Abstract
Three cytokines use the IL-12p40 cytokine subunit namely: IL-12p70 (IL-12-comprised of IL-12p40 and IL-12p35), IL-23 (comprised of the IL-12p40 and IL-23p19 subunits) and homodimeric IL-12p40 (IL-12(p40)2). Following activation, immature dendritic cells (DCs) upregulate the chemokine receptor Chemokine-C-Receptor 7 (CCR7), and migrate in response to homeostatic chemokines such as chemokine (C-C motif) ligand 19 (CCL19). Induction of the cytokine IL-12p40 in response to pathogen-exposure, likely in its homodimeric form, is one of the primary events that mediates migration of DCs in response to CCL19. Here we show that following exposure to Francisella tularensis Live Vaccine Strain (LVS), DCs produce IL-12p40 and promote the migration of DCs to the chemokine CCL19 in an IL-12Rβ1- and IL-12p(40)2-dependent manner. Induction of IL-12p40 and resulting chemokine responsiveness in DCs is TLR2-dependent and coincides with the uptake of F. tularensis LVS and activation of DCs. Importantly, we show that IL-12Rβ1 signaling is required for DC migration from the lung to the draining lymph node following F. tularensis LVS exposure and coincides with accumulation of IL-12p40 expressing DCs in the draining lymph nodes. Together, these findings illustrate that IL-12p40 is induced rapidly in response to F. tularensis LVS and is required for DC migration through an IL-12Rβ1-IL-12(p40)2 dependent mechanism.
Keywords: Tularemia, Dendritic cells, Intracellular pathogens, IL-12p40
Introduction
Francisella tularensis, a gram-negative facultative intracellular bacteria, is a highly infectious pathogen known to be the causative agent of the zoonotic disease, tularemia. The routes of Francisella infection include contact, ingestion or inhalation. However, inhalation with even low doses of airborne bacteria (<10 colony forming units, CFU) causes serious illness and therefore projects its use as a possible bioterrorism tool [1]. An F. tularensis Live Vaccine Strain (LVS) has been developed from the F. tularensis SCHU S4 strain, but is currently not licensed for use in humans [1]. Due to the absence of licensed vaccines against tularemia, much effort is directed towards understanding immune regulation in response to F. tularensis.
Three cytokines use the Interleukin (IL)-12p40 cytokine subunit namely IL-12p70 (IL-12-comprised of IL-12p40 and IL-12p35), IL-23 (comprised of the IL-12p40 and IL-23p19 subunits) and homodimeric IL-12p40 (IL-12(p40)2). The IL-12p40 subunit cytokines play critical and distinct functions in the generation of adaptive T cell responses to pathogens. For example, IL-12(p40)2 is required for DC migration and initiation of adaptive immune responses [2–3], while IL-12 and IL-23 are critical for generation of distinct T helper cell (Th) responses. The initiation of the host immune response to pathogen exposure is the activation of antigen presenting cells (APCs), mainly dendritic cells (DCs) by pathogen associated receptors and production of cytokines. Activated DCs upregulate chemokine receptors, specifically Chemokine-C-Receptor 7 (CCR7) and migrate to secondary lymphoid organs in response to the homeostatic chemokine Chemokine (C-C- motif) ligand 19 (CCL19 [4–5]. The migration of pathogen-activated DCs in response to CCL19 is critical for the generation of an effective adaptive immune response [6–7]. Recently, we and others have shown that following bacterial stimulation, DCs deficient in IL-12p40 fail to migrate toward CCL19 [2–3, 8] and do not generate effective adaptive T cell responses [2, 8]. Furthermore, migration of pathogen-exposed IL-12p40 gene-deficient DCs can be rescued by addition of IL-12(p40)2 [2] and is mediated by its receptor IL-12 Receptor β1 (IL-12Rβ1) [9]. These data suggest that production of IL-12(p40)2 by pathogen-activated DCs is a crucial first step in initiation of the host immune response [2]. Downstream of DC activation and migration to the secondary lymphoid organs, production of DC-derived IL-12p40 dependent cytokines is crucial for generation of T cell responses, specifically IL-12 is critical for the induction of IFNγ responses [10], while the production of DC-derived IL-23 is required for generation and maintenance of Th17 cells [11]. Accordingly, cellular immunity against F. tularensis requires the induction of both T helper 1 (Th1) [12–13] and T helper 17 (Th17) cells [14]. Infection of mouse bone marrow derived DCs (BMDCs) with F. tularensis LVS induces the production of IL-12p40 and IL-12 [15], while infection of macrophages with F. tularensis LVS induces IL-12p40 and IL-12p35 mRNA [16] and IL-12p40 protein in human monocyte derived macrophages [17]. In vitro infection of human monocytes with the virulent strain of F. tularensis SCHU S4 induces IL-23 [18]. These studies suggest that all three IL-12p40 subunit cytokines are induced in response to Francisella infection. However, it is not known whether IL-12p40 induced in response to F. tularensis LVS exposure has a role to play in chemokine responsiveness to CCL19 and in DC migration from the lung to the draining lymph nodes (DLN). In this study, using in vitro chemotaxis assays we show that Francisella-activated DCs induce TLR2-dependent IL-12p40 production and migrate in response to the homeostatic chemokine CCL19 in an IL-12p(40)2 and IL-12Rβ1-dependent manner. Furthermore, we show that migration of DCs from the Francisella-exposed lung in vivo is also mediated by IL-12Rβ1 signaling and this coincides with rapid accumulation of IL-12p40-expressing DCs in the DLNs. Our data therefore suggest that IL-12p40 expression by DCs is the first crucial step in initiation of downstream immune responses in pulmonary tularemia.
Materials and Methods
Mice
C57BL/6 (B6) were purchased from Taconic Laboratory (Hudson, NY). IL-12p40-IRES-GFP reporter (yet40), IL-12p40−/− mice and TLR2−/− mice originated from The Jackson Laboratory (Bar Harbor, ME). IL-12Rβ1−/− mice were used as previously described [19] and were a generous gift from Dr. Michael J Walter, Washington University School of Medicine. Experimental mice were used between the ages of six to eight weeks. All mice were treated in accordance to University of Pittsburgh IACUC guidelines.
Bacteria
The LVS strain of F. tularensis (BEI) was grown in Mueller–Hinton (MH) broth or cultured on MH agar, supplemented with ferric pyrophosphate and isovitalex [12]. Bacteria were grown to mid-log phase at 37°C and the CFU was determined by plating the bacterial stocks on MH agar plates. Bacterial colony formation was counted after 3 days of incubation at 37°C. F. tularensis LVS stocks were then heat inactivated by incubating bacterial stocks grown to mid-log phase at 60°C for one hour. Bacteria were frozen in 1 ml aliquots without glycerol at −70°C until needed. Total bacterial protein was determined with the Pierce BCA protein Assay Kit (Thermo Scientific) following the manufacturer’s protocol. Irradiated M. tuberculosis H37RV whole cells was obtained under National Institutes of Health (NIH) contract AI-75320.
Generation of bone marrow-derived dendritic cells (BMDCs)
BMDCs were generated from the bone marrow cells of mice as previously described [14]. Briefly, cells were extracted from mouse femurs and 1 × 107 cells were plated with 10 ml of DMEM supplemented with 10% FBS (complete DMEM (cDMEM) containing 20 ng/ml recombinant murine GM-CSF (rmGM-CSF; Peprotech). Cells were cultured for 3 days at 37°C in 5% CO2, after which an additional 10 ml of cDMEM containing 20 ng/ml rmGM-CSF was added. On day 7, the non-adherent cells were collected by centrifugation, counted and used as BMDCs.
Exposure of BMDCs to F. tularensis LVS
BMDCs from 7-day cultures were placed in 24-well plates at a concentration of 1 × 106 cells/well. Cells were treated with heat-inactivated (100 μg/ml) or live F. tularensis LVS grown at 37°C at a multiplicity of infection (MOI) of 100 bacteria per cell in 1 ml of cDMEM. Irradiated M. tuberculosis (100 μg/ml) was used as a positive control [2]. Cells were incubated for 24 hours at followed by centrifugation and removal of the supernatants for protein analysis. 37°C in 5% CO2 In some wells, B6 DCs were treated with either Goat IgG isotype control or IL-12p40 neutralizing antibody (both from R and D Biosystems-100 ng/ml), while IL-12p40−/− DCs were treated with IL-12(p40)2 (500 ng/ml) for 24 hours. Untreated and treated BMDCs were washed extensively and used in assays described below.
BMDC uptake of F. tularensis LVS
F. tularensis LVS was conjugated to Alexa Fluor 488 following the manufacturer’s protocol (Invitrogen). Air-dried heat inactivated F. tularensis LVS were suspended in 0.1% sodium bicarbonate buffer containing 200μg/ml Alexa Fluor dye and labeled at 37°C, 500 rpm for one hour. Conjugated F. tularensis LVS was then washed twice in PBS and resuspended in cDMEM. BMDCs were treated with labeled F. tularensis (100μg/ml). Cells were incubated for 24 hours at 37°C in 5% CO2 and the percentage of CD11c+ cells that phagocytized labeled F. tularensis was determined using flow cytometry.
In vivo tracking of BMDCs
Day 7 in vitro-generated BMDCs were used for in vivo tracking as described previously by us [2]. In brief, BMDCS were activated with F. tularensis LVS for 3 hours or left untreated. Cells were washed and stained with 6 μg/ml TAMRA orange (Invitrogen) for 3 mins at 37°C, where upon they were washed and resuspended in PBS. 5×106 cells were delivered intratracheally into B6 mice and after 18 hours post instillation, the lungs and DLN were harvested, processed into single cell suspensions, and the number of CD11c+ TAMRA+ cells determined by flow cytometry.
In vivo tracking of lung CD11c+ DCs
B6 or IL-12Rβ1−/− mice each received a suspension of 10 μg of heat inactivated LVS in a 5mM CFSE (Invitrogen) solution delivered intratracheally. Control mice received CFSE alone. In some experiments, IL-12p40 reporter mice received PBS or 10 μg of heat inactivated LVS delivered in PBS. 18–24 hours after instillation, lungs and DLNs were harvested and single cell suspensions prepared. Flow cytometry was used to determine the frequency of CFSE-labeled CD11c+ cells or IL-12p40+ expressing CD11c+ cells within the DLNs.
Chemotaxis Assay
The responsiveness of treated and untreated BMDCs to CCL19 was determined as previously described [2]. Treated and untreated BMDCs were resuspended at a concentration of 1 × 106 cells/ml in 1× Hanks Balanced Salt Solution (HBSS) without calcium and magnesium (mediatech-Cellgro) plus 1% heat inactivated Fetal Bovine Serum (FBS) (Sigma-Aldrich). 100μl of the cell suspension was added to the upper chamber of a transwell in a 24 well plate (Fisher Scientific) while the lower chamber contained 25 ng/ml CCL19 (R&D Biosystems) in 600 μl of HBSS. Transwell plates were incubated at 37°C for 90 mins following which the transmigrated cells from the lower chamber were fixed by addition of 1% formaldehyde. A standard number of 20 μm size fluorescent microspheres (Polysciences Inc.) were added to each sample and the cells were counted on a Becton Dickinson FACSCalibur flow cytometer using Cell Quest software. Chemotaxis index was then calculated by dividing the normalized cell count by the average cell count for untreated controls with non-specific migration toward HBSS. Cells were defined by their forward and side scatter characteristics while beads were defined by their size and fluorescent intensity.
Determination of protein levels
ELISA antibody pairs were used to detect IL-12p40, IL-12p70 and IL-23 cytokine levels (BD Pharmingen) in the culture supernatants. Recombinant cytokines were used to generate standard curves.
Flow cytometry
For cell surface staining experiments, single cell suspensions were stained with fluorochrome-labeled antibodies specific for CD80 (Clone 16-10A1), CD86 (Clone GL1), CD11c (Clone HL3), (from BD Biosciences). Cells were collected on a Becton Dickinson LSRII flow cytometer using DIVA software. Cells were gated based on their forward and side scatter characteristics and the frequency of specific cell types was determined using FlowJo (Tree Star Inc, CA).
RT-PCR
RNA was extracted from BMDCs using commercial RNA extraction kit (Qiagen) as described [14]. RNA was treated with DNAse and reverse transcribed and cDNA was amplified with FAM-labeled probe and PCR primers on ABI Prism 7900 detection system. The fold-increase in signal over that derived from uninfected samples was determined by calculating the 2−Δ Δct as before [2, 20]. The primer and probes sequences have been previously published for IL-12p40, IL-12p35, and IL-23p19 [20].
Statistical Analysis
Differences between the means of experimental groups were analyzed using the two tailed Student’s t-test. Differences were considered significant when p ≤ 0.05.
Results
F. tularensis LVS induces IL-12p40 cytokine members in Dendritic cells
Mice that lack IL-12p40 are extremely susceptible to pulmonary F. tularensis LVS infection [12, 14]. DCs are one of the likely producers of IL-12p40 cytokine members in response to infection [15]. Therefore, to determine whether F. tularensis LVS exposure induces IL-12p40 cytokine members in DCs, we treated bone marrow dendritic cells (BMDCs) generated from B6 mice with live or heat inactivated F. tularensis LVS and determined the levels of IL-12p40 in culture supernatants. We found that DCs treated with either live or heat inactivated F. tularensis LVS similarly induced IL-12p40 protein levels in culture supernatants when compared to untreated BMDC cultures (Figure 1A). Therefore in all future experiments heat inactivated F. tularensis LVS treatment was used. Furthermore, levels of IL-12p40 induced by F. tularensis LVS were comparable to IL-12p40 levels induced by stimulation of BMDCs with irradiated M. tuberculosis. Since the IL-12p40 subunit is a component of two other cytokines, namely IL-23 and IL-12p70 (IL-12), we then determined the levels of IL-12 and IL-23 in BMDC-stimulated culture supernatants and found that the F. tularensis LVS treatment induced IL-12 (Figure 1B). We also found that the levels of IL-12p40 and IL-12 induced by heat inactivated F. tularensis LVS was similar to levels induced by treatment with irradiated M. tuberculosis (Figure 1A, B). Further, although IL-23 protein in supernatants from F. tularensis LVS activated DCs was below detectable levels using commercially available kits (data not shown), we detected induction of IL-23p19 and IL-12p40 mRNA in BMDCs treated with F. tularensis LVS (Figure 1C), suggesting that exposure to F. tularensis LVS likely also induces IL-23. To further assess if F. tularensis LVS could induce the transcription of IL-12p40 cytokines, we exposed BMDCs generated from IL-12p40 reporter mice (yet40 reporter mice) to F. tularensis LVS. The yet40 reporter mice have been used to determine IL-12p40 transcription both in vitro and in vivo [3, 14, 21]. Accordingly, BMDCs exposed to F. tularensis LVS and M. tuberculosis resulted in significant induction of CD11c+ IL-12p40 YFP+ DCs indicating IL-12p40 transcription was taking place following pathogen exposure (Figure 1D, E). These data suggest that F. tularensis LVS induces IL-12p40 transcription and production of IL-12p40-dependent cytokines in DCs rapidly following pathogen exposure.
Figure 1. F. tularensis upregulates production of IL-12p40 cytokine members in dendritic cells.
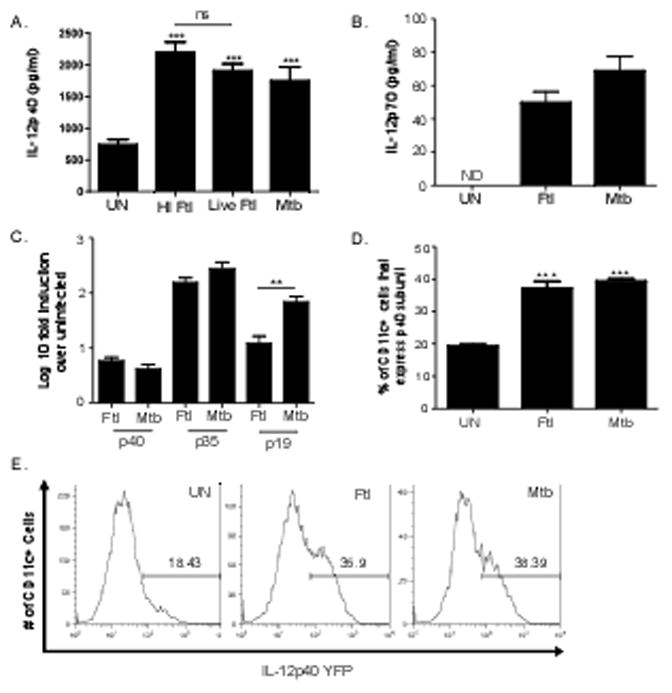
B6 BMDCs were treated with either heat inactivated F. tularensis (HI Ftl) (100 μg/ml), live F. tularensis (Live Ftl) (MOI 1:100) or irradiated M. tuberculosis (Mtb) (100 μg/ml) for 24 hours or left untreated (UN) and culture supernatants were analyzed for IL-12p40 (A) or IL-12 (B). RNA was isolated from untreated BMDCS, heat inactivated F. tularensis LVS-treated (Ftl) (100 μg/ml) or irradiated M. tuberculosis (Mtb) (100 μg/ml)-treated BMDCs. Log 10 fold induction of IL-12p40, IL-12p35, and IL-23p19 induction over untreated BMDCs was assessed by RT-PCR using the 2−ΔΔct calculation (C). BMDCs from yet40 reporter mice were left untreated (UN) or treated with either heat inactivated F. tularensis (Ftl) (100 μg/ml) or irradiated M. tuberculosis (Mtb) (100 μg/ml) for 24 hours and the percentage of CD11c+ cells expressing IL-12p40 reporter was determined by flow cytometry (D, E). The data points represent the mean and SD of four samples. (*p<0.005, **p<0.005, *** p<0.005) One experiment representative of at least two shown. ND- protein Not Detectable in culture supernatants.
Induction of IL-12p40 in DCs coincides with pathogen uptake and activation of DCs
To further determine whether DC uptake of F. tularensis LVS coincides with IL-12p40 cytokine production, we labeled F. tularensis LVS with Alexa Flour 488 and stimulated BMDCs for 24 hours with labeled F. tularensis LVS and determined the uptake of labeled bacteria by BMDCs using flow cytometry. We show that approximately 75% of BMDCs take up F. tularensis LVS (Figure 2A), suggesting that uptake of F. tularensis LVS coincides with induction of IL-12p40 cytokines. To further determine whether induction of IL-12p40 cytokines correlated with the ability of F. tularensis LVS to activate BMDCs, we compared the expression of the known surface activation markers CD80 and CD86 on F. tularensis LVS treated BMDCs. We observed a significant upregulation of both CD80 and CD86 expression on CD11c+ cells when exposed to F. tularensis LVS and M. tuberculosis (Figure 2B–D). These data suggest that DCs uptake F. tularensis LVS, are activated and produce IL-12p40 cytokines rapidly following pathogen exposure. That M. tuberculosis-activated DCs exhibited higher levels of activation when compared to F. tularensis-activated DCs further suggests that different intracellular bacteria likely induce different levels of DC activation as would be expected of inherently distinct bacterial pathogens.
Figure 2. F. tularensis uptake and surface expression of activation markers in BMDCs coincide with IL-12p40 induction.
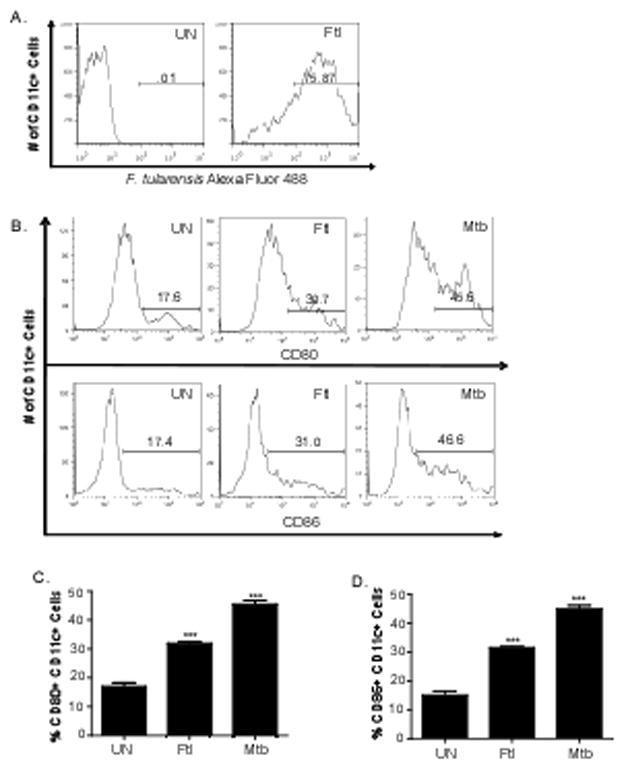
B6 BMDCs were left untreated (UN) or treated with heat inactivated F. tularensis (Ftl) (100 μg/ml) that were conjugated to Alexa Fluor 488. After 24 hours in culture, cells were washed, surface stained for CD11c and the percentage of CD11c+ cells also positive for Alexa Fluor 488 was determined using flow cytometry (A). Surface expression of activation markers, CD80 and CD86, on CD11c+ cells untreated (UN) or treated with heat inactivated F. tularensis (Ftl) (100 μg/ml) or irradiated M. tuberculosis (Mtb) (100 μg/ml) was determined by flow cytometry and shown as histogram plots (B) or frequency (C, D). The data points represent the mean and SD of three samples. (**p<0.005, *** p<0.005). One experiment representative of two shown.
F. tularensis LVS exposure elicits CCL19 dependent chemotaxis of DCs
In addition to production of IL-12p40 and upregulation of activation markers, DCs respond to pathogen exposure by becoming responsive to homeostatic chemokines such as CCL19 [2–3]. Accordingly, it has been shown that pulmonary DCs migrate from the lung to the DLNs in response to F. tularensis LVS exposure [22]. Therefore, we next tested if F. tularensis LVS-activated DCs are able to migrate in response to the homeostatic chemokine CCL19 and if this was dependent on IL-12p40 and IL-12Rβ1 signaling. Therefore, we treated DCs with F. tularensis LVS and using in vitro chemotaxis assays show that pathogen-activated DCs but not untreated DCs migrate in response to the homeostatic chemokine CCL19 (Figure 3A). Furthermore, the ability of F. tularensis LVS-activated DCs to migrate in response to CCL19 was comparable to migration of M. tuberculosis-activated DCs (Figure 3A), suggesting that pathogen exposure induces migration of DCs. The migration of M. tuberculosis-activated DCs in response to CCL19 is mediated through IL-12p40 [2–3] and its receptor molecule IL-12Rβ1[9]. Therefore, we next addressed if the migration of F. tularensis LVS–activated DCs in response to homeostatic chemokine CCL19 was also IL-12Rβ1 and IL-12p40 dependent. We also report that F. tularensis LVS-activated BMDCs generated from IL-12Rβ1−/− mice did not migrate in response to CCL19 following F. tularensis LVS exposure (Figure 3B). To further address whether the IL-12Rβ1-dependent chemokine responsiveness was mediated through the production of IL-12p40, we treated F. tularensis-stimulated B6 BMDCs with IL-12p40 neutralizing antibody and found that this effectively abrogated the ability of BMDCs to migrate in response to CCL19 (Figure 3C). Furthermore, we also found that BMDCs derived from IL-12p40−/− mice upon exposure to Francisella were unable to migrate in response to CCL19 (Figure 3D). Importantly, we show that exogenous treatment of F. tularensis-activated IL-12p40−/− BMDCs with recombinant IL-12p(40)2 was able to effectively rescue the ability of IL-12p40−/− BMDCs to migrate in response to CCL19 (Figure 3D). These data effectively show that F. tularensis LVS exposure results in induction of IL-12p40, likely as a homodimer, and mediates DC migration towards CCL19 through its receptor IL-12Rβ1.
Figure 3. F. tularensis activates BMDCs to respond to the chemokine CCL19 via the IL-12p40/IL-12Rβ1 axis.
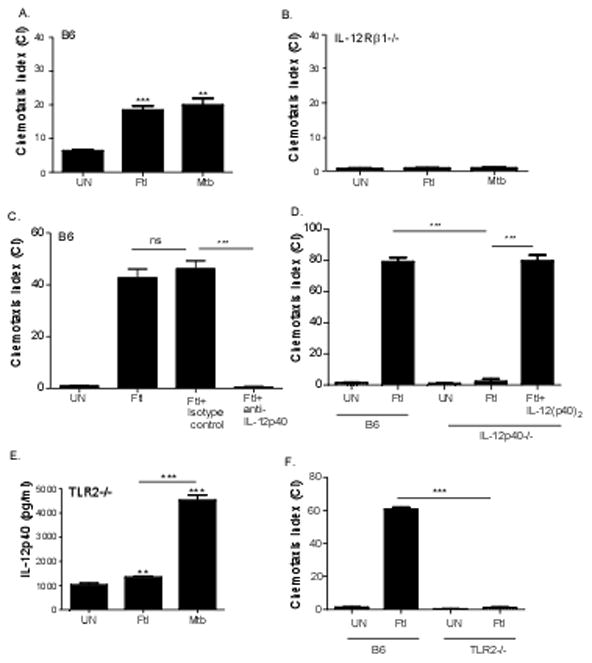
B6 BMDCs (A) and IL-12Rβ1−/− BMDCs (B) were left untreated (UN) or treated with heat inactivated F. tularensis (Ftl) (100 μg/ml) or irradiated M. tuberculosis (Mtb) (100 μg/ml) and incubated for 24 hours. B6 BMDCs were left untreated (UN) or treated with heat inactivated F. tularensis (Ftl) (100 μg/ml) (C, D) or treated with heat inactivated F. tularensis (Ftl) (100 μg/ml) and isotype control (Ftl+Isotype control) or IL-12p40 neutralizing antibody (Ftl+ anti-IL-12p40) for 24 hours (C). IL-12p40−/− BMDCs were left untreated, treated with heat inactivated F. tularensis (Ftl) alone or with recombinant IL-12p(40)2 for 24 hours (D). Cells were washed, collected, and chemotaxis assay towards CCL19 (25 ng/ml) was carried out in a 24 well transwell plate. Cell counts were normalized and chemotaxis index was calculated as described under Methods. TLR2−/− BMDCs were left untreated or incubated with heat inactivated F. tularensis (Ftl) (100 μg/ml) or irradiated M. tuberculosis (Mtb) (100 μg/ml) for 24 hours. Culture supernatants were analyzed for IL-12p40 (E) and ability to migrate to CCL19 in a chemotaxis assay was determined as described above (F). The data points represent the mean and SD of 3–4 samples.. (*p<0.005, **p<0.005, *** p<0.005). One experiment representative of at least two shown.
TLR2 is required for the inflammatory response to F. tularensis LVS in BMDCs [23] and macrophages [24]. To address whether TLR2 is required for F. tularensis LVS induced IL-12p40-dependent chemotaxis, we treated BMDCs from TLR2−/− with F. tularensis LVS and determined levels of IL-12p40 cytokine in culture supernatants. Consistent with published studies [15], our data shows that BMDCs derived from TLR2−/− mice (Figure 3E) produced reduced levels of IL-12p40 in response to F. tularensis LVS treatment. As reported before, the IL-12p40 cytokine production in response to M. tuberculosis stimulation is not TLR2 dependent [25], since TLR2−/− BMDCs stimulated with M. tuberculosis induced high levels of IL-12p40 protein in DC culture supernatants. Furthermore, we found that DCs that lack the adapter molecule MyD88 did not induce IL-12p40 in response to both F. tularensis LVS and M. tuberculosis (data not shown). Importantly, we show that the reduced ability of TLR2−/− BMDCs to produce IL-12p40 also coincided with decreased ability of F. tularensis-stimulated TLR2−/− BMDCs to migrate in response to CCL19 (Figure 3F). These data suggest that F. tularensis LVS induced IL-12p40 cytokine production in DCs is TLR2 and MyD88-dependent, and mediates chemotaxis to CCL19 through its receptor IL-12Rβ1.
IL-12Rβ1 is required for migration of F. tularensis LVS activated DCs from the lungs to the DLNs in vivo
Our in vitro data suggests that F. tularensis-induced IL-12p40 production is required for chemokine responsiveness of DCs to CCL19 through its receptor IL-12Rβ1. To test the relevance of our findings in migration of DCs in vivo, we activated B6 and IL-12Rβ1−/− BMDCs with F. tularensis LVS in vitro and labeled them with TAMRA orange as described before [2]. Labeled cells were then delivered intratracheally into the lungs of B6 mice and we tracked migration of TAMRA-labeled CD11c+ in the DLN by flow cytometry [2]. We found there was significantly more B6 F. tularensis-stimulated DCs than untreated B6 BMDCs in the DLNs, 18 hours after transfer (Figure 4A). Importantly, we show that there were fewer F. tularensis-activated IL-12Rβ1−/− BMDCs in the DLNs when compared to F. tularensis-activated B6 BMDCs (Figure 4A). The fact that higher numbers of F. tularensis–activated IL-12Rβ1−/− BMDCs were detected in the lungs when compared to F. tularensis-activated B6 BMDCs, supports our hypothesis that these cells were not able to migrate from the lung to the DLN (Figure 4B). Furthermore, retention of IL-12Rβ1−/− F. tularensis BMDCs in the lungs was similar to the retention seen in mice that received untreated B6 DCs (Figure 4B), suggesting that the migration of B6 BMDCs from the lungs to the DLNs was dependent on F. tularensis exposure and IL-12Rβ1 signaling.
Figure 4. IL-12RBβ1 is required for DC migration from the lung to the DLNs following F. tularensis exposure.
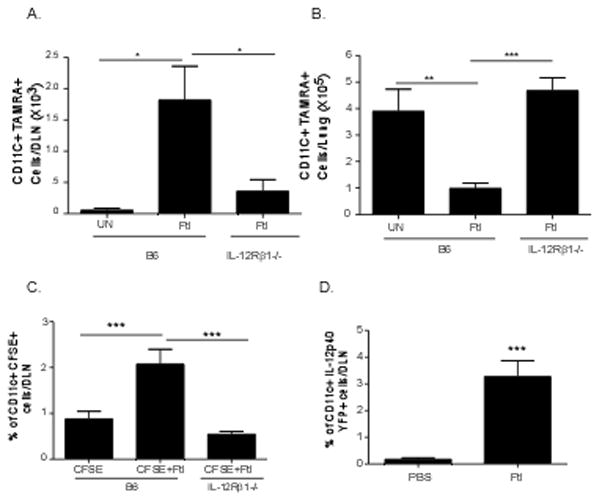
B6 BMDCs and IL-12Rβ1−/− BMDCs were treated with heat inactivated F. tularensis (Ftl) (100 μg/ml) for 24 hours, following which cells were washed and labeled with TAMRA orange. 5×106 labeled BMDCs were intratracheally transferred into B6 mice and the number of CD11c+ TAMRA+ cells were determined in DLNs (A) and lung (B) at 18 hours after transfer by flow cytometry. B6 and IL-12Rβ1−/− (C) received 10 μg of heat inactivated F. tularensis intratracheally along with CFSE as described under methods. 18 hours later, the frequency of CD11c+ CFSE+ cells was determined in the DLNs using flow cytometry. Yet40 reporter mice received 10 μg of heat inactivated F. tularensis intratracheally as described under methods (D). Control yet40 reporter mice received the same volume of PBS intra-tracheally. 24 hours later, the frequency of CD11c+ IL-12p40 YFP+ cells were determined in the DLNs using flow cytometry. The data points represent the mean and SD of 5–10 samples. (*p<0.005, **p<0.005, *** p<0.005).
Since the above data using BMDCs suggests that DCs require IL-12Rβ1 signaling to migrate from the lung to the DLN, we then determined whether lung resident DCs require IL-12Rβ1 signaling to respond to F. tularensis exposure and migrate from the lung to the DLN. Therefore, we labeled cells in the airways of B6 and IL-12Rβ1−/− mice with CFSE [2] and activated lung DCs by administering F. tularensis LVS intratracheally. We then determined the frequency of CD11c+ CFSE+ DCs within the DLN, 18 hrs after delivery. We found that the frequency of CD11c+ CFSE+ DCs in the DLNs of B6 mice exposed to F. tularensis LVS was significantly higher when compared to control B6 mice that received CFSE alone (Figure 4C). However, there was a significantly reduced frequency of CD11c+ CFSE+ DCs in the DLNs of IL-12Rβ1−/− mice exposed to F. tularensis LVS (Figure 4C). These data together demonstrate that in the absence of IL-12Rβ1 signaling, lungs DCs activated by F. tularensis LVS are less able to migrate from the lung to the DLNs. To further determine whether pulmonary F. tularensis LVS exposure resulted in accumulation of IL-12p40 expressing DCs in the DLNs in vivo, we treated yet40 reporter mice intratracheally with F. tularensis LVS or PBS and found that exposure to F. tularensis LVS resulted in accumulation of CD11c+ IL-12p40 YFP+ DCs in the DLNs, 24 hours later. These data together suggest that IL-12p40 expressing DCs are migratory and require IL-12Rβ1 signaling to migrate from the lungs to the DLNs following F. tularensis LVS exposure.
Discussion
We have previously shown that following exposure to pathogens such as M. tuberculosis and Y. pestis, the production of IL-12p40 by activated DCs is one of the early events that is required for chemokine responsiveness and initiation of adaptive immune responses [2–3]. We now demonstrate that F. tularensis LVS-stimulated DCs also produce IL-12p40 rapidly and mediate chemokine responsiveness to CCL19, through its receptor IL-12Rβ1. The requirement for IL-12p40 does not appear to reflect the need for other IL-12p40-dependent cytokines such as IL-12 or IL-23, since the migratory phenotype in BMDCs lacking IL-12p40 can be rescued by providing IL-12p(40)2 exogenously. Importantly, our data also show that ability of F. tularensis-activated lung DCs to migrate to the DLNs is also dependent on IL-12Rβ1, suggesting that production of IL-12p40 by DCs is a crucial first step in generation of rapid immune responses following pulmonary tularemia. Our data show that induction of IL-12p40, IL-12p35 and IL-23p19 mRNA takes place in DCs following F. tularensis LVS exposure, suggesting that all three of the IL-12p40 cytokines are induced in response to pathogen stimulation. The production of effector cytokines IFNγ and IL-17 is critical for protective immunity to F. tularensis LVS [12, 14]. IFNγ production is critical for macrophage activation and control of intracellular pathogens. More recently, we described a critical role for IL-17 in induction of IL-12 and in driving Th1 responses during F. tularensis LVS pulmonary infection [14]. Furthermore, IL-17 can also directly act on macrophages and enhance intracellular pathogen control [14]. These studies suggest that both the Th1 and Th17 responses are crucial for control of F. tularensis LVS. Accordingly, we show that the inducible expression of IL-12p40 and IL-12p35 mRNA and presence of IL-12 protein (Figure 1), suggests that the Th1 polarizing cytokine IL-12 can function to drive Th1 responses. The inducible gene expression for IL-12p40 and IL-23p19 mRNA in Francisella-treated DCs suggests the induction of IL-23, allows for generation of Th17 responses and subsequent IL-17 dependent Th1 induction. Consistent with these data, we recently showed that F. tularensis infected IL-12p35−/− mice have reduced induction of IFNγ, while IL-23p19−/− mice had reduced induction of IL-17 and IFNγ responses in the lung [14]. Therefore, our data suggests that following F. tularensis LVS exposure, DCs produce IL-12p(40)2 to initiate chemokine responsiveness and migration to the DLNs, while IL-12 and IL-23 production likely play important roles in initiation of Th1 and Th17 responses respectively.
The IL-12 receptor family consists of the IL-12Rβ1, IL-12Rβ2 and IL-23R. The IL-12 cytokine binds to the IL-12 receptor comprised of IL-12Rβ1 and IL-12Rβ2, while IL-23 binds to the IL-23R comprised of IL-12Rβ1 and IL-23R. IL-12p40 binds to IL-12Rβ1 and interferes with binding of IL-12 [26]. In addition, IL-12(p40)2 functions as a macrophage chemoattractant [27–28] and this activity is blocked when anti-IL-12Rβ1 antibody [27] or IL-12Rβ1 gene deficient mice were used [28]. The signaling induced by IL-12(p40)2 in IL-12Rβ1 is thought to be mediated by NFκB DNA binding [29], since treatment of BMDCs with IL-12p(40)2 alone was able to stimulate NF-κβ nuclear migration in DCs [9]. Consistent with this, the IL-12p40-dependent pathogen-dependent migration of M. tuberculosis activated-DCs [2] was IL-12Rβ1-dependent and was associated with impaired NF-κβ-dependent gene activation [9]. In the current study, we show that IL-12Rβ1 expression on DCs is important for the CCL19 chemokine responsiveness of F. tularensis LVS-activated BMDCs. Importantly, neutralization of IL-12p40 in F. tularensis-activated DCs resulted in complete abrogation of chemokine responsiveness to CCL19, further validating a role for IL-12p40-IL-12Rβ1 axis in DC migration following pathogen exposure. We also show that exposure to F. tularensis LVS and resulting IL-12p40 cytokine production coincides with uptake of the bacteria and upregulation of activation markers on DCs. However, it is possible that not all the DCs that have taken up the bacteria have completely acquired activation status at this time, since we found that a smaller proportion of DCs were activated compared to higher proportion of DCs that have taken up bacteria.
DCs are infected following pulmonary infection with F. tularensis LVS in the lung [22] and our data suggest that the production of IL-12p40 likely mediates the migration of DCs from the infected lung via an IL-12Rβ1-dependent pathway for initiation of adaptive immune responses. Consistent with our hypothesis that the migratory phenotype of lung DCs following F. tularensis LVS exposure is dependent on IL-12Rβ1, we show that F. tularensis-induced migration of lung DCs to the DLNs in IL-12Rβ1−/− mice is defective. Furthermore, our data that transfer of F. tularensis-activated IL-12Rβ1−/− BMDCs into B6 mice also results in defective BMDC migration to LNs suggesting that it is the IL-12p40-IL-12Rβ1 signaling in DCs that is crucial for migration of pathogen exposed DCs to the DLNs. Furthermore, our data that CD11c+ IL-12p40 YFP+ DCs accumulate in the DLNs within 24 hours following F. tularensis exposure, project that DC migration is a rapid first step in initiation of immune responses. This is consistent with other reports showing that in yet40 reporter mice, IL-12p40-expressing DCs are detected in DLNs following activation with bacteria and can promote differentiation of T cells [21]. These studies provide a rationale for the production of IL-12p40 cytokine production as one of the earliest events following F. tularensis-induced DC activation [30–31] and for the fact that IL-12p40 is produced in excess of IL-12p70 (Figure 1). Accordingly, neutralization of IL-12p40 as well as IL-12p40 deficiency in DCs results in abrogation of F. tularensis-induced chemokine responsiveness to the homeostatic chemokine, CCL19. Our data that the F. tularensis-stimulated IL-12p40−/− DC chemokine responsiveness to CCL19 can be rescued with treatment with IL-12p(40)2 further validates a crucial role for IL-12p(40)2 in DC migration following pathogen exposure [2]. It is known that TLR2 signaling is important for induction of IL-12p40 cytokines following F. tularensis LVS exposure [15]. However, our observation that TLR2 is essential for IL-12p40-mediated chemokine responsiveness to CCL19, suggests that TLR2 dependent innate signaling is one of the early steps in initiating immune responses during tularemia.
In conclusion, our data demonstrate that F. tularensis LVS induced innate production of IL-12p(40)2 is required for DC migration. We also describe that chemokine responsiveness to CCL19 and ability to mediate migration of DCs from the lung to the DLNs following F. tularensis LVS exposure is IL-12Rβ1 dependent. The data presented here therefore impact our understanding of the earliest events taking place in the DCs following F. tularensis LVS infection.
Highlights.
Following exposure to F. tularensis LVS, DCs migrate to the chemokine CCL19.
DC chemokine responsiveness is IL-12Rβ1- and IL-12p(40)2-dependent.
IL-12Rβ1 signaling is required for DC migration from the lung to the draining lymph node following F. tularensis LVS exposure.
Acknowledgments
We thank Dr. Toni Darville for providing TLR2−/− mice. This work was supported by Children’s Hospital of Pittsburgh and NIH grants AI083541 and HL105427-01 to SAK; a Research Advisory Committee Grants from Children’s Hospital of Pittsburgh of the UPMC Health System to SRS and YL. All authors have no conflicting financial interests.
Abbreviations
- Th
T helper
- BMDCs
Bone-marrow Dendritic Cells
- IFNγ
Interferon gamma
- IL-17
Interleukin 17
- LVS
Live Vaccine Strain
- DLNs
Draining Lymph Nodes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. Jama. 2001;285:2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 2.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–15. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson RT, Khader SA, Locksley RM, Lien E, Smiley ST, Cooper AM. Yersinia pestis evades TLR4-dependent induction of IL-12(p40)2 by dendritic cells and subsequent cell migration. J Immunol. 2008;181:5560–7. doi: 10.4049/jimmunol.181.8.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–21. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 7.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–15. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormick S, Santosuosso M, Small CL, Shaler CR, Zhang X, Jeyanathan M, et al. Mucosally delivered dendritic cells activate T cells independently of IL-12 and endogenous APCs. J Immunol. 2008;181:2356–67. doi: 10.4049/jimmunol.181.4.2356. [DOI] [PubMed] [Google Scholar]
- 9.Robinson RT, Khader SA, Martino CA, Fountain JJ, Teixeira-Coelho M, Pearl JE, et al. Mycobacterium tuberculosis infection induces il12rb1 splicing to generate a novel IL-12Rbeta1 isoform that enhances DC migration. J Exp Med. 2010;207:591–605. doi: 10.1084/jem.20091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trinchieri G. Interleukin 12: a cytokine at the interface of inflammation and immunity. Advances in Immunology. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 11.Oppmann B, Lesley R, Blom B, Timans J, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 12.Duckett NS, Olmos S, Durrant DM, Metzger DW. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun. 2005;73:2306–11. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkins KL, Cooper A, Colombini SM, Cowley SC, Kieffer TL. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect Immun. 2002;70:1936–48. doi: 10.1128/IAI.70.4.1936-1948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 Is Required for T Helper 1 Cell Immunity and Host Resistance to the Intracellular Pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong KJ, Wickstrum JR, Yeh HW, Parmely MJ. Toll-like receptor 2 controls the gamma interferon response to Francisella tularensis by mouse liver lymphocytes. Infect Immun. 2007;75:5338–45. doi: 10.1128/IAI.00561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole LE, Santiago A, Barry E, Kang TJ, Shirey KA, Roberts ZJ, et al. Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J Immunol. 2008;180:6885–91. doi: 10.4049/jimmunol.180.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loegering DJ, Drake JR, Banas JA, McNealy TL, Mc Arthur DG, Webster LM, et al. Francisella tularensis LVS grown in macrophages has reduced ability to stimulate the secretion of inflammatory cytokines by macrophages in vitro. Microb Pathog. 2006;41:218–25. doi: 10.1016/j.micpath.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, et al. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–54. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- 19.Piccotti J, Li K, Chan S, Eichwald E, Bishop D. Interleukin-12 (IL-12)-driven alloimmune responses in vitro and in vivo: requirement for beta1 subunit of the IL-12 receptor. Transplantation. 1999;67:1453–60. doi: 10.1097/00007890-199906150-00011. [DOI] [PubMed] [Google Scholar]
- 20.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 21.Reinhardt R, Hong S, Kang S, Wang Z, Locksley R. Visualization of IL-12/23p40 In Vivo Reveals Immunostimulatory Dendritic Cell Migrants that Promote Th1 Differentiation. Journal of Immunology. 2006;177:1618–27. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- 22.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, et al. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun. 2008;76:5843–52. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74:2809–16. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, et al. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun. 2007;75:4127–37. doi: 10.1128/IAI.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pompei L, Jang S, Zamlynny B, Ravikumar S, McBride A, Hickman SP, et al. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J Immunol. 2007;178:5192–9. doi: 10.4049/jimmunol.178.8.5192. [DOI] [PubMed] [Google Scholar]
- 26.Presky D, Minetti L, Gillessen S, Wilkinson V, Wu C, Gubler U, et al. Analysis of the multiple interactions between IL-12 and the high affinity IL-12 receptor complex. Journal of Immunology. 1998;160:2174–9. [PubMed] [Google Scholar]
- 27.Ha S, Lee CH, Lee SB, Kim CM, Jang KL, Shin HS, et al. A novel function of IL-12p40 as a chemotactic molecule for macropahges. Journal of Immunology. 1999;163:2902–8. [PubMed] [Google Scholar]
- 28.Russell TD, Yan Q, Fan G, Khalifah AP, Bishop DK, Brody SL, et al. IL-12p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor b1. Journal of Immunology. 2003;171:6866–74. doi: 10.4049/jimmunol.171.12.6866. [DOI] [PubMed] [Google Scholar]
- 29.Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide synthase and activation of NF-kB by interleukin-12 p40 in microglial cells. Journal of Biological Chemistry. 2001;276:7899–905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinzel FP, Rerko RM, Ling P, Hakimi J, Schoenhaut DS. Interleukin-12 is produced in vivo during endotoxemia and stimulates synthesis of g interferon. Infection and Immunity. 1994;62:4244. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. Journal of Experimental Medicine. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]


