Abstract
A well established functional polymorphism of the human androgen receptor (hAR) is the length of AR’s N-terminal glutamine tract (Q-tract). This tract is encoded by a CAG trinucleotide repeat and varies from 8 to 33 codons in the healthy population. Q-tract length is inversely correlated with AR transcriptional activity in vitro, but whether endogenous androgen action is affected is not consistently supported by results of clinical and epidemiological studies. To test whether Q-tract length influences androgen sensitivity in vivo, we examined effects of controlled androgen exposure in “humanized” mice with hAR knock-in alleles bearing 12, 21 or 48 CAGs. Mature male mice were analyzed before or 2 weeks after orchidectomy, with or without a subdermal dihydrotestosterone (DHT) implant to attain stable levels of this non-aromatizable androgen. The validity of this DHT clamp was demonstrated by similar serum levels of DHT and its two primary 3α/β-Diol metabolites, regardless of AR Q-tract length. Q-tract length was inversely related to DHT-induced suppression of castrate serum LH (p=0.005), as well as seminal vesicle (SV) weight (p=0.005) and prostate lobe weights (p<0.006). This confirms that the hAR Q-tract polymorphism mediates in vivo tissue androgen sensitivity by impacting negative hypothalamic feedback and trophic androgen effects on target organs. In this manner, AR Q-tract length variation may influence numerous aspects of male health, from virilization to fertility, as well as androgen-dependent diseases, such as prostate cancer.
Keywords: AR, CAG repeat, glutamine tract, humanized mouse model
1. Introduction
The androgen receptor (AR) is a ligand-activated transcription factor that is crucial in male sexual differentiation and development (Quigley et al., 1995), including formation of the prostate gland and its late-life pathology (Cunha et al., 2004). A well established genetic polymorphism that influences AR activity is the CAG triplet repeat in Ar’s exon 1, which encompasses the receptor’s N-terminal transactivation domain. This CAG repeat encodes a glutamine tract (Q-tract) that varies in length from 8 to 33 repeats (median 21) in the normal population (Rajender et al., 2007). Pathological Q-tract expansion causes the rare X-linked neurodegenerative disease, spinobulbar muscular atrophy (Kennedy syndrome), resulting from a toxic gain-of-function mechanism, as well as mild androgen insensitivity (Thomas et al., 2006; Lieberman and Robins, 2008).
ARs with shorter Q-tract lengths exhibit increased transactivation of androgen-responsive reporter genes in vitro (Chamberlain et al., 1994; Tut et al., 1997; Beilin et al., 2000), supporting the notion that shorter Q-tract ARs are more active at a given ligand level. However, in vivo androgen action is complicated by pre-receptor hormone activation, post-receptor co-regulator proteins and systemic and tissue steroid metabolism. In vivo, an AR with stronger transcriptional activity (short Q-tract length) would be predicted to produce greater negative feedback suppression of pituitary LH secretion and more potent androgenic trophic effects. However, studies of the relationship between AR Q-tract length and the hypothalamic-pituitary-testicular (HPT) axis in humans have produced conflicting data (Van Pottelbergh et al., 2001; Walsh et al., 2005; Stanworth et al., 2008; Huhtaniemi et al., 2009). Confounding factors that may mask underlying relationships include relatively small effects of Q-tract length, compensation by negative feedback, misclassification of clinical endpoints and interactions with numerous other genetic and physiological pathways.
In order to test directly whether AR Q tract length mediates in vivo androgen sensitivity, we used an engineered mouse model with human AR (hAR) alleles varying in Q-tract length (Albertelli et al., 2006). In these “humanized” mice, the hAR N-terminal domain (NTD) replaces that of the mouse, eliminating the 15% amino acid difference of the murine NTD as well as its displaced Q tract. Alleles with short (12Q), median (21Q) or long (48Q) glutamine tracts represent the average and extremes in Q-tract length within the human population. These AR alleles in mice produce differences in target gene transcription and prostate cancer progression but systemic effects on the androgen sensitivity have not been investigated in detail (Albertelli et al., 2006; Albertelli et al., 2008). To determine androgen effects without the confounding influences of variable circulating levels of testosterone (T) or aromatization of T to estradiol (Huhtaniemi et al., 2009), dihydrotestosterone (DHT) implants were used to “clamp” levels of pure androgen following orchidectomy. The short-term use of the DHT clamp paradigm permits causal relationships to be elucidated unambiguously using an openloop system compared with the inevitable confounding in the native closed-loop system. This reveals the AR Q tract length influence on the key androgen sensitive endpoints of negative hypothalamic-pituitary feedback on gonadotropin secretion and trophic effects on accessory glands.
2. Materials and Methods
2.1 Animals and DHT treatment
Mice with “humanized” AR alleles containing polyamino acid tracts of 12, 21 or 48 Qs were generated and genotyped as previously described (Albertelli et al., 2006). All mouse procedures were approved by the University of Michigan Committee on Use and Care of Animals, in accord with the NIH Guidelines for the Care and Use of Experimental Animals. Male mice were orchidectomized at 8 weeks of age and nine days later treated by subdermal implantation of 0.5 cm silastic tubing filled with ~5 mg crystalline DHT (Singh et al., 1995) for an additional 5 days. DHT was used since it is non-aromatizable unlike testosterone. A DHT dose was chosen, based on previous experience (Simanainen et al., 2009; Allan et al., 2010), to produce partial suppression (~50%) of post-castration increases in serum gonadotrophin levels.
2.2 Sample collection
Mice were killed by cardiac exsanguination under isoflurane anaesthesia. Seminal vesicles were dissected from the urogenital track and freshly weighed both before and after emptying the gland by manual expression of fluid secretions. The amount of secretions was defined as the difference between weights of intact and emptied seminal vesicles. The remaining urogenital track including bladder, urethra, and surrounding prostate lobes was fixed in 4% paraformaldehyde over night at 4°C for histology. Following fixation, individual prostate lobes were dissected free of fat and connective tissue and weighed separately.
2.3 Hormone Assays
All assays were performed in a single batch. Mouse serum LH was analyzed using an immunofluorometric assay as previously described (Jimenez et al., 2005), but using specific antibodies for mLH. The capture antibody used is the anti-LH antibody (5303 SPRN-1, Medix Biochemica, Turku, Finland) and the detection antibody is the anti-LH antibody (MAb 518B7, supplied by Dr J Roser, Dept of Animal Science, UC Davis, (Spearow and Trost, 1987)) directly labeled with a Europium chelate using the DELFIA Eu-labelling kit (Perkin Elmer, City, Country) as per suppliers methodology. For the mLH assay, the detection limit was 0.02 ng/ml, the quantification limit 0.05 ng/ml and the within-assay QC was 6.8% at low (0.25 ng/ml), 4.7% at mid (0.49 ng/ml) and 7.4% at high (1.18 ng/ml) range. Mouse serum FSH was determined using a specific immunofluorometric assay as described and validated previously (Jimenez et al., 2005).
Serum levels of T, DHT and its two principal metabolites 5α-androstane-3α,17β-diol (3αDiol) and 5α-androstane-3β,17β-diol (3βDiol) were measured in extracts of 50 µl (intact) or 100 µl (DHT-treated) of mouse serum by liquid chromatography tandem mass spectrometry (LC-MS/MS) (Harwood and Handelsman, 2009) as adapted for mouse serum and tissues (McNamara et al., 2010). Serum was extracted with 3:2 (volume:volume) of hexane:ethyl acetate fortified with testosterone-1,2,3-d3 (d3-T), dihydrotestosterone-16,16,17-d3 (d3-DHT), 5α-Androstane-3α,17β-diol-16,16,17-d3 (d3-3αDiol) and 5α-Androstane-3β,17β-diol-16,16,17-d3 (d3-3βDiol) as internal standards. The organic layer, separated by freezing the aqueous layer, was dried and reconstituted in 1.2 ml of 20% methanol in PBS prior to injection onto the C8 column for analysis (1 ml). The level of quantification (LOQ) for T, DHT, 3αDiol and 3βDiol were 20, 100, 400 and 400 pg, respectively.
2.4 Histology
Paraffin embedded, fixed prostate lobes were sectioned at 5µm and slides were stained with hematoxylin and eosin for basic histological analysis.
2.5 Detection of proliferation and apoptosis
Cell proliferation was determined using a proliferating cell nuclear antigen (PCNA) kit (Zymed, San Francisco, CA) and apoptosis by in situ detection of TUNEL staining of nuclear DNA fragmentation using the ApopTag kit (Chemicon) as previously described (Simanainen et al., 2009). CASTGRID V1.10 (Olympus Corp. Albertslund, Denmark) software was used for unbiased stereological analysis of prostate epithelial proliferation index based on counting of at least 500 cells as previously described (Simanainen et al., 2007; Simanainen et al., 2009).
2.6 Statistics
Statistical analysis was performed using two-way analysis of variance (ANOVA) with treatment group (intact, castrate, castrate+DHT) and Q-tract length as the main fixed factors using the least significant difference (LSD) method as a post hoc test and suitable linear contrasts for Q-tract trend or specific a priori comparisons. In the case of significant main factor interactions, the simple effects within the main factor were compared by one-way ANOVA with the LSD method as a post hoc test. Statistical analyses were performed using SPSS (SPSS Inc, Chicago, IL) and NCSS (Kaysville, UT) software. Data is expressed as mean and standard error of the mean (SE) unless otherwise specified and P values less than 0.05 were considered statistically significant.
3. Results
3.1 Validation of the DHT clamp
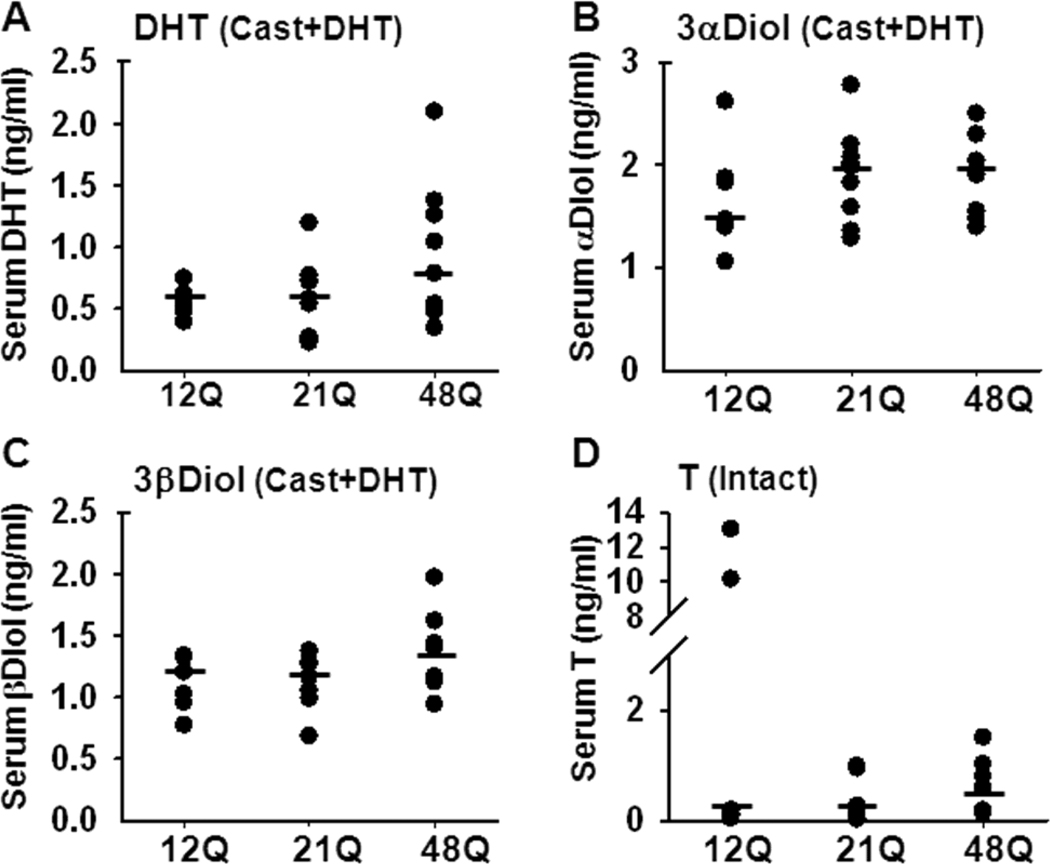
Mature “humanized” AR males at 8 weeks of age underwent orchidectomy to ablate both the androgenic support of target organs and the negative feedback regulation on gonadotrophins. After 9 days, orchidectomized males were treated with subdermal DHT implants for 5 days to fix the androgen delivery at a constant level. The validity of this DHT clamp was determined by analyzing the circulating androgen levels using a LC-MS/MS based method tested previously for mouse samples (McNamara et al., 2010). In orchidectomized, DHT-treated males, serum levels of DHT (Fig 1A) and its metabolites, 3αDiol and 3βDiol (Fig 1B,C), were similar in mice regardless of AR Q-tract length. Serum T was undetectable in all samples from orchidectomized males, with or without DHT treatment. In the intact males, median serum T did not significantly differ with Q-tract length, with the median (range) being 0.11 (0.03–13), 0.13 (0.01–0.98) and 0.38 (0.13–1.5) ng/ml for 12Q, 21Q and 48Q, respectively (Fig 1D).
Figure 1. Serum steroid hormone levels.
The Q-tract length does not significantly influence serum hormone levels. Serum from hAR 12Q, 21Q and 48Q mice was analyzed for DHT (A), 3αDiol (B) and 3βDiol (C) levels in castrated and DHT-treated males and for T in intact males (D), by the LC-MS/MS based method. DHT, 3αDiol and 3βDiol were undetectable in intact males, and no T was detected in serum of DHT-treated males (not shown). Each dot represents the serum steroid concentration of one animal. The median concentrations are shown by horizontal lines. N=8–10 for all groups.
3.2 AR-dependent negative feedback regulation of serum LH, but not FSH, is influenced by AR Q-tract length
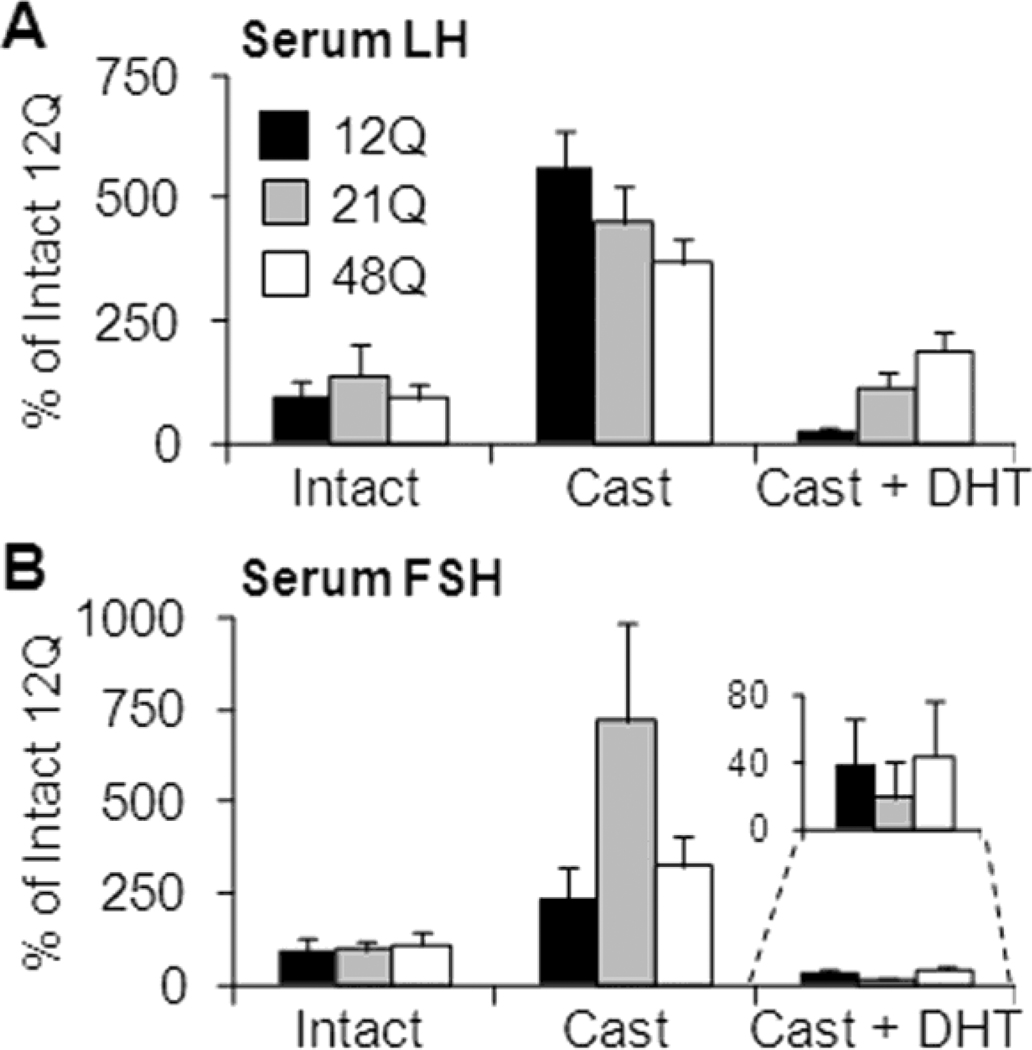
DHT-induced suppression of castrate LH levels was greatest for the shorter Q-tract length (p<0.005 test for trend), with DHT-suppressed serum LH levels being 6, 26 and 48% of castrate levels in 12Q, 21Q and 48Q males, respectively (Fig. 2A). Furthermore, following DHT treatment, the suppressed LH levels remained markedly higher than intact levels in 48Q males (65% higher than intact) while 21Q males were restored to intact levels (15% lower). In 12Q males the DHT treatment reduced serum LH levels below the intact level (70% lower). AR Q-tract length had no influence on serum LH or FSH in intact or untreated orchidectomized mice or on serum FSH levels in DHT-treated orchidectomized mice (Fig. 2B).
Figure 2. Serum gonadotrophin levels.
Feedback on gonadotrophin secretion in Q tract variant mice. Serum gonadotrophins LH (A) and FSH (B) were analyzed using mouse-specific immunofluorometric assays. The levels are shown, relative to intact 12Q males, for intact, castrated (9 days) + DHT (5 days) or castrated (14 days) mice. The LH and FSH levels were similar between intact males with different AR Q-tract lengths. The DHT treatment restored the serum LH from castrate level to intact in 12Q and 21Q males, but not in 48Q males, with a significant (p=0.005) trend observed between Q-tract length and serum LH. Castration significantly increased the LH levels in all males, with the magnitude of increase following the Q-tract length with 12Q highest and 48Q lowest. Serum FSH levels were not statistically different between 12Q, 21Q and 48Q males in any treatment group. Data shown as Mean±SE. N=9–10 for all groups. For reference, the intact levels are shown as Supplemental data in Table 1.
3.3 Androgen-dependent sex accessory organ weights correlate with AR Q-tract length
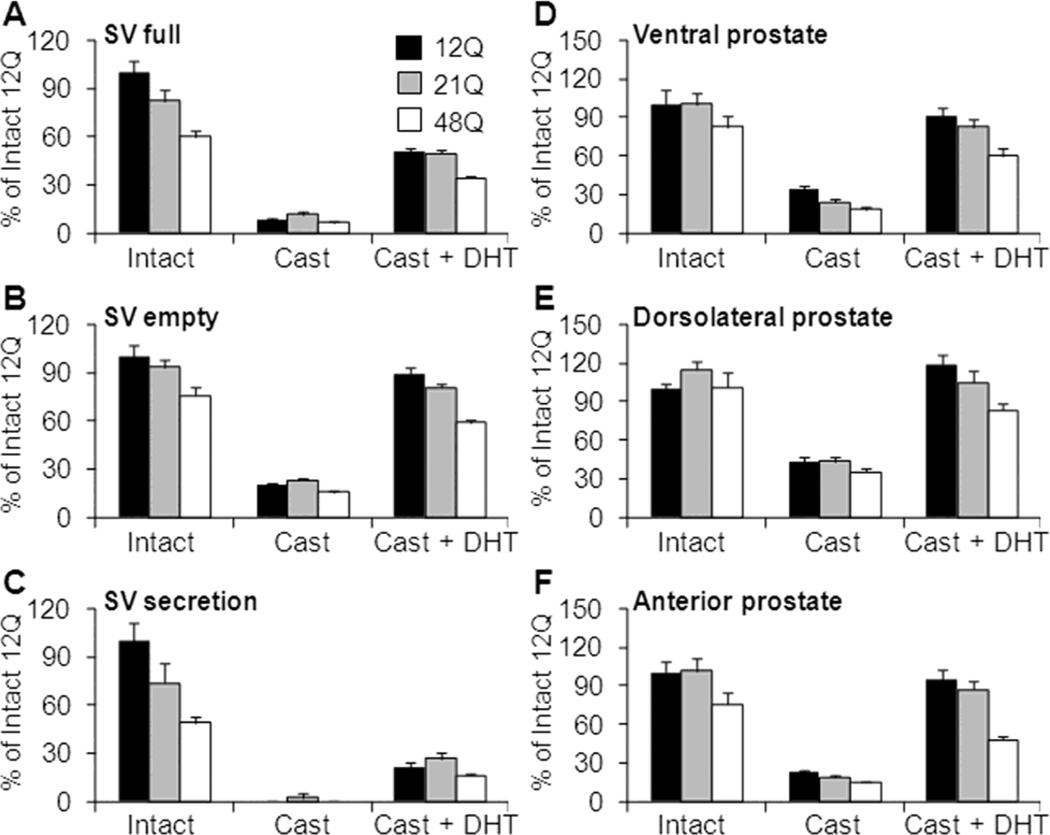
Seminal vesicle (SV) weights in intact males followed the Q-tract length order 12Q>21Q>48Q. The trend was highly significant for full (tissue + secretion; p<0.001) and empty (manually emptied gland; p=0.008) SV weights as well as SV secretions (p=0.001). Therefore the smaller full SV weights in 21Q (82±7% of 12Q) and 48Q (60±6% of 12Q) males were due to AR Q-tract effects on both tissue weight and secretion volume (Fig. 3A–C). In all males, orchidectomy-induced involution reduced the full SV mass by an average 90% of the intact weight and followed the Q-tract order (12Q>21Q>48Q). The tissue weight was reduced to 80% of intact weights and significantly (p=0.001) followed the trend in Q-tract length. The volume of SV secretion was mostly non-detectable following castration. DHT treatment after castration restored SV weights to about 60% of the respective intact weights within the 5 days of treatment. Moreover, the SV weights followed the Q-tract order with a significant trend for full (p<0.001) and empty (p=0.008) glands and a non-significant trend for weight of the secretions (p=0.063).
Figure 3. Seminal vesicle and prostate lobe weights.
Q tract length affects AR trophic function. Full seminal vesicle (SV; A), emptied SV (manually emptied; B) and secretion (full SV – tissue weight; C) weights shown as relative (%) to intact 12Q weights. Relative data is shown for intact, castration (9 days) + DHT (5 days) treatment or castration (14 days) only. The SV weights followed the expected sensitivity difference with 48Q males having smaller androgen dependent sex accessory organs followed by 21Q and 12Q males. Data shown as Mean±SE. N=9–10 for all groups. For reference, the intact levels are shown as Supplemental data in Table 1.
Ventral (VP; D), dorsolateral (DLP; E) and anterior (AP; F) prostate lobe weights in hAR mice with 12Q, 21Q or 48Q-tract lengths, shown relative (%) to intact 12Q weights. Relative data is shown for intact, castration (9 days) + DHT (5 days) treatment or castration (14 days) only. The prostate weights followed the expected sensitivity difference with 48Q males having smaller androgen dependent sex accessory organs followed by 21Q and 12Q males.
The prostate anterior (AP), ventral (VP) and dorsolateral (DLP) lobes were analyzed separately from intact, castrate or castrate + DHT treated hAR male mice. In intact males, there was no significant Q-tract dependent trend for prostate lobe weights, although the VP and AP lobes were smallest in 48Q males, suggestive of their lower androgen responsiveness (Fig. 3D–E). As expected, orchidectomy reduced (<35% of intact), and short-term DHT administration restored (>60% of intact), the weight of all prostate lobes. For both castrate and DHT-treated orchidectomized mice, AR Q-tract length influenced prostate lobe regression and regrowth consistently and significantly (12Q>21Q>48Q; p<0.05). The trend was most prominent following DHT stimulation for all lobes (p≤0.005) with the greatest regrowth achieved for the DLP. Weights in 12Q, 21Q and 48Q mice were 119, 91 and 82% relative to the intact lobe, while the VP weights were 91, 82 and 70%, and AP weights 95, 68 and 63%, relative to intact weight. Similarly, following castration the weights correlated inversely with Q-tract length (12Q>21Q>48Q), with significant trends observed for VP (p=0.004) and AP (p=0.013), and a non-significant (p=0.075) trend in DLP (Fig 3D–E).
3.4 Proliferation and apoptosis reveal lobe-specific AR Q-tract dependence in DHT responses
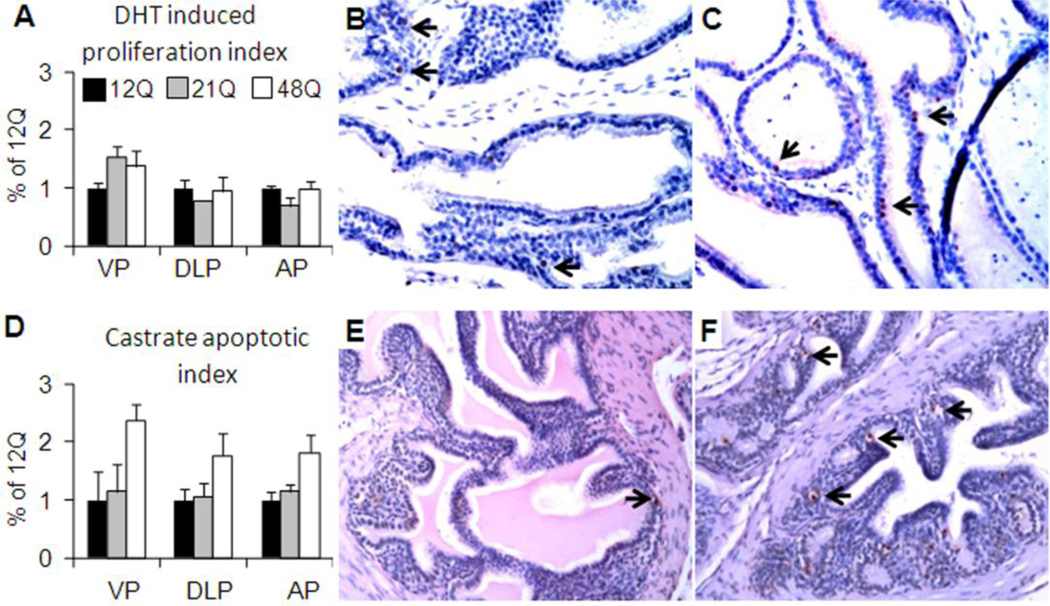
Epithelial proliferation was quantified by stereological determination of the proportion of PCNA immunopositive cells within the prostate epithelia in intact and DHT-treated males (Fig. 4A–C). In intact mice the prostate epithelial proliferation was low (<3% of epithelial cells) in all prostate lobes for all groups and was not detected after castration (not shown). With five days of DHT treatment, the epithelial proliferation increased significantly (p<0.05) above intact level in all lobes. Particularly in the VP, epithelial proliferation was dependent on the Q-tract length and was higher in 48Q and 21Q males compared to 12Q males. In DLP and AP the epithelial proliferation did not differ significantly between the Q-tract lengths (Fig. 4A).
Figure 4. Prostate epithelial cell proliferation and apoptosis.
Proliferating cell nuclear antigen (PCNA) immunostaining was performed to detect proliferative cells (epithelial proliferation index) in VP, DLP and AP and quantified by stereology. A) The DHT-induced proliferation in 12Q, 21Q and 48Q males is shown relative to 12Q. Representative photos of PCNA immunopositivity in VP of 12Q (B) and 48Q (C) males. Examples of positive staining are indicated by arrows.
Tunel in situ staining was performed to detect apoptotic cells (apoptotic index) following castration in VP, DLP and AP, and quantified by stereology. D) The apoptotic index in castrated 12Q, 21Q and 48Q males is shown as relative to 12Q. Representative photos of Tunel positivity in AP of 12Q (E) and 48Q (F) males. Examples of positive staining are indicated by arrows.
Prostate epithelial apoptosis was determined by stereological quantification of Tunel-positive epithelial cells. After castration, epithelial apoptosis was markedly increased above levels in intact mice and depended on Q-tract length, with 48Q significantly (p=0.002) higher than 12Q and 21Q (Fig. 4D–F). Apoptosis was very low (<1%) or undetectable in prostate lobes of all intact and DHT treated males (not shown).
4. Discussion
By use of a DHT clamp, we show that systemic AR activity correlates inversely with Q-tract length and can significantly influence androgen dependent physiological processes. In the humanized AR mice, acute DHT-induced suppression of post-castration serum LH level is greatest in 12Q males and least in 48Q mice. Similarly, the post-castration re-growth of the prostate and seminal vesicles positively correlates with the AR Q-tract length. The experimental paradigm of the DHT clamp eliminates differential hormone metabolism as an explanation for Q tract effects and allows detection of small differences that may be less evident at the molecular level in intact mice (Albertelli et al., 2006).
These findings agree with some epidemiological studies showing AR Q-tract length dependent variation in serum LH and/or testosterone levels (Walsh et al., 2005; Stanworth et al., 2008; Huhtaniemi et al., 2009; Lindstrom et al., 2010). However, although androgen sensitivity in vivo is largely a product of androgen levels, AR expression, and transcriptional activity, it can be significantly modified by other hormones and physiological as well as study limitations that are difficult to assess or control in clinical settings. Thus, there are conflicting results on whether AR Q-tract length affects clinical endpoints (Van Pottelbergh et al., 2001; Zitzmann et al., 2001). Complexity is evident in the present study since a Q-tract length effect is not seen for serum FSH, which is subject to more powerful regulation by inhibin (Hayes et al., 2001a) and estradiol (Hayes et al., 2001b), or for serum LH in intact males (with a closed loop feedback system) or orchidectomized mice (with complete androgen deficiency). However the effects that are seen here are androgen and AR mediated. Interestingly, the AR Q-tract affect appears to influence post-castration LH levels. It may be that the LH promoter is subject to differing intensity of androgenic negative feedback dictated by the Q tract length. Some of the differences from clinical results may be species-specific. For example, serum T in mice varies greatly between individuals and over time, in part due to lack of the dampening effects of circulating SHBG as in men (Bartke and Dalterio, 1975; Coquelin and Desjardins, 1982). Even with more stable serum T in man, large sample sizes are required to demonstrate differences in negative feedback set-point (Krithivas et al., 1999; Crabbe et al., 2007; Huhtaniemi et al., 2009).
Complementary evidence for Q-tract length effects on androgen sensitivity is provided by the effects of DHT on seminal vesicle and prostate weights after orchidectomy. The weights significantly follow the Q-tract length order, with greatest growth in 12Q and least in 48Q mice. These findings are similar to the influence of CAG repeat length on prostate growth in hypogonadal men treated with T (Zitzmann et al., 2003). Prostate epithelial cell renewal is androgen dependent (Cunha et al., 1987; Heinlein and Chang, 2004), but the level of proliferation in prostate lobes five days after androgen replacement does not follow the expected Q-tract length order. However, while androgen action in the prostate is often thought to be only proliferative and anti-apoptotic, recent studies reveal an anti-proliferative role of androgens in promoting epithelial cell differentiation and thereby suppressing proliferation (Simanainen et al., 2007; Wu et al., 2007). Transient rapid induction of proliferation upon androgen replacement followed by a steady low level of epithelial proliferation has been shown previously in rodent prostate (Chen et al., 1996). Therefore, the 12Q AR may be more potent in promoting differentiation following androgen replacement leading to lower epithelial proliferation compared to 21Q and 48Q. However, the effects on tissue weights demonstrate that although the Q-tract effects may be small, sustained subtle differences in AR activity may be cumulative over time. These slight differences in AR activity over a lifetime may impact androgen dependent organs and susceptibility to late-life hormone-dependent diseases.
This study clarifies some previous conflicts in epidemiological studies and suggests that the effects of Q-tract length are genuine but of a magnitude that may be masked by diverse genetic and/or environmental factors. This is especially problematic for observational studies that typically are not randomized or adjusted to control for unknown as well as known risk factors. In the homogeneous genetic background of the hAR mice, Q tract length effects on prostate cancer progression and response to androgen deprivation therapy are more readily detected than in human populations (Albertelli et al., 2008). Prostate tumors arise earlier in 12Q than in 48Q mice, but disease progresses slowly, with a well-differentiated phenotype, especially following castration. Along with data here, this suggests that AR Q-tract length affects may be most significant in situations of low or changing androgen levels, as in prostate cancer treatment or in aging men in general (Kaufman and Vermeulen, 2005).
In conclusion, the humanized AR mouse model offers direct experimental evidence that androgen sensitivity is reduced with increasing Q-tract length, indicated by reduced negative feedback on LH secretion and reduced trophic effects on sex accessory glands. Future epidemiological studies may resolve Q-tract length effects if the groups studied are sufficiently well defined, homogeneous and large. Furthermore, while modest differences in AR Q tract length may not measurably affect most aspects of male health, there may be greater affects in individuals with lengths at the extremes of the normal range or when circulating hormone levels are changing, such as during puberty, aging or treatment with exogenous androgens.
Highlights.
We determine the in vivo influence of AR Q-tract length on androgen sensitivity
We utilize DHT clamp and “humanized” AR mouse model with 12, 21 or 48 CAG repeats
In vivo AR activity correlates inversely with Q-tract length
Q-tract length influences negative hypothalamic feedback
Q-tract length modifies trophic androgen effects on target organs
Supplementary Material
Acknowledgements
We thank Dweepanita Das for assistance with mouse surgery and prostate dissections and Natalie Farrawell for technical help with steroid assays.
Grants or fellowships supporting the research:
Cancer Institute NSW Early Career Fellowship (US).
DOD17-02-1-0099 and NCI-P50 CA69568 (DMR). Additional support came from the University of Michigan Cancer Center Support Grant (5 P30 CA46592).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: Authors have nothing to disclose
References
- Albertelli MA, O'Mahony OA, Brogley M, Tosoian J, Steinkamp M, Daignault S, Wojno K, Robins DM. Glutamine tract length of human androgen receptors affects hormone-dependent and -independent prostate cancer in mice. Hum Mol Genet. 2008;17:98–110. doi: 10.1093/hmg/ddm287. [DOI] [PubMed] [Google Scholar]
- Albertelli MA, Scheller A, Brogley M, Robins DM. Replacing the mouse androgen receptor with human alleles demonstrates glutamine tract length-dependent effects on physiology and tumorigenesis in mice. Mol Endocrinol. 2006;20:1248–1260. doi: 10.1210/me.2006-0021. [DOI] [PubMed] [Google Scholar]
- Allan CM, Couse JF, Simanainen U, Spaliviero J, Jimenez M, Rodriguez K, Korach KS, Handelsman DJ. Estradiol induction of spermatogenesis is mediated via an estrogen receptor-{alpha} mechanism involving neuroendocrine activation of follicle-stimulating hormone secretion. Endocrinology. 2010;151:2800–2810. doi: 10.1210/en.2009-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Dalterio S. Evidence for episodic secretion of testosterone in laboratory mice. Steroids. 1975;26:749–756. doi: 10.1016/0039-128x(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Beilin J, Ball EM, Favaloro JM, Zajac JD. Effect of the androgen receptor CAG repeat polymorphism on transcriptional activity: specificity in prostate and non-prostate cell lines. J Mol Endocrinol. 2000;25:85–96. doi: 10.1677/jme.0.0250085. [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Robles AI, Martinez LA, Liu F, Gimenez-Conti IB, Conti CJ. Expression of G1 cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in androgen-induced prostate proliferation in castrated rats. Cell Growth Differ. 1996;7:1571–1578. [PubMed] [Google Scholar]
- Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol. 1982;243:E257–E263. doi: 10.1152/ajpendo.1982.243.3.E257. [DOI] [PubMed] [Google Scholar]
- Crabbe P, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab. 2007;92:3604–3610. doi: 10.1210/jc.2007-0117. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, Wang YZ, Donjacour AA, Kurita T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol. 2004;92:221–236. doi: 10.1016/j.jsbmb.2004.10.017. Epub 2004 Dec 21. [DOI] [PubMed] [Google Scholar]
- Harwood DT, Handelsman DJ. Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta. 2009;409:78–84. doi: 10.1016/j.cca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Hayes FJ, DeCruz S, Seminara SB, Boepple PA, Crowley WF., Jr. Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J Clin Endocrinol Metab. 2001b;86:53–58. doi: 10.1210/jcem.86.1.7101. [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Pitteloud N, DeCruz S, Crowley WF, Jr., Boepple PA. Importance of inhibin B in the regulation of FSH secretion in the human male. J Clin Endocrinol Metab. 2001a;86:5541–5546. doi: 10.1210/jcem.86.11.8031. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Pye SR, Limer KL, Thomson W, O'Neill TW, Platt H, Payne D, John SL, Jiang M, Boonen S, Borghs H, Vanderschueren D, Adams JE, Ward KA, Bartfai G, Casanueva F, Finn JD, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Silman AJ, Wu FC. Increased estrogen rather than decreased androgen action is associated with longer androgen receptor CAG repeats. J Clin Endocrinol Metab. 2009;94:277–284. doi: 10.1210/jc.2008-0848. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Spaliviero JA, Grootenhuis AJ, Verhagen J, Allan CM, Handelsman DJ. Validation of an ultrasensitive and specific immunofluorometric assay for mouse follicle-stimulating hormone. Biol Reprod. 2005;72:78–85. doi: 10.1095/biolreprod.104.033654. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- Krithivas K, Yurgalevitch SM, Mohr BA, Wilcox CJ, Batter SJ, Brown M, Longcope C, McKinlay JB, Kantoff PW. Evidence that the CAG repeat in the androgen receptor gene is associated with the age-related decline in serum androgen levels in men. J Endocrinol. 1999;162:137–142. doi: 10.1677/joe.0.1620137. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Robins DM. The androgen receptor's CAG/glutamine tract in mouse models of neurological disease and cancer. J Alzheimers Dis. 2008;14:247–255. doi: 10.3233/jad-2008-14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom S, Ma J, Altshuler D, Giovannucci E, Riboli E, Albanes D, Allen NE, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Chanock SJ, Dunning AM, Feigelson HS, Gaziano JM, Haiman CA, Hayes RB, Henderson BE, Hunter DJ, Kaaks R, Kolonel LN, Le Marchand L, Martinez C, Overvad K, Siddiq A, Stampfer M, Stattin P, Stram DO, Thun MJ, Trichopoulos D, Tumino R, Virtamo J, Weinstein SJ, Yeager M, Kraft P, Freedman ML. A Large Study of Androgen Receptor Germline Variants and Their Relation to Sex Hormone Levels and Prostate Cancer Risk. Results from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara KM, Harwood DT, Simanainen U, Walters KA, Jimenez M, Handelsman DJ. Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2010 doi: 10.1016/j.jsbmb.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- Rajender S, Singh L, Thangaraj K. Phenotypic heterogeneity of mutations in androgen receptor gene. Asian J Androl. 2007;9:147–179. doi: 10.1111/j.1745-7262.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- Simanainen U, Allan CM, Lim P, McPherson S, Jimenez M, Zajac JD, Davey RA, Handelsman DJ. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology. 2007;148:2264–2272. doi: 10.1210/en.2006-1223. [DOI] [PubMed] [Google Scholar]
- Simanainen U, McNamara K, Gao YR, Handelsman DJ. Androgen sensitivity of prostate epithelium is enhanced by postnatal androgen receptor inactivation. Am J Physiol Endocrinol Metab. 2009;296:E1335–E1343. doi: 10.1152/ajpendo.00017.2009. [DOI] [PubMed] [Google Scholar]
- Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136:5311–5321. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Trost BA. Development of a sensitive enzyme-linked immunosorbent assay for cattle, sheep, rat, and mouse luteinizing hormone. Biol Reprod. 1987;37:595–605. doi: 10.1095/biolreprod37.3.595. [DOI] [PubMed] [Google Scholar]
- Stanworth RD, Kapoor D, Channer KS, Jones TH. Androgen receptor CAG repeat polymorphism is associated with serum testosterone levels, obesity and serum leptin in men with type 2 diabetes. Eur J Endocrinol. 2008;159:739–746. doi: 10.1530/EJE-08-0266. [DOI] [PubMed] [Google Scholar]
- Thomas PS, Jr., Fraley GS, Damian V, Woodke LB, Zapata F, Sopher BL, Plymate SR, La Spada AR. Loss of endogenous androgen receptor protein accelerates motor neuron degeneration and accentuates androgen insensitivity in a mouse model of X-linked spinal and bulbar muscular atrophy. Hum Mol Genet. 2006;15:2225–2238. doi: 10.1093/hmg/ddl148. [DOI] [PubMed] [Google Scholar]
- Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab. 1997;82:3777–3782. doi: 10.1210/jcem.82.11.4385. [DOI] [PubMed] [Google Scholar]
- Van Pottelbergh I, Lumbroso S, Goemaere S, Sultan C, Kaufman JM. Lack of influence of the androgen receptor gene CAG-repeat polymorphism on sex steroid status and bone metabolism in elderly men. Clin Endocrinol (Oxf) 2001;55:659–666. doi: 10.1046/j.1365-2265.2001.01403.x. [DOI] [PubMed] [Google Scholar]
- Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, Ferrell RE, Roth SM. Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J Appl Physiol. 2005;98:132–137. doi: 10.1152/japplphysiol.00537.2004. [DOI] [PubMed] [Google Scholar]
- Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104:12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann M, Brune M, Kornmann B, Gromoll J, von Eckardstein S, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasoreactivity. J Clin Endocrinol Metab. 2001;86:4867–4873. doi: 10.1210/jcem.86.10.7889. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab. 2003;88:2049–2054. doi: 10.1210/jc.2002-021947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.