.Dotter CT, Rosch J, Seaman AJ. Selective clot lysis with low-dose streptokinase. Radiology. 1974;111:31–37. doi: 10.1148/111.1.31. [DOI] [PubMed] [Google Scholar]
- 20.Agnelli G, Buchanan MR, Fernandez F, Van Ryn J, Hirsh J. Sustained thrombolysis with DNA recombinant type tissue plasminogen activator in rabbits. Blood. 1985;66:399–401. [PubMed] [Google Scholar]
- 21.Agnelli G. Rationale for bolus t-PA therapy to improve efficacy and safety. Chest. 1990;97(4) Suppl:161S–167S. doi: 10.1378/chest.97.4_supplement.161s. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Shapiro WB, Porush JG. The Use of tissue plasminogen activator to declot arteriovenous access in hemodialysis patients. Amer J Kidney Diseases. 1993;21:38–43. doi: 10.1016/s0272-6386(12)80718-9. [DOI] [PubMed] [Google Scholar]
- 23.Vlassopoulos D, Logothetis E, Arvanitis C, et al. Local thrombolysis with recombinant tissue plasminogen activator for thrombosed vascular access in hemodialysis patients (Letter to editor) Clinical. Nephrology. 1996;46:77–78. [PubMed] [Google Scholar]
- 24.Boobes Y, Hassan HA, Neglen P, Obeid K, Denour N. Recombinant tissue plasminogen activator to declot dialysis fistulas. J of Nephrology. 1997;10:107–110. [PubMed] [Google Scholar]
- 25.Falk A, Mitty H, Guller J, Teodorescu V, Uribarri J, Vassalotti J. Thrombolysis of clotted hemodialysis grafts with tissue plasminogen activator. J Vasc Interv Radiol. 2001;12:305–311. doi: 10.1016/s1051-0443(07)61908-3. [DOI] [PubMed] [Google Scholar]