Abstract
Objective
This study investigated the role of the prostaglandin (PG) pathway in locally-applied, simvastatin-induced oral bone growth. The possibility of enhancing long-term bone augmentation with an alendronate-based carrier was initiated.
Methods
Mandibles of 44 mature female rats were treated bilaterally with the following combinations: 2 mg simvastatin in ethanol (SIM-EtOH), EtOH, 2 mg simvastatin acid complexed with alendronate-beta-cyclodextrin conjugate (SIM/ALN-CD), ALN-CD, or ALN. Bone wash technology (injection of PBS and recollection by suction) was used to sample injection sites at baseline (day 0), and 3, 7, 14 and 21 days post-treatment. After 21-24 or 48 days, histomorphometric analysis was done. The amount of PGE2 in bone wash fluid was measured by ELISA, normalized by total protein, and compared between high and low bone growth groups (ANOVA) and correlated with subsequent bone histology at 21 days (Spearman). SIM-stimulated PGE2 synthase and EP4 receptor mRNA in murine osteoblast and fibroblast cell lines were evaluated with real-time PCR.
Results
Single injections of 2 mg SIM-EtOH induced significantly more new bone than control side after 21 days. PGE2/protein ratios peaked at day 7 and were correlated with the subsequent 21-day new bone width. The correlations at day 14 between PGE2 and new bone width changed to a negative relationship in the test group. SIM-stimulated osteoblasts expressed increased mRNA levels of PGE receptor EP4, while SIM activated PGE synthesis in fibroblasts. SIM/ALN-CD tended to preserve bone long-term.
Conclusion
Findings suggest that PGE pathway activation and higher levels of PGE2 during the first week following SIM-induced bone growth are desirable, and alendronate-beta-cyclodextrin conjugates not only act as tissue-specific carriers, but preserve new bone.
Keywords: PGE2, simvastatin, alendronate, cyclodextrin, bone growth
Introduction
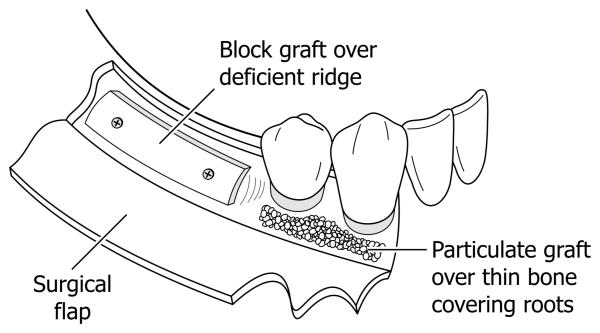
Oral and maxillofacial tumors, trauma, and extraction of teeth may result in severe loss of alveolar and mandibular bone, and there is increasing need for regeneration of bone for esthetic and dental implant therapy. Using exogenous grafts and growth factors (including bone morphogenetic protein [BMP]-2), trials to augment and induce local bone formation in the oral and maxillofacial area have been successful. (1) Primary examples would be adding bone for sinus augmentation or to increase deficient alveolar ridge width (Figure 1). Alveolar ridge defects are currently treated by surgically reflecting a flap of soft tissue (gingiva and mucosa) from the underlying bone, and then placing a bone graft (block or particles) onto the defect. The graft may be supplemented with growth factors such as BMP-2. The surgical flap is then replaced over the graft (perhaps with a membrane), sutured and allowed to heal. Significant cost and side-effects have been associated with this surgical approach. Less complicated, more reliable and predictable materials and therapeutic strategies are needed. The use of local bone anabolic injections could provide a cost-effective and patient-friendly approach to produce new bone in bone defects of the mouth and skull.
Figure 1.
Graphic of human mandibular defects which are currently treated by osseous block grafts (e.g. deficient, narrow alveolar ridge) prior to dental implants, or particulate osseous grafts (e.g. over thin bone covering tooth roots). Both require surgical flap reflection with attendant morbidity and expense.
Locally-applied hydrophobic statins (specific inhibitors of 3-hydroxy-3 methylglutaryl coenzyme A [HMG-CoA] reductase in the production of cholesterol) were discovered to be potent stimulators of bone formation. (2) In a recent study, locally-applied statin preserved greater residual alveolar ridge 2 weeks after treatment of extraction sockets and increased the bone mineral density after 4 weeks compared to the control group. (3) Stimulation of endogenous BMP-2 has long been claimed as the primary mechanism by which statins stimulate osteogenesis. (2)
Stein et al. (4) and Tylin et al. (5) showed that locally-injected simvastatin induced new bone formation in rodent models in vivo. They demonstrated that locally-applied simvastatin increased tissue swelling in a dose dependent manner, and the swelling was reduced by lowering the dose without effecting the bone formation. However, further reduction of inflammation with the cyclooxygenase (COX)- and prostaglandin (PG)-inhibiting nonsteroidal anti-inflammatory drug indomethacin nearly eliminated new bone growth, (4) suggesting that simvastatin-induced bone growth involved the prostaglandin pathway.
From a mechanism perspective, Maeda et al. (6) demonstrated that simvastatin increased the expression of BMP-2 and vascular endothelial growth factor (VEGF) in vitro. VEGF, BMP-2 and Cbfal also were expressed one day earlier in statin-treated sides than the other side treated with collagen alone in vivo. (7) Bradley et al. (8) found that a cyclooxygenase-2 inhibitor reduced both bone formation and BMP-2 production caused by simvastatin in a rat mandible model, which suggests cross-talk between BMP-2 and COX-2/PGE pathways in statin-induced bone formation. Obtaining direct evidence of the role of COX-2/PGE is clinically relevant since the most common pain medications prescribed following dental bone augmentation procedures contain COX inhibitors (e.g. aspirin, ibuprofen).
From a clinical perspective, simvastatin has been dissolved in an ethanol carrier to allow injection through small gauge needles, necessary within firm tissue overlying oral bone (e.g. gingiva and keratinized mucosa). (9,10) However, use of ethanol may be problematic due to pain at the injection site and dispersion of simvastatin-ethanol carrier into unwanted areas beyond the site of injection. (10) It has also been found that simvastatin-induced bone growth (new bone area and new bone width) after 24 days was resorbed over time, and only 23-55% of the new bone remained after 90 days. (11) Attenuation of this later resorption is critical in translation of local statin injections into clinically-relevant dental bone augmentation.
To address these issues encountered in the clinical development of injectable statin therapy, the following experiments were undertaken. To provide direct evidence of the role of PGE2 in simvastatin-induced bone augmentation, we analyzed in vivo PGE2 production following simvastatin injections (using bone wash methodology), then correlated these values to subsequent bone histology in a rat model. In addition, in vitro studies of osteoblast and fibroblast cell lines were used to dissect how simvastatin may activate PGE2-associated genes in cells from the periosteal microenvironment. Finally, the possibility of using the molecular complex of simvastatin acid/alendronate-beta-cyclodextrin conjugates to deliver simvastatin acid and to preserve new bone formation in the jaw was explored.
Experimental Section
Preparation of the Molecular Complex of Simvastatin Acid/Alendronate-Beta-Cyclodextrin Conjugates
Methods modified from Yoshinari et al. (12) were used to prepare the simvastatin acid/beta-cyclodextrin complexes. To obtain simvastatin acid, sodium hydroxide (0.1 N, 9 ml) was added to 6 ml of a SIM solution in ethanol (42 g/l). The resulting solution was stirred at 50° C for 2 h to open up the lactone ring of SIM. Residual ethanol was removed under vacuum. The resulting solution was neutralized to pH 6.0 with HCl (0.1 M) and then was freeze-dried. The resulting dry powder was dispersed in acetone and centrifuged to remove NaCl. Simvastatin acid was then isolated from the supernatant. To prepare the molecular complex between simvastatin acid and alendronate-beta-cyclodextrin (ALN-CD [13]), simvastatin acid and ALN-CD were mixed in de-ionized water at the final concentrations of simvastatin acid 10 mg/ml and ALN-CD 75 mg/ml. The complex (pH 6.0) was stirred at room temperature for 1 h and then sterilized by filtration (0.22 μm) for the animal study.
Animal Procedures
All animal procedures were approved by the Institutional Animal Care & Use Committee at the University of Nebraska in accordance with National Institutes of Health guidelines. A bilateral mandible model (11) using 44 mature female Sprague Dawley rats (Harlan Teklad, Madison, WI, USA) was used for these experiments. Prior to injections, all rats were sedated and maintained by isoflurane inhalation anesthesia.
Active drug treatments were randomized to right and left sides, and injections were placed over the lateral aspect of the mandible near the inferior angle. A 25 gauge 1 cm long needle was inserted 6 mm intramuscularly at the angle of the mandible, and the solution was deposited supraperiosteally. Short-term Experiments: 2 mg simvastatin dissolved in 50 μl of 70% ethanol was injected on one side of the mandible (SIM-EtOH), and 50 μl of 70% ethanol alone was injected on the other (EtOH control) (n = 20), as described previously. (11) A pilot study showed that a 2 mg simvastatin dose produced significant bone growth after 3 weeks without systemic complications. After healing (21 days) was attained, the animals were euthanized by CO2 asphyxiation. Additional rats (n = 12) were injected with alendronate-beta-cyclodextrin conjugates (ALN-CD) or alendronate in water (ALN) on one side of the mandible, versus water alone on the contralateral mandible (control), for additional bone wash studies. Long-term Experiments: To evaluate the ability of SIM/ALN-CD to create and preserve new bone compared to SIM-EtOH, right and left sides of the mandible were randomly injected with these two drug preparations as described above, then histologic new bone was evaluated following euthanization at 48 days (n = 12).
Bone Wash Sampling
Bone wash sampling was accomplished using the device modified from previous reports, (14) consisting of a 20 gauge outer cannula containing 27 gauge inner cannula attached to a syringe. The outer cannula penetrated the skin and muscle until it reached the bone surface at the site of experimental drug/control injections, where it formed a seal against the bone. 500 μl of PBS were injected onto the bone surface through the inner cannula, and the fluid containing bone wash then was collected using suction force through the outer cannular into sterile vials. Samples were obtained at baseline, day 7 and/or day 21 in one set of rats (SIM-EtOH & EtOH, ALN-CD & H2O, ALN & H2O; n = 22), and at day 3 and day 14 in the other set (SIM-EtOH & EtOH; n = 10) to provide at least a 7-day interval between sampling to minimize the impact of sampling trauma on subsequent samples.
Histomorphometric Analysis of Decalcified Specimens
To determine amount of bone growth and surrounding inflammation, samples were prepared for decalcified sections that included both sides of the mandible and overlying soft tissue. (4) The specimens were decalcified in 5% formic acid, embedded in paraffin and subsequently 5 μm thick sections were cut at 200 μm intervals to give in-depth evaluation of the mandibular bone surface, and stained with hematoxylin-eosin.
Three sections for each animal (both sides in the same section) were analyzed without knowledge of group using a light microscope and a digital camera interfaced with Sigma Scan Pro 5 software (SPSS Inc., Chicago, IL, USA). As described previously, (11) mandibular width of old and new bone (micrographs previously shown in references 4 & 11) was calculated as the average of three levels measured 1 mm apart beginning at the base of the mandible, and mandibular area of old and new bone was measured. Percent osteoblast surface (forming surface), flat-lining cell surface and osteoclast surface (resorbing surface) on the bone periphery were measured under 20× power after confirmation of cell type under 400×. Morphometric analysis of cell types in adjacent soft tissue was accomplished by grid intersection point counting at 400× power, yielding the percent of plasma cells, lymphocytes, neutrophils, macrophages, monocytes, muscle, fibroblasts, collagen and blood vessels.
Analysis of Biomarkers
Total Protein
The amount of total protein in bone wash samples was quantified using a BCA protein assay kit (Pierce Chemical, Rockford, IL, USA). Twenty five μl of each standard or unknown sample were used and the working range was 20-2000 μg/ml. The absorbance was measured at 562 mm using a microplate reader (BioTek, North Seattle, WA, USA) and the concentration of total protein in each bone wash sample was calculated.
Prostaglandin E2
A Luminex Prostaglandin E2 kit (Cayman Chemical, Ann Arbor, MI, USA) was used to quantify the amount of PGE2 in samples. This assay is based on the competition between PGE2 of unknown concentration in sample and phycoerytherin-conjugated PGE2 tracer of known concentration for a limited amount of PGE2 monoclonal antibody binding site on the microsphere beads. In brief, 100 μl of standard or sample were placed into appropriated wells into which phycoerytherin-conjugated PGE2 tracer and beads coupled with monoclonal antibody for PGE2 were added. Raw data were reported as fluorescence intensity (FI) using the Bio-Plex reader. The standard curve and the concentration of samples were calculated using data analysis tools provided by Cayman Chemical (sensitivity = 35 pg/ml).
Cell Culture
In order to explore potential mechanisms of PGE2 production, prominent cells of the periosteal microenvironment (osteoblasts and fibroblasts) stimulated by simvastatin were evaluated for activation of genes important to the PGE2 bone anabolic pathway. Murine calvarial preosteoblasts MC3T3-E1 subclone 14 (CRL-2594, Manassas, VA, USA) and murine embryonic fibroblasts (CF1, ATCC # SCRC-1040) were cultured in α-MEM (Hyclone, Logan, UT) with 1 mM sodium pyruvate containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/ml streptomycin and 0.5 μg/ml amphotericin B (Invitrogen, Carlsbad, CA, USA) under a humidified 5% CO2 atmosphere. Cells were subcultured and seeded in 6 well cell culture plates at a concentration of 15,000 cells/cm2. When cells reached approximately 80% confluence, the concentration of FBS was decreased to 2% in media before the treatment.
Real-time (semi-quantitative) PCR
The cells were incubated with and without 10−7 M simvastatin dissolved in ethanol carrier. The concentration of ethanol was 0.01% in media. Total RNA was extracted 6-72 h after treatment using TRLzol® (Invitrogen) and isolated according to the manufacturer's protocol. An RNA pellet was dissolved in diethyopyrocarbonate, treated with (DEPC) H2O and stored at −70° C. cDNA was synthesized using the Superscrip III first-strand synthesis system (Invitrogen, Carlsbad, CA, USA) by manufacturer's instruction. The real time PCR was performed in triplicate using Real master mix SYBR Rox (Fisher Scientific, Pittsburgh, PA, USA). One μl cDNA was amplified in a 20 μl master mix containing hot master Tag DNA polymerase, 10 mM magnesium acetate, 1.0 mM dNTPs with dUTP and SYBR fluorescent dye solution. The oligonucleotide primers were designed by Plexor primer design software (Promega, Madison, WI, USA) and the primers used for microsomal PGE2 synthase and EP4 had following sequences: mPGE2 synthase: forward, 5′-ACTTCATCTCCTCCGTCCTGG-3′; reverse 5′-AAGGAGGTGACCGAGTTTTGC-3′; EP4: forward, 5′-CCTTCATCCTACTTTTCTTCGGTCTG-3′; reverse, 5′-TGTAGAAGTAGGCGTGGTTGATGG-3′ (UNMC Eppley Molecular Biology Core Laboratory, Omaha, NE, USA). β-Actin was used for the housekeeping gene. Each primer was used at a 300 nM concentration. Amplication protocol was as follows: initial temperature denaturation at 94-95° C for 1 min, 40 cycles of 94° C for 20 seconds for cycled temperature denaturation; 55° C for 20 sec for annealing; and 68° C for 30 sec for extension. Dissociation curve was obtained after last cycle to validate the specificity of the primer. The comparative ΔΔCt method was used to assess relative changes in mRNA levels amongst samples.
Statistical Analyses
Histological measurements within animals (between injection groups) were compared using analysis of variance (ANOVA). The concentration of PGE2 was normalized by total protein in each sample. PGE2 levels among bone wash sampling times (baseline, 3, 7, 14 and 21 days), between injection groups and between animals with high eventual bone growth (area of new bone > 0.5 mm2) and low eventual bone growth (area of new bone < 0.5 mm2) were compared using repeated measures ANOVA. Spearman Correlation Coefficients were used to compare earlier biomarker levels (conventional and rank-transformed data [15]) with subsequent bone histology at 21 days. mRNA levels were compared using ANOVA and Bonferroni post hoc tests.
Results
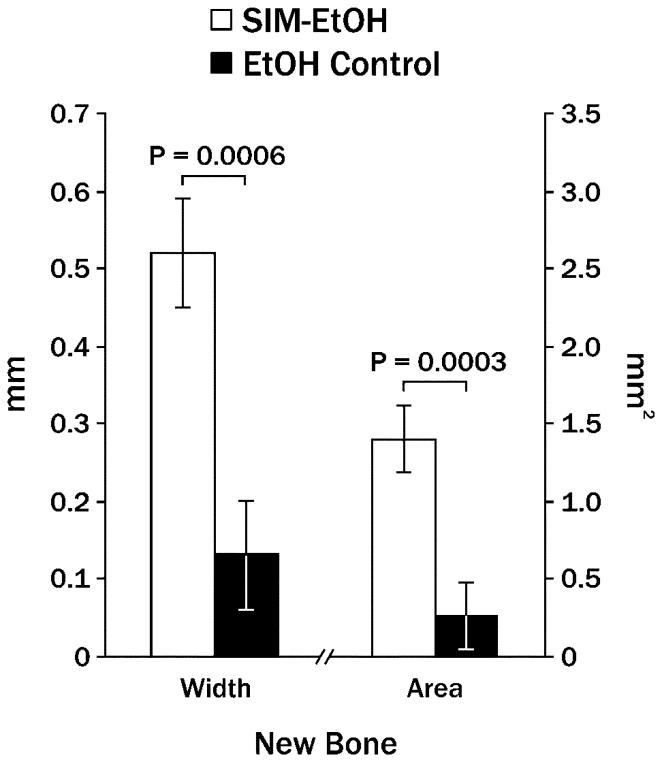
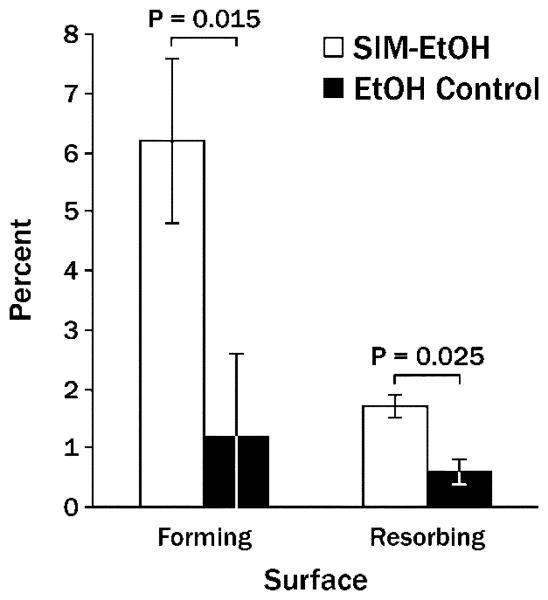
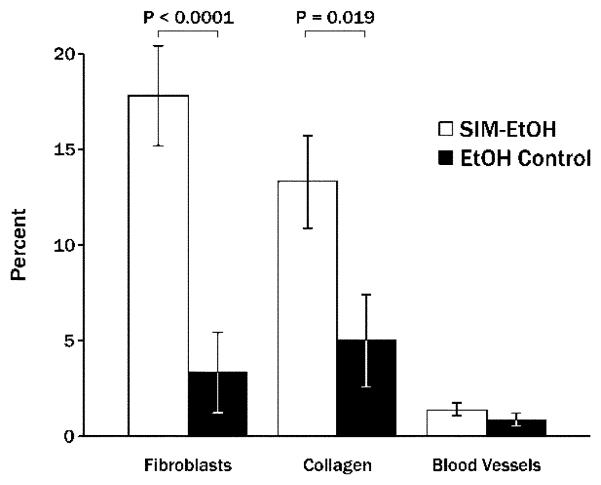
Single injections of 2 mg simvastatin in ethanol on one side of the mandible produced significantly more new bone width and new bone area than contralateral ethanol alone after 21 days (Figure 2). At that point in time, the ratio of bone forming to bone resorbing surface was 3.65:1 around simvastatin injections and 2.0:1 around control injections (Figure 3). The area adjacent to the bone (displacing muscle tissue) was composed primarily of fibroblast-like cells and collagen (Figure 4), with significantly higher values on the simvastatin side.
Figure 2.
Mean new mandibular bone width and area (± standard error of the mean) 21 days following a single injection of 2 mg simvastatin in 70% ethanol (SIM-EtOH) compared to contralateral ethanol alone (EtOH).
Figure 3.
Mean percent (± SEM) of bone forming and bone resorbing surface 21 days following a single injection of 2 mg simvastatin in ethanol (SIM-EtOH) compared to contralateral ethanol alone (EtOH).
Figure 4.
Mean percent (± SEM) of area occupied by fibroblast-like cells, collagen-like tissue and blood vessels displacing muscle adjacent to the injection site 21 days following 2 mg simvastatin/ethanol injections (SIM-EtOH) compared to contralateral ethanol alone (EtOH).
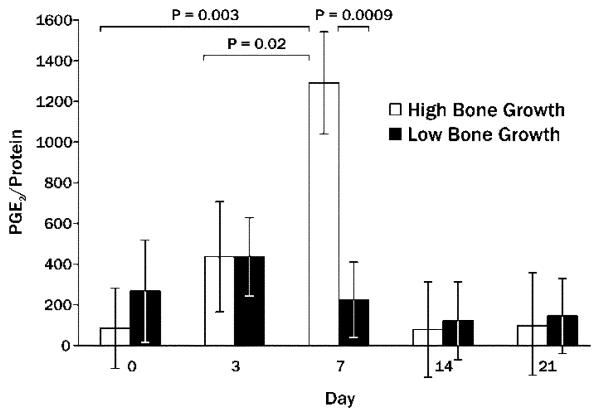
PGE2 was detectable in all bone wash samples. PGE2/protein ratios peaked at day 7 in both simvastatin (2822.5 ± 673.2) and control (246.7 ± 673.2) sites, but were not statistically different between simvastatin and control groups. However, when sites that showed higher levels of bone growth (>0.5 mm2 new bone area) at 21 days post-injection were compared to sites that showed limited bone growth (<0.5 mm2 new bone area), PGE2/protein was significantly higher in the high growth sites after 7 days (Figure 5). Day 7 levels of PGE2/protein trended toward a positive correlation (Spearman) with the amount of 21-day new bone width (Table 1).
Figure 5.
Mean PGE2/protein ratio (± SEM) at various days post-injection preceding 21-day determination of high amounts of mandibular new bone area (>0.5 mm2; High Bone Growth) compared to limited new bone area (<0.5 mm2; Low Bone Growth).
Table 1.
Significant Spearman Correlations Between Simvastatin-stimulated Early PGE2 Levels and Subsequent Bone Histology
| Day |
Side |
Histology Measure |
R Value/p Value |
|---|---|---|---|
| 0 | SIM-EtOH | % blood vessel | −.94/0.005 |
| 7 | SIM-EtOH | New bone width | .58/0.07 |
| SIM-EtOH | % resorb. surface | .75/0.01 | |
| SIM-EtOH | % collagen | .68/0.03 | |
| SIM-EtOH | % blood vessel | .73/0.02 | |
| 14 | SIM-EtOH | New bone width | −.74/0.01 |
All other significant correlations between early SIM-stimulated PGE2 and subsequent histology are outlined in Table 1. The percentage of blood vessels around bone was negatively correlated with PGE2/protein in the simvastatin specimens at baseline. This changed to a significant positive correlation for PGE2/protein at day 7. Finally, the correlation between day 14 PGE2/protein and day 21 new bone width changed to a negative relationship in the SIM group. Pearson Correlation Coefficients were similar to the Spearman correlations, but with generally higher R values (data not shown). Furthermore, correlations using the rank-transformed bone wash data showed a similar pattern, strongly confirming the impact of PGE2 at day 7 on day 21 new bone width (r = 0.63, P = 0.003), new bone area (r = 0.59, P = 0.006) and percent resorbing surface (r = 0.58, P = 0.008).
Data shown in Figure 5 and Table 1 suggest a close relationship between day 7 amounts of PGE2 and subsequent bone growth. However, in the following week the PGE2 levels had a negative impact on this future bone growth in the simvastatin group. Using linear regression analysis, 77% of new bone area variability was explained by PGE2/protein (R2 = 0.769, P = 0.01) on simvastatin sides. This association held for PGE2/protein in the control specimens for new bone width (R2 = 0.920, P = 0.0001) and new bone area (R2 = 0.730, P = 0.0046).
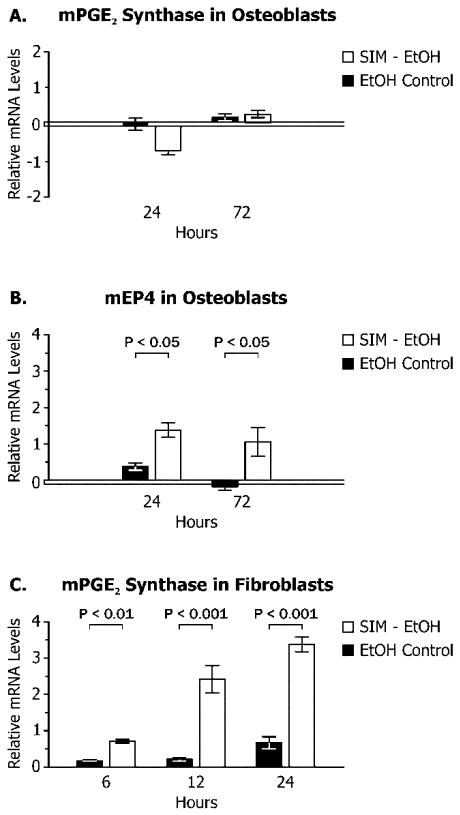
When preosteoblasts were stimulated with simvastatin in culture, the relative amount of mRNA for PGE2 synthase, the primary enzyme driving PGE2 production, was not increased (Figure 6A). However, significant increase in the EP4 mRNA, the primary PGE2 receptor in this cell line, was noted after 24 h and 72 h (over 2 fold, Figure 6B). In contrast to the osteoblasts, a fibroblast cell line representing the main cellular component of the periosteal microenvironment showed an increase in the mRNA for PGE2 synthase after 6-24 h (5 fold over control at 24 h, Figure 6C).
Figure 6.
PGE2 synthase (A) and EP4 (B) mRNA expression in rodent preosteoblasts, and PGE2 synthase mRNA expression in rodent fibroblasts (C) in cell culture following stimulation with simvastatin/ethanol (SIM-EtOH) compared to ethanol alone (EtOH control) (mean relative change from zero at baseline ± standard deviation).
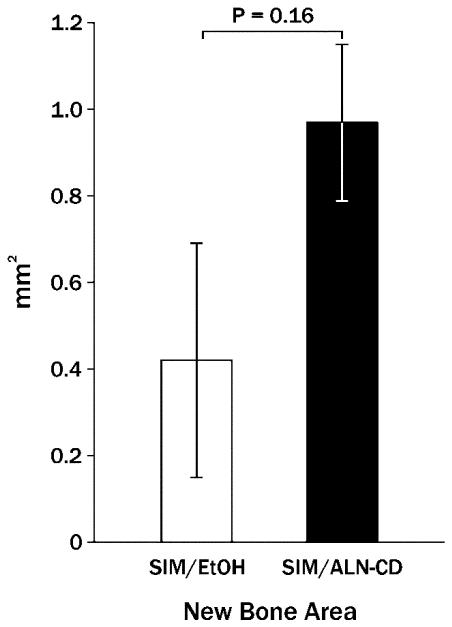
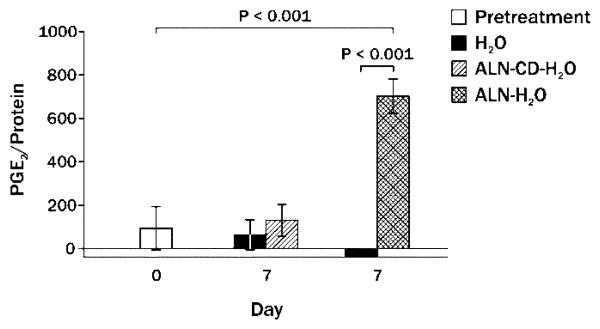
The previous data indicate that injectable simvastatin is capable of stimulating new bone formation associated with PGE2 pathway activation, but the new bone is susceptible to subsequent resorption and lack of localization to the site of injection. (11) Therefore, simvastatin acid (active form of simvastatin) was complexed with alendronate-beta-cyclodextrin conjugate (ALN-CD), an osteotropic drug carrier containing the anti-resorption drug alendronate. Long-term (48 day) results of single injections of 2 mg simvastatin/alendronate-beta-cyclodextrin conjugate on rat mandibles compared with 2 mg simvastatin in ethanol (same preparation as the 21-day experiments) showed a trend toward more new bone area surviving in SIM/ALN-CD group (Figure 7). At an earlier time period (24 days), the bone at the SIM/ALN-CD injection site had a lower percent osteoclast/mm of bone surface than SIM-EtOH (0.3 ± 0.2 vs. 1.0 ± 0.2%, P = 0.007) and number of osteoclasts/mm (0.14 ± 0.09 vs. 0.50 ± 0.09, P = 0.009). At the 48 day time, both SIM-EtOH and SIM/ALN-CD had minimal forming or resorbing bone surface (mostly quiescent bone) without elevated fibroblasts, collagen or signs of inflammation in surrounding soft tissue. Injection of the ALN-CD component of the complex did not stimulate any more PGE2 production in bone washes after 7 days than water injections alone, although ALN in water caused a significant increase in 7-day PGE2 (Figure 8).
Figure 7.
Mean new mandibular bone area (± standard error of the mean) 48 days following a single injection of 2 mg simvastatin in 70% ethanol (SIM-EtOH) compared to 2 mg simvastatin in alendronate-cyclodextrin (SIM/ALN-CD).
Figure 8.
Mean PGE2/protein ratio (± SEM) prior to and 7 days post-injection of alendronate (ALN) or alendronate-beta-cyclodextrin (ALN-CD) both diluted in water compared to water alone.
Discussion
PGE2 is a major product of the COX pathway and has been proposed as a mediator of both bone formation and resorption. (16-18) PGE2 also appears to be associated with angiogenesis (Table 1). Statins are known to regulate COX-2 expression in different ways depending on the amount of local inflammation. For instance, statins stimulated cells of the monocyte lineage to express COX-2 gene and produce PGE2 by controlling small G protein after the blockade of the mevalonate pathway. (19) However, LPS-stimulated expression of both COX-2 and PGE2 was suppressed by statins in a Rac and NF-kB-dependent manner. (20) Statin-induced PGE2 production in the bone microenvironment was a unique aspect of the current study. This is the first investigation to our knowledge to follow PGE2 levels chronologically after local simvastatin application, and relate this pattern to eventual bone growth. Seven days after SIM injections, the bone wash PGE2 levels from the surrounding area were significantly correlated with subsequent new bone width. Sites with higher bone growth had significantly more PGE2/protein than sites with low bone growth. In fact, 77% of the variability of new bone was explained by PGE2/protein levels around simvastatin injections in the current study. PGE2 levels also correlated with the percent of resorbing surfaces seen in the 21-day histology.
Limitations of the bone wash technique include: 1) contamination of the sample with blood products; 2) slight variability in the amount of wash retrieved; and 3) sampling at a single point on the periosteum without intrabony access. Bone wash PGE2 levels in the current study have been normalized with essentially albumin, which is not locally produced and helps standardize for differences in sample volume and bleeding contamination. Bone wash measurements used here are not intended to represent definitive values, but rather to rank low versus high PGE2 amounts, then compare these to low versus high subsequent bone growth in the same area. Therefore, correlations also were analyzed using rank transformation of bone wash data, where values were ranked from low to high. Such data calculation avoided much of the potential variability around the continuous biomarker measurements. Results confirmed the correlations between day 7 PGE2 and subsequent new bone width and percent resorbing surface.
Alternate sampling methods to assess bone PGE2 levels would include obtaining bone and periosteal biopsies, with the disadvantages of very small sample amounts disrupting the experimental site and jeopardizing subsequent biochemical or histologic measurements. Unpublished pilot studies in our laboratory have shown that harvesting bone and periosteum postmortem around SIM injection sites in rat palates resulted in PGE2 levels undetectable by ELISA. Larger mandibular specimens containing portions of the overlying masseter muscle have been sufficient for biochemical analysis, (8) but these animals were euthanized. Gingival crevicular fluid (GCF) samples are another option in humans though not rats, but a previous human study showed that GCF and bone wash measurements of bone turnover markers were significantly different, presumably because GCF is mostly produced further from the bone surface. (14) However, multiple bone wash measurements of untreated areas over an 8-20 day period were not different, supporting the reproducibility of the method.
The possible sources of PGE2 production and its interaction with bone forming cells remain a matter of speculation. However, increased in vivo osteoblasts and bone-forming surface were noted in the simvastatin injection sites (Figure 3). In addition, fibroblasts and collagen were enhanced adjacent to the bone forming surfaces as a result of SIM injections (Figure 4). Our in vitro studies confirmed that rodent fibroblast cell lines stimulated with SIM, but not preosteoblast cells, showed elevated activation of PGE2 synthase within the first 24 h. According to our other experiments, expression of mPGE2 synthase and release of PGE2 in osteoblast and fibroblast cell culture were correlated (data not shown). Furthermore, EP4, the primary PGE2 receptor on the osteoblasts, was up-regulated within 24 h. These results suggest that simvastatin stimulation of PGE2 synthesis by fibroblasts and activation of PGE2 receptor in osteoblasts may be one of the mechanisms of osteogenesis by local statin delivery. While systemic doses of statin have generally been shown to down-regulate PGE2 production in rat tissues (21) and humans, (22) local application of statins and prostaglandin E receptor agonists (i.e. PGE up-regulation) have shown promise in stimulating bone grown in animal models. (4, 5, 8, 11, 23) In vitro statin-induced stimulation of COX-2 gene transcription and PGE2 formation have been demonstrated in other cell types, such as macrophages (18) and keratinocytes. (24) Taken together, these findings are consistent with locally-applied simvastatin enhancing the PGE pathway among cells in the bone microenvironment.
Even though PGE2 levels were considerably lower 14 days after SIM injections, PGE2 values at that time point were negatively correlated with new bone width one week later. These findings suggest that a transition was occurring between day 7 and 14 whereby the timing and local concentration of PGE2 in the bone turnover cycle may be important to eventual bone augmentation. It has been found previously that PGE can have both anabolic and catabolic activity depending on its local concentration. (25) In a recent review, (26) it was concluded that PGE2 contributed to catabolic or anabolic actions dependent on the factor inducing PG production and the local cellular milieu. For instance, PGE2 stimulation of bone resorption in rodents is primarily dependent on Gαs activation in the EP4 receptor, particularly among inflammatory cells where proinflammatory cytokines IL-1, IL-6, IL-11, IL-17 and tumor necrosis factor-α drive continuous PG production and upregulate receptor activator of nuclear factor kB ligand (RANKL). On the other hand, bone formation is enhanced by intermittent higher dose pulses of PGE (potentially induced by intermittent simvastatin [11]) via EP2 or EP4 receptors on osteoblasts, and cAMP/PKA or EPAC/NFAT, CREB or AP-1 pathways. Our previous studies indicated that PGE2 inhibition during the first week following simvastatin application caused reduced local bone formation and BMP-2 secretion. (4, 8) Alternatively, reduction of PGE2 at the 14-day time point may be important for improved odds of bone preservation.
The delivery system for simvastatin appears to be crucial to achieving clinically-relevant bone growth in the oral/facial region. The standard carrier for simvastatin injections in the current rat bilateral mandible study was 70% ethanol, compared to earlier studies using a simvastatin suspension in methylcellulose gel. (11) The advantages of the ethanol carrier over methylcellulose include: 1) less viscosity allowing use of small gauge needles for less trauma and leakage in dense tissue (e.g., masseter muscle or gingival papilla), 2) solution versus suspension to allow better distribution of the drug, and 3) better dissipation of the carrier. However, clinical concerns with the ethanol carrier, such as potential pain at the site of injection, dispersion of the drugs away from the treatment site of injection, and need to preserve new bone over time, make evaluation of carrier alternatives important.
While SIM-EtOH caused significant new bone growth with efficient injections, increased resorbing bone surface at injection sites was noted after 21-24 days compared to EtOH alone (Figure 3), suggesting potential bone resorption in the future. The longer-term (48 days) evaluations indicated that only 30% of the early bone formation (21 days) stimulated by 2 mg SIM-EtOH was retained (Figures 2 and 7). This is similar to the SIM-methylcellulose preparations after 90 days. (11) By contrast, the same dose of SIM in a ALN-CD delivery system averaged nearly 70% retention of early bone (Figures 2 and 7). This is consistent with the lower percent of osteoclasts/mm and number of osteoclasts/mm seen at SIM/ALN-CD versus SIM-EtOH sites at 21 days, suggesting the likelihood that reduced subsequent resorption (between days 21 & 48) would occur. In addition, the ALN-CD conjugate itself did not stimulate significant PGE2 after 7 days in vivo, while ALN alone stimulated a highly significant increase in PGE2 (Figure 8). Interestingly, we have reported that ALN-CD has an anabolic effect on rat mandibular bone, probably by sequestering endogenous PGE2 from the bone microenvironment. (13) PGE2 may complex readily with the cyclodextrin conjugate and form a reservoir for sustained release of PGE2. In the current study, simvastatin acid occupied much of the CD cavities, thereby preventing the PGE2 sequestration potential. The data also suggest that ALN conjugated to cyclodextrin could not stimulate the PGE2 bone anabolic pathway during the first week, yet the anabolic effects of simvastatin acid that complexed with the cyclodextrin were intact. Probably, ALN-CD was gradually metabolized and alendronate or its derivative might be released locally, thereby inhibiting osteoclast activity and bone resorption. Additional effort has been undertaken in our laboratories to better understand the local metabolism of ALN-CD. In addition, longer term studies evaluating new bone in function (e.g. around an implant) using histomorphometry and bone stress tests should be performed.
We conclude that: 1) simvastatin in ethanol carrier is a potent inducer of mandibular bone growth; 2) there is direct evidence for the importance of PGE2 in the early bone anabolic effects of locally-applied simvastatin; and 3) the use of ALN-CD carriers hold promise to enhance simvastatin bone augmentation procedures because they reduce potential side-effects (pain), may target/retain drug to the desirable bone surface, and preserve the newly-formed bone.
Acknowledgements
These studies were supported by USPHS Research Grants DE-015096 (RAR) and AR-053325 (DW) from the National Institutes of Health, Bethesda, MD 20892. The authors thank Marian Schmid for animal technology support; Christopher Fries, Miles Berg and Aaron Bradley for laboratory procedures; Susan McCoy for manuscript preparation and Kim Theesen for graphic design.
References
- 1.Jung RE, Windisch SI, Eggenschwiler AM, Thoma DS, Weber FE, Hämmerle CH. A randomized-controlled clinical trial evaluating clinical and radiological outcomes after 3 and 5 years of dental implants placed in bone regenerated by means of GBR techniques with or without addition of BMP-2. Clin. Oral Implants Res. 2009;20:660–666. doi: 10.1111/j.1600-0501.2008.01648.x. [DOI] [PubMed] [Google Scholar]
- 2.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, Liu C, Zang G, Sun H. The effect of simvastatin on remodeling of the alveolar bone following tooth extraction. Int. J. Oral Maxillofac. Surg. 2008;37:170–176. doi: 10.1016/j.ijom.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Stein D, Lee Y, Schmid MJ, Killpack B, Genrich MA, Narayana N, Marx DB, Reinhardt RA. Local simvastatin effects on mandibular bone growth and inflammation. J. Periodontol. 2005;76:1861–1870. doi: 10.1902/jop.2005.76.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thylin MR, McConnell JC, Schmid MJ, Reckling RR, Ojha J, Bhattacharyya I, Marx DB, Reinhardt RA. Effects of statin gels on murine calvarial bone. J. Periodontol. 2002;73:1141–1148. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 6.Maeda T, Kawane T, Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology. 2003;144:681–692. doi: 10.1210/en.2002-220682. [DOI] [PubMed] [Google Scholar]
- 7.Wong RW, Rabie AB. Statin collagen grafts used to repair defects in the parietal bone of rabbits. Br. J. Oral Maxillofac. Surg. 2003;41:244–248. doi: 10.1016/s0266-4356(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 8.Bradley JD, Cleverly DG, Burns AM, Helm NB, Schmid MJ, Marx DB, Cullen DM, Reinhardt RA. Cyclooxygenase-2 inhibitor reduces simvastatin-induced bone morphogenetic protein-2 and bone formation in vivo. J. Periodont. Res. 2007;42:267–273. doi: 10.1111/j.1600-0765.2006.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris MS, Lee Y, Lavin MT, Giannini PJ, Schmid MJ, Marx DB, Reinhardt RA. Injectable simvastatin in periodontal defects and alveolar ridges: pilot studies. J. Periodontol. 2008;79:1465–1473. doi: 10.1902/jop.2008.070659. [DOI] [PubMed] [Google Scholar]
- 10.Rutledge J, Schieber MD, Chamberlain JM, Byarlay M, Killeen AC, Giannini PJ, Marx DB, Reinhardt RA. Simvastatin application to augment facial jaw bone in a dog model: pilot study. J. Periodontol. 2010 Nov 2; doi: 10.1902/jop.2010.100214. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Schmid MJ, Marx DB, Beatty MW, Cullen DM, Collins ME, Reinhardt RA. The effect of local simvastatin delivery strategies on mandibular bone formation in vivo. Biomaterials. 2008;29:1940–1949. doi: 10.1016/j.biomaterials.2007.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshinari M, Matsuzaka K, Hashimoto S, Ishihara K, Inoue T, Oda Y, Ide T, Tanaka T. Controlled release of simvastatin acid using cyclodextrin inclusion system. Dent. Mater. J. 2007;26:451–456. doi: 10.4012/dmj.26.451. [DOI] [PubMed] [Google Scholar]
- 13.Liu X-M, Wiswall AT, Rutledge JE, Akhter MP, Cullen DM, Reinhardt RA, Wang D. Osteotropic β-cyclodextrin for local bone regeneration. Biomaterials. 2008;29:1686–1692. doi: 10.1016/j.biomaterials.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson AN, Schmid MJ, Marx DB, Reinhardt RA. Bone turnover markers in serum and periodontal microenvironments. J. Periodont. Res. 2003;38:355–361. doi: 10.1034/j.1600-0765.2003.02002.x. [DOI] [PubMed] [Google Scholar]
- 15.Conover WJ. Practical Non-parametric Statistics. Wiley; New York: 1971. p. 337. [Google Scholar]
- 16.Yoshida K, Oida H, Kobayashi T, Maruyama T, Tanaka M, Katayama T, Yamaguchi K, Segi E, Tsuboyama T, Matsushita M, Ito K, Ito Y, Sugimoto Y, Ushikubi F, Ohuchida S, Kondo K, Nakamura T, Narumiya S. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. USA. 2002;99:4580–4585. doi: 10.1073/pnas.062053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez-Yañez GO, Seymour GJ, Walsh LJ, Forwood MR, Symons AL. Prostaglandin E2 enhances alveolar bone formation in the rat mandible. Bone. 2004;35:1361–1368. doi: 10.1016/j.bone.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa} B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–1998. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 19.Chen JC, Huang KC, Wingerd B, Wu WT, Lin WW. HMG-CoA reductase inhibitors induce COX-2 gene expression in murine macrophages: role of MAPK cascades and promoter elements for CREB and C/EBP beta. Exp. Cell. Res. 2004;301:305–319. doi: 10.1016/j.yexcr.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 20.Habib A, Shamseddeen I, Nasrallah MS, Antoun TA, Nemer G, Bertoglio J, Badreddine R, Badr KF. Modulation of COX-2 expression by statins in human monocytic cells. FASEB J. 2007;21:1665–1674. doi: 10.1096/fj.06-6766com. [DOI] [PubMed] [Google Scholar]
- 21.Nassar PO, Nassar CA, Guimaraes MR, Acquino SG, Andia DC, Muscara MN, Spolidorio DM, Rossa C, Jr., Spolidorio LC. Simvastatin therapy in cyclosporine A-induced alveolar bone loss in rats. J. Periodontol. Res. 2009;44:479–488. doi: 10.1111/j.1600-0765.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Hernandez A, Sanchez-Galan E, Ortego M, Martin-Ventura JL, Blanco-Colio LM, Tarin-Vincente N, Jimenez-Nacher JJ, Lopez-Bescos L, Egido V, Tunon J. Effect of intensive atorvastatin therapy on prostaglandin E2 levels and metalloproteinase-9 activity in the plasma of patients with non-ST-elevation acute coronary syndrome. Am. J. Cardiol. 2008;102:12–18. doi: 10.1016/j.amjcard.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 23.Axelrad TW, Kakar S, Einhorn TA. New technologies for the enhancement of skeletal repair. Injury. 2007;38(Suppl):549–562. doi: 10.1016/j.injury.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Coutant KD, Wolff-Winiski B, Ryder NS. Fluvastatin enhances receptor-stimulated intracellular Ca2+ release in human keratinocytes. Biochem. Bisphys. Res. Commun. 1998;245:307–312. doi: 10.1006/bbrc.1998.8429. [DOI] [PubMed] [Google Scholar]
- 25.Miller SC, Marks SC., Jr. Local stimulation of new bone formation by prostaglandin E1: quantitative histomorphometry and comparison of delivery by minipumps and controlled-release pellets. Bone. 1993;14:143–151. doi: 10.1016/8756-3282(93)90241-2. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]