Abstract
Protein kinase C-zeta (PKCζ), an atypical isoform of the PKC family of protein serine/threonine kinases, is expressed in human platelets. However, the mechanisms of its activation and the regulation of its activity in platelets are not known. We have found that under basal resting conditions, PKCζ has a high phosphorylation status at the activation loop threonine 410 (T410) and the turn motif (autophosphorylation site) threonine 560 (T560), both of which have been shown to be important for its catalytic activity. After stimulation with agonist under stirring conditions, the T410 residue was dephosphorylated in a time- and concentration-dependent manner, while the T560 phosphorylation remained unaffected. The T410 dephosphorylation could be significantly prevented by blocking the binding of fibrinogen to integrin αIIbβ3 with an antagonist, SC-57101; or by okadaic acid used at concentrations that inhibits protein serine/threonine phosphatases PP1 and PP2A in vitro. The dephosphorylation of T410 residue on PKCζ was also observed in PP1cγ null murine platelets after agonist stimulation, suggesting that other isoforms of PP1c or another phosphatase could be responsible for this dephosphorylation event. We conclude that human platelets express PKCζ, and it may be constitutively phosphorylated at the activation loop threonine 410 and the turn motif threonine 560 under basal resting conditions, which are differentially dephosphorylated by outside-in signaling. This differential dephosphorylation of PKCζ might be an important regulatory mechanism for platelet functional responses.
Keywords: Protein kinase C, phosphorylation, dephosphorylation, phosphatase, integrin
1. Introduction
In most cell types, extracellular signals are relayed from the surface receptors to intracellular signaling molecules, such as the protein kinase C (PKC) family of serine/threonine kinases. These PKCs phosphorylate key serine and threonine residues present in target proteins leading to conformational changes resulting in alteration of their functions, which will eventually dictate the cellular responses [1, 2]. Thus, PKCs are an integral part of cellular signal transduction processes. Various members of the classical and novel classes of PKC have been shown to be important for platelet functional responses such as aggregation, granule secretion, and thromboxane generation [3–7]. PKC-zeta (PKCζ) is a member of the atypical class of the PKC family [8]. Unlike the conventional and the novel isoforms, the atypical PKCs do not respond to second messenger diacylglycerol (DAG) because its C1 domain is modified to contain only one zinc-finger motif [8]. They also do not bind calcium because they lack the calcium binding C2 domain [8]. They are, however, sensitive to phosphoinositide 3-kinase (PI3-K) lipid product phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and are activated by 3-phosphoinositide dependent protein kinase-1 (PDK1) in a PIP3-enhanced manner [9]. PKCζ, in addition, can be activated by phospholipase D2 (PLD2) via protein-protein interaction [10]. Phosphorylation of the threonine 410 residue present in the activation loop and autophosphorylation of threonine 560 residue present in the turn motif, of the kinase domain of the enzyme, have been shown to be important for its kinase activity [10–12]. However, evidence also exists that it can be catalytically active in the absence of threonine 410 phosphorylation [13].
PKCζ functions as a MEK1 kinase in human alveolar macrophages [14]. Atypical PKCs transduce signals from the receptors of TNFα and IL-1 to the activation sites of NFκB in vivo [15]. It has recently been shown that PKCζ phosphorylates its upstream target, insulin receptor substrate-1, -3 and −4, but not −2, and this represents a negative feedback mechanism that may contribute to signal specificity in insulin action [16]. Furthermore, the polarity complex PAR-3/PAR-6/PKCζ plays an essential role in polarization in epithelial cells and migrating astrocytes [17]. An alternatively spliced variant PKMζ is involved in long-term potentiation (LTP) maintenance in hippocampal pyramidal cells [18]. Thus, this enzyme is involved in many functional responses in various types of cells.
In mice deficient in PKCζ, phenotypic alterations are seen in the secondary lymphoid organs such as spleen and lymph nodes [19]. The genetic deletion of PKCζ results in an impairment of B-cell receptor signaling and defects in the transcription of NFκB-dependent genes [20]. PKCζ knock-outs also have a defective T-cell-dependent immune response [20]. However, these mice are grossly normal [19].
PKCζ has been shown to be expressed in human platelets [21–23], although it is not known whether it gets phosphorylated and/or activated downstream of receptor activation by physiological agonists. The molecular mechanisms of its regulation as well as the physiological functions of this kinase, in platelets, are also unknown. Since this enzyme is expressed in both platelets and immune cells, it can be speculated that it might play a role in linking immune responses with thrombosis. In this study, we investigated the mechanism of regulation of PKCζ activation in platelets. We have confirmed its expression in human platelets, and have also found it to be basally phosphorylated at the activation loop threonine 410 and the turn motif threonine 560 under resting conditions. Upon agonist stimulation, the threonine 410 residue gets dephosphorylated, possibly in an integrin αIIbβ3-dependent manner, whereas the phosphorylated threonine 560 remains unaffected. This differential dephosphorylation might be important for platelet functional responses.
2. Materials and methods
2.1 Materials
Apyrase (type VII), bovine serum albumin (fraction V), and acetylsalicylic acid were obtained from Sigma (St Louis, MO, USA). PGE1 was purchased from Enzo Life Sciences (Plymouth Meeting, PA, USA). AYPGKF, a PAR-4 agonist, was custom synthesized at Invitrogen (Carlsbad, CA, USA). SC-57101 was a gift from Searle and Co (Greenwich, CT, USA). Ethyl alcohol (Absolute, 200 proof) was purchased from Aaper Alcohol Co. (Shelbyville, KY, USA). The β-actin antibody was purchased from Cell Signaling Technology (Beverly, MA, USA). The phospho-PKC ζ/λ (Thr410/403) (Cell Signaling Technology, Beverly, MA, USA) [24–26] and phospho-PKCζ (pT560) (Epitomics, Burlingame, CA, USA) [27, 28] antibodies have been validated by other investigators, as well as by the company (product datasheets). Furthermore, platelets do not express PKC λ isoform [29, 30], so the phospho-PKC ζ/λ (Thr410/403) antibody will detect PKCζ in platelets only when it is phosphorylated at Thr410. Both antibodies have also been shown to be reactive in both human and mouse tissues (company product datasheets). Total PKC ζ antibody, okadaic acid (a general serine/threonine inhibitor) and HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Chemiluminescent HRP-substrate was from Millipore (Billerica, MA, USA). Ketamine (Ketaset) was purchased from Fort Dodge Animal Health (Fort Dodge, IA, USA). All the other reagents were of reagent grade, and de-ionized water was used throughout.
2.2 Animals
CD-1 mice carrying PP1cγ-null mutation and subsequently backcrossed for 10 generations onto Balb/C background were generated in the laboratory of Susannah Varmuza (University of Toronto, Toronto, Canada) [31].
2.3 Isolation of human platelets
All experiments using human subjects were performed in accordance with the Declaration of Helsinki. Whole blood was drawn from healthy consenting human volunteers into tubes containing one-sixth volume of ACD (2.5 g of sodium citrate, 1.5 g of citric acid, 2 g of glucose in 100 ml of deionized water). Blood was centrifuged (Eppendorf 5810R centrifuge) at 230 g for 20 min at room temperature (25°C) to obtain PRP (platelet-rich plasma). PRP was incubated (or not) with 1 mM aspirin for 30 min at 37°C. The PRP was then centrifuged for 10 min at 980 g at room temperature to pellet the platelets. Platelets were resuspended in Tyrode's buffer pH 6.5 (138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM PIPES, and pH 6.5) containing 0.1 U/mL apyrase and 1 μM PGE1 (or not). The platelets were also prepared without aspirin and PGE1 treatment for some experiments. The platelet suspension was then centrifuged for 10 min at 980 g at room temperature to pellet the platelets. Platelets were then resuspended in Tyrode's buffer pH 7.4 (138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM Hepes, and pH 7.4) containing 0.1 U/mL apyrase. Platelets were counted using the Hemavet (Drew Scientific Inc., Dallas, TX, USA) and concentration of cells was adjusted to 2 × 108 platelets/mL. The washed platelet suspension contained approximately 200,000 platelets/μl, less than 100 leukocytes/μl and RBCs were not detectable. All experiments using isolated platelets were performed within 4 hours after drawing blood without or with (for some experiments) added extracellular calcium (1mM).
2.4 Okadaic acid stock solutions preparation
A master stock solution (1 mM) of okadaic acid was first prepared by dissolving it in 200 proof absolute ethanol. This was serially diluted in absolute ethanol to get stock solutions of 250, 100, 50 and 5 μM.
2.5 Isolation of murine platelets
Blood was collected from ketamine-anesthetized mice by cardiac puncture into syringes containing 3.8% sodium citrate as anticoagulant. The whole blood was centrifuged (IEC Micromax Centrifuge, International Equipment Components, CA, USA) at 100g for 10 minutes to isolate the PRP. Prostaglandin E1 (1 μM) was added to PRP. The platelets were centrifuged at 400g for 10 minutes, and the pellet was resuspended in Tyrode's buffer pH 7.4 containing 0.1U/mL apyrase.
2.6 Platelet aggregation
Platelet aggregation was measured using a lumi-aggregometer (Chrono-Log, Haverton, PA, USA) at 37°C under stirring conditions (900 rpm). A 0.5 mL sample of aspirin-treated (or non-aspirin -treated) washed human platelets was stimulated with 500 μM AYPGKF and the change in light transmission was measured. In okadaic acid studies, platelets were pre-incubated with 1 μl okadaic acid stock solutions of 5, 50, 100, and 250 μM (giving final concentrations of 10, 100, 200 and 500 nM, respectively) or 1 μl ethanol for 5 min at 37°C before agonist stimulation. The chart recorder (Kipp and Zonen, Bohemia, NY, USA) was set at 0.2 mm/s.
2.7 Western Immunoblot analysis
Platelets were stimulated with AYPGKF and the reaction was stopped by the addition of SDS–Laemmli buffer (1× is 10% by volume glycerol, 62.5 mM Tris-HCL, pH 6.8, 2% SDS, 0.01 mg/ml bromophenol blue, 1 mM Dithiothreitol (DTT)). Proteins were separated by 10% SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked by incubation with Tris (tris(hydroxymethyl)aminomethane)-buffered saline (TBS: 20 mM Tris, 140 mM NaCl) containing 3% (wt/vol) bovine serum albumin (BSA) for 1 hour at room temperature. Membranes were incubated overnight at 4°C with the primary antibody (1:1000 dilution) in loading buffer (TBS with 3% BSA) with gentle agitation. After three washes for 5 minutes each with Tris-Buffered Saline Tween-20 (TBS-T), the membranes were probed with an HRP-conjugated secondary antibodies (1:10,000 dilution) in TBS-T for 1 hour at room temperature. After additional washing steps, membranes were then incubated with the chemiluminescent HRP-substrate and immunoreactivity was detected using a Fuji Film Luminescent Image Analyzer (LAS-3000 CH, Tokyo, Japan).
2.8 Statistical analysis
Western blot data were compiled from at least three independent experimental results. Densitometric analyses of the phospho-PKCζ immunoreactive bands were performed using Fuji Film Science Lab 2003 Image Gauge Version 4.22 software and were normalized to their corresponding β-actin bands, and then the percent change (of the normalized values) was calculated taking the unstimulated samples as 100%. The results were expressed as mean ± SEM. Statistical significance was tested by One-way ANOVA followed by Bonferroni post hoc analysis. P values <0.05 was considered statistically significant, which are indicated by *, ** or *** for P <0.05, <0.01 or <0.001, respectively; and non-significance is indicated by N.S.
3. Results
3.1 PKCζ is expressed in human platelets and is phosphorylated under basal resting conditions
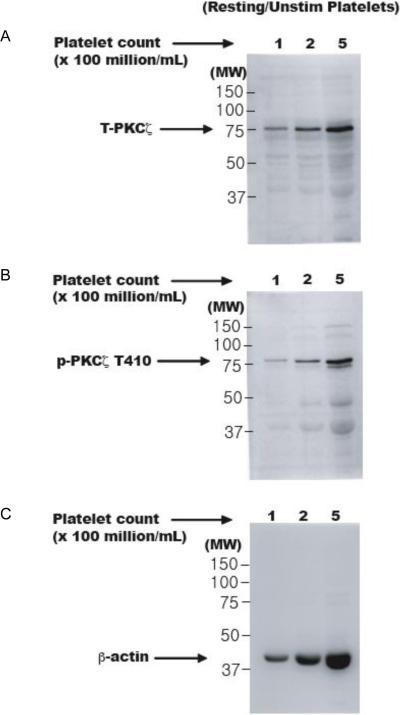
PKCζ has been shown to be expressed in human platelets [21–23]. In this study, we have confirmed the presence of this enzyme in human platelets by immunoblotting for total protein. Different concentrations of unstimulated platelet samples (100, 200 and 500 million per ml) were first prepared and whole cell lysates were analyzed by Western blotting for the total protein. As shown in figure 1A, the signal strength of the protein of ~ 75 kDa increased with increasing concentrations and we conclude that PKCζ is expressed in human platelets.
Figure 1. Expression of PKCζ in human platelets.
Whole cell lysates of various concentrations of unstimulated human platelet samples (100, 200 and 500 million platelets per mL of Tyrode's buffer) were prepared and run on an SDS-PAGE. Western blots, representative of three independent experimental results, for total PKCζ (Panel A) and phospho-PKCζ T410 (Panel B). Corresponding lane loading controls with β-actin are also shown (Panel C). Unstim: unstimulated; MW: molecular weight in kDa; T-PKCζ: total-PKCζ; p-PKCζ T410: phosphorylated PKCζ at threonine 410.
The T410 residue present in the activation loop has been shown to be important for its kinase activity [10–12]. We probed the unstimulated platelet lysates with a phospho-specific antibody and our results show that PKCζ is phosphorylated at T410 under basal resting conditions in platelets (Fig. 1B). We did not observe any phosphorylation of other enzymes, such as Erk, in these resting platelet samples (data not shown), which verifies that the high basal phosphorylation of PKCζ was not a consequence of inadvertent platelet activation during blood collection and platelet isolation.
Since phosphorylation of this residue has been shown to be important for its activity, we speculate that PKCζ may be constitutively active under resting conditions in platelets. One potential mechanism for this is via protein-protein interaction. It was shown that the phox homology (PX) domain of phospholipase D2 (PLD2) directly interacts with the kinase domain of PKCζ, and this association results in phosphorylation and activation of PKCζ, which is independent of PLD2's lipase activity [10]. Of note, PLD2 has been shown to be present in platelets [32]. In co-immunoprecipitation experiments, we did not observe an association between these two proteins (data not shown). A plausible explanation could be that these two proteins dissociate after activation and hence we did not detect their association.
Additionally, there are reports that cleavage of PKCζ by caspases could result in generation of a catalytically active subunit (the kinase domain) of this enzyme [12]. We have used a pan-caspase inhibitor in our experiments but did not observe any effect on the dephosphorylation event (data not shown), suggesting that caspase-mediated cleavage of PKCζ probably does not occur in platelets.
3.2 Dephosphorylation of T410 residue upon agonist stimulation of platelets
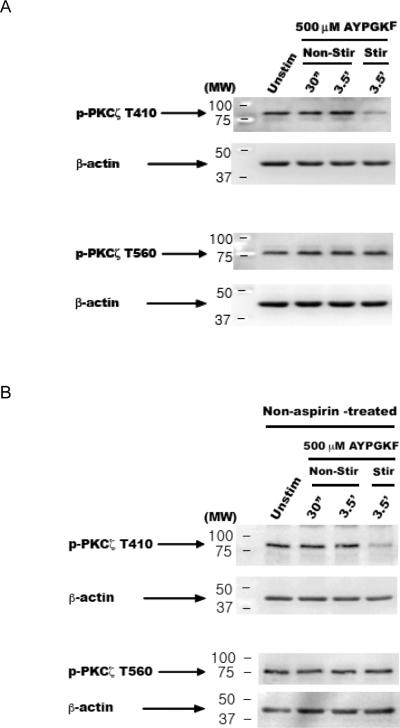
We observed that PKCζ has a high basal phosphorylation. For most other enzymes, agonist stimulation leads to an increase in the levels of phosphorylation and/or activity from a low basal state. A high basal phosphorylation could mean a constitutively phosphorylated state, which could decrease with agonist treatment. To test this hypothesis, we activated platelets with a PAR4 agonist AYPGKF (500 μM) under both non-stirring and stirring conditions, and have found that the T410 residue gets dephosphorylated upon agonist stimulation only under stirring conditions (Fig. 2A). T560, another residue present in the turn motif of the kinase domain, also has been shown to be important for its activity [8]. Surprisingly, T560 phosphorylation was not affected by agonist stimulation, either under non-stirring or stirring conditions (Fig. 2A). We have also performed the same experiments using non-aspirin, non-PGE1, - treated platelets (Fig. 2B), as well as in the presence of 1 mM extracellular calcium (data not shown), and observed the similar differential dephosphorylation responses.
Figure 2. Dephosphorylation of T410 residue of PKCζ with agonist stimulation under stirring conditions.
Washed human platelets were stimulated with PAR4 agonist AYPGKF (500 μM) for various time-points under non-stirring (non-stir) and stirring (stir) conditions, and lysates prepared. Western blots, representative of three independent experimental results, for phospho-PKCζ T410 and phospho-PKCζ T560. Aspirin-treated (Panel A); and non-aspirin, non-PGE1 -treated (Panel B) samples. Corresponding lane loading controls with β-actin are also shown. Unstim: unstimulated.
3.3 T410 becomes dephosphorylated in an aggregation- and integrin αIIbβ3-dependent manner
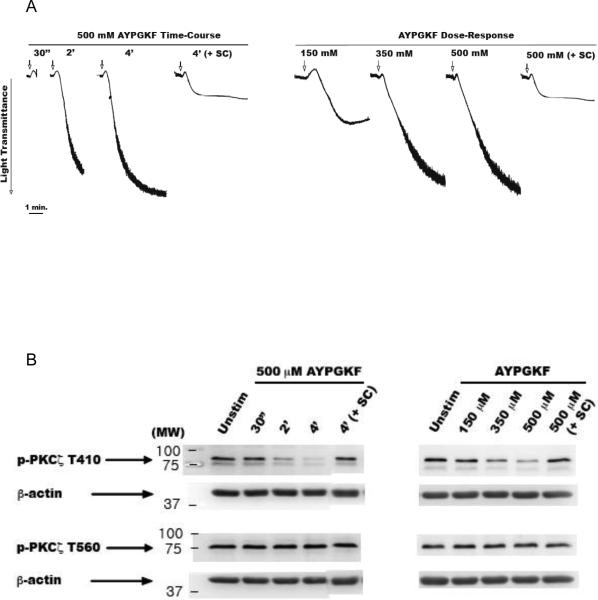
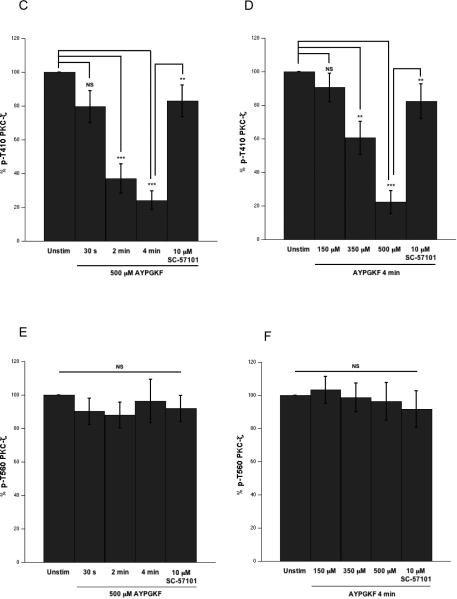
Next, we performed a full panel of time-course and dose-response of agonist stimulation under stirring conditions (Fig. 3A) to further evaluate the dephosphorylation event. Platelets were stimulated with 500 μM of AYPGKF for various time points (0–4 min) and lysates were analyzed by Western blotting (Fig. 3B). We observed that T410 gets dramatically dephosphorylated in a time-dependent manner (~80%, at 4 min) (Fig. 3B and C). Platelets were also stimulated with varying concentrations of AYPGKF (100–500 μM) for 4 mins. Again, the T410 residue was found to get dephosphorylated in a concentration-dependent manner (Fig. 3B and D). Therefore, the T410 dephosphorylation is dependent on aggregation. The T560 phosphorylation, on the other hand, remained unaffected by aggregation (Fig. 3B, E and F).
Figure 3. Differential dephosphorylation of PKCζ via agonist-stimulated platelet aggregation and prevention of T410 dephosphorylation by integrin αIIbβ3 blockade.
Representative aggregation tracings of washed and aspirin-treated human platelets stimulated under stirring conditions, with 500 μM AYPGKF for various time-points and for 4 mins in the presence of 10 μM of integrin αIIbβ3 antagonist SC-57101 (panel A left); and with varying concentrations of AYPGKF for 4 mins and with 500 μM AYPGKF for 4 mins in the presence of 10 μM of integrin αIIbβ3 antagonist SC-57101 (panel A right). Representative Western blots of the lysates probed with phospho-PKCζ T410 and phospho-PKCζ T560 antibodies (panel B). Densitometric analysis of the phospho-PKCζ T410 blots from the time-course (panel C) and concentration-response (panel D) experiments. Densitometric analysis of the phospho-PKCζ T560 blots from the time-course (panel E) and concentration-response (panel F) experiments. Data compiled from three independent experimental results. Corresponding lane loading controls with β-actin are also shown. Unstim: unstimulated, SC: SC-57101, *: p<0.5, **: p<0.01, ***: p<0.001, NS: non-significant.
From the previous observation, it was evident that the T410 dephosphorylation could be mediated via fibrinogen binding to activated integrin αIIbβ3 (outside-in signaling). To confirm this hypothesis, we blocked the integrin with an antagonist, SC-57101 (10 μM) and stimulated the platelets with the agonist for 4 mins (Fig. 3A). The T410 dephosphorylation was significantly prevented by blocking the interaction of integrin with fibrinogen and the subsequent outside-in signaling (Fig. 3B–D). This result strongly suggests that αIIbβ3 activation-mediated signaling leads to dephosphorylation of T410 on PKCζ. Alternatively, other potential signaling (besides αIIbβ3 activation-mediated signaling) that could arise from cell-cell contact could contribute to the dephosphorylation. It is worth mentioning here that basal T560 phosphorylation is not affected either by agonist stimulation or by integrin blockade (Fig. 3B, E and F). The differential dephosphorylation of PKCζ was also observed with other agonists, such as SFLLRN (acting on PAR1) and convulxin (acting on GPVI) (data not shown).
3.4 Dephosphorylation of T410 is possibly mediated by αIIbβ3-associated ser/thr phosphatases
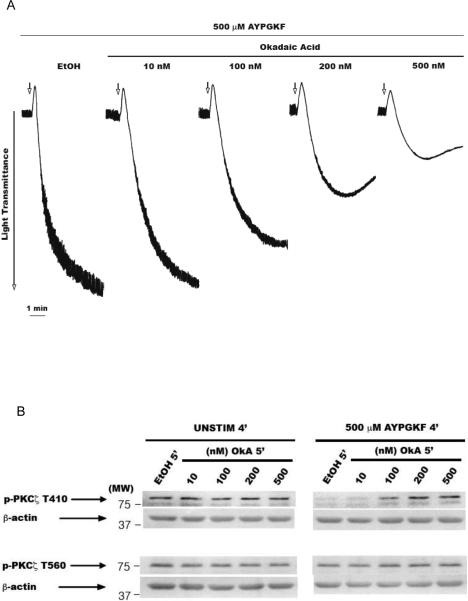
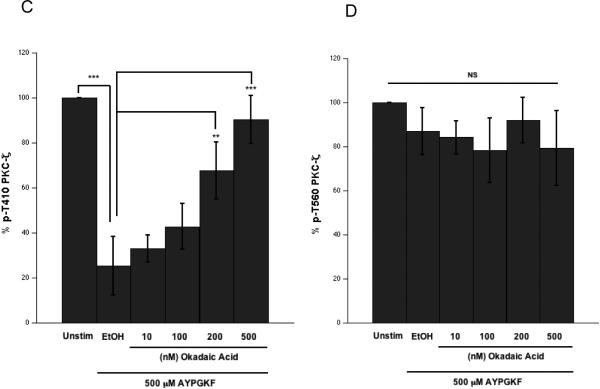
Our results showed that the integrin αIIbβ3 outside-in signaling possibly mediates the dephosphorylation of T410 residue. Although, αIIbβ3 does not have any intrinsic phosphatase activity, the catalytic subunits of protein phosphatase 1 (PP1c) [33] and protein phosphatase 2A (PP2Ac) [34] associate constitutively with the cytoplasmic tail of integrin αIIb subunit. Upon integrin engagement, PP1c dissociates from the integrin complex and gets activated [33]. In contrast, fibrinogen binding to the integrin does not alter PP2Ac-αIIb interaction and moderately reduces PP2Ac activity [34]. In order to determine if these phosphatases participated in the dephosphorylation of T410 residue on PKCζ, we employed okadaic acid, a generic Ser/Thr phosphatase inhibitor [35]. Platelets were pre-incubated with various concentrations (1–500 nM) of okadaic acid for 5 mins at 37°C and then stimulated with the agonist for 4 mins (Fig. 4A). Lysates were analyzed by Western blotting. Okadaic acid by itself did not affect the basal phosphorylation levels of T410 and T560 (Fig. 4B). We observed that the agonist-dependent dephosphorylation of T410 could be significantly prevented by okadaic acid concentrations of 200 nM and above (Fig. 4B and C), a concentration range that inhibits PP1 and PP2A in cell-free systems in vitro [35]. This suggested that the agonist-mediated T410 dephosphorylation is mediated by serine/threonine protein phosphatases. Alternatively, since okadaic acid inhibited aggregation, it is equally possible that the prevention of T410 dephosphorylation was a consequence of inhibition of integrin-fibrinogen interaction, in addition to its ability to inhibit the phosphatases. However, the basal T560 phosphorylation remained unaffected in platelets treated with okadaic acid and stimulated with the agonist (Fig. 4B and D).
Figure 4. Prevention of T410 dephosphorylation by okadaic acid.
Washed and aspirin-treated human platelets were preincubated with 1 μl absolute ethanol or various concentrations of okadaic acid (10–500 nM) at 37° C for 5 mins under stirring conditions, and then stimulated with 500 μM AYPGKF for 4 mins under stirring conditions. Representative aggregation tracings (panel A). Representative Western blots of the lysates probed with phospho-PKCζ T410 and phospho-PKCζ T560 antibodies (panel B). Unstimulated (panel B left) and stimulated with 500 μM AYPGKF (panel B right) samples. Densitometric analysis of the phospho-PKCζ T410 blots, with each phospho-PKCζ T410 signal in the AYPGKF-stimulated sample normalized to its corresponding phospho-PKCζ signal in the unstimulated sample (panel C). Densitometric analysis of the phospho-PKCζ T560 blots, with each phospho-PKCζ T560 signal in the AYPGKF-stimulated sample normalized to its corresponding phospho-PKCζ signal in the unstimulated sample (panel D). Corresponding lane loading controls with β-actin are also shown. Data compiled from three independent experimental results. Unstim: unstimulated, EtOH: ethyl alcohol (200 proof), OkA: okadaic acid, *: p<0.5, **: p<0.01, ***: p<0.001, NS: non-significant.
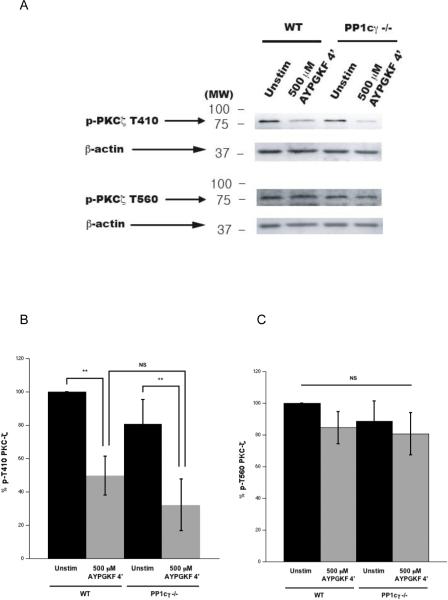
Since the catalytic subunit of protein phosphatase 1 gamma isoform (PP1cγ) is required for thrombin-induced platelet integrin activation [33], we considered whether PP1cγ may participate in dephosphorylating PKCζ T410 downstream of integrin engagement. We used a genetic approach wherein, the dephosphorylation of T410 in PKC ζ was analyzed using platelets from PP1cγ null mice [31]. In our experimental conditions, the agonist-mediated dephosphorylation of T410 on PKCζ still occurred in PP1cγ null platelets (Fig. 5A and B), suggesting that other isoforms of PP1c or another phosphatase could be mediating this event. The basally phosphorylated state of T560 residue remained unaffected after agonist stimulation, even in PP1cγ null platelets (Fig. 5A and C).
Figure 5. Inability of PP1cγ deficient platelets to prevent the T410 dephosphorylation.
Washed and aspirin-treated platelets from wild type or PP1cγ null mice were stimulated with 500 μM APGKF for 4 mins under stirring conditions, and lysates were prepared. Representative Western blots of the lysates probed with phospho-PKCζ T410 and phospho-PKCζ T560 antibodies (panel A). Densitometric analyses of the phospho-PKCζ T410 blots (panel B) and the phospho-PKCζ T560 blots (panel C). Corresponding lane loading controls with β-actin are also shown. Data compiled from three independent experimental results. Unstim: unstimulated, WT: wild type, PP1cγ−/−: PP1cγ null mice, **: p<0.01, NS: non-significant.
We have tried to measure the enzymatic activity of PKCζ by performing an invitro kinase assay on the immuoprecipitated protein, but were unsuccessful (data not shown). Unfortunately, the functional roles of PKCζ in platelets could not be evaluated at this time. Addition of the myristoylated peudosubstrate inhibitory peptide into the platelet suspension led to destruction/agglutination of platelets.
4. Discussion
The PKC family of serine/threonine kinases mediates a number of physiological functions in platelets such as aggregation, secretion and thromboxane generation [3–7]. The novel and the conventional classes of PKC have been thoroughly studied in this regard [3–7]. However, nothing is known regarding the regulation and the physiological role of the atypical member PKCζ in platelets [21–23]. The current study was undertaken to investigate the regulatory mechanism of this enzyme in platelets.
PKCζ was detected by Western blot analysis of the lysates prepared from washed platelets, and is basally phosphorylated on threonine 410 (T410) and threonie 560 (T560) residues. At least in some cell types, PKCζ is known to be constitutively phosphorylated and active under basal resting conditions [36] Agonist-stimulation (only under stirring conditions) led to dephosphorylation of the T410 residue but not the T560 residue, suggesting that integrin activation and/or signaling might be necessary for T410 dephosphorylation. Consistently, T410 dephosphorylation could be significantly prevented by blocking fibrinogen binding to the integrin, even when the platelets were stimulated with the agonist.
Integrins do not have any intrinsic phosphatase activity but a pool of Ser/Thr phosphatases interact with the integrin complex. We investigated a role for such phosphatases in the dephosphorylation of T410 residue. Okadaic acid (a ser/thr phosphatase inhibitor) by itself could alter the basal phosphorylation levels of many proteins because the inhibition of phosphatases could shift the equilibrium towards kinase mediated phosphorylation events. Therefore, control unstimulated platelet samples treated with the corresponding concentrations of okadaic acid were prepared, and the final PKCζ phosphorylation levels were calculated by normalizing to the control unstimulated samples. Okadaic acid by itself did not affect the basal phosphorylation status of PKCζ but prevented the agonist-mediated dephosphorylation of T410 on PKCζ strongly suggesting that PP1 and/or PP2A could be the phosphatases responsible for T410 dephosphorylation.
Protein phosphatase 1 (PP1) which is constitutively associated with the integrin and gets activated in an integrin-dependent manner [33] could be one of the possible candidates. The γ isoform of the catalytic subunit of PP1 has been shown to one of the phosphatases responsible for the 2MeSADP-mediated dephosphorylation of PKCη [4]. However, no significant difference in the level of T410 dephosphorylation of PKCζ was observed in the wild-type versus the PP1cγ knock-out mouse platelets, suggesting that this isoform may not be involved in the dephosphorylation process. Other isoforms of the catalytic subunit of PP1 (α, β/δ) or PP2A or another phosphatase, either alone or collectively, could be responsible for this phenomenon. However, such studies could not be conducted at the present time due to the lack of PP1c isoform-specific pharmacological inhibitors, embryonic lethality of PP2Acα null mice [37] and the unavailability of PP1cα or PP1cβ null mice. Of note, the basal phosphorylation of T560 remains unaffected under all the conditions used in this study.
The activation loop threonine 410 of PKCζ is phosphorylated by PDK-1 (PIP2-dependent kinase-1) in many cell systems, and the turn motif threonine 560 is an autophosphorylation site. The phosphorylation of both these two residues have been shown to be required for its kinase activity. However, there is also evidence that this enzyme can still be fully active even in the absence of the T410 phosphorylation site [13]. Tyrosine phosphorylation was observed in mutants where T410 was deleted, but which showed similar kinase activity as the wild-type protein. It was suggested that phosphorylation of tyrosine 428 present near the activation loop, could result in a similar active conformation as the threonine 410 phosphorylated protein [13].
The physiological significance of the basally phosphorylated state of PKCζ and the subsequent dephosphorylation of T410 in an integrin-dependent manner, is not known at the current time. One plausible explanation could be that this enzyme is constitutively active and might act as a negative regulator of platelet functional responses, and integrin-mediated (outside-in) signaling leads to its deactivation. The T560 autophosphorylation signal alone might not be sufficient to maintain its full kinase activity. Studies in PKCζ knockout mice might hopefully shed some lights into the physiological role/s of this protein in platelets.
In summary, we have demonstrated that, in platelets, the atypical PKC isoform zeta (ζ) may be constitutively phosphorylated at the activation loop threonine 410 residue as well as the turn motif threonine 560 residue. Only the threonine 410 gets dephosphorylated, possibly in an integrin αIIbβ3-dependent manner (Fig. 6). Although serine/threonine phosphatases PP1c and PP2Ac could possibly regulate this dephosphorylation, PP1cγ isoform is not involved in this event.
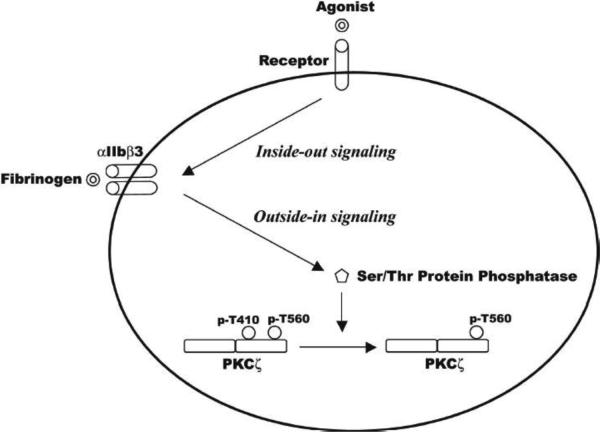
Figure 6. Hypothetical model of regulation of PKCζ in platelets.
PKCζ is expressed in human platelets and is basally phosphorylated on threonine 410 and 560 residues. Agonist stimulation, via aggregation- and activated integrin-mediated outside-in signaling that activates an unidentified serine/threonine phosphatase, leads to dephosphorylation of the T410 residue, but not the T560 residue.
Acknowledgements
The authors would like to sincerely thank Nawaf Alrehani (Baylor College of Medicine, TX), Dr. Soochong Kim and Monica Dupon (Temple University) for the transport and maintenance of the mice; Dr. Jianguo Jin (Temple University) for drawing blood from mice; and Carol Dangelmaier and Todd Getz (Temple University) for their valuable technical suggestions.
FUNDING This work was supported by HL81322, HL93231 and HL60683 from National Institutes of Health to SPK, and HL081613 to KVV.
Abbreviations
- PKC
Protein kinase C
- DAG
diacylglycerol
- PI3-K
phosphoinositide 3-kinase
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PKD1
3-phosphoinositide dependent protein kinase-1
- PLD2
phospholipase D2
- PP1
serine/threonine protein phosphatase 1
- PP2A
serine/threonine protein phosphatase 2A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors declare that there is no conflict of interest.
REFERENCES
- [1].Pfeilschifter J, Huwiler A. Regulatory functions of protein kinase C isoenzymes in purinoceptor signalling in mesangial cells. J Auton Pharmacol. 1996;16:315–8. doi: 10.1111/j.1474-8673.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- [2].Rainbow RD, Norman RI, Everitt DE, Brignell JL, Davies NW, Standen NB. Endothelin-I and angiotensin II inhibit arterial voltage-gated K+ channels through different protein kinase C isoenzymes. Cardiovasc Res. 2009;83:493–500. doi: 10.1093/cvr/cvp143. [DOI] [PubMed] [Google Scholar]
- [3].Quinton TM, Kim S, Dangelmaier C, Dorsam RT, Jin J, Daniel JL, et al. Protein kinase C- and calcium-regulated pathways independently synergize with Gi pathways in agonist-induced fibrinogen receptor activation. The Biochemical journal. 2002;368:535–43. doi: 10.1042/BJ20020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bynagari YS, Nagy B, Jr., Tuluc F, Bhavaraju K, Kim S, Vijayan KV, et al. Mechanism of activation and functional role of protein kinase Ceta in human platelets. The Journal of biological chemistry. 2009;284:13413–21. doi: 10.1074/jbc.M808970200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Murugappan S, Tuluc F, Dorsam RT, Shankar H, Kunapuli SP. Differential role of protein kinase C delta isoform in agonist-induced dense granule secretion in human platelets. The Journal of biological chemistry. 2004;279:2360–7. doi: 10.1074/jbc.M306960200. [DOI] [PubMed] [Google Scholar]
- [6].Yacoub D, Theoret JF, Villeneuve L, Abou-Saleh H, Mourad W, Allen BG, et al. Essential role of protein kinase C delta in platelet signaling, alpha IIb beta 3 activation, and thromboxane A2 release. The Journal of biological chemistry. 2006;281:30024–35. doi: 10.1074/jbc.M604504200. [DOI] [PubMed] [Google Scholar]
- [7].Nagy B, Jr., Bhavaraju K, Getz T, Bynagari YS, Kim S, Kunapuli SP. Impaired activation of platelets lacking protein kinase C-theta isoform. Blood. 2009;113:2557–67. doi: 10.1182/blood-2008-07-169268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem. 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- [9].Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, et al. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–77. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- [10].Kim JH, Kim JH, Ohba M, Suh PG, Ryu SH. Novel functions of the phospholipase D2-Phox homology domain in protein kinase Czeta activation. Mol Cell Biol. 2005;25:3194–208. doi: 10.1128/MCB.25.8.3194-3208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, et al. Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. The Journal of biological chemistry. 1999;274:25308–16. doi: 10.1074/jbc.274.36.25308. [DOI] [PubMed] [Google Scholar]
- [12].Smith L, Wang Z, Smith JB. Caspase processing activates atypical protein kinase C zeta by relieving autoinhibition and destabilizes the protein. The Biochemical journal. 2003;375:663–71. doi: 10.1042/BJ20030926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ranganathan S, Wang Y, Kern FG, Qu Z, Li R. Activation loop phosphorylation-independent kinase activity of human protein kinase C zeta. Proteins. 2007;67:709–19. doi: 10.1002/prot.21348. [DOI] [PubMed] [Google Scholar]
- [14].Monick MM, Carter AB, Flaherty DM, Peterson MW, Hunninghake GW. Protein kinase C zeta plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J Immunol. 2000;165:4632–9. doi: 10.4049/jimmunol.165.8.4632. [DOI] [PubMed] [Google Scholar]
- [15].Sanz L, Diaz-Meco MT, Nakano H, Moscat J. The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway. Embo J. 2000;19:1576–86. doi: 10.1093/emboj/19.7.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee S, Lynn EG, Kim JA, Quon MJ. Protein kinase C-zeta phosphorylates insulin receptor substrate-1, -3, and -4 but not -2: isoform specific determinants of specificity in insulin signaling. Endocrinology. 2008;149:2451–8. doi: 10.1210/en.2007-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–7. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- [18].Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, et al. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008;28:7820–7. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, et al. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–80. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- [20].Martin P, Duran A, Minguet S, Gaspar ML, Diaz-Meco MT, Rennert P, et al. Role of zeta PKC in B-cell signaling and function. Embo J. 2002;21:4049–57. doi: 10.1093/emboj/cdf407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sun L, Mao G, Rao AK. Association of CBFA2 mutation with decreased platelet PKC-theta and impaired receptor-mediated activation of GPIIb-IIIa and pleckstrin phosphorylation: proteins regulated by CBFA2 play a role in GPIIb-IIIa activation. Blood. 2004;103:948–54. doi: 10.1182/blood-2003-07-2299. [DOI] [PubMed] [Google Scholar]
- [22].Wang F, Naik UP, Ehrlich YH, Osada S, Ohno S, Kornecki E. Stimulatory antibody-induced activation and selective translocation of protein kinase C isoenzymes in human platelets. The Biochemical journal. 1995;311(Pt 2):401–6. doi: 10.1042/bj3110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baldassare JJ, Henderson PA, Burns D, Loomis C, Fisher GJ. Translocation of protein kinase C isozymes in thrombin-stimulated human platelets. Correlation with 1,2-diacylglycerol levels. The Journal of biological chemistry. 1992;267:15585–90. [PubMed] [Google Scholar]
- [24].Zhang Q, Adiseshaiah P, Kalvakolanu DV, Reddy SP. A Phosphatidylinositol 3-kinase-regulated Akt-independent signaling promotes cigarette smoke-induced FRA-1 expression. The Journal of biological chemistry. 2006;281:10174–81. doi: 10.1074/jbc.M513008200. [DOI] [PubMed] [Google Scholar]
- [25].Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. Journal of virology. 2003;77:1524–39. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xie Z, Singh M, Siwik DA, Joyner WL, Singh K. Osteopontin inhibits interleukin-1beta-stimulated increases in matrix metalloproteinase activity in adult rat cardiac fibroblasts: role of protein kinase C-zeta. The Journal of biological chemistry. 2003;278:48546–52. doi: 10.1074/jbc.M302727200. [DOI] [PubMed] [Google Scholar]
- [27].Kawata K, Kubota S, Eguchi T, Moritani NH, Shimo T, Kondo S, et al. Role of the low-density lipoprotein receptor-related protein-1 in regulation of chondrocyte differentiation. Journal of cellular physiology. 222:138–48. doi: 10.1002/jcp.21930. [DOI] [PubMed] [Google Scholar]
- [28].Lee JO, Lee SK, Jung JH, Kim JH, You GY, Kim SJ, et al. Metformin induces Rab4 through AMPK and modulates GLUT4 translocation in skeletal muscle cells. Journal of cellular physiology. 226:974–81. doi: 10.1002/jcp.22410. [DOI] [PubMed] [Google Scholar]
- [29].Gilio K, Harper MT, Cosemans JM, Konopatskaya O, Munnix IC, Prinzen L, et al. Functional divergence of platelet protein kinase C (PKC) isoforms in thrombus formation on collagen. The Journal of biological chemistry. 285:23410–9. doi: 10.1074/jbc.M110.136176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bynagari-Settipalli YS, Chari R, Kilpatrick L, Kunapuli SP. Protein kinase C - possible therapeutic target to treat cardiovascular diseases. Cardiovascular & hematological disorders drug targets. 10:292–308. doi: 10.2174/187152910793743869. [DOI] [PubMed] [Google Scholar]
- [31].Varmuza S, Jurisicova A, Okano K, Hudson J, Boekelheide K, Shipp EB. Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1cgamma gene. Dev Biol. 1999;205:98–110. doi: 10.1006/dbio.1998.9100. [DOI] [PubMed] [Google Scholar]
- [32].Vorland M, Holmsen H. Phospholipase D in human platelets: presence of isoenzymes and participation of autocrine stimulation during thrombin activation. Platelets. 2008;19:211–24. doi: 10.1080/09537100701777329. [DOI] [PubMed] [Google Scholar]
- [33].Vijayan KV, Liu Y, Li TT, Bray PF. Protein phosphatase 1 associates with the integrin alphaIIb subunit and regulates signaling. The Journal of biological chemistry. 2004;279:33039–42. doi: 10.1074/jbc.C400239200. [DOI] [PubMed] [Google Scholar]
- [34].Gushiken FC, Patel V, Liu Y, Pradhan S, Bergeron AL, Peng Y, et al. Protein phosphatase 2A negatively regulates integrin alpha(IIb)beta(3) signaling. The Journal of biological chemistry. 2008;283:12862–9. doi: 10.1074/jbc.M708804200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rossini GP, Hess P. Phycotoxins: chemistry, mechanisms of action and shellfish poisoning. Exs. 100:65–122. doi: 10.1007/978-3-7643-8338-1_3. [DOI] [PubMed] [Google Scholar]
- [36].Luiken JJ, Ouwens DM, Habets DD, van der Zon GC, Coumans WA, Schwenk RW, et al. Permissive action of protein kinase C-zeta in insulin-induced CD36- and GLUT4 translocation in cardiac myocytes. J Endocrinol. 2009;201:199–209. doi: 10.1677/JOE-09-0046. [DOI] [PubMed] [Google Scholar]
- [37].Gotz J, Probst A, Ehler E, Hemmings B, Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12370–5. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]