Abstract
Dendritic spines of striatal Medium Spiny Neurons (MSNs) receive converging dopaminergic and glutamatergic inputs. These spines undergo experience-dependent structural plasticity following repeated drug administration and during disease states like Huntington’s and Parkinson’s. Thus, understanding the molecular mechanisms leading to structural plasticity is an important step toward establishing a clear relationship between spine structure and function, and will ultimately contribute to understanding how changes in dendritic spine structure relate to behaviors or diseases. One major difficulty faced when studying MSN development is the lack of a detailed, standardized in vitro model system that produces MSNs with in vivo-like morphologies. For example, unlike cultured pyramidal neurons, MSNs grown in mono-cultures display stunted dendritic arborization and fail to develop a full cohort of mature dendritic spines.
Here we report the generation of an embryonic mouse cortical-striatal co-culture that generates high cell yields from a single embryo. Unlike MSNs in striatal monoculture, MSNs in co-culture develop in vivo-like morphologies and high densities of dendritic spines. Morphological identification of co-cultured MSNs expressing a soluble fluorescent protein can be confirmed by immunochemical detection of DARPP-32 (Dopamine and cyclic AMP regulated phosphoprotein of 32 kDa). Additionally, co-cultured MSN spines contain PSD-95 puncta and are apposed to SV2 puncta, indicating the spines express synaptic machinery. Finally, whole-cell recordings of co-cultured MSNs exhibit higher mEPSC frequency compared to mono-cultured MSNs, suggesting that the spines are functionally mature. These studies establish that this co-culture system is suitable for studying the morphological and physiological development and function of MSN dendritic spines.
Keywords: Medium Spiny Neurons, striatum, dendritic spines, cortex, primary cell culture
1. Introduction
GABAergic medium spiny neurons (MSNs) are the major type of neuron in the striatum (Kemp, 1968; Kemp and Powell, 1971; Graveland and DiFiglia, 1985; Rafols et al., 1989; Matamales et al., 2009) and are characterized by medium-sized cell bodies, complex dendritic arbors, and a high density of dendritic spines that receive both glutamatergic and dopaminergic inputs (Rafols et al., 1989; Gertler et al., 2008). In vivo, MSN dendritic spines undergo density and morphology changes during development and in association with numerous disease and experience-dependent states (Robinson and Kolb, 2004; Deutch et al., 2007; van Spronsen and Hoogenraad, 2010).
Previous research in the well-established cortical and hippocampal neuron culture system has provided significant information regarding the mechanisms of spinogenesis and plasticity in pyramidal neurons (see Ethell and Pasquale, 2005; Tada and Sheng, 2006; Schubert and Dotti, 2007 for review). These studies have been aided by the reproducible nature of spine development in these culture systems and the detailed protocols available. In contrast, commonly used protocols for the culturing of MSNs often rely on striatal mono-culture (e.g. Ventimiglia and Kindsay, 1998), a system that generally produces morphologically immature MSNs with low densities of dendritic spines when compared to MSNs co-cultured with glutamatergic neurons (Segal et al, 2003; Tian et al, 2010). Importantly, it appears that excitatory afferent activity, produced by the inclusion of cortical or hippocampal pyramidal neurons, is required for the development of MSN dendritic spines in culture (Segal et al, 2003).
Here we report a detailed method that can be used to generate cortical-striatal co-cultures that support the survival and development of MSNs with in vivo-like characteristics. This method takes advantage of the large number and relative robustness of striatal cells in the embryonic striatum (a.k.a. the ganglionic eminence), making it possible to produce multiple dishes of primary neurons from a single embryo, supported by media conditioned on previously prepared astroglia cultures. This straightforward protocol provides an in vitro system that can be applied to a wide variety of investigations into the molecular mechanisms that regulate MSN dendritic spine morphology and plasticity.
2. Materials and Methods
2.1. Animals
Animal procedures were performed at the University of Minnesota in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and in accordance with protocols approved by the University of Minnesota IACUC, as well as the principles outlined in the National Institute of Health Guide for the Care and Use of Laboratory animals.
2.2. Preparation of Coverslips
German glass 12mm coverslips (Bellco; 1943-10012) were acid washed in a 1M HCl solution overnight at 55 °C, washed twice for 30 minutes with distilled water and then rinsed 30 minutes each in 50%, 75%, and 90% solutions of ethanol. Following washing, coverslips were dried and maintained in at 225 °C oven until immediately prior to coating. Observations regarding the importance of the source, washing and storage of coverslips for cell adherence, survival, and development are detailed in supplemental table 1.
One to two days before cultures were prepared, acid-washed coverslips were transferred to 35mm petri dishes (5 coverslips per 35mm dish) and coated with a mixture of 100μg/ml Poly-D-Lysine (PDL, Sigma; p7886) and 4μg/ml laminin (Invitrogen; 23017015). PDL was prepared by dissolving 100mg of PDL in 0.1M borate buffer (pH 8.5). PDL/borate solution was then sterilized through a 0.2μm filter, aliquoted and stored at −80°. Laminin was thawed on ice, aliquoted and stored at −80°. Immediately prior to coverslip coating, PDL was thawed at 32° and Laminin was thawed on ice. When working with laminin, care must be taken to avoid warming the solution too quickly, which can cause laminin to aggregate, reducing its ability to serve as a substrate for cells. After overlaying 50μl of PDL/laminin on each coverslip, the dishes were sealed with parafilm to prevent evaporation and coverslips were incubated overnight at room temperature. Coverslips were then rinsed three times with sterile water. Each 35mm dish was filled with 2ml of neuronal plating media (see section 2.4) and stored in the tissue culture incubator until needed.
2.3. Dissection of striatal and cortical tissues
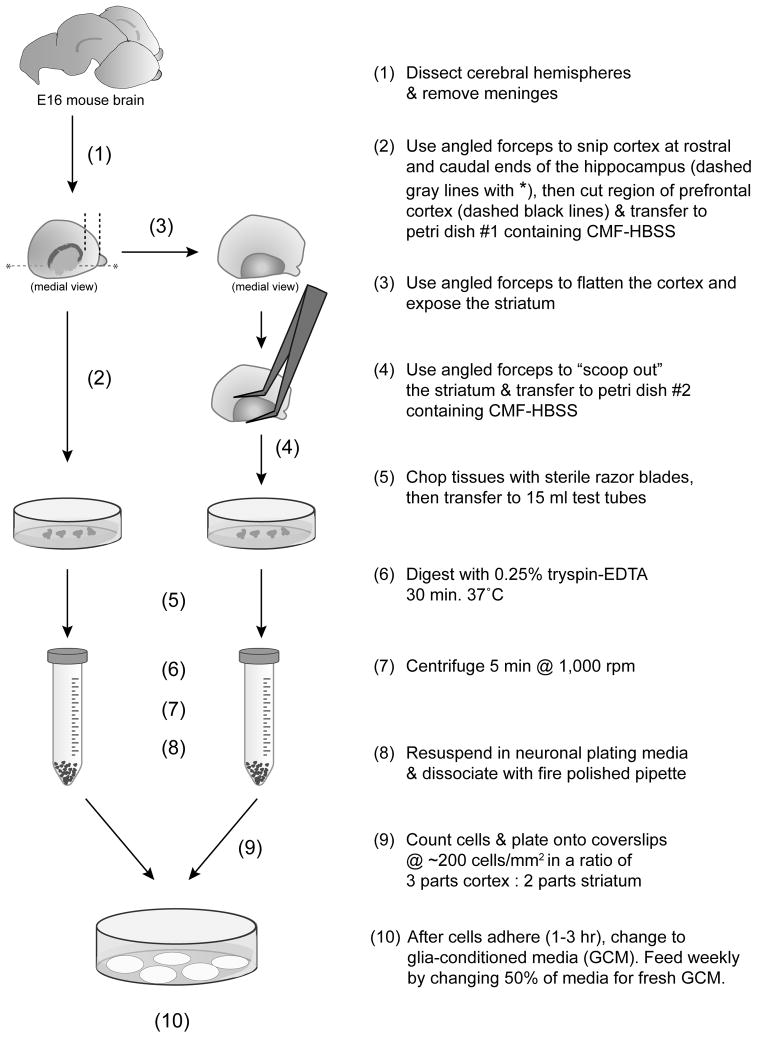
Figure 1 presents a flow chart of the culturing protocol. Embryonic day 16 (E16) pregnant mice were euthanized by CO2 asphyxiation and the uterus was removed. Within a sterile dissecting hood, embryos were removed from the uterus and decapitated. Heads were stored in room temperature Ca2+/Mg2+-free Hank’s Balance Salt Solution with 10mM HEPES (CMF-HBSS, the same solution in which all dissections took place). All dissecting equipment was sterilized in 70% ethanol and was routinely dipped in 70% ethanol during the dissection, especially during transitions from exterior structures to interior structures (i.e. between removing brain from skull and working with isolated brain). These steps were undertaken to maintain the sterile environment and avoid contamination. Routine use of this procedure eliminates the need for antibiotic administration at any point during the preparation or maintenance of the cultures. Brains were removed from the skull using forceps and transferred to a wax bottom 35mm petri dish (5ml of paraffin wax in the bottom of dissecting dishes helps protect the tips of delicate tools). The hemispheres were removed from the cerebral peduncles with a sharp forceps. The meninges were removed and hemispheres were placed medial surface up. A region of cortex, roughly corresponding to the pre-frontal cortex, was cut from the medial side of the anterior aspect of the cortex. The remaining cortex overlying the lateral ventricle was folded away to expose the ventricle and ganglionic eminences. The ganglionic eminences (medial and lateral; lateral being the presumptive striatum (Wichterle et al., 2001)) were removed using angled forceps to “scoop out” the entire eminence from the lateral ventricle and separate it from the overlying cortex. The cortex and ganglionic eminence regions were transferred to separate CMF-HBSS containing petri dishes. After dissection of all pups, tissue was minced using a clean, sterile razor blade. Videos of the dissection procedure are available upon request.
Figure 1.
Schematic illustrating the major steps in the cortical-striatal co-culture procedure.
2.4. Tissue dissociation
Minced cortical and striatal tissue was transferred to separate sterile 15ml conical tubes and allowed to settle to the bottom of the tube. CMF-HBSS was removed and replaced by either papain or trypsin digestion solutions. For papain treatment, minced tissue pieces were resuspended in 5 ml of 20–25 units/ml papain (Worthington, LS003126) solution including 1.1mM EDTA and 5.5mM cysteine in CMF-HBSS and incubated for 30 minutes at 37 °C. Cells were allowed to settle to the bottom of the tube, digestion solution was removed and 5ml of inhibition solution (750μg/ml DNAse1, 10mg/ml BSA, and 10mg/ml Type II-O Trypsin inhibitor) was then added and the incubation continued for 15 minutes. Tissue was then centrifuged at 1000g (~3000rpm) for five minutes, resuspended in neuronal plating media (10mM HEPES, 10mM sodium pyruvate, 0.5mM glutamine, 12.5μM glutamate, 10% Newborn Calf Serum, 0.6% Glucose in EMEM (Minimal Essential Media, plus Earl’s salt)) and triturated no more than ten times with a fire polished Pasteur pipette. For trypsin digestion, 10X Trypsin-EDTA (Sigma-Aldrich, T4174) was added to the cell suspension at a final concentration of 0.25%Trypsin and incubated for 30 minutes at 37 °C with occasional gentle agitation. Tissue was monitored during this period and 3mg/ml DNAse1 (Sigma-Aldrich, DN25) added if tissue pieces appear to be connected by a stringy mass of DNA. After trypsinization, cells were centrifuged at 1000g for five minutes, solution was removed and replaced with neuronal plating media and triturated no more than ten times using sterile flame-polished glass pipettes. For either protease treatment, properly digested tissue should be fully dissociated after 10 triturations. Excessive trituration will reduce cell viability, as will introduction of air/bubbles during the trituration. Following dissociation, cells were counted using trypan blue (to determine cell viability) and a hemocytometer. Cell viability should be ≥ 90%.
2.5. Plating and maintenance of neuronal cultures
Cells were plated at a total density of 2×105 cells/35mm dish. For co-cultures, a ratio of 2 parts striatal to 3 parts cortical cells was used. One to three hours after plating the neuronal plating media was replaced with neuronal growth media (50× B27, 0.5mM glutamine, Neurobasal) that had been conditioned 24–48hrs on confluent glia cultures (see 2.7). Every seven days one half of the media was replaced with new glial conditioned neuronal growth media.
2.6 Plasmid DNAs
Three different types of plasmids were used to test DNA transduction methods: (1) pEGFP-C1 (Clontech) expresses enhanced green fluorescent protein (EGFP) under the control of the CMV promoter, (2) pCAG-EGFP (constructed in the Lanier lab) expresses EGFP under the control of the CMV immediate early enhancer and the chicken beta actin promoter (pCAG, Alexopoulou et al, 2008), (3) EGFP was cloned into pTRE-tight (Clontech) and co-transfected with pCAG-rtTA-advanced (produced by subcloning the rtTA-advanced tet transactivator from pTet-On-Advanced (Clontech) into pCAG). Additional details about plasmid construction are available on request.
2.7. Electroporations
For some experiments, striatal cells were electroporated using the Lonza Nucleofector system and the mouse neuron transfection reagent (cat. VAPG-1001). We have found that best results are obtained if the two parts of the transfection solution are mixed on the day of transfection (rather than mixed in advance as per the manufacturer’s instructions) and if the transfection kit is used within 3 months of purchase. For this reason, we buy the smaller kit (10 reactions) unless we plan to do more than 10 transfections in 2 months time. For transfection, 1×106 dissociated striatal cells were transferred to a microfuge tube and centrifuged at 1000g for 5 minutes, then plating media was removed and cells were gently resuspended in 100μl of complete transfection reagent containing 10–12μg of pCAG-EGFP or pEGFP-C1 or a mix of 10μg of pTRE-tight-EGFP and 17μg of pCAG-rtTA plasmid DNA per 1 million cells (keep total DNA volume < 10 μl). Following electroporation, pre-warmed plating media was added to the cuvette, cells were quickly transferred to 2ml of pre-warmed plating media and samples were taken for hemocytometer counting using trypan blue. Cell viability should be ≥90%. We have found that the inclusion of unelectroporated neurons at plating helps the survival and development of electroporated neurons. Therefore, in the co-culture condition, cortical cells were unelectroporated and the striatal cells were electroporated. In the mono-culture condition electroporated striatal cells are mixed 1:1 with unelectroporated striatal neurons at plating.
2.8. Glia Cultures
Glia cultures were prepared from cortices of postnatal (P1-2) mice with the striatal tissue removed. Minced tissue was incubated in CMF-HBSS plus 0.25% trypsin-EDTA and DNAse (either 3mg/ml DNAseI or 1μl/ml Benzonase (Novagen, 70664-3)) at 37°C for 30 minutes. After trypsinization, an equal volume of glia plating media (EMEM, 10mM HEPES, 1mM Sodium Pyruvate, 0.2mM glutamine, 10% NCS, 0.6% Glucose, 1× Penicillin-Streptomycin) was added to inhibit the trypsin and the tissue was collected by centrifugation (1000g for 2 minutes). Tissue was resuspended in glia plating media, mechanically dissociated using a flame-polished glass pipette and filtered through a 0.7μm cell strainer (BD Biosciences, 352350). Cells from one litter of pups were plated one pup per dish onto 10 cm tissue culture dishes (untreated, because coating with PDL does not increase the yield). Glia plating media was replaced one day after culturing and once per week each subsequent week. The first day after plating dishes tend to have a large amount of debris and significant cell division does not occur until approximately 3 days in vitro (div). After 7–10div plates have reached 70–100% confluence and are suitable for use in the preparation of conditioned media. These cultures will be >90% astroglia and appear as large, flat cells that can be somewhat difficult to view under phase contrast.
Alternatively, confluent dishes of glial cells can be frozen and stored in liquid nitrogen following standard protocols. Briefly, glia cultures are trypsinized, resuspended in an equal volume of glia plating media, centrifuged and resuspended in cold freezing media (MEM/20% NCS/10% DMSO). Glia should be aliquoted one 10 cm dish of cultured cells per freezing vial. Vials are transferred to a styrofoam container to slow freeze overnight at −80, and then transferred to liquid nitrogen. To thaw glia, agitate one vial of cells in a 37° water bath until the contents are just thawed (do not let the vial warm up). Immediately transfer the cell suspension to a tube containing 5 volumes of warm glia plating media, centrifuge and resuspend cells in warm glia plating media. Plate one vial of cells per 10 cm dish. If freezing and storage was done properly, >90% of the cells should survive. It should take about 2 weeks for the glia to reach confluence and be good for conditioning.
Glial-conditioned growth media (GCM) was prepared by removing the glia plating media and replacing with 7–10ml of neuronal growth media for 24–48 hrs. Once glial conditioning of neuronal growth media was complete, the GCM was removed and replaced with glia plating media. GCM is used within 24hrs after it is reclaimed from glial plates and stored in the 37°C/5% CO2 incubator when not in use. Glial plates are typically used only once per week for conditioning media and, if used to condition multiple times in one week, given at least 48hrs in glia plating media between conditioning sessions. Confluent glia plates can be maintained in this fashion for three or more months and are discarded if significant microglial proliferation is observed (microglia appear as bright, refractile cells, usually growing on top of the astroglia).
2.9. Immunofluorescence
At 14 and 21div cultures were processed for immunofluorescent imaging. Coverslips were fixed in 4% Paraformaldehyde/PHEM (60mM PIPES pH 7.0, 25mM K-HEPES pH 7.0, 10mM EGTA, 2mM MgCl2)/0.12M sucrose buffered fixative for 15–20 minutes at 4°C. Following fixation, cells were rinsed with Phosphate Buffered Saline (PBS) and blocked in 3% fatty acid free bovine serum albumin (BSA; Roche 03117057001) in PBS for 30 minutes at room temperature or overnight at 4°C. The use of a BSA blocking step before and after permeabilization enhances preservation of fine cell structures, especially cytoskeletal components. In addition, use of BSA, rather than serum, makes the blocking compatible with phospho-epitope specific antibody staining. Cells were permeabilized for 10 minutes at room temperature in 0.2% Triton X-100 in PBS after which coverslips were rinsed for 5 minutes in room temperature PBS. After permeabilization, cells were again blocked in 3% BSA in PBS for a minimum of 15 minutes at room temperature. Coverslips were incubated overnight at 4°C with 50μl of primary antibody mixture per slip in 1% BSA in PBS. The following primary antibodies were used: Rabbit anti-DARPP-32 1:250 (Cell Signaling, 2302), mouse anti-βIII Tubulin 1:2000 (Promega, G7121), mouse anti-SV2 1:100 (DSHB, SV2), and monoclonal mouse anti-PSD-95 1:100 (Chemicon, MAB1598). The protocol described above was used for most combinations of antibodies. The only exception was in staining using the PSD-95 antibody. For best exposure of this epitope, cultures were fixed as described above and then treated for 5 minutes with −20°C Methanol. Following overnight incubation at 4°C with primary antibody, coverslips were washed in room temperature PBS and incubated for 1 hour at room temperature with secondary antibody and phalloidin combinations in 1% BSA in PBS. The following secondary antibodies were used: donkey anti-rabbit and anti-mouse conjugated to AMCA, Texas Red, FITC, TRITC, or Cy5 (Jackson Immunoresearch), all used at 1:100 dilutions. Alexa-488 or -594 phalloidins (Molecular Probes) were diluted 1:200. Following the secondary incubation period, coverslips were washed for 5 minutes in room temperature PBS. For nuclear staining, coverslips were submerged in 1μg/ml DAPI in PBS for 30 seconds and rinsed in room temperature PBS for 5 minutes before mounting. Coverslips were mounted on glass slides with 2.5% 1,4-Diazabicyclo-[2.2.2]Octane, 150mM Tris pH 8.0, and 80% glycerol mountant to reduce photobleaching.
2.10. Imaging, Quantification, and Statistical Analysis
Images for Sholl analysis and antibody localization were collected using a Zeiss Axiovert 200M microscope and Openlab software (Improvision/Perkin Elmer). Image adjustments were made using Photoshop CS3 (Adobe) with brightness and intensity changes standardized for each experiment (all conditions). All image analysis was conducted using one or more plugin for ImageJ (NIH, http://rsbweb.nih.gov/ij/; detailed below). All statistical analysis was conducted using GraphPad Prism v4.0 (GraphPad Software).
For experiments calculating the percentage of DARPP-32 positive neurons, a minimum of 20 fields per coverslip was imaged at 10× magnification. Exposure times for each channel were standard across conditions. Multi-channel images of the same field were merged into a single “stacked” image in ImageJ and the cell-counter plug-in (http://rsbweb.nih.gov/ij/plugins/cell-counter.html) was used to count and label DAPI stained nuclei, βIII-Tubulin-positive neurons, and DARPP-32 staining. To be counted as DARPP-32 positive, complete soma staining and a visible staining in the dendrites was required. This inclusion criteria is relatively strict when compared to other reports that require detection of DARPP-32 only in the soma (e.g. Ivkovic and Ehrlich, 1999). The percentage of DARPP-32 positive cells per field was calculated and each field was treated as an independent observation. Replications from 2 independent cultures were pooled. Percentages were compared between time points using Mann-Whitney U to compare two groups of non-Gaussian distribution, p<0.05 was considered significant.
For experiments investigating the morphological complexity of DARPP-32 positive neurons, Sholl analysis was performed (Sholl, 1953). All isolated DARPP-32 positive neurons on the coverslip were imaged at 20× magnification using standard exposure time across conditions. The ImageJ concentric rings plugin (http://rsbweb.nih.gov/ij/plugins/concentric-circles.html) was used to place concentric rings every 10μm out to 150μm from the cell’s center. The cell-counter plugin was used to count and label processes (axons and dendrites) crossing each ring starting 20μm from the center of the cell. Replications from 3 independent cultures were pooled. Crossings were compared across the distance measured and between conditions using a two-way ANOVA with Bonferonni’s post-test to determine distances of significant difference, p<0.05 was considered significant.
For experiments determining spine density and morphology in striatal monoculture and striatal-cortical co-culture, EGFP positive, DARPP-32 positive neurons were imaged at 100× magnification using a standard exposure time across conditions. Images were collected using a Personal Deltavision Microscope and softWoRx software (Applied Precision). Terminal tips of isolated dendrites were identified and z-stack images were collected at 0.15–0.2μm intervals through the dendrite and the stacks were deconvolved using softWoRx software (Applied Precision). The deconvolved tiff files were imported into Neuronstudio (Mount Sinai School of Medicine, Rodriguez et al 2008) and subjected to semi-automated spine analysis. Densities calculated from individual segments of dendrite were treated as an independent observation and densities calculated from 3 independent culture preps were pooled. For dendritic spine morphology comparisons, a trained analyst, blind to condition, confirmed that spines identified by Neuronstudio met criteria for inclusion (observable neck connected to the dendrite shaft). Neuronstudio collects three parameters used to automatically qualify dendritic spines into morphological categories; spine head diameter (hd), spine neck length (nl), and spine neck diameter (nd). In step one, spines with a neck ratio (hd:nd) greater than 1.1 will be thin or mushroom and spines with a neck ratio less than 1.1 will be thin or stubby. For spines that have been identified as either thin or stubby, spines with a thin ratio (nl:hd) greater than 2.5 will be classified as thin and spines with a thin ratio of less than 2.5 will be classified as stubby. For spines that have been identified as either thin or mushroom, spines with head diameters greater than 0.35μm will be classified as mushroom, spines with head diameters less than 0.35μm are classified as stubby. Occasionally Neuronstudio identified spines but was unable to collect the spine neck diameter. In these cases spines were manually categorized based on the nl:hd ratio and head diameter. Spines were categorized as mushroom if they had a neck length to head diameter ratio of less than 1 and an absolute head diameter greater than 0.35μm (nl:hd <1, hd >0.35μm). Spines with a neck length to head diameter ratio of greater than 2.5 were classified as thin (nl:hd >2.5) and all other spines were classified as stubby (nl:hd >1, <2.5). Densities and morphological measurements were compared between conditions using Mann-Whitney U to compare two groups of non-Gaussian distribution, p<0.05 was considered significant. Measurements are reported in the results section and displayed in figures as mean ± SEM.
2.11. Electrophysiology
Coverslips were transferred to a recording chamber superfused with artificial cerebral spinofluid (ACSF) at 22–23°C saturated with 95% O2/5% CO2 and containing 119mM NaCl, 2.5mM KCl, 1.0mM NaH2PO4, 1.3mM MgSO4, 2.5mM CaCl2, 26.2mM NaHCO3, and 11mM glucose. Picrotoxin (100 mM) was added to block GABA-A receptor-mediated IPSCs (inhibitory post-synaptic currents). Cells were visualized using infrared-differential interference contrast (DIC) optics. MSNs were identified by their morphology (i.e. soma size, dendrite organization, dendritic spines) and hyperpolarized resting membrane potential. In some cultures, DIC observations were confirmed by EGFP expression (Fig. 5b, left panel)
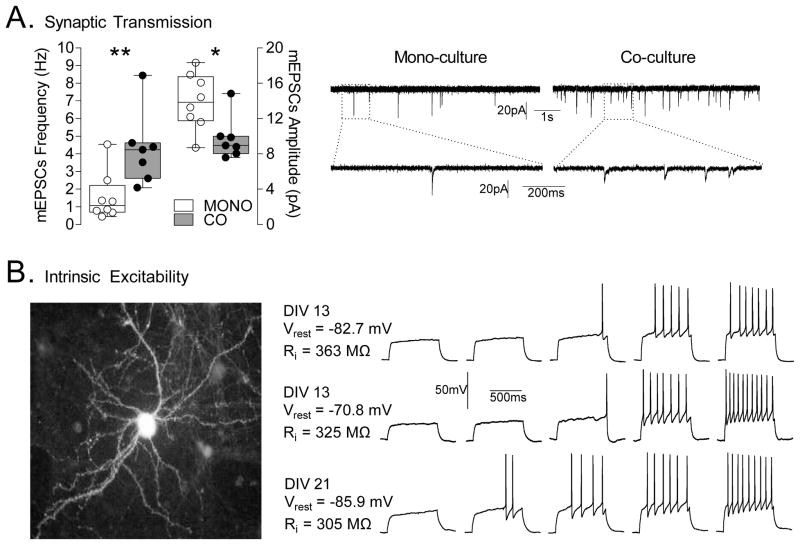
Figure 5.
Basic electrophysiological characterization of mono-and co-cultured MSNs. (A) Analysis of synaptic transmission. Left, co-cultured MSNs (CO, n = 7 cells) exhibit a significantly greater mEPSC frequency and lower amplitude compared to mono-cultured MSNs (MONO, n = 8 cells). Right, sample traces of mEPSCs from mono- and co-cultured MSNs. Calibration: 1 s, 20 pA and 200 ms, 20 pA. The lower traces on each side show an expanded sweep (1 s duration) of a portion of one of the upper traces. *p<0.05, **p<0.01. (B) Analysis of intrinsic excitability. Left, image of one of the recorded EGFP-expressing MSNs taken directly from the recording rig. Right, Sample traces from co-cultured MSNs at 13div (top, at 100, 120, 140, 180 and 200 pA; middle, at 80, 100, 120, 160 and 200 pA) and 21div (bottom, at 80, 100, 120, 140 and 160 pA). Calibration: 500 ms, 50 mV.
To assess excitatory synaptic transmission, neurons were voltage clamped at −70 mV using a Multiclamp 700A amplifier (Molecular Devices, Foster City, CA) and miniature EPSCs (excitatory post-synaptic currents) were collected in the presence of lidocaine hydrochloride (1mM). Electrodes (3–5 MΩ) contained 117mM cesium gluconate, 2.8mM NaCl, 20mM HEPES, 0.4mM EGTA, 5mM tetraethylammonium-Cl, 2mM MgATP, and 0.3mM MgGTP, pH 7.2–7.4 (285–295 mOsm). Series resistance (10–30 MΩ) and input resistance were monitored online with a 4 mV depolarizing step (100 ms) at 0.1 Hz. Data were low-pass filtered at 2 kHz, digitized at 10 kHz, and collected and analyzed using custom software (Igor Pro; Wavemetrics, Lake Oswego, OR). Quantal events were analyzed using Minianalysis software (Synaptosoft, Decatur, GA) and verified by eye.
To assess firing properties, kynurenic acid (2mM) was used to block glutamatergic transmission during recording. Whole-cell current-clamp recordings were performed with electrodes (3–5 MΩ) containing 120mM K-gluconate, 20mM KCl, 10mM HEPES, 0.2mM EGTA, 2mM MgCl2, 4mM Na2ATP, and 0.3mM Tris–GTP. Data were low-pass filtered at 5 kHz, digitized at 10 kHz, and collected and analyzed using custom software (Igor Pro; Wavemetrics). Membrane potentials were held at approximately −70 mV. Series resistances ranged from 10 to 18MΩ and input resistances (Ri) were monitored on-line with a +40pA, 150ms current injection given before every 800ms current injection stimulus. Firing was obtained through a series of hyperpolarizing and depolarizing current injections (800 ms duration at 0.1 Hz, −20 to +260pA range with a 20pA step increment). Resting membrane potentials were corrected for liquid junction potential (~ 14mV).
The mEPSC amplitudes and frequencies are presented as mean ± SEM in the results section and shown in a box-and-whisker plot in Fig. 5A. The box-and-whisker plot shows maximum, upper quartile, median, lower quartile and minimum. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software; La Jolla, CA). Statistical significance was assessed using two-tailed Student’s t tests, p<0.05 was considered significant.
3. Results
3.1. Optimized dissection protocol
A flow-chart detailing the optimized dissection protocol is presented in Figure 1.
3.2. Qualitative evaluation of reagents
Previous reports on the co-culture of striatal and cortical neurons have used different ages and species of animals, as well as different matrices, medias, and cell densities (Segal et al., 2003; Shen et al., 2007; Day et al., 2008; Gertler et al., 2008; Sun et al., 2008; Sun and Wolf, 2009; Tian et al., 2010). We sought to establish a streamlined procedure in which all tissue was collected from a single animal or litter at an embryonic stage compatible with other commonly used hippocampal and cortical culture techniques. In the process, many of the published parameters were tested and their affect on the survival and development of DARPP-32+ MSNs monitored over a month in culture (Summarized in Supplemental Table 1).
3.2.1. Source of tissue
The source of tissue is an important consideration because it can affect the yield of cells, the relative enrichment of particular types of cells, and the resiliency of neurons. Published striatal mono-culture methods have used a variety of pre- or postnatal stages, while the published co-culture methods have combined pre- and postnatal tissues, sometimes even using a combination of mouse and rat tissues. In an attempt to develop a streamlined method, several developmental stages were tested to determine if both cortical and striatal tissue could be harvested from the same animal. When early postnatal (P1 or P2 mouse) tissues were used, live cell yields were high at plating, but these postnatal cultures consistently showed poor adhesion and experienced survival issues, with the majority of neurons dying before a week in culture. When working with postnatal tissues, increases in plating density (up to five times higher than that used with embryonic tissue) appeared to improve survival, but cultures never appeared to be as robust as those derived from prenatal tissue. These problems were eliminated when using embryonic tissues. The use of embryonic tissues made it possible to harvest cortical and striatal tissues from the same animal and improved cell yields at plating, allowing for more coverslips to be generated from a single litter and for single pups to generate sufficient tissue to be plated in isolation. Finally, the use of embryonic day 16 (E16) tissue made the protocol compatible with currently used hippocampal culture techniques, allowing for multiple types of cultures to be generated from single litters.
3.2.2. Matrix
The type of matrix used to support neurons in culture can have significant effects on adherence, survival, and rate of development. Therefore, several matrices were tested for their effect on embryonic tissues. Matrigel (1:50 in plating media, BD Biosciences 354234) allowed for adherence and survival of striatal and cortical neurons, but morphological development was no better than on PDL/laminin and the thick nature of Matrigel made imaging studies more difficult. In addition, the undefined nature of Matrigel would preclude future studies in the role of matrix on MSN development. Laminin or Poly-D-Lysine alone gave poor adherence and growth. In contrast, high levels of adherence, survival, and development were achieved with a mixture of 4μg/ml laminin and 100μg/ml poly-D-lysine, with the additional improvement that cells developed in a near mono-layer, allowing for easier imaging of dendritic arbors. Increasing laminin concentrations (from 4μg/ml to 20μg/ml) did not improve adherence, survival, or development above the lower concentration.
3.2.3. Digestion Solution
In the absence of protease treatment, mechanical dissociation of brain tissue caused significant cell death. Therefore, the efficacies of papain and trypsin digestion were compared. For postnatal tissue, papain digestion was more effective and yielded higher viability than trypsin. For embryonic tissues, papain and trypsin gave identical results, both in terms of cell viability immediately after dissociation and subsequent long-term survival and differentiation (data not shown). Because of its significantly lower cost, ease of preparation and routine usage in other types of cell culture, trypsin dissociation became the method of choice.
3.2.4. Glial conditioning and growth factors
The embryonic cortex and striatum have a high density of glia cells that can proliferate in culture. These glia cells secrete factors that enhance neuronal survival and development, including Brain Derived Neurotrophic Factor (BDNF), which has been shown to promote survival and development of striatal neurons in culture (Mizuno et al., 1994; Widmer and Hefti, 1994; Nakao et al., 1995; Ventimiglia et al., 1995; Ivkovic and Ehrlich, 1999). Despite the presence of some glia in both the mono and co-culture conditions, routine feeding of glia-conditioned media (GCM) improved the survival and development of MSNs when compared to unconditioned neuronal growth media. Inhibition of glial proliferation with anti-mitotic agents had a negative impact on MSN survival and BDNF application did not improve the survival or development of MSNs above the level produced by GCM (see Supplemental Fig. 1)
3.2.5. DNA transduction
The ability to transduce MSNs with exogenous DNA will be critical for future experiments investigating the development and plasticity of MSN dendritic spines. Several transduction methods were tested in the co-culture condition, with the goal of finding a method that efficiently transduced MSNs. Electroporation of plasmid DNA using the Lonza/Amaxa system prior to plating transduced approximately 10–20% of MSNs in culture with no detectable effect on survival or differentiation. Furthermore, using plasmids with a hybrid CMV early enhancer/chicken beta-actin promoter (pCAG-EGFP), expression was maintained for more than 21 days. In contrast, expression from pEGFP-C1 (CMV promoter) decreased markedly during the first week in culture and was barely detectable by 21 div (data not shown). In addition to successfully transducing a constitutively active plasmid, we have had success using a two-plasmid, tet-ON system to allow temporally restricted expression of EGFP. When using the tet-ON system, the transactivator plasmid (driven by the constitutively active CAG promoter) and a plasmid containing the tet-response element (expressing soluble EGFP) were simultaneously electroporated (see section 2.6). Expression of EGFP was induced by maintaining cultures in GCM containing 1μg/ml doxycycline and reached maximal levels after about 4–7 days. Expression could be maintained following induction for more than 21 days (e.g. Figure 3A).
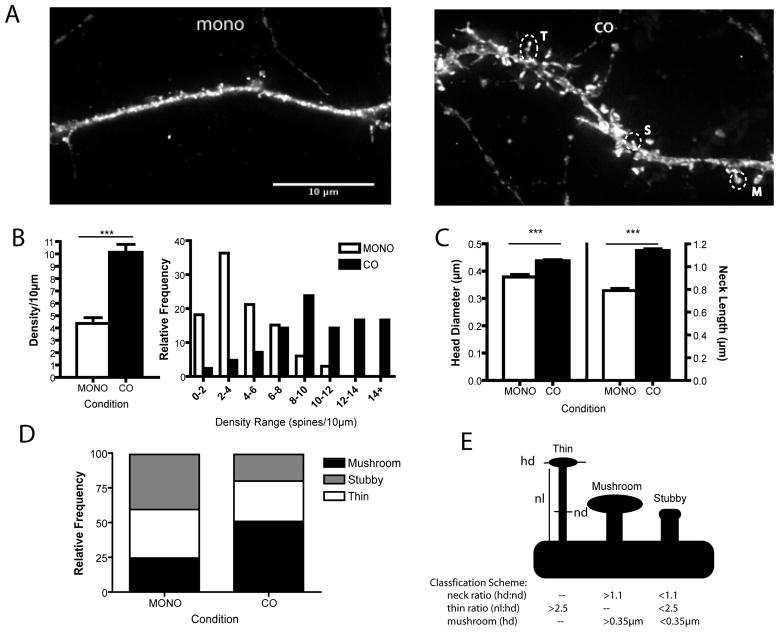
Figure 3.
Dendritic spine density and morphology comparison between culture conditions. Cultures were co-electroporated with pCAG-rtTA and pTRE-tight-EGFP before plating and expression was induced by addition of 1μg/ml Doxycycline at 14div. (A) Representative images of 21div EGFP expressing MSN dendrites in mono-culture (left) and co-culture (right). Overlayed circles indicate spines of the different morphological types (T: thin, S: stubby, M: mushroom). Scale bar = 10μm. (B) There were significantly more spines in the co-culture condition and co-cultured MSNs had relatively higher frequencies of high densities. (C) Co-cultured MSNs have significantly larger spine heads and longer spine necks. For (B-D) means are displayed ± SEM, ***= P<0.001. (D) Relative frequencies of thin, stubby and mushroom type spines. (E) Schematic of morphology types as assessed by Neuronstudio, displaying measurements (hd: head diameter, nl: neck length, nd: neck diameter) and criteria for inclusion used by Neuronstudio.
In contrast to electroporation, calcium phosphate transfection, although effective at transducing cortical pyramidal neurons on the coverslip, proved inappropriate for use in the MSN co-cultures. Calcium phosphate simply failed to transfect MSNs at any time point (≪1% transfection or 1–2 MSNs per coverslip). In addition, both adenovirus and adeno-associated viruses 5 (AAV5), yielded high levels of glia infection that obscured the visualization of infected MSNs. Initial tests with Adeno-associated virus 2 (AAV2) was efficacious at transducing the pyramidal neurons but failed to transduce significant numbers of MSNs. Tests are currently underway to determine if another AAV serotype might be more efficacious for MSN transduction.
3.3. Effect of culture condition on the development of DARPP-32+ expressing MSNs
The survival and development of MSNs were compared between the co-culture and mono-culture conditions in order to determine when the number of DARPP-32 expressing MSNs was maximal and dendritic arborization and spine density had reached mature levels. In both culture conditions, DARPP-32 expression was first detectable at 9div, but cells were still morphologically immature (data not shown). By 14div, DARPP-32 expression had increased and was easily detectable in morphologically complex neurons. Using βIII Tubulin to identify neurons and DAPI to identify all cellular nuclei, the percentage of neurons in the culture was analyzed (Table 1). At 14div there was no significant difference in the percentage of neurons between culture conditions. However, when DARPP-32 antibody staining was used to specifically identify MSNs, it was revealed that there was a significantly higher percentage of neurons that were MSNs in the co-culture condition (6.08% co vs. 3.08% mono, n = 100, p<.001 two-tailed). After 21div, the percentage of total cells that were neurons was significantly lower in the co-culture compared to the mono-culture (39.04% co vs. 56.05% mono, n = 100, p<.001 two-tailed), likely due to the proliferation of glia in the co-culture condition. In contrast, at 21div the percentage of total cells that were MSNs was not significantly different between culture conditions (1.94% co vs. 2.39% mono, n = 100, p=0.1590 two-tailed). In the co-cultures, the proliferation of the glia, rather than the dying of MSNs, likely accounts for the apparent decrease from 14 to 21 div in the percentage of total cells that were MSNs. This percent of MSNs in the co-culture is consistent with published estimates of the number of MSNs in the developing striatum (see discussion).
Table 1.
Effect of culture condition of the yield of MSNs
| % neuron of total cells | % MSN of total neuron | % MSN of total cells | Cells/mm2 a | Neurons/mm2 | MSN/mm2 | ||
|---|---|---|---|---|---|---|---|
| MONO | 14div | 51.33 | 3.08 | 1.67 | 228.60 | 186.91 | 4.03 |
| 21div | 56.05 | 3.58 | 2.39 | 229.97 | 115.59 | 3.36 | |
|
| |||||||
| CO | 14div | 53.93 | 6.08 | 3.13 | 143.41 | 113.18 | 5.52 |
| 21div | 39.04 | 3.88 | 1.94 | 255.67 | 82.56 | 3.47d | |
|
| |||||||
| Published Reports | Adult Striatum | 20b | ~99b | ~20% | - | - | - |
| Embryonic LGE | ~55c | ~23c | ~12.7 | - | - | - | |
density at plating = ~200 cells/mm2
Schröder et al. J Hirnforsch (1975) vol. 16 (4) pp. 333–50
Skogh et al. Neuroscience (2003) vol. 120 (2) pp. 379–85
equivalent to ~ 329 MSNs on a 12mm coverslip.
3.4. Morphological development of MSNs in different culture conditions
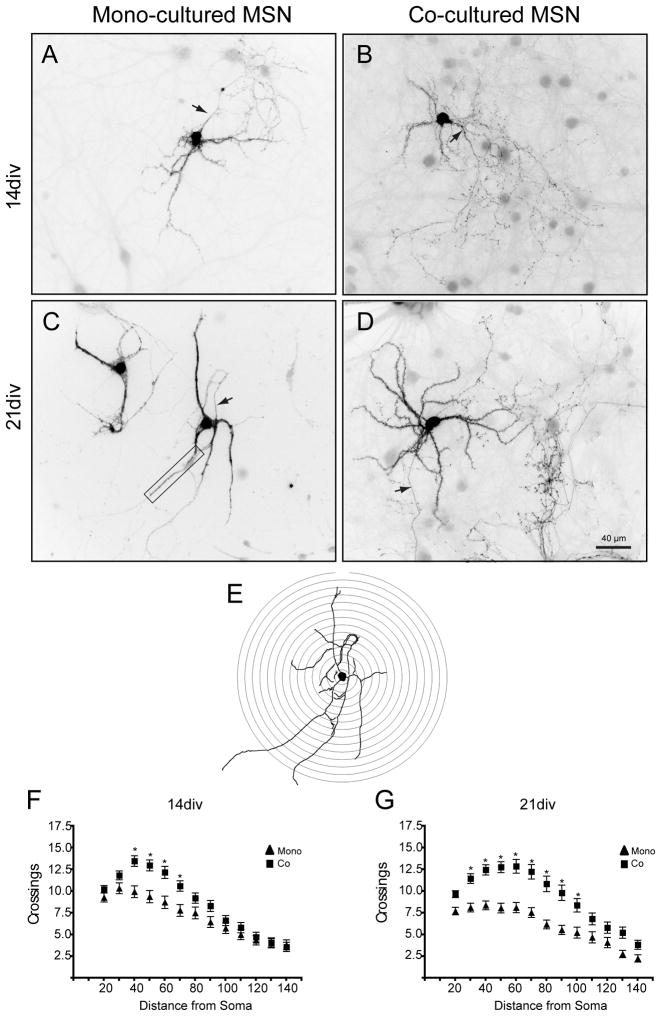
Culture condition can affect the rate and complexity of morphological development. To assess the complexity of MSN processes, a Sholl analysis (Fig. 2E) was performed on DARPP-32+ neurons from both culture conditions. At 14div, morphological complexity was greater in the co-culture condition, with significantly more crossings at 40–70μm from the cell body (Fig. 2A, B, F; bonferroni post-hocs allowed paired comparisons, p<0.05). The increased complexity in the co-culture condition was maintained at 21div, with significantly more crossings at 30–100μm from the cell body (Fig. 2C, D, G). Although the co-cultures showed increased complexity of the more distal dendrites from 14 to 21div, there were no significant differences in complexity between time-points within a given condition. The apparent decrease in complexity in the mono-culture from 14 to 21div was due, at least in part, to increased fasciculation in mono-cultured MSN dendrites (for example, see Fig. 2C). As mono-cultured MSNs developed, their dendrites tended to bundle together, making it difficult to resolve single dendrites and their associated spines. In the co-culture condition MSN dendrites remained spatially separated from one another.
Figure 2.
Comparison of MSN complexity between culture conditions. (A–D) Sample images of DARPP-32+ staining of MSNs in mono- and co-culture. In these images, the fluorescent signal has been inverted and contrast enhanced to increase visibility. Arrows point to the axon. Under the conditions used for Sholl analysis, only the part of the axon closest to the cell body is general visible and it thus contributes minimally to the counts. (A) 14div mono-cultured MSN, (B) 14div co-cultured MSN, (C) 21div monocultured MSN (boxed area shows a region of fasiculation), (D) 21div co-cultured MSN. Arbor complexity was assessed using Sholl analysis. (E) The diagram illustrates concentric rings overlaid on a tracing of the neuron in (C). Crossings were quantified at (F) 14div and (G) 21div. Co-cultured MSNs were more complex than mono-cultured MSNs at both time points (Significant main effect of condition, distance, and significant interaction in Two-Way ANOVA; bonferroni post-hocs allowed paired comparison, * = p<0.05).
3.5. Quantification of dendritic spines in different culture conditions
Medium spiny neurons are so named due to the high density of spines that stud their dendritic arbors. To assess the density of dendritic spines, EGFP filled, DARPP-32+ MSNs were examined at 21div, a time point where morphological maturation was maximal and DARPP-32 expression levels were high. At this time point, there was a significantly higher density of dendritic spines in the co-culture condition (10.12 ± 0.7 spines/10μm co vs. 4.36 ± 0.5 spines/10μm mono, nco = 42 segments, nmono = 33 segments, p<.0001, two-tailed). This significant difference in spine densities can be seen as an increase in the relative frequency of spine densities greater than 10 spines/10μm in the co-culture conditions (Fig. 3C).
In addition to an increased density of dendritic spines produced by co-culturing, the morphology of spines was significantly different between conditions. Morphometric analyses showed that spine head diameter was significantly larger in co-cultured MSNs (Fig. 3D, 0.44 ± 0.004μm co vs. 0.38 ± 0.008μm mono, nco = 42 segments, nmono = 33 segments, p<.0001, two-tailed). In addition to larger heads, co-cultured MSN dendritic spines had significantly longer spine necks than mono-cultured MSN spines (Fig. 3E, 1.143 ± 0.01μm co vs. 0.79 ± 0.02μm mono, nco = 42 segments, nmono = 33 segments, p<.0001, two-tailed). Spines can be categorized based on their morphologies into varied classifications including mushroom, thin, and stubby. In the mono-culture condition approximately 25% of spines had mushroom-like morphologies (neck length to head diameter less than one and head diameter greater than 0.35μm), nearly 35% of spines had thin-like morphologies (neck length to head diameter greater than 2.5), and the remaining 40% of spines had a stubby-like morphology. The co-culture condition had a greater percentage of spines with mushroom-like morphologies (52%) and smaller proportions of thin (28%) and stubby (20%) morphologies (Fig. 3D).
3.6. Immunochemical analysis of dendritic spines
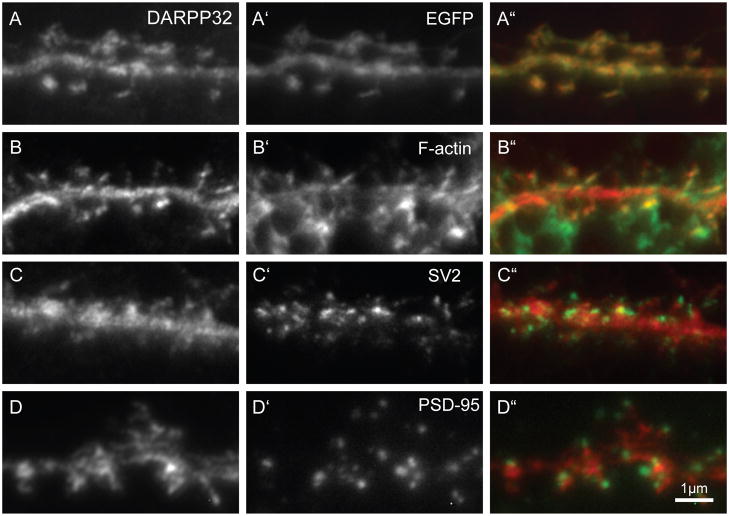
In order for dendritic spines to receive and respond to stimulation they must have intact pre- and post-synaptic machinery. Because co-cultured MSNs were significantly more developed than those in mono-cultures, immunocytochemical analysis of dendritic spines was restricted to 21div co-cultures. In the co-cultures, DARPP-32 was detected in all of the dendritic spines, where it appeared to partially co-localize with EGFP (Fig. 4A). Spine heads were filled with filamentous actin (f-actin; Fig. 4B), were apposed to SV2 containing pre-synaptic compartment (Fig. 4C) and contained a PSD-95 rich post-synaptic density (Fig. 4D), indicative of the presence of functional excitatory synapses. It should be noted that although DARPP-32 co-localized with EGFP, DARPP32 only partially overlapped with PSD-95. Whether this is an artifact of the methanol treatment used for the PSD-95 staining or reflects the underlying biology remains to be determined.
Figure 4.
Localization of pre- and -postsynaptic markers in DARPP32+ co-cultured MSNs. Left most column (A–D) shows DARPP32 staining. (A-A″) DARPP-32 antibody staining fills dendritic shafts and spines and appears to colocalize with EGFP (A′, expressed from pCAG-EGFP). The DARPP32+ dendritic spines have (B′) actin-rich heads and are associated with (C′) pre-synaptic SV2 and (D′) post- synaptic PSD95 puncta, indicating a morphologically mature synapse. The right column shows a merge of the left and middle panels with DARPP32 staining in red and (A″) EGFP, (B″) F-actin, (C″) SV2 or (D″) PSD95 in green. Scale bar = 1μm.
3.7. Electrophysiological characteristics of MSNs in co- and mono-culture conditions
The presence of PSD-95 and SV2 puncta indicate that dendritic spines of co-cultured MSNs could be functional. In order to compare the synaptic function of mono- and co-cultured MSNs, whole-cell patch clamp recordings were conducted on 19–24div cultures (Fig. 5A). MSNs were identified by morphology (i.e. soma size, dendrite organization) as well as by their hyperpolarized resting membrane potential. Specifically, compared to interneurons (GABA and ACh interneurons), which exhibit a depolarized resting potential (Vrest, around −50/−60 mV), MSNs exhibit a hyperpolarized Vrest (around −80 mV). However, another type of striatal neurons, fast spiking interneurons (FSNs), exhibit a relatively hyperpolarized Vrest (around −74 mV), which might lead to potential confusion with MSNs. Nonetheless, FSNs represent less than 5% of total number of striatal neurons in the adult striatum and possess a morphology (number of dendritic branches and dendritic architecture) distinguishable from MSNs (Kreitzer, 2009; Govindaiah et al., 2010), which make the level of possible contamination extremely low. Co-cultured MSNs had significantly higher mEPSC frequency (4.28 ± 0.48 Hz co vs. 1.56 ± 0.48 Hz mono, t13 = 3.05, p < 0.01), consistent with the higher numbers of functional dendritic spines in co-cultures. Unexpectedly, mono-cultured MSNs exhibited higher mEPSC amplitude compared to co-cultured MSNs (9.73 ± 0.92 pA co vs. 13.95 ± 1.11 pA mono, t13 = 2.87, p < 0.05). Due to different experimental conditions between brain slices and cultured neurons, it is difficult to compare electrophysiological results obtained in these two preparations. However, it is noteworthy to mention that the level of mEPSC amplitude and frequency in co-cultured conditions are comparable to those observed in in vitro sagittal striatal slice recordings (in which glutamatergic fibers from the PFC are left intact) made in the presence of lidocaine to isolate responses that reflect only activity-independent glutamate release (e.g. Kourrich et al., 2007).
Overall neuronal excitability, and thus neuronal function, depends on both synaptic and intrinsic factors (i.e. voltage-gated ion channel conductances). To test if characteristics of intrinsic excitability have properly developed in our co-culture model, we assessed firing properties of co-cultured MSNs. Recordings showed firing characteristics typical of MSNs (Fig. 5B) and comparable to those recorded in vivo (O’Donnell and Grace, 1995) and in mature brain slices (Nisenbaum et al., 1994; Kreitzer, 2009; Kasanetz and Manzoni, 2009; Kourrich and Thomas, 2009). Specifically, co-cultured MSNs displayed a hyperpolarized resting membrane potential, a slowly depolarizing ramp when depolarized close to or slightly above firing threshold, regular spiking and a delayed firing latency during incremental depolarizing steps (Kita et al., 1985; Bargas et al., 1989; Nisenbaum et al., 1994; Kawaguchi, 1997; Belleau and Warren, 2000).
4. Discussion
After testing myriad conditions, it was determined that optimal MSN development and survival were obtained when MSNs from the embryonic striatum were co-cultured with embryonic cortical neurons, plated on a mixture of poly-D-Lysine and laminin and grown in glial-conditioned Neurobasal/B27 media. This culture system takes advantage of the high viability of neurons in the embryonic striatum, enabling plating of large numbers of coverslips from a single litter. Additionally, the technique described in this paper can be adapted for use with transgenic animals, with the cells from single pups contributing to several dishes of coverslips.
MSNs in co-culture appeared to be quite refractory to most methods of DNA transduction, such that only electroporation of the striatal cell suspension immediately prior to plating reliably transduced a significant number of MSNs. Fortunately, using a strong CAG promoter, it was possible to maintain high levels of gene expression for >21 days, with no effect on cell health. By comparison, expression from the CMV promoter gradually decreased and was generally lost by 14div (data not shown). Using electroporation, it was also possible to co-transfect a tet-on expression plasmid with a tet-transactivator expressed from a CAG promoter and induce the tet promoter expression of EGFP at any time from 3–21div (e.g. Fig 3A). Robust levels of expression were achieved within 72 hours of induction with 1μg/ml doxycyclin and could be maintained for at least 21 days with weekly feeding of fresh doxycyclin-free glial conditioned media. Thus, both constitutive and inducible gene expression can be obtained in the co-culture system.
Interestingly, development of the MSNs in co- or mono-culture appeared to require the physical presence of glia in the dish; addition of anti-mitotic agents to limit glial expansion affected both the development and survival of MSNs and the requirement for glia could not be substituted for with glial-conditioned media. Despite the presence of glia in the in culture dish, glial-conditioned media improved survival and development of MSNs beyond what is seen in dishes maintained in unconditioned media. It would appear that both physical contact with the glia in the dish and secreted factors provided by the glial-conditioned media are supporting enhanced survival and development. The most likely explanation for the need for GCM is that during early development in vitro the number of glial cells in the culture dish is not sufficient to provide the secreted factors necessary to support the MSNs. This conclusion is in agreement with previous reports indicating that the presence of a small number of proliferating astrocytes enhances the survival and differentiation of striatal neurons in culture (Sebben et al., 1990; Skogh and Campbell, 2003).
In vivo, the number of DARPP-32+ cells increases significantly during the first few weeks of postnatal development (Foster et al, 1987). Our finding that the number of DARP32+ MSNs did not increase from 14–21 div (roughly corresponding to postnatal days 10–17) suggests that dissociation of the tissue disrupted signaling events that are critical to MSN differentiation and/or DARPP-32 expression (Jain et al, 2001). Although is has been reported that inclusion of BDNF in E17 striatal mono-culture increases the number of DARPP32+ cells (Ivkovic and Ehrlich, 1999), we found no such effect in the co-culture system.
At first glance, the fact that only ~2% of the cells in the co-culture were MSNs might seem unexpectedly low given the often quoted fact that in the adult striatum, ≥95% of the neurons are MSNs (Table 1); however, it is important to remember that due to the abundance of glia cells, only 20–55% of the total cells in the adult striatum are in fact MSNs (the value depends on whether the analysis is limited to the caudate (Kataoka et al., 2010) or includes the entire striatum (Schroder et al., 1975) and likely varies between species). In the co-culture, the entire ganglionic eminence (GE) was dissected as a source of MSNs, but the majority of DARPP32+ cells are found in the lateral ganglionic eminence (LGE; (Deacon et al., 1994)). It has been estimated that the LGE comprises about 50% of the total GE and that ~12.7% of LGE cells are DARPP32+ (Skogh et al., 2003). Since only 40% of the cells in the co-culture came from the ganglionic eminence, at plating only ~2.5% of cells should be MSNs (2.5%= 50% of GE is LGE × 12.7% of LGE cells are MSNs × 40% of plated cells are from GE). This yield of MSNs is similar to that reported for other embryonic striatal culture systems (Watts et al., 1997). Thus, the current yield of MSNs suggests that maximal survival of MSNs has been achieved in the co-culture.
Although mono-culture is capable of supporting the survival of MSNs, our data indicate that MSNs in the mono-culture condition were less morphologically complex than those in the co-culture condition. The fasciculation of dendrites in the monoculture condition could complicate further experiments designed to investigate the development and plasticity of dendritic spines by limiting the sampling area to only the terminal tips of dendrites. MSNs grown in co-culture had 10.12 ± 0.7 spines/10μm, a value remarkably similar to that reported for in vivo analysis of mature MSNs in adult mouse and hamster striatum (Staffend and Meisel, 2011; Jedynak et al, 2007; Neely et al, 2007). In addition to having a significantly greater density of dendritic spines, spines in the co-culture condition were morphologically more like MSNs in vivo. Spines on co-cultured MSNs had larger heads and longer necks than those on mono-cultured MSNs. This difference in spine morphologies was reflected as a change in the relative proportion of different spine-types. In mono-cultured MSNs, only about a quarter of spines had mushroom-like morphologies, contrasted with more than half of all spines being this type in co-culture. Importantly, using similar morphometric classification, MSNs in adult mouse striatum were shown to have similar ratios of mushroom, thin and stubby spines (Jedynak et al 2007).
The presence of any spines or excitatory post-synaptic currents in the monocultured MSNs is likely still dependent on cortical innervation. Given the method of collection of the striatal neurons (removal of the eminence from over-lying cortex) it is likely that there is contamination by cortical pyramidal neurons in the mono-culture and that the variable presence of this glutamatergic input contributes to the development of spines in mono-culture. Whether the morphology of dendritic spines in the monoculture represent an immature morphology or a compensatory change due to decreased afferent activity is beyond the scope of the current study.
Electrophysiological characterization of co-cultured MSNs demonstrated that the basic synaptic and intrinsic properties are comparable to those observed in mature MSNs recorded in slice and in in vivo preparations (O’Donnell and Grace, 1995; Belleau and Warren, 2000; Kasanetz and Manzoni, 2009). These results suggest that the present co-culture method provides appropriate conditions for supporting the development of mature, in vivo-like MSNs. Previous research indicated that monocultured MSNs grown in Neurobasal/B27 media fail to develop mature firing properties, but that MSNs grown in a serum containing DMEM media are able to develop mature firing properties (Rubini et al., 2006). In the current co-culture system, MSNs grown in Neurobasal/B27 media do develop mature firing properties, suggesting that in previous experiments factors other than the media were allowing for mature intrinsic property development. Since the inclusion of serum in the media will enhance glial survival and proliferation (Noble and Mayer-Pröschel, 1998), it is possible that the reported effect of media may have been due to the influence of glia on MSN development.
Despite to obvious differences in the two preparations, it is striking that mEPSC frequency and amplitude measured in co-cultured MSNs were similar to those observed in slices in which the glutamatergic fibers are left intact (e.g. Kourrich et al., 2007). Unexpectedly, mono-cultured MSNs exhibited higher mEPSCs amplitude when compared to co-cultured MSNs. This increased mEPSC amplitude in mono-cultures may reflect a developmental homeostatic adaptation, such as synaptic scaling (Turrigiano et al., 1998; Turrigiano, 2008), intended to compensate for the low level of excitatory afferents produced by low levels of cortical contamination in the mono-culture condition. Previous research has shown that reductions in network activity, produced by changes in excitatory input density (Ivenshitz and Segal, 2010) or chronic blockade with TTX (Segal et al., 2003; Fishbein and Segal, 2011), produce significant increases in mEPSC amplitude. Previous reports have demonstrated a correlation between increased AMPAR insertion and increased mEPSC amplitude (Matsuzaki et al 2004); however, given that MSNs in mono-culture have smaller spine head diameters than those in co-culture, it seems unlikely that the increased mEPSC amplitude seen monocultured MSN is due entirely to increased AMPR insertion. This may suggest there is a mechanism other than AMPA receptor insertion for increasing mEPSC amplitude in mono-cultured MSNs or that the correlation between AMPA receptor complement and spine head size is not as tightly coupled in MSNs in vitro.
Alternatively, it is possible that, rather than a change in post-synaptic strength, the enhanced mEPSC amplitude seen in the mono-cultured MSNs is related to the significant reduction in spine neck length. Previous research has demonstrated that the spine neck serves as a barrier to the diffusion of calcium produced by depolarization of the spine head (e.g. Schmidt and Eilers, 2009) and that there is an inverse correlation between neck length and depolarization at the soma (e.g. Araya et al., 2006). Finally, it is quite possible that a combination of synaptic scaling and changes in calcium diffusion could contribute to increasing the amplitude of mEPCSs in mono-cultured MSNs.
In summary, we describe and characterize a simple, streamlined culture system capable of supporting the development and maturation of in vivo-like MSNs, complete with high densities of dendritic spines. These MSNs survive for more than a month in vitro and display electrophysiological and morphological characteristics consistent with mature MSNs. Future research in this system can be used to address mechanisms of MSN dendritic spine development and plasticity.
Supplementary Material
Research highlights.
Use embryonic striatal and cortical tissue to culture Medium Spiny Neurons (MSNs)
Cultures can be made from a single embryo - good for studies with transgenics
Electroporated MSNs can retain expression for more than 21 days
Produces MSNs with complex dendritic morphology and abundant dendritic spines
MSNs have in vivo-like morphological and electrophysiological characteristics
Acknowledgments
We would like to thank Kacey Rajkovich and Arman Cicic for their efforts on data analysis and Teresa Nick for careful review of this manuscript. This project was supported by grants T32 GM008471, NIDA training grant T32 DA007234 and the Academic Health Center of the University of Minnesota (for RP), R01DA019666 (to MT) and R01NS049178 (to LML). The funding agencies played no role in the design of the study or the writing of the manuscript. The mouse anti-SV2 monoclonal was developed by K.M. Buckley and obtained from the Developmental Studies Hybridoma Bank under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Appendix A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulou AN, Couchman JR, Whiteford JR. The CMV early enhancer/chick beta actin (CAG) promoter can be used to drive transgene expression during the differentiation of murine embryonic stem cells into vascular progenitors. BMC Cell Biology. 2008;9:2–11. doi: 10.1186/1471-2121-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. PNAS. 2006;103:17961–66. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargas J, Galarraga E, Aceves J. An early outward conductance modulates the firing latency and frequency of neostriatal neurons of the rat brain. Experimental brain research Experimentelle Hirnforschung Expérimentation cérébrale. 1989;75:146–56. doi: 10.1007/BF00248538. [DOI] [PubMed] [Google Scholar]
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol. 2000;84:2204–16. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–9. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–14. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW, Pakzaban P, Isacson O. The lateral ganglionic eminence is the origin of cells committed to striatal phenotypes: neural transplantation and developmental evidence. Brain Res. 1994;668:211–9. doi: 10.1016/0006-8993(94)90526-6. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat Disord. 2007;13 (Suppl 3):S251–8. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Segal M. Active cortical innervation protects striatal neurons from slow degeneration in culture. J Neural Trans. 2011;118:445–51. doi: 10.1007/s00702-010-0505-5. [DOI] [PubMed] [Google Scholar]
- Foster GA, Schultzberg M, Hökfelt T, Goldstein M, Hemmings HC, Ouimet CC, Walaas SI, Greengard P. cyclic adenosine 3“:5-”monophosphate-regulated phosphoprotein (DARPP-32) in the prenatal rat central nervous system, and its relationship to the arrival of presumptive dopaminergic innervation. J Neurosci. 1987;7:1994–2018. doi: 10.1523/JNEUROSCI.07-07-01994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–24. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Wang Y, Cox CL. Substance P selectively modulates GABA(A) receptor-mediated synaptic transmission in striatal cholinergic interneurons. Neuropharmacology. 2010;58:413–22. doi: 10.1016/j.neuropharm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–11. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Ehrlich ME, Trivedi P, Greengard P. Developmental regulation of phosphoprotein gene expression in the caudate-putamen of rat: an in situ hybridization study. Neuroscience. 1992;51:65–75. doi: 10.1016/0306-4522(92)90471-d. [DOI] [PubMed] [Google Scholar]
- Ivenshitz M, Segal M. Neuronal density determines network connectivty and spontaneous activity in cultured hippocampus. J Neurophysiol. 2010;104:1052–1060. doi: 10.1152/jn.00914.2009. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Ehrlich ME. Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J Neurosci. 1999;19:5409–19. doi: 10.1523/JNEUROSCI.19-13-05409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Armstrong RJ, Barker RA, Rosser AE. Cellular and molecular aspects of striatal development. Brain Research Bulletin. 2001;55:533–40. doi: 10.1016/s0361-9230(01)00555-x. [DOI] [PubMed] [Google Scholar]
- Jedynak J, Uslaner J, Esteban J, Robinson T. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–53. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Manzoni OJ. Maturation of excitatory synaptic transmission of the rat nucleus accumbens from juvenile to adult. J Neurophysiol. 2009;101:2516–27. doi: 10.1152/jn.91039.2008. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–91. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Kemp JM. Observations on the caudate nucleus of the cat impregnated with the Golgi method. Brain Res. 1968;11:467–70. doi: 10.1016/0006-8993(68)90043-7. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond, B, Biol Sci. 1971;262:429–39. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- Kita H, Kita T, Kitai ST. Active membrane properties of rat neostriatal neurons in an in vitro slice preparation. Experimental brain research Experimentelle Hirnforschung Expérimentation cérébrale. 1985;60:54–62. doi: 10.1007/BF00237018. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–8. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–83. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer A. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–47. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau J-M, Valjent E, Hervé D, Girault J-A. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS ONE. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies G, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–92. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–56. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Nakao N, Brundin P, Funa K, Lindvall O, Odin P. Trophic and protective actions of brain-derived neurotrophic factor on striatal DARPP-32-containing neurons in vitro. Brain Res Dev Brain Res. 1995;90:92–101. doi: 10.1016/0165-3806(96)83489-4. [DOI] [PubMed] [Google Scholar]
- Nakao N, Odin P, Brundin P. Selective sub-dissection of the striatal primordium fr cultures affects the yield of DARPP-32 containing neurons. Neuroreport. 1994;5:1081–84. doi: 10.1097/00001756-199405000-00015. [DOI] [PubMed] [Google Scholar]
- Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spine neurons. Neuroscience. 2007;149:457–464. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Xu ZC, Wilson CJ. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J Neurophysiol. 1994;71:1174–89. doi: 10.1152/jn.1994.71.3.1174. [DOI] [PubMed] [Google Scholar]
- Noble M, Mayer-Pröschel M. Culture of astrocytes, oligodendrocytes, and O-2A progenitor cells. Culturing Nerve Cells. 1998:499–543. [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafols JA, Cheng HW, McNeill TH. Golgi study of the mouse striatum: age-related dendritic changes in different neuronal populations. J Comp Neurol. 1989;279:212–27. doi: 10.1002/cne.902790205. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 (Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS ONE. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubini P, Pinkwart C, Franke H, Gerevich Z, Norenberg W, Illes P. Regulation of intracellular Ca2+ by P2Y1 receptors may depend on the developmental stage of cultured rat striatal neurons. J Cell Physiol. 2006;209:81–93. doi: 10.1002/jcp.20705. [DOI] [PubMed] [Google Scholar]
- Schroder KF, Hopf A, Lange H, Thorner G. Morphometrical-statistical structure analysis of human striatum, pallidum and subthalamic nucleus. J Hirnforsch. 1975;16:333–50. [PubMed] [Google Scholar]
- Schmidt H, Eilers J. Spine neck geometry determines spino-dendritic cross-talk in the presence of mobile endogenous calcium binding proteins. J Comput Neurosci. 2009;27:229–43. doi: 10.1007/s10827-009-0139-5. [DOI] [PubMed] [Google Scholar]
- Schubert V, Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–12. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- Sebben M, Gabrion J, Manzoni O, Sladeczek F, Gril C, Bockaert J, Dumuis A. Establishment of a long-term primary culture of striatal neurons. Brain Res Dev Brain Res. 1990;52:229–39. doi: 10.1016/0165-3806(90)90239-u. [DOI] [PubMed] [Google Scholar]
- Segal M, Greenberger V, Korkotian E. Formation of dendritic spines in cultured striatal neurons depends on excitatory afferent activity. Eur J Neurosci. 2003;17:2573–85. doi: 10.1046/j.1460-9568.2003.02696.x. [DOI] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10:1458–66. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Skogh C, Campbell K. Homotopic glial regulation of striatal projection neuron differentiation. Neuroreport. 2003;14:1037–40. doi: 10.1097/01.wnr.0000073424.02536.22. [DOI] [PubMed] [Google Scholar]
- Skogh C, Parmar M, Campbell K. The differentiation potential of precursor cells from the mouse lateral ganglionic eminence is restricted by in vitro expansion. Neuroscience. 2003;120:379–85. doi: 10.1016/s0306-4522(03)00427-5. [DOI] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. DiOlistic labeling of neurons in tissue slices: a qualitative and quantatative analysis of methodological variations. Front Neuroanat. 2011;5:14. doi: 10.3389/fnana.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–30. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wolf ME. Nucleus accumbens neurons exhibit synaptic scaling that is occluded by repeated dopamine pre-exposure. Eur J Neurosci. 2009;30:539–50. doi: 10.1111/j.1460-9568.2009.06852.x. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tian X, Kai L, Hockberger PE, Wokosin DL, Surmeier DJ. MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons. Mol Cell Neurosci. 2010;44:94–108. doi: 10.1016/j.mcn.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–35. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–6. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10:207–14. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventimiglia R, Kindsay RM. Rat Striatal Neurons in Low-Density, Serum-Free Culture. In: Goslin GBaK., editor. Culturing Nerve Cells. MIT Press; Cambridge, MA: 1998. pp. 371–93. [Google Scholar]
- Ventimiglia R, Mather PE, Jones BE, Lindsay RM. The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci. 1995;7:213–22. doi: 10.1111/j.1460-9568.1995.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Watts C, Dunnett SB, Rosser AE. Effect of embryonic donor age and dissection on the DARPP-32 content of cell suspensions used for interstriatal transplantation. Exp Neurol. 1997;148:271–280. doi: 10.1006/exnr.1997.6646. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–71. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Widmer HR, Hefti F. Neurotrophin-4/5 promotes survival and differentiation of rat striatal neurons developing in culture. Eur J Neurosci. 1994;6:1669–79. doi: 10.1111/j.1460-9568.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.